Abstract
Cancer therapy using oncolytic viruses represents a promising new approach for controlling ovarian cancer. In this study, we have circumvented the limitation of repeated vaccination by employing different virus vectors, Semliki Forest Virus (SFV) and vaccinia virus (VV) for boosting the immune response. We found that infection of tumor-bearing mice with VV followed by infection with SFV or vice versa leads to enhanced antitumor effects against murine ovarian surface epithelial carcinoma (MOSEC) tumors. Furthermore, infection with VV-ovalbumin (OVA) followed by infection with SFV-OVA or vice versa was found to lead to enhanced OVA-specific CD8+ T-cell immune responses. In addition, we found that infection with SFV-OVA followed by infection with VV-OVA leads to enhanced antitumor effects in vivo and enhanced tumor killing in vitro through a combination of viral oncolysis and antigen-specific immunity. The clinical implications of this study are discussed.
Introduction
Ovarian cancer is the sixth most common malignancy in women and the leading cause of death from all gynecological cancers in the United States.1,2 Although significant advancement has occurred in both surgical and chemotherapeutic techniques, the overall 5-year survival rate for all stages remains <50% (refs. 2,3,4). Current therapies such as surgery, chemotherapy, and radiotherapy usually fail to control advanced stages of the disease. Therefore, alternative therapeutic approaches may serve as an important method to control these advanced stage ovarian tumors.
Cancer therapy using oncolytic viruses represents a promising new approach for controlling tumors (for review see refs. 5,6). Oncolytic viruses such as measles,7,8 vaccinia virus (VV),9,10,11,12 and Sindbis viruses13,14 have been employed for virotherapy and shown to target and lyse tumor cells directly. The viruses are also capable of spreading to adjacent tumor cells, thereby eradicating the target cells without seriously harming the normal tissues. In addition, as the cellular pathways by which these viruses lyse the cells are highly complex, the emergence of virus-resistant tumor cells is unlikely. Therefore, the selective targeting and replication of these viruses offer a potentially safe and effective alternative for combating cancers, such as ovarian cancer.
Recently, it has been demonstrated that the VV can infect and kill both human and murine ovarian surface epithelial carcinoma (MOSEC) in vitro and, when injected intraperitoneally (i.p.) into mice, the virus preferentially infects tumor cells but not normal tissue.15 This work was done with the same strain used in this study.15 The use of the oncolytic VV is a potentially effective strategy for controlling ovarian cancer. The ability of VV to preferentially infect ovarian cancer cells in vivo creates the opportunity to incorporate genes that are capable of generating potent tumor-specific immunity to further enhance the therapeutic effects of VV.
Oncolytic therapy using recombinant VVs most likely requires repeated treatment, as the virus may infect only a portion of tumor cells in vivo. However, the presence of neutralizing antibodies specific to the VV vector would prohibit the booster effect of the recombinant VV vector and prevent successful vaccination with VV.16 Neutralizing antibodies have also been shown to be critically inhibitory with other viral vectors such as herpes simplex virus, which is much more sensitive to innate immune–mediated clearance, and antibody clearance, than VV.17,18 As the VV has been used for the eradication of smallpox, a significant population has previously been immunized with VV. These individuals may have a preexisting immunity against the VV and may not be suitable candidates for the treatment with the same kind of VV vector. Therefore, it is useful to find conditions for repeated vaccination and crucial to identify strategies that are able to circumvent this limitation.
In this study, we have circumvented the limitation of repeated vaccination by employing a different virus vector, Semliki Forest Virus (SFV) to generate antitumor immune responses. We found that infection of tumor-bearing mice with VV followed by infection with SFV or vice versa leads to enhanced antitumor effects against MOSEC tumors. Furthermore, infection with VV-OVA followed by infection with SFV-OVA or vice versa was found to lead to enhanced OVA-specific CD8+ T-cell immune responses. In addition, we found that infection with SFV-OVA followed by infection with VV-OVA leads to enhanced antitumor effects in vivo and enhanced tumor killing in vitro through a combination of viral oncolysis and antigen-specific immunity.
Results
Infection of tumor-bearing mice with one oncolytic virus prevent reinfection with the same virus
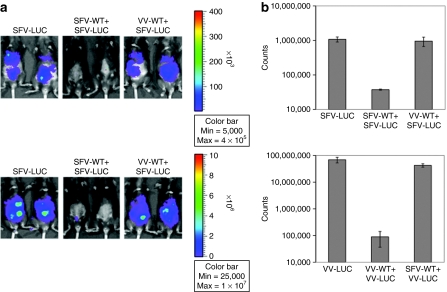
It has been shown that infection with oncolytic virus leads to induction of neutralizing antibodies. In order to determine whether mice infected with one oncolytic virus can be reinfected with the same virus, C57BL/6 mice were injected i.p. with MOSEC cells on day 0. Mice were then infected with SFV-wild-type (WT) or VV-WT on day 3 followed by SFV-luc or VV-luc on day 17. The virus infection was characterized by luciferase expression using luminescence imaging on day 19. As shown in Figure 1a,b, mice reinfected with the same virus demonstrated a significant decrease in infection as depicted by the decrease in luminescence intensity as compared to mice infected with a different virus (P < 0.05). We have previously shown that control imaging of tumor-free mice infected with VV or SFV demonstrated background infection levels.14,19 Thus, our data indicate that infection of tumor-bearing mice with one oncolytic virus prevents reinfection with the same virus.
Figure 1.
In vivo luminescence imaging to demonstrate the luciferase expression in tumor-bearing mice infected with SFV or VV. C57BL/6 mice were injected intraperitoneally with 5 × 105/mouse of MOSEC cells on day 0. Mice were then infected with 2 × 106/mouse of SFV-WT or VV-WT on day 3, and the same dose of SFV-luc or VV-luc on day 17. The virus infection was characterized by luciferase expression using luminescence imaging on day 19. (a) Representative luminescence images depicting the fluorescence intensity in tumor-bearing mice infected with SFV/VV. (b) Bar graph depicting the fluorescence intensity in tumor-bearing mice infected with SFV/VV. MOSEC, murine ovarian surface epithelial carcinoma; SFV, Semliki Forest Virus; VV, vaccinia virus; WT, wild type.
Infection with SFV followed by infection with VV leads to enhanced antitumor effects against MOSEC tumors compared to repeated infection with the same virus
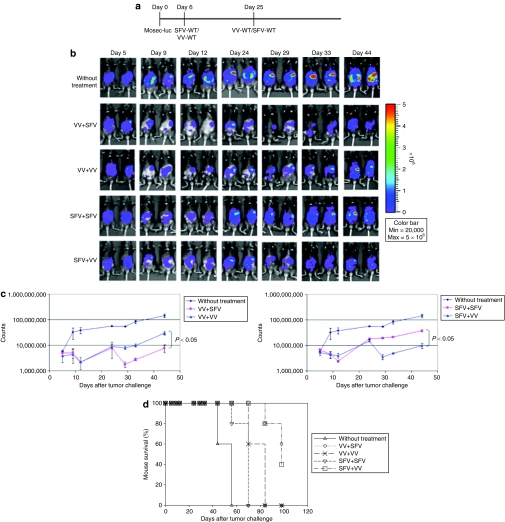
In order to determine whether infection with one virus followed by infection with a different virus would lead to enhanced antitumor effects in tumor-bearing mice, C57BL/6 mice were injected i.p. with luciferase-expressing MOSEC tumor cells on day 0. Mice were then infected with SFV-WT or VV-WT followed by infection with SFV-WT or VV-WT as depicted in Figure 2a. The antitumor effects were characterized by luciferase expression using luminescence imaging. As shown in Figure 2b,c, mice infected with VV followed by SFV or vice versa demonstrated a significant decrease in tumor load depicted by the decrease in luminescence intensity as compared to mice reinfected with the same virus (P < 0.05). Furthermore, mice infected with VV followed by SFV or vice versa demonstrated prolonged survival compared to mice reinfected with the same virus (Figure 2d). Thus, our data indicate that infection with one oncolytic virus followed by infection with a different virus leads to enhanced antitumor effects against MOSEC tumors.
Figure 2.
In vivo luminescence imaging to demonstrate the antitumor effects generated by infection with SFV or VV in tumor-bearing mice. C57BL/6 mice were injected intraperitoneally with 5 × 105/mouse of MOSEC-luciferase cells on day 0. Mice were then infected with 2 × 106/mouse of SFV-WT or VV-WT on day 6, and the same dose of SFV-WT or VV-WT on day 25. The antitumor effects were characterized by luciferase expression using luminescence imaging. (a) Schematic diagram demonstrating the regimen of infection with SFV or VV. (b) Representative luminescence images depicting the antitumor effects in tumor-bearing mice infected with SFV/VV. (c) Line graphs depicting the fluorescence intensity in tumor-bearing mice infected with SFV/VV. (d) Kaplan–Meier survival analysis of MOSEC-luc tumor-bearing mice infected with SFV/VV. MOSEC, murine ovarian surface epithelial carcinoma; SFV, Semliki Forest Virus; VV, vaccinia virus; WT, wild type.
Infection with SFV-OVA followed by infection with VV-OVA or vice versa leads to enhanced OVA-specific CD8+ T-cell immune responses compared to treatment with WT-SFV and WT-OVA
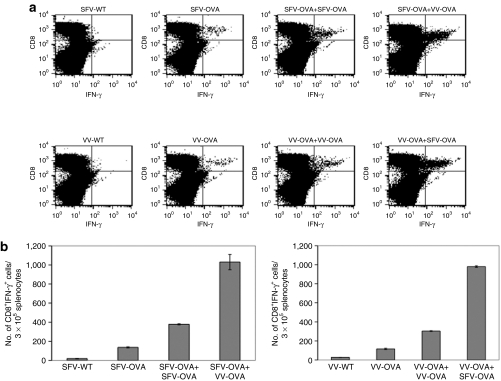
In order to determine whether infection with one virus expressing a specific antigen followed by infection with a different virus expressing the same antigen would lead to enhanced antigen-specific immune responses in tumor-bearing mice, C57BL/6 mice were i.p. injected with MOSEC cells on day 0. Mice were then infected with SFV-OVA or VV-OVA on day 3, followed by the same dose of SFV-OVA or VV-OVA on day 17. The control group was injected with a single dose of SFV-WT, VV-WT, SFV-OVA, or VV-OVA on day 3. The spleens were harvested on day 24 and the OVA-specific T-cell immune responses were characterized using intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 3, mice infected with VV-OVA followed by SFV-OVA or vice versa demonstrated a significant enhancement in the OVA-specific CD8+ T-cell immune responses as compared to mice reinfected with the same virus (P < 0.05). Thus, our data indicate that infection with a virus expressing a particular antigen followed by infection with another virus expressing the same antigen leads to enhanced antigen-specific CD8+ T-cell immune responses.
Figure 3.
Infection with SFV-OVA followed by VV-OVA or vice versa leads to enhanced OVA-specific CD8+ T-cell immune responses. C57BL/6 mice were intraperitoneally injected with 5 × 105/mouse of MOSEC cells on day 0. Mice were then infected with 2 × 106 /mouse of SFV-OVA or VV-OVA on day 3, and the same dose of SFV-OVA or VV-OVA on day 17. The control group was injected with a single dose of SFV-WT, VV-WT, SFV-OVA, or VV-OVA on day 3. The spleens were harvested on day 24, the splenocytes were restimulated with OVA peptide SIINFEKL and the OVA-specific T-cell immune responses were characterized using intracellular cytokine staining followed by flow cytometry analysis. (a) Representative flow cytometry data depicting the number of OVA-specific CD8+ T-cells in the various groups. (b) Bar graph representing the number of OVA-specific CD8+ T-cells/3 × 105 splenocytes (mean ± SE). Data shown are representative of two experiments performed. IFN-γ, interferon-γ MOSEC, murine ovarian surface epithelial carcinoma; OVA, ovalbumin; SFV, Semliki Forest Virus; VV, vaccinia virus; WT, wild type.
Infection with SFV-OVA followed by infection with VV-OVA leads to further enhanced antitumor effects compared to infection with SFV-WT followed by infection with VV-WT
To further increase the antitumor effects of oncolytic viruses, we used viruses encoding a foreign antigen, OVA. We expect that when tumor cells are infected with SFV-OVA they will present OVA antigen on the cell surface. The subsequent infection with VV-OVA will induce a potent OVA-specific T-cell immune response, which will result in tumor cell killing. Thus, we expect that the combination of viral oncolysis and antigen-specific immunity will potentially generate further increased antitumor effects.
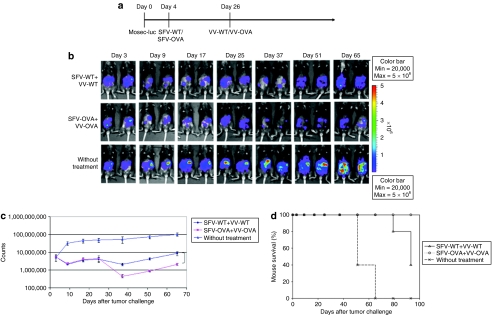
In order to determine whether infection with SFV-OVA followed by infection with VV-OVA can lead to further enhanced antitumor effects, C57BL/6 mice were injected intraperitoneally with MOSEC-luciferase cells on day 0 and then infected with SFV-WT or SFV-OVA on day 4, followed by the same dose of VV-WT or VV-OVA on day 26. The treatment regimen is depicted in Figure 4a. The antitumor effects were then characterized over time by luminescence imaging. As shown in Figure 4b,c, mice infected with SFV-OVA followed by VV-OVA demonstrated a significant decrease in tumor load compared to mice infected with SFV-WT followed by VV-WT (P < 0.05). The median survival of the mice infected with SFV-WT followed by VV-WT was 93 days, whereas the mice infected with SFV-OVA followed by VV-OVA were all alive at the end of month 4. Furthermore, mice infected with SFV-OVA followed by VV-OVA demonstrated prolonged survival compared to mice infected with SFV-WT followed by VV-WT (P = 0.0026) (Figure 4d). Thus, our data indicate that infection with an oncolytic virus carrying a foreign antigen followed by infection with a different oncolytic virus carrying the same foreign antigen leads to enhanced antitumor effects compared to infection with WT viruses, presumably through a combination of viral oncolysis and antigen-specific immunity.
Figure 4.
Infection with SFV-OVA followed by infection with VV-OVA leads to further enhanced antitumor effects. C57BL/6 mice were injected intraperitoneally with 5 × 105/mouse of MOSEC-luciferase cells on day 0. Mice were then infected with 2 × 106/mouse of SFV-WT or SFV-OVA on day 4, and the same dose of VV-WT or VV-OVA on day 26. The antitumor effects were characterized by luminescence imaging. (a) Schematic diagram demonstrating the regimen of infection with SFV or VV. (b) Representative luminescence images depicting the fluorescence intensity in tumor-bearing mice infected with SFV-OVA and VV-OVA compared to SFV-WT and VV-WT. (c) Line graph depicting the fluorescence intensity in tumor-bearing mice infected with SFV-OVA and VV-OVA compared to SFV-WT and VV-WT. (d) Kaplan–Meier survival analysis of MOSEC-luc tumor-bearing mice infected with SFV-OVA and VV-OVA compared to SFV-WT and VV-WT (P = 0.0026). MOSEC, murine ovarian surface epithelial carcinoma; OVA, ovalbumin; SFV, Semliki Forest Virus; VV, vaccinia virus; WT, wild type.
Infection with SFV-OVA followed by infection with VV-OVA leads to enhanced tumor killing by a combination of viral oncolysis and OVA-specific CD8+ T-cells
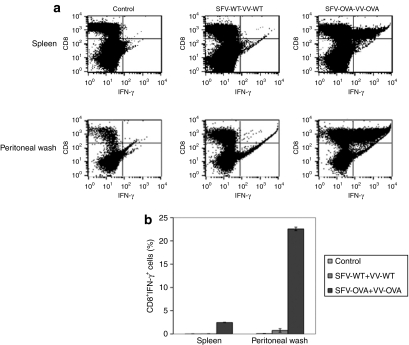
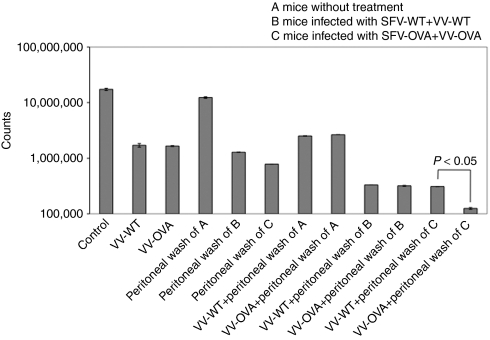
In order to determine whether infection with SFV-OVA followed by VV-OVA would lead to accumulation of OVA-specific T-cells in the spleens and peritoneal cavity of tumor-bearing mice, C57BL/6 mice were i.p. injected with MOSEC cells on day 0. Mice were then infected with SFV-WT or SFV-OVA on day 3, followed by the same dose of VV-WT or VV-OVA on day 13. The cells from the spleens and peritoneal wash were harvested on day 19 and the OVA-specific T-cell immune responses were characterized using intracellular cytokine staining followed by flow cytometry analysis. As shown in Figure 5, mice infected with SFV-OVA followed by VV-OVA demonstrated a significant enhancement in the OVA-specific CD8+ T-cell in the peritoneal wash as well as the spleens, suggesting accumulation of antigen-specific T-cells around the tumor site. We incubated the peritoneal wash from infected mice with MOSEC-luciferase cells which were infected with VV-WT or VV-OVA and MOSEC-luc tumor cell lysis was examined by luminescence imaging. As shown in Figure 6, cells infected with VV-OVA and incubated with the peritoneal wash of mice treated with SFV-OVA+VV-OVA demonstrated the highest level of MOSEC-luc tumor cell lysis compared to cells incubated with the peritoneal wash of mice treated with SFV-WT+VV-WT or cells infected with VV-WT (P < 0.05). This indicates that the MOSEC-luc tumor killing was due to direct oncolysis of VV-OVA as well as OVA-specific T-cell-mediated killing from the cells in the peritoneal wash. Taken together, our data indicate that infection with SFV-OVA followed by infection with VV-OVA leads to enhanced tumor killing by a combination of viral oncolysis and OVA-specific CD8+ T-cells.
Figure 5.
Infection with SFV-OVA followed by VV-OVA leads to enhanced tumor killing by a combination of viral oncolysis and OVA-specific CD8+ T-cells. C57BL/6 mice were intraperitoneally injected with 2 × 106/mouse of MOSEC-luciferase cells on day 0. Mice were then infected with 2 × 106 SFV-WT or SFV-OVA on day 3, and the same dose of VV-WT or VV-OVA on day 13. On day 19, mice were killed, and the peritoneal wash and splenocytes were harvested and the OVA-specific T-cell immune responses were characterized using intracellular cytokine staining followed by flow cytometry analysis. (a) Representative flow cytometry data depicting the number of OVA-specific CD8+ T-cells in the spleens and in the peritoneal wash of infected mice. (b) Bar graph representing the number of OVA-specific CD8+ T-cells/3 × 105 splenocytes (mean ± SE). Data shown are representative of two experiments performed. IFN-γ, interferon-γ MOSEC, murine ovarian surface epithelial carcinoma; OVA, ovalbumin; SFV, Semliki Forest Virus; VV, vaccinia virus; WT, wild type.
Figure 6.
In vitro luminescence imaging to demonstrate the cell lysis of MOSEC-luc tumor cells infected with VV-WT or VV-OVA incubated with cells from peritoneal wash of infected mice. MOSEC-luciferase cells were seeded in 24-well plate at 5 × 105 cells/well. A volume of 2 × 106 VV-WT or VV-OVA was added to each well. After 24 hours, the cells were washed with PBS and incubated with 1/8 of the peritoneal wash from the infected mice described in Figure 5 in each well. After another 24 hours, luciferin was added to each well, and the cell lysis was determined by luminescence imaging. Representative bar graph depicting the luciferase expression of MOSEC-luc cells infected with VV-WT or VV-OVA incubated with cells from peritoneal wash of infected mice. MOSEC, murine ovarian surface epithelial carcinoma; OVA, ovalbumin; PBS, phosphate-buffered saline; SFV, Semliki Forest Virus; VV, vaccinia virus; WT, wild type.
Discussion
In this study, we have circumvented the limitation of repeated treatment of ovarian cancer with the same viral vector by employing a different ovarian-tropic viral vector for boosting the therapeutic effects generated by virotherapy. We found that infection of tumor-bearing mice with VV-WT followed by infection with SFV-WT or vice versa leads to enhanced antitumor effects against MOSEC tumors. Furthermore, infection with SFV encoding a foreign antigen (SFV-OVA) followed by infection with VV encoding the same foreign antigen (VV-OVA) was found to lead to further enhancement in the therapeutic effect generated by virotherapy using WT viral vectors. This enhancement in antitumor effects was due to a combination of viral oncolysis and antigen-specific immunity. Thus, the combination of the two mechanisms of tumor killing leads to a significantly improved survival in the treated mice.
In this study, we have observed that treatment with an oncolytic viral vector followed by infection with another oncolytic viral vector leads to enhanced antitumor effects (see Figures 2 and 4). The observed effect is most likely due to the fact that additional treatment with a different viral vector would not be inhibited by neutralizing antibodies generated by the first treatment with oncolytic virus. This is supported by our data (see Figure 1) as well as other studies.16,20 It has been shown that the presence of neutralizing antibodies specific to a particular viral vector would compromise the subsequent infection with the same viral vector, thus reducing the efficacy of the treatment.16 However, we cannot exclude the possibility that factors other than neutralizing antibodies, such as T-cell-mediated immunity may contribute to the reduced therapeutic antitumor effect on subsequent infection with the same oncolytic virus.
In this study, we have employed ovalbumin antigen as a model antigen to be encoded by the viral vector for our approach. Ovalbumin is a highly immunogenic foreign antigen and can therefore bypass the immune tolerance. A similar approach can be used by using viral vectors encoding other potent foreign antigens that can trigger a potent immune response without having issue of immune tolerance. So far, only a limited number of ovarian tumor antigens have been reported with many having potential issues of immune tolerance. The employment of ovarian-tropic viral vectors encoding a potent immunogenic foreign antigen can potentially provide the tumor cell with a clear tumor antigen without concerns for immune tolerance. The destruction of the tumor cell by the combination of virotherapy and immunotherapy may potentially lead to antigenic epitope spreading, resulting in the control of other ovarian tumor cells not infected by the virus. Ovarian tumor treatment using the principle of antigenic epitope spreading21,22,23,24 from ovarian tumors caused by lysis of a portion of ovarian tumor cells may prove more effective and broadly applicable for the generation of ovarian tumor–specific immunity.
We expect that tumor-tropic virotherapy using viral vectors encoding a foreign antigen may also generate inflammation in the tumor microenvironment and activate antigen-presenting cells in the presence of ovarian tumor–associated antigens. The ovarian tumor cell death caused by oncolytic virotherapy as well as the antigen-specific CD8+ T-cell-mediated killing would release ovarian tumor antigens, which will be processed and presented by dendritic cells to T cells, resulting in de novo activation of ovarian tumor–specific immunity. This would lead to therapeutic antitumor effects even in ovarian tumor cells not infected by oncolytic viruses.
In summary, our study demonstrates that infection with a viral vector encoding a foreign antigen followed by infection with another viral vector encoding the same antigen leads to enhanced antitumor effects in vivo and enhanced tumor killing in vitro through a combination of viral oncolysis and antigen-specific immunity. Our study may serve as an important foundation for future clinical translation.
Materials and Methods
Mice. Female C57BL6 mice, 5–8 weeks of age, were purchased from National Cancer Institute, and were used in compliance with institutional animal health-care regulations. All animal experimental procedures were approved by the Johns Hopkins Institutional Animal Care and Committee.
VV and SFV viruses. The VV-WT, and VV expressing luciferase (VV-luc), or full-length chicken OVA (VV-OVA) were prepared as described previously. The SFV-WT and SFV expressing luciferase (SFV-luc) or OVA (SFV-OVA) were generated following Gibco instruction manual (Gibco, Carlsbad, CA) for SFV gene expression system and previously described protocol.
BHK21 cells were cultured in IMDM (Gibco) supplemented with 5% fetal bovine serum. Two micrograms of pSCAβ, pSCAβ-luciferase, or pSCAβ-OVA together with 1.2 µg of pSCA Helper were transfected into a 10-cm plate of BHK21 cells by lipofectamine transfection (Invitrogen, Carlsbad, CA). Medium was changed after 6 hours, and the supernatant was collected and aliquoted after 36 hours. To activate SFV virus before infection, α-chymotrypsin (Sigma, St Louis, MO) with a final concentration of 0.5 µg/µl was incubated with the virus at room temperature for 45 minutes.
Cell lines. We employed a transplantable murine ovarian surface epithelial carcinoma (MOSEC) developed by Roby et al.,25 which is syngeneic to immunocompetent C57BL/6 mice. The MOSEC and MOSEC cells expressing luciferase were grown in RPMI medium (Gibco) supplemented with 10% fetal bovine serum, 1× nonessential amino acid, L-glutamine, sodium pyruvate, and penicillin–streptomycin, and 0.1% β-mercaptoethanol.
Characterization of antitumor effects of SFV and VV. MOSEC-luciferase cells were washed and resuspended in Hanks balanced salt solution buffer, and i.p. injected into mice at a dose of 5 × 105 tumor cells/ mouse. A volume of 2 × 106 of the WT, or OVA-expressing SFV or VV was i.p. injected, and the luciferase level was examined on the days indicated. The mice were i.p. injected with 200 µl of 15 µg/µl luciferin (Promega, Madison, WI), after 10 minutes, luminescence imaging was conducted on a cryogenically cooled IVIS system (Xenogen/Caliper Life Sciences, Alameda, CA), and an acquisition time of 1 minute was used. The signal intensity was analyzed by Living Image software 2.5 (Xenogen/Caliper Life Sciences).
Intracellular cytokine staining and flow cytometry analysis. For characterization of OVA-specific CD8+ T-cells, splenocytes were harvested 7 days after the second injection of SFV-OVA or VV-OVA. A volume of 5 × 106 splenocytes were incubated with 1 µg/ml H-2Kb-restricted OVA peptide (SIINFEKL) and 1 µl/ml GolgiPlug (BD Cytofix/Cytoperm Kit; BD Biosciences, San Jose, CA) overnight. Cells were then harvested, stained for CD8 and interferon-γ following standard protocol, and analyzed on a FACSCalibur flow cytometer, using CellQuest software (Becton Dickinson, San Jose, CA). The analyses were carried out with gated lymphocyte populations.
Peritoneal wash and cell lysis assay. Peritoneal wash was performed by i.p. injection of mouse with 8 ml phosphate-buffered saline, and draining through 18G needle. Cells were collected by centrifugation at 1,600 rpm for 5 minutes, and 1/8 of the cells were used to examine OVA-specific CD8+ T-cells.
For cell lysis assay, MOSEC-luciferase cells were seeded in 24-well plate at a concentration of 5 × 105 cells/ well. A volume of 2 × 106 VV-WT or VV-OVA was added to each well; 24 hours later, the cells were washed with phosphate-buffered saline and incubated with 1/8 of the peritoneal wash each well. After another 24 hours, 20 µl of 15 µg/µl luciferin was added to each well, and the cell killing effect was determined by luminescence imaging with an acquisition time of 1 minute.
Statistical analysis. All data expressed as means ± SD are representative of at least two independent experiments. Comparisons between individual data points were made using Student's t-test.
Acknowledgments
This work was supported by the NCDGG (1U19 CA113341-01), the American Cancer Society (ACS), National Cancer Institute SPORE in Cervical Cancer P50 CA098252, and the 1 RO1 CA114425-01.
REFERENCES
- Greenlee RT, Murray T, Bolden S., and , Wingo PA. Cancer statistics, 2000. CA Cancer J Clin. 2000;50:7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Baum M, Ebb S., and , Brooks M. Biological fall out from trials of adjuvant tamoxifen in early ovarian cancer. In: Salmon SE (ed.). Adjuvant therapy of cancer V1. Saunders: Philadelphia, pp 269–274; 1990. [Google Scholar]
- Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. doi: 10.1007/978-1-4757-3587-1_4. [DOI] [PubMed] [Google Scholar]
- Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA., and , Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–1588. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestwich RJ, Harrington KJ, Vile RG., and , Melcher AA. Immunotherapeutic potential of oncolytic virotherapy. Lancet Oncol. 2008;9:610–612. doi: 10.1016/S1470-2045(08)70163-3. [DOI] [PubMed] [Google Scholar]
- Russell SJ., and , Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–241. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC., and , Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer Res. 2002;62:4656–4662. [PubMed] [Google Scholar]
- Lin CT, Hung CF, Juang J, He L, Lin KY, Kim TW, et al. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies and antitumor effects of HPV-16 E7-expressing Sindbis virus replicon particles. Mol Ther. 2003;8:559–566. doi: 10.1016/s1525-0016(03)00238-7. [DOI] [PubMed] [Google Scholar]
- Chen CH, Wang TL, Hung CF, Pardoll DM., and , Wu TC. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines. Vaccine. 2000;18:2015–2022. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]
- Chen CH, Wang TL, Ji H, Hung CF, Pardoll DM, Cheng WF, et al. Recombinant DNA vaccines protect against tumors that are resistant to recombinant vaccinia vaccines containing the same gene. Gene Ther. 2001;8:128–138. doi: 10.1038/sj.gt.3301370. [DOI] [PubMed] [Google Scholar]
- Gilbert PA., and , McFadden G. Poxvirus cancer therapy. Recent Pat Antiinfect Drug Discov. 2006;1:309–321. doi: 10.2174/157489106778777592. [DOI] [PubMed] [Google Scholar]
- Unno Y, Shino Y, Kondo F, Igarashi N, Wang G, Shimura R, et al. Oncolytic viral therapy for cervical and ovarian cancer cells by Sindbis virus AR339 strain. Clin Cancer Res. 2005;11:4553–4560. doi: 10.1158/1078-0432.CCR-04-2610. [DOI] [PubMed] [Google Scholar]
- Tseng JC, Hurtado A, Yee H, Levin B, Boivin C, Benet M, et al. Using sindbis viral vectors for specific detection and suppression of advanced ovarian cancer in animal models. Cancer Res. 2004;64:6684–6692. doi: 10.1158/0008-5472.CAN-04-1924. [DOI] [PubMed] [Google Scholar]
- Hung CF, Tsai YC, He L, Coukos G, Fodor I, Qin L, et al. Vaccinia virus preferentially infects and controls human and murine ovarian tumors in mice. Gene Ther. 2007;14:20–29. doi: 10.1038/sj.gt.3302840. [DOI] [PubMed] [Google Scholar]
- Kirn DH, Wang Y, Liang W, Contag CH., and , Thorne SH. Enhancing poxvirus oncolytic effects through increased spread and immune evasion. Cancer Res. 2008;68:2071–2075. doi: 10.1158/0008-5472.CAN-07-6515. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- Wakimoto H, Johnson PR, Knipe DM., and , Chiocca EA. Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells. Gene Ther. 2003;10:983–990. doi: 10.1038/sj.gt.3302038. [DOI] [PubMed] [Google Scholar]
- Chang CL, Ma B, Pang X, Wu TC., and , Hung CF. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Mol Ther. 2009;17:1365–1372. doi: 10.1038/mt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast WM. VEEV replicon-based vaccines used in heterologous prime boost strategies induce lifelong protection against cancer and therapy of cervical cancer in mice and robust cell-mediated immunity in rhesus macaques. In: Vaccine Technology II P09, 1–6 June 2008, Algarve, Portugal.; 2008. [Google Scholar]
- Nava-Parada P, Forni G, Knutson KL, Pease LR., and , Celis E. Peptide vaccine given with a Toll-like receptor agonist is effective for the treatment and prevention of spontaneous breast tumors. Cancer Res. 2007;67:1326–1334. doi: 10.1158/0008-5472.CAN-06-3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa H, Sato E, Briones G, Chen LM, Matsuo M, Nagata Y, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilon SA, Kelly C., and , Wei WZ. Broadening of epitope recognition during immune rejection of ErbB-2-positive tumor prevents growth of ErbB-2-negative tumor. J Immunol. 2003;170:1202–1208. doi: 10.4049/jimmunol.170.3.1202. [DOI] [PubMed] [Google Scholar]
- Wierecky J, Müller MR, Wirths S, Halder-Oehler E, Dörfel D, Schmidt SM, et al. Immunologic and clinical responses after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res. 2006;66:5910–5918. doi: 10.1158/0008-5472.CAN-05-3905. [DOI] [PubMed] [Google Scholar]
- Roby KF, Taylor CC, Sweetwood JP, Cheng Y, Pace JL, Tawfik O, et al. Development of a syngeneic mouse model for events related to ovarian cancer. Carcinogenesis. 2000;21:585–591. doi: 10.1093/carcin/21.4.585. [DOI] [PubMed] [Google Scholar]