Abstract
Transposons are promising systems for somatic gene integration because they can not only integrate exogenous genes efficiently, but also be delivered to a variety of organs using a range of transfection methods. piggyBac (PB) transposon has a high transposability in mammalian cells in vitro, and has been used for genetic and preclinical studies. However, the transposability of PB in mammalian somatic cells in vivo has not been demonstrated yet. Here, we demonstrated PB-mediated sustained gene expression in adult mice. We constructed PB-based plasmid DNA (pDNA) containing reporter [firefly and Gaussia luciferase (Gluc)] genes. Mice were transfected by injection of these pDNAs using a hydrodynamics-based procedure, and the conditions for high-level sustained gene expression were examined. Consequently, gene expressions were sustained over 2 months. Our results suggest that PB is useful for organ-selective somatic integration and sustained gene expression in mammals, and will contribute to basic genetic studies and gene therapies.
Introduction
Nonviral vectors for gene transfer are promising tools for genetic studies and therapies because of their high productivity and high safety.1,2 Because conventional plasmid DNA (pDNA)-based nonviral vectors have no tendency for chromosomal integration, gene expression from these vectors is transient. However, some diseases such as hereditary or chronic diseases need sustained therapeutic gene expression.
One of the approaches to overcome this limitation is utilization of transposons.3 Transposons are mobile genetic elements that transpose between or within vectors, and chromosomes. In this transposition, transposase recognizes transposon-specific inverted terminal repeat sequences (IRs) located on both ends of the transposons, and removed from their original sites and integrated into other sites. Because of this feature, transposons containing genes of interest between their two IRs are able to carry the genes from vectors to chromosomes.
The transposability of a few transposons has been demonstrated in mammalian cells. After molecular reconstruction of Sleeping Beauty (SB) transposon,4 SB has been widely used for mammalian genetic5,6 and preclinical studies7 because of its high transposability in mammalian cells. Recently, piggyBac (PB), a transposon derived from cabbage looper moth Trichoplusia ni,8 was shown to transpose more efficiently than other transposons including Tol2 (refs. 9,10), passport,10 and two hyperactive versions of SB9,10,11 in mammalian cells. In addition, PB can integrate up to 9.1 kilobases (kb) of foreign sequence without significant reduction in transposition efficiency,12 whereas the transposition efficiency of SB is reduced in a size-dependent manner (about 50% when the size of transposon reaches 6 kb).13 Because of its high cargo capacity and high transposition efficiency in mammalian cells, PB is regarded as a promising tool for basic genetic studies and gene therapies. PB has been used for chromosomal integration in mammalian germ lines,12 embryonic stem cells,14 and tumor xenograft.15 In addition, PB has also been used for induction of pluripotency.16,17,18 However, the transposability of PB in mammalian somatic cells in vivo has not been demonstrated yet. An in vivo transposition investigation of PB is needed for in vivo genetic applications, such as preclinical studies of gene therapies or organ-specific tumor model establishment.19,20
In the present study, we investigated and demonstrated PB-mediated sustained gene expression in adult mice in vivo. At first, we constructed PB-based pDNA containing reporter [firefly and Gaussia luciferase (Gluc)] genes. Mice were transfected by injection of these pDNAs using a hydrodynamics-based procedure, and the conditions for sustained gene expression were optimized. Consequently, gene expressions were sustained over 2 months. Our results suggest that PB is useful for organ-selective somatic integration and sustained gene expression in mammals, and will contribute to basic genetic studies and gene therapies.
Results
Transposition in human hepatocyte-derived cell lines
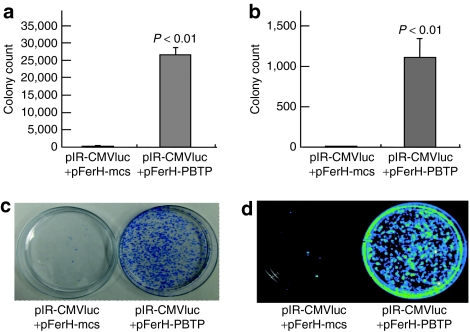
Initially, we created two pDNAs. One contains an expression cassette of PB transposase (pFerH-PBTP), whereas the other contains expression cassettes of firefly luciferase (Fluc) and neomycin-resistance genes flanked with PB IRs and internal sequences necessary for efficient chromosomal integration21 (pIR-CMVluc) (Figure 1). To examine the transposition activity by the constructed pDNAs, we transfected these pDNAs to human hepatocellular liver carcinoma cell lines, HepG2 and Hep3B. We selected these cells because the liver is the major target organ of hydrodynamics-based transfection procedure.22,23 To investigate chromosomal integration and sustained expression of neomycin-resistance gene, transfected cells were incubated in G418-containing medium for 2 weeks. The transposase groups (pIR-CMVluc and pFerH-PBTP) of HepG2 and Hep3B formed 147-fold and 71-fold more colonies than the control groups (pIR-CMVluc and negative control pDNA; pFerH-mcs; Figure 1), respectively (Figure 2a–c). In addition, these colonies showed luciferase luminescence (Figure 2d). These results indicated that both Fluc and neomycin-resistance genes were integrated into chromosomes by the constructed pDNAs in mammalian hepatocyte-derived cells.
Figure 1.
Plasmid DNA construction. BlastR, blasticidin-resistance gene; CMV, cytomegalovirus promoter; EF1α, human elongation factor 1α promoter; EM7, bacterial EM7 promoter; Fluc, firefly luciferase gene; Gluc, Gaussia luciferase gene; hFerH, human ferritin heavy chain promoter; HGF, human hepatocyte growth factor gene; NeoR, neomycin-resistance gene; PBIR; piggyBac terminal inverted repeat sequence; PBTP, piggyBac transposase gene; SV40, simian virus 40 promoter; ori, E. coli origin of replication.
Figure 2.
Sustained gene expression in vitro. (a,b) In vitro transposition study. Hep3B (a) and HepG2 (b) cells (2 × 105 cells/well) were transfected with 0.67 µg pIR-CMVluc and 0.33 µg pFerH-mcs (left bar) or pFerH-PBTP (right bar). The number of colonies was counted by methylene blue staining after 2 weeks' selection with G418. Each value represents the mean ± SD (n = 4). (c,d) An image of Hep3B colonies. The colonies were stained with methylene blue (c). 0.3 mmol/l -luciferin was added to the colonies (d). Both images were captured after 2 weeks' selection with G418.
Prolonged firefly luciferase expression in vivo
We next transfected these pDNAs to adult mice by a hydrodynamics-based procedure to determine the transposability of PB in vivo. Because expression by this procedure in liver is much higher (>1,000-fold) than that in other organs,22 we measured Fluc expression in livers. The Fluc activity of the transposase group (pIR-CMVluc and pFerH-PBTP) did not decrease from 5 to 8 days after transfection, whereas that of the control group (pIR-CMVluc and pFerH-mcs) decreased to about 1/4 during the same time period (Figure 3a). PB transposase did not increase expression from conventional pDNA under these experimental conditions both 1 and 8 days after transfection (Figure 3b).
Figure 3.
Sustained Fluc expression in vivo. Expression time course of Fluc from PB-based (a) or conventional (b) pDNA. 25 µg pIR-CMVluc and 1 µg pFerH-PBTP (gray bar) or pFerH-mcs (white bar) were injected (a). 25 µg pCMV-luc and 1 µg pFerH-PBTP (gray bar) or pFerH-mcs (white bar) were injected (b). After pDNA injection, livers were collected at the indicated time points, and Fluc activities were measured. Each value represents mean ± SD (n = 4–8). RLU, relative light unit.
Prolonged secreted protein expression in vivo
For a longer investigation of PB-mediated sustained exogenous gene expression, we next created another pDNA containing the Gluc24 expression cassette flanked with PB IRs and the same internal sequences as pIR-CMVluc (pIR-CMVGluc) (Figure 1). We selected Gluc because it is secreted in blood and enables continuous measurement of the expression level in the same mice,25 and because it can be expressed without being compromised by neutralizing antibodies for at least 3 weeks.26 In addition, because the half-life of Gluc in blood is about 20 minutes,25 Gluc activity in serum correlates well with the real-time expression. In the transposase group (pIR-CMVGluc and pFerH-PBTP), the Gluc expression decreased rapidly until 1 day after transfection, but the rate of decrease became slow, and Gluc expression was still detected at 80 days after transfection. In contrast, in the control group (pIR-CMVGluc and pFerH-mcs), Gluc expression decreased rapidly until 7 days after transfection by which time the Gluc expression had reached background level (Figure 4a). As in the case of Fluc, PB transposase did not affect Gluc expression from conventional pDNA (pCMV-Gluc; Figure 1) (Figure 4b). These results indicated that expression from pDNA containing PB IRs is prolonged over 2 months by PB transposase in mice.
Figure 4.
Sustained Gluc expression in vivo. Expression time course of Gluc from PB-based (a,c) or conventional (b) pDNA. 25 µg pIR-CMVGluc and 1 µg pFerH-PBTP (closed rhombuses), or pFerH-mcs (closed squares) were injected (a). 25 µg pCMV-Gluc and 1 µg pFerH-PBTP (closed rhombuses), or pFerH-mcs (closed squares) were injected (b). 25 µg pIR-EF1Gluc and 1 µg pFerH-PBTP (closed rhombuses), or pFerH-mcs (closed squares) were injected (c). Blood samples were collected at the indicated time points, and Gluc activities in serum were measured. Each value represents mean ± SD (n = 4–6). RLU, relative light unit.
Effect of promoters on gene expression in vivo
Although Gluc expression from pIR-CMVGluc was prolonged when pIR-CMVGluc was co-transfected with pFerH-PBTP, Gluc expression decreased gradually. We assumed that the gradual decrease in Gluc expression resulted from postintegrative gene silencing because the CMV promoter is susceptible to gene silencing.27 A previous study about the postintegrative gene silencing of SB showed that the EF1 promoter was less susceptible to postintegrative gene silencing.28 Therefore, we created a new pDNA (pIR-EF1Gluc; Figure 1) by exchanging the CMV promoter of pIR-CMVGluc for the human EF1 promoter. In the transposase group (pIR-EF1Gluc and pFerH-PBTP), Gluc expression decreased until 10 days after transfection, but no apparent decrease was observed from 10 to 55 days after transfection. In contrast, in the control group (pIR-EF1Gluc and pFerH-mcs), Gluc expression resulted in a near background level at 14 days after transfection (Figure 4c).
Molecular confirmation of transposition
To confirm that chromosomal integration resulted from transposition and not from recombination, we performed plasmid excision assay using PCR. In transposition, PB transposon is excised from donor plasmid before integration. Therefore, if transposition occurred, the shorter version of the donor plasmid should be produced (Figure 5a). The excision-dependent PCR products were detected only in the transposase groups both in Hep3B, HepG2 (Figure 5b), and mouse livers (Figure 5c). These results suggested that chromosomal integration resulted from transposition.
Figure 5.
Molecular confirmations of the excisions of donor plasmids. (a) Schematic diagram of plasmid excision assay. (b) pIR-CMVluc (lane 1), DNA isolated from HepG2 cells transfected with pIR-CMVluc and pFerH-PBTP (lane 3), or pFerH-mcs (lane 4), Hep3B cells transfected with pIR-CMV luc and pFerH-PBTP (lane 5), or pFerH-mcs (lane 6) were used as a template of PCR, respectively. (c) pIR-EF1Gluc (lane 1), DNA isolated from the livers of mouse transfected with pIR-EF1Gluc and pFerH-PBTP (lane 3), or pFerH-mcs (lane 4) were used as a template of PCR, respectively.
For further confirmation of transposition, we examined the sequence of integration sites by plasmid rescue. In accord with previous studies,9,10,11,12 PB was integrated into only TTAA sequences (Table 1).
Table 1. piggyBac integration sites in Hep3B cells.
Effect of the amount of transposase on transposition in vitro
We next investigated the effect of the amount of pFerH-PBTP with regard to the transposition efficiency in vitro. The number of G418-resistant colonies increased in a pFerH-PBTP-dependent manner over the range of 0–250 ng, but a further increase in pFerH-PBTP to 500 ng resulted in a decrease in the number of G418-resistant colonies in both 1 × 105 HepG2 and Hep3B (Figure 6a,b). To examine whether the cytotoxicity of transposase contributed to this decrease, we investigated the cell viability with a constant amount of pIR-CMVluc and an increasing amount of pFerH-PBTP. The cell viability decreased in a pFerH-PBTP-dependent manner (Figure 6c). In addition, we also examined whether the cytotoxicity depends on transposase itself or transposition catalyzed by transposase. When pFerH-PBTP was co-transfected with conventional pDNA (pCMV-luc), the cell viability slightly decreased, but no statistical significance was observed. In contrast, when pFerH-PBTP was co-transfected with pIR-CMVluc, the cell viability decreased with statistical significance. These results suggest that the cytotoxicity may be partially caused by transposase itself, but mainly caused by transposition.
Figure 6.
Effect of the amount of transposase. (a,b) Effect of the amount of transposase versus the transposition in vitro. Hep3B (a) and HepG2 (b) cells (1 × 105 cells/well) were transfected with 100 ng pIR-CMVluc and the indicated amount of pFerH-PBTP, respectively. Total DNA amount was adjusted by adding pFerH-mcs. The number of colonies was counted by methylene blue staining after 2 weeks' selection with G418. (c) Effect of the amount of transposase versus the cell viability in vitro. Hep3B cells (1 × 104 cells/well) were transfected with 10 ng pIR-CMVluc and the indicated amount of pFerH-PBTP. Cell viability was determined at 2 days after transfection, and represented by % of cells without transfection. Each value represents the mean ± SD (n = 4–6). Asterisks (* and **) indicate t-test statistically different (P < 0.05 and P < 0.01, respectively) from the peak point of each graphs (i.e., 250 ng of pFerH-PBTP in (a) and (b), 0 ng of pFerH-PBTP in (c)). (d) Effect of tranposase versus cell viability in vitro. Hep3B cells (1 × 104 cells/well) were transfected with 10 ng pIR-CMVluc (left) or pCMV-luc (right), and 50 ng pFerH-mcs (white bar) or pFerH-PBTP (gray bar), respectively. Cell viability was determined at 2 days after transfection and represented by % of cells without transfection. Each value represents the mean ± SD (n = 6).
Effect of the amount of transposase on transposition in vivo
To investigate the ideal amount of pFerH-PBTP in vivo, we co-transfected mice with a constant amount of 25 µg pIR-EF1Gluc and a variable amount of pFerH-PBTP from 1 to 50 µg using the hydrodynamics-based transfection procedure. The sustained Gluc expression level increased in a pFerH-PBTP-dependent manner over the range of 1–25 µg, but a further increase in pFerH-PBTP to 50 µg resulted in a reduction of the sustained Gluc expression level (Figure 7).
Figure 7.
Effect of the amount of transposase versus the transposition in vivo. (a) Expression time course. Mice were injected with 25 µg pIR-EF1Gluc and 1 (open rhombuses), 5 (open squares), 10 (open triangles), 25 (closed rhombuses), and 50 (closed squares) µg pFerH-PBTP, respectively. Blood was collected at the indicated time points, and Gaussia luciferase activity in serum was measured. Each value represents the mean ± SD (n = 4–6). (b) Percentage of sustained gene expression. Gaussia luciferase activities 78 days after injection were divided by those 1 day after injection and multiplied by 100. Each value represents the mean ± SD (n = 4–6). *t-test statistically different (P < 0.05) from the peak point (i.e., 25 µg of pFerH-PBTP).
Discussion
Here, we demonstrated that PB can prolong gene expression from pDNA in vivo. Exogenous gene expression was prolonged by PB transposase in vivo only when pDNA contains PB IRs (Figures 3 and 4). When we injected PB-based pDNA containing Gluc gene under EF1 promoter control, expression levels did not apparently decrease from 10 days after injection (Figure 4c). These results support a previous report showing that an SB-based pDNA containing human factor IX under EF1 promoter control succeeded in producing long-lasting expression without apparent decrease in the expression level.29
It was previously reported that gene expression from conventional pDNA containing mammalian promoters persists longer than pDNA containing viral promoters.27 In our study, although expression from PB-based pDNA containing Gluc gene under CMV promoter control was prolonged, Gluc expression decreased gradually. In addition, when pDNA containing PB IRs was injected without pFerH-PBTP, Gluc expression by EF1 promoter was detected 10 days after injection, whereas that by CMV promoter had reached background level 7 days after injection (Figure 4a,c). These results and previous studies27,28 suggest that mammalian promoters (ubiquitin C and EF1) are less susceptible to gene silencing than viral promoters,28 and tend to express longer than viral promoters (CMV, RSV, and SV40)27,28 not only in the episomal state27 but also when integrated by transposons (Figure 4).28 Therefore, mammalian promoters could be suitable for sustained gene expression regardless of whether the vectors are integrative or not.
Sustained Gluc expression ranged from 0.8 to 5.3% of the initial Gluc expression (Figure 7b). However, the initial Gluc expression may not reflect the actual amount of the expression cassette because the hydrodynamics-based procedure activates transcription.30 Therefore, the initial expression may be lower if transcription was not highly activated by the hydrodynamics-based procedure and the actual percentage of integration may be higher than the percentage shown in Figure 7b. Interestingly, Gluc expression became stable earlier in higher pFerH-PBTP groups (Figure 7a). The initial decrease in Gluc expression may be partially explained by the decrease in the remaining episomal expression cassettes. Therefore, this earlier stabilization of Gluc expression in higher pFerH-PBTP groups may be due to fewer remaining episomal expression cassettes. In addition, the peak expression was also higher when more pFerH-PBTP was transfected (Figure 7a). This may result from more efficient expression from chromosomes than from pDNA.
Although IRs of PB possess promoter or enhancer effects,31,32 the initial expressions from pIR-CMVluc and pIR-CMVGluc were lower than those from pCMV-luc and pCMV-Gluc, respectively (Figures 3a,b and 4a,b). These differences may be explained by the differences of plasmid backbones. For example, the number of CG motif, which may cause gene silencing, in pIR-CMVluc is about one hundred more than that in pCMV-luc. Because the construct outside of the transposon is not necessary for transposition, transferring the transposon into other constructs could solve this lower expression. Although SB transposase was reported to increase expression from conventional pDNA,33 PB transposase did not affect expression from conventional pDNA in our experimental conditions (Figures 3b and 4b).
In the case of some transposons including SB, transposition efficiency decreases in the presence of an excess of transposase.9,11,13,29 This decrease is called “overproduction inhibition.” A decrease in transposition efficiency could result in a low level of sustained transgene expression29 and therapeutic effect. Avoiding “overproduction inhibition” and identifying the ideal amount of transposase expressing pDNA may be necessary to achieve high-level sustained transgene expression. In our study, both the numbers of colonies in vitro (Figure 6a,b) and sustained gene expression levels in vivo (Figure 7a,b) decreased in the presence of an excess of pFerH-PBTP. In addition, cell viability decreased when the amount of tranfected pFerH-PBTP increased (Figure 6c). Although it is still unclear whether other factors such as transposase–transposase interaction can contribute to the decrease of the numbers of colonies and sustained expression level, this result suggests that the cytotoxicity of PB transposase can partially contribute to the decrease of sustained gene expression level. From this viewpoint, high transfection efficiency may increase the amount of PB transposase and decrease sustained gene expression levels. Moreover, the promoter strength in transfected cells may also affect sustained gene expression level. Because transcription factor expression may differ among cell types, cell types may affect not only the transfection efficiency but also the transcription activity of promoters controlling transposase expression. In a previous study showing “overproduction inhibition” of PB,9 the maximal number of colonies was about 5,000 when the amount of transposase was increased. On the other hand, in two other studies showing no “overproduction inhibition” of PB,11,31 the maximal number of colonies was about 400 and 100, respectively, when the amount of transposase was increased. This difference also suggests that a higher transfection efficiency could induce “overproduction inhibition.” The transfection efficiency is affected by both the transfection methods and the cell types. Therefore, adjustment of the optimum amount of transposase for each transfection method and cell type may be needed to achieve high transposition efficiency.
Transposon-based vectors still have problems for therapeutic applications because transposons can integrate into or nearby the coding region, and affect endogenous gene expression. In the case of integrative viral vectors, cancer produced by insertional mutagenesis has been reported.34 Although PB has a lower tendency to integrate into or nearby genes than lentivirus, the tendency of PB to integrate into or nearby genes is higher than random integration and SB,11 and the risk of insertional mutagenesis remains. Site-specific integration using sequence-specific DNA-binding proteins is one approach to avoid insertional mutagenesis.35,36,37 PB is suitable for site-specific integration because DNA-binding protein-transposase chimera is active as native transposase, whereas the chimeric transposase of SB and Tol2 is inactive9 or low active.36,37 Moreover, the chimeric PB transposase was reported to integrate 67% of PB transposons into a single target site on a pDNA in mosquito embryos.35
During preparation of this article, a new hyperactive SB transposase named SB100X, which has higher transposability than PB, has been reported.38 Because use of PB for mammals is relatively new, such hyperactive versions of PB transposase have not been reported yet. However, PB may be improved as use of PB increases.
In the present study, we used a hydrodynamics-based procedure to introduce transposon-based pDNA into mice. Systemic injection of such a high volume solution is not suitable for clinical applications. However, the organ-restricted hydrodynamics-based procedure that injects solution into a specific vein of an expandable organ using a balloon catheter may be suitable for clinical treatment.2 In contrast to viral vectors, transposon-based pDNA can be transfected by various conventional nonviral methods such as lipoplex, polyplex, electroporation, and mechanical massage.1,2 Therefore, transposon-based pDNA can easily be adapted to a variety of organs such as the lung,39,40 liver,29 kidney,41,42 or spleen43 using a suitable transfection method for each target, whereas the targets of viral vectors may be limited by the nature of each virus. In addition, when the target organs are susceptible to some viral vectors, the transposon can also be loaded on viral vectors such as adenovirus,44 herpes simplex virus,45 or integrase-defective lentivirus.46,47
In conclusion, we succeeded in prolonged gene expression by co-transfection of pDNA containing the PB transposase expression cassette and pDNA containing PB IRs. The present study is an initial report that demonstrates PB-mediated sustained gene expression in vivo, and provides evidence that PB is a promising tool for various in vivo genetic applications such as gene therapies. In addition, the present study showed “overproduction inhibition” of PB in vivo, suggesting that optimization of the amount of PB transposase is necessary for high-level sustained gene expression. Improvement of both transposon systems and gene delivery methods will develop new therapy to overcome refractory diseases. We believe that the present study will encourage the development of PB-based vectors, and contribute to future basic genetic studies and studies of gene therapies.
Materials and Methods
Animals. Female ICR mice (5-week-old) were purchased from the Shizuoka Agricultural Cooperative Association for Laboratory Animals (Shizuoka, Japan). All the animals were housed with free access to food and water. The light (dark/light cycle was 2/12 hours), temperature, and humidity were kept constant throughout the experiments. All protocols for animal experiments were carried out with the approval of the Animal Experimentation Committee of the Graduate School of Pharmaceutical Sciences, Kyoto University.
pDNA construction. To create pFerH-mcs, the portion of pVIVO2-mcs (InvivoGen, San Diego, CA) from the pMB1 replication origin to EF1polyA was amplified by PCR using primer1 and primer2 (primer sequences are listed below). The PCR fragment was purified, and self-ligated using Mighty Cloning Kit (blunt end) (Takara Bio, Ōtsu, Japan). To create pFerH-PBTP, PB transposase ORF was amplified using p3E1.2 (a gift from Prof. Hajime Mori, Kyoto Institute of Technology, Kyoto, Japan) as a template, and primer3 and primer4. The PCR product was cloned into pFerH-mcs using In-Fusion PCR cloning kit (Takara Bio). The PCR product including PB transposon IRs (p3EIR) was created using p3E1.2 as a template, primer5, and primer6. The expression cassette including the firefly luciferase gene under CMV promoter control and neomycin-resistance gene under SV40 promoter control was amplified by PCR using pCMV-luc as a template, primer7, and primer8. To create pIR-CMVluc, these two PCR products were ligated using Mighty Cloning Kit (blunt end). The expression cassette including Gluc gene under CMV promoter control was amplified by PCR using pCMV-Gluc Control Plasmid (New England BioLabs Japan, Tokyo, Japan) as a template, primer9, and primer10. This PCR product was ligated with p3EIR to create pIR-CMVGluc. hEF1 promoter was amplified by PCR using pBLAST49-hHGF as a template, primer11, and primer12. The PB transposon including the Gluc expression cassette without CMV promoter was created by PCR using pIR-CMVGluc as a template, primer13, and primer14. To create pIR-hEF1Gluc, this PCR product was ligated with hEF1 promoter PCR product. To create pIR-blastHGF, pBLAST49-hHGF (InvivoGen) was digested by restriction enzyme SgfI and ligated with p3EIR using Mighty Cloning Kit (blunt end). KOD-FX or KOD-plus ver.2 (Toyobo, Osaka, Japan) was used for all PCRs, and High Pure PCR Product Purification Kit (Roche Diagnostics, Tokyo, Japan) or gel indicator DNA extraction kit (Biodynamics Laboratory, Tokyo, Japan) was used for purification of PCR products. All pDNAs were amplified in the E. coli strain DH5α, isolated and purified using QIAprep Spin Miniprep Kit (Qiagen, Tokyo, Japan) or JETSTAR 2.0 Plasmid Giga Kit (GenoMed, Lohne, Germany).

Cell culture. HepG2 was maintained in Dulbecco's modified Eagle's essential medium containing 10% fetal bovine serum. Hep3B was maintained in Eagle's minimum essential medium containing 2 mmol/l glutamine, 1% nonessential amino acids, 1 mmol/l sodium pyruvate, and 10% fetal bovine serum.
In vitro transposition study. The indicated numbers of cells were seeded into individual wells of 6- or 12-well plates 18 hours before transfection. Cells were transfected with the indicated amount of pDNA by FuGENE6 (Roche Diagnostics). Two days after transfection, cells were harvested, and 1/10~1/100th of the cells were transferred to 100 mm plates or 6-well plates, and maintained in medium containing 800 µg/ml G418 (Nacalai Tesque, Kyoto, Japan) for 2 weeks. For luciferase imaging, 0.3 mmol/l -luciferin (Promega, Tokyo, Japan) in PBS was added to the cells, and then luminescence was captured for 5 minutes using a NightOwl NC320 Molecular Light Imager (Berthold Technologies, Bad Wildbad, Germany). To count G418-resistant colonies, cells were fixed with 4% paraformaldehyde in PBS (Wako Pure Chemical Industries, Osaka, Japan) for 10 minutes and stained with 0.2% methylene blue (Wako Pure Chemical Industries) in PBS. The numbers of colonies were corrected by the dilution ratio.
Cell viability assay. Hep3B (1 × 104) cells were seeded into individual wells of 96-well plates 18 hours before transfection. Cells were transfected with the indicated amount of pDNA by FuGENE6. Two days after transfection, viability was determined using Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan).
Plasmid excision assay in vitro. Hep3B and HepG2 (2 × 105) cells were seeded into individual wells of 6-well plates 18 hours before transfection. Cells were transfected with 0.67 µg pIR-CMVluc and 0.33 µg pFerH-PBTP of pFerH-mcs. Two days after transfection, cells were harvested, and DNA was isolated using Genelute mammalian genomic DNA extraction kit (Sigma-Aldrich Japan, Tokyo, Japan). PCR amplification was performed using the isolated DNA as templates, primer15, primer16, and PrimeSTAR GXL DNA Polymerase (Takara Bio) PCR products were electrophoresed on 1% agarose S (Nippon Gene, Tokyo, Japan) gel.
Analysis of transposon-chromosome junctions via plasmid rescue. Hep3B (2 × 105) cells were seeded into individual wells of 6-well plates 18 hours before transfection. Cells were transfected with 0.67 µg pIR-blastHGF and 0.33 µg pFerH-PBTP. Two days after transfection, cells were harvested, transferred to 100 mm plates and propagated in medium containing 3 µg/ml blasticidin S (InvivoGen). DNA was isolated from these cells using Genelute mammalian genomic DNA extraction kit, and digested by restriction enzyme BglII (Takara Bio) and BamHI (Toyobo). After digestion by restriction enzymes, DNA was purified using High Pure PCR Product Purification Kit and ligated using Ligation-Convenience Kit (Nippon Gene). The ligation products were used to transform E. coli Competent Quick DH5α (Toyobo) or E. coli HST08 Premium Competent Cells (Takara Bio). pDNA was isolated and purified using QIAprep Spin Miniprep Kit. Nucleotide sequences of the pDNA were sequenced by Fasmac sequencing service (Fasmac, Atsugi, Japan). UC Santa Cruz BLAT was used to map PB integration sites.
Assay of firefly luciferase activity in liver. Mice were injected intravenously via the tail vein with 1.6 ml saline containing the indicated amount of pDNA. At the indicated time points, mice were killed, and livers were harvested. The livers were homogenized by adding lysis buffer (0.05% Triton X-100, 2 mmol/l EDTA, 0.1 mol/l Tris, pH 7.8). The homogenate was centrifuged at 16,060 g for 10 minutes at 4 °C. The firefly luciferase activity of the supernatant was measured using Picagene luciferase substrate (Toyo Ink, Tokyo, Japan) and Lumat LB 9507 (EG&G Berthold, Bad Wildbad, Germany).
Assay of Gluc activity in serum. Mice were injected intravenously via the tail vein with 1.6 ml saline containing the indicated amount of pDNA. At the indicated time points, blood was collected via the tail vein. The blood samples were put on ice for 30 minutes and centrifuged at 16,060 g for 10 minutes at 4 °C. The Gluc activity of the supernatant was measured using Gluc assay kit (New England BioLabs Japan) and Lumat LB 9507.
Plasmid excision assay in vivo. Mice were injected intravenously via the tail vein with 1.6 ml saline containing the 25 µg pIR-EF1Gluc, and 25 µg pFerH-PBTP or pFerH-mcs. Three days after injection, livers were harvested, and DNA was isolated using Genelute mammalian genomic DNA extraction kit. PCR amplification was performed using the isolated DNA as templates, primer15, primer16, and PrimeSTAR GXL DNA Polymerase PCR products were electrophoresed on 1% agarose S gel.
Acknowledgments
We thank Prof. Hajime Mori (Kyoto Institute of Technology, Kyoto, Japan) for p3E1.2 (a plasmid DNA containing the piggyBac original fragment).
REFERENCES
- Li SD., and, Huang L. Non-viral is superior to viral gene delivery. J Control Release. 2007;123:181–183. doi: 10.1016/j.jconrel.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Higuchi Y., and, Hashida M. Nonviral approaches for targeted delivery of plasmid DNA and oligonucleotide. J Pharm Sci. 2008;97:726–745. doi: 10.1002/jps.21024. [DOI] [PubMed] [Google Scholar]
- Ivics Z., and, Izsvák Z. Transposons for gene therapy! Curr Gene Ther. 2006;6:593–607. doi: 10.2174/156652306778520647. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Hackett PB, Plasterk RH., and, Izsvák Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Dupuy AJ, Jenkins NA., and, Copeland NG. Sleeping beauty: a novel cancer gene discovery tool. Hum Mol Genet. 2006;15 Spec No 1:R75–R79. doi: 10.1093/hmg/ddl061. [DOI] [PubMed] [Google Scholar]
- Takeda J, Keng VW., and, Horie K. Germline mutagenesis mediated by Sleeping Beauty transposon system in mice. Genome Biol. 2007;8 Suppl 1:S14. doi: 10.1186/gb-2007-8-s1-s14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izsvák Z., and, Ivics Z. Sleeping beauty transposition: biology and applications for molecular therapy. Mol Ther. 2004;9:147–156. doi: 10.1016/j.ymthe.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Cary LC, Goebel M, Corsaro BG, Wang HG, Rosen E., and, Fraser MJ. Transposon mutagenesis of baculoviruses: analysis of Trichoplusia ni transposon IFP2 insertions within the FP-locus of nuclear polyhedrosis viruses. Virology. 1989;172:156–169. doi: 10.1016/0042-6822(89)90117-7. [DOI] [PubMed] [Google Scholar]
- Wu SC, Meir YJ, Coates CJ, Handler AM, Pelczar P, Moisyadi S, et al. piggyBac is a flexible and highly active transposon as compared to sleeping beauty, Tol2, and Mos1 in mammalian cells. Proc Natl Acad Sci USA. 2006;103:15008–15013. doi: 10.1073/pnas.0606979103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KJ, Carlson DF, Foster LK, Kong BW, Foster DN., and, Fahrenkrug SC. Enzymatic engineering of the porcine genome with transposons and recombinases. BMC Biotechnol. 2007;7:42. doi: 10.1186/1472-6750-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MH, Coates CJ., and, George AL., Jr PiggyBac transposon-mediated gene transfer in human cells. Mol Ther. 2007;15:139–145. doi: 10.1038/sj.mt.6300028. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu X, Li G, Han M, Zhuang Y., and, Xu T. Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell. 2005;122:473–483. doi: 10.1016/j.cell.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Geurts AM, Yang Y, Clark KJ, Liu G, Cui Z, Dupuy AJ, et al. Gene transfer into genomes of human cells by the sleeping beauty transposon system. Mol Ther. 2003;8:108–117. doi: 10.1016/s1525-0016(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Wang W, Lin C, Lu D, Ning Z, Cox T, Melvin D, et al. Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:9290–9295. doi: 10.1073/pnas.0801017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Zhang X, Jiang W, Wu C, Chen C, Zheng Y, et al. Tumor-directed gene therapy in mice using a composite nonviral gene delivery system consisting of the piggyBac transposon and polyethylenimine. BMC Cancer. 2009;9:126. doi: 10.1186/1471-2407-9-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltjen K, Michael IP, Mohseni P, Desai R, Mileikovsky M, Hämäläinen R, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaji K, Norrby K, Paca A, Mileikovsky M, Mohseni P., and, Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusa K, Rad R, Takeda J., and, Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods. 2009;6:363–369. doi: 10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson CM, Frandsen JL, Kirchhof N, McIvor RS., and, Largaespada DA. Somatic integration of an oncogene-harboring Sleeping Beauty transposon models liver tumor development in the mouse. Proc Natl Acad Sci USA. 2005;102:17059–17064. doi: 10.1073/pnas.0502974102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesner SM, Decker SA, Larson JD, Ericson K, Forster C, Gallardo JL, et al. De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res. 2009;69:431–439. doi: 10.1158/0008-5472.CAN-08-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Harrell RA, Handler AM, Beam T, Hennessy K., and, Fraser MJ., Jr piggyBac internal sequences are necessary for efficient transformation of target genomes. Insect Mol Biol. 2005;14:17–30. doi: 10.1111/j.1365-2583.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Song Y., and, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- Zhang G, Budker V., and, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- Tannous BA, Kim DE, Fernandez JL, Weissleder R., and, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11:435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO, et al. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat Methods. 2008;5:171–173. doi: 10.1038/nmeth.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannous BA. Gaussia luciferase reporter assay for monitoring biological processes in culture and in vivo. Nat Protoc. 2009;4:582–591. doi: 10.1038/nprot.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DR, Smyth SE, Goddard CA, Pringle IA, Higgins CF, Colledge WH, et al. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther. 2001;8:1539–1546. doi: 10.1038/sj.gt.3301561. [DOI] [PubMed] [Google Scholar]
- Garrison BS, Yant SR, Mikkelsen JG., and, Kay MA. Postintegrative gene silencing within the Sleeping Beauty transposition system. Mol Cell Biol. 2007;27:8824–8833. doi: 10.1128/MCB.00498-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant SR, Meuse L, Chiu W, Ivics Z, Izsvak Z., and, Kay MA. Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat Genet. 2000;25:35–41. doi: 10.1038/75568. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Nakayama A, Takahashi Y, Fukuhara Y., and, Takakura Y. Reactivation of silenced transgene expression in mouse liver by rapid, large-volume injection of isotonic solution. Hum Gene Ther. 2008;19:1009–1020. doi: 10.1089/hum.2008.020. [DOI] [PubMed] [Google Scholar]
- Cadiñanos J., and, Bradley A. Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res. 2007;35:e87. doi: 10.1093/nar/gkm446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Harrison RL, Hollister JR, Mohammed A, Fraser MJ., Jr, and, Jarvis DL. Construction and characterization of new piggyBac vectors for constitutive or inducible expression of heterologous gene pairs and the identification of a previously unrecognized activator sequence in piggyBac. BMC Biotechnol. 2007;7:5. doi: 10.1186/1472-6750-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda K, Yamamoto S, Endoh M., and, Kaneda Y. Transposon-independent increase of transcription by the Sleeping Beauty transposase. Biochem Biophys Res Commun. 2004;317:796–800. doi: 10.1016/j.bbrc.2004.03.116. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Maragathavally KJ, Kaminski JM., and, Coates CJ. Chimeric Mos1 and piggyBac transposases result in site-directed integration. FASEB J. 2006;20:1880–1882. doi: 10.1096/fj.05-5485fje. [DOI] [PubMed] [Google Scholar]
- Yant SR, Huang Y, Akache B., and, Kay MA. Site-directed transposon integration in human cells. Nucleic Acids Res. 2007;35:e50. doi: 10.1093/nar/gkm089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivics Z, Katzer A, Stüwe EE, Fiedler D, Knespel S., and, Izsvák Z. Targeted Sleeping Beauty transposition in human cells. Mol Ther. 2007;15:1137–1144. doi: 10.1038/sj.mt.6300169. [DOI] [PubMed] [Google Scholar]
- Mátés L, Chuah MK, Belay E, Jerchow B, Manoj N, Acosta-Sanchez A, et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet. 2009;41:753–761. doi: 10.1038/ng.343. [DOI] [PubMed] [Google Scholar]
- Belur LR, Frandsen JL, Dupuy AJ, Ingbar DH, Largaespada DA, Hackett PB, et al. Gene insertion and long-term expression in lung mediated by the Sleeping Beauty transposon system. Mol Ther. 2003;8:501–507. doi: 10.1016/s1525-0016(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu L, Fletcher BS., and, Visner GA. Sleeping Beauty-based gene therapy with indoleamine 2,3-dioxygenase inhibits lung allograft fibrosis. FASEB J. 2006;20:2384–2386. doi: 10.1096/fj.06-6228fje. [DOI] [PubMed] [Google Scholar]
- Fujii N, Isaka Y, Takabatake Y, Mizui M, Suzuki C, Takahara S, et al. Targeting of interstitial cells using a simple gene-transfer strategy. Nephrol Dial Transplant. 2006;21:2745–2753. doi: 10.1093/ndt/gfl327. [DOI] [PubMed] [Google Scholar]
- Mukai H, Kawakami S., and, Hashida M. Renal press-mediated transfection method for plasmid DNA and siRNA to the kidney. Biochem Biophys Res Commun. 2008;372:383–387. doi: 10.1016/j.bbrc.2008.04.097. [DOI] [PubMed] [Google Scholar]
- Mukai H, Kawakami S, Kamiya Y, Ma F, Takahashi H, Satake K, et al. Pressure-mediated transfection of murine spleen and liver. Hum Gene Ther. 2009;20:1157–1167. doi: 10.1089/hum.2008.213. [DOI] [PubMed] [Google Scholar]
- Yant SR, Ehrhardt A, Mikkelsen JG, Meuse L, Pham T., and, Kay MA. Transposition from a gutless adeno-transposon vector stabilizes transgene expression in vivo. Nat Biotechnol. 2002;20:999–1005. doi: 10.1038/nbt738. [DOI] [PubMed] [Google Scholar]
- Bowers WJ, Mastrangelo MA, Howard DF, Southerland HA, Maguire-Zeiss KA., and, Federoff HJ. Neuronal precursor-restricted transduction via in utero CNS gene delivery of a novel bipartite HSV amplicon/transposase hybrid vector. Mol Ther. 2006;13:580–588. doi: 10.1016/j.ymthe.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Staunstrup NH, Moldt B, Mátés L, Villesen P, Jakobsen M, Ivics Z, et al. Hybrid lentivirus-transposon vectors with a random integration profile in human cells. Mol Ther. 2009;17:1205–1214. doi: 10.1038/mt.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vink CA, Gaspar HB, Gabriel R, Schmidt M, McIvor RS, Thrasher AJ, et al. Sleeping beauty transposition from nonintegrating lentivirus. Mol Ther. 2009;17:1197–1204. doi: 10.1038/mt.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]