Abstract
Hydrogels can provide a controllable cell microenvironment for numerous applications in regenerative medicine, and delivery of gene therapy vectors can be employed to enhance their bioactivity. We investigated the delivery of lentiviral vectors from hydrogels, and employed the immobilization of lentivirus to hydroxylapatite (HA) nanoparticles as a means to retain and stabilize vectors within hydrogels, and thereby increase delivery efficiency. Entrapment of the vector alone within the hydrogel maintained the activity of the virus more effectively compared to the absence of a hydrogel, and release was slowed with an increasing solid content of the hydrogel. Association of the lentivirus with HA increased the activity of the complexes, with HA increasing the virus activity for 72 hours. Cells seeded onto lentivirus–HA-loaded hydrogels had a decreased number of infected cells outside of the hydrogel relative to the absence of HA. In vivo studies with collagen hydrogels loaded with lentivirus and HA-lentivirus demonstrated sustained and localized transgene expression for at least 4 weeks, with increased expression using the lentivirus–HA complex. This strategy of nanoparticle immobilization to stabilize and retain vectors is broadly applicable to hydrogels, and may provide a versatile tool to combine gene therapy and biomaterials for applications in regenerative medicine.
Introduction
Hydrogels have broad applicability to regenerative medicine, having been used in applications such as bone and nerve regeneration, islet transplantation, and ovarian follicle maturation.1,2,3,4 For these and other applications, the hydrogel must create an environment that presents the appropriate combination of molecular cues to promote tissue formation. Hydrogels are frequently designed to mimic the numerous properties (e.g., mechanics) and functions (e.g., cell adhesion) of the natural extracellular matrix. Hydrogels are composed of hydrophilic polymers, either natural (e.g., collagen and hyaluronic acid) or synthetic [e.g., polyethylene glycol (PEG)],2,5 that can self-assemble or be chemically crosslinked into 3D structures. The stability of the hydrogel can be regulated through its solid content or degradation rate, the latter of which is typically controlled by varying the extent of crosslinking or self-assembly.6,7,8,9
Gene delivery represents a powerful and versatile tool for inducing the expression of tissue inductive factors, with the primary challenge being delivery. Although hydrogels can readily present stimuli to cells that are immobilized to the polymer, providing soluble growth factors for extended time periods is a significant challenge.10 The high water content of the hydrogels releases most entrapped proteins rapidly, though several strategies based on incorporation of growth factor–binding sites (e.g., heparin) are being developed. The alternative to delivering proteins is nucleic acids, which can induce the production of inductive factors that synergize with the biological stimuli within the hydrogel. Relative to proteins, gene delivery may provide more sustained availability of the protein, as cells expressing the transgene serve as bioreactors for localized protein production.1 Hydrogels can deliver both viral and nonviral vectors, and generally address the extracellular barriers to gene delivery. Encapsulation of vectors within the hydrogel aims to maintain the local concentration of the vector through a sustained release approach. Alternatively, the hydrogel may limit release either through the mesh size or modification of the polymer with binding sites for the vector.11,12 In this latter approach, polymers can be modified with antibodies that bind viruses.12 These approaches localize gene delivery, though the modification of the material or the vector complicates the widespread use of this strategy.
In this report, we investigated the association of lentiviral vectors with nanoparticles as a simple strategy to enhance and localize delivery from a hydrogel. These studies employed collagen hydrogels, which form under relatively mild conditions that do not diminish the activity of the lentiviral vector while also supporting cell infiltration in vitro and in vivo. Initial studies were performed with entrapment of lentivirus in collagen gels of varying density, with characterization of release and vector stability, and investigated the relationship between cell migration and infection. Subsequent studies investigated the association of the lentiviral vectors with hydroxylapatite (HA) nanoparticles, which were incorporated to bind and retain the virus. Disks from HA have been previously used for adenovirus binding,13 and this report investigated the inclusion of small quantities of HA nanoparticles into the collagen gel to promote lentivirus binding and retention within the hydrogel. The enhancement in activity resulting from immobilization to HA was investigated in vitro and in vivo. Strategies that bind and retain lentiviral vectors within hydrogels can be employed to enhance and control gene transfer for numerous applications in regenerative medicine and in model systems that investigate tissue formation.
Results
Lentivirus in collagen gel
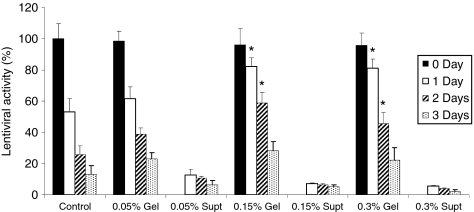
The activity of lentivirus retained in and released from the collagen gel was initially investigated. Lenti-luciferase-loaded collagen gels were incubated with cell culture medium (Figure 1), with a control for virus activity being the suspension of viruses within media alone. The control viruses incubated in medium alone had activity reduced by ~50% during the first day, which decreased further through 3 days. This extent of decrease in activity with time was consistent with the previous reports for adenovirus and VSV-G pseudotyped lentivirus.14,15,16 The collagen gels, however, reduced the decline in virus activity. In 0.15 and 0.3% collagen gels, the virus activity at days 1 and 2 was significantly greater than that observed within the control (P < 0.05). Release of the lentivirus from the gel was also observed, though the total amount released was relatively low. For the 0.05, 0.15, and 0.3% collagen gel, approximately 13, 7, and 5%, respectively, of virus particles were released from the collagen gel at the first day. This result suggested that lentivirus was retained, and the activity of the virus was stabilized for collagen gels with a concentration ≥0.15%.
Figure 1.
Lentivirus in collagen gel. Lentivirus encoding luciferase was loaded into collagen gels and incubated with 10% FBS-DMEM media at 37 °C. Active amounts of viruses from the gel and supernatant media were measured at different gel concentrations and time points. For the measurement of viral activity inside the gels, the gels were solubilized by the treatment of collagenase (1 mg/ml) for 1 hour. Virus in 10% FBS-DMEM was used as control. Values are mean ± SD. *Statistical significance relative to control group at P < 0.01. supt, supernatant. DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum.
In vitro transduction in virus-loaded collagen gel
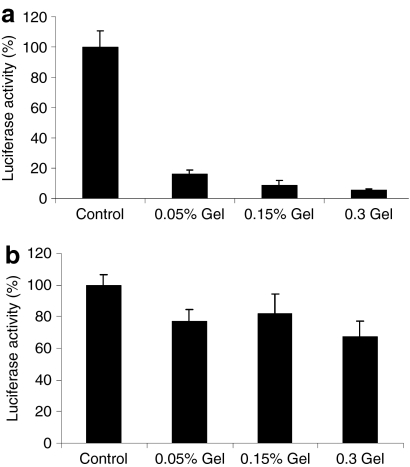
We subsequently investigated transduction in vitro as a function of cell infiltration into the collagen gel. Hydrogels used for regenerative medicine would be implanted into an injury site, and cells from the surrounding tissue would infiltrate. These in vitro studies simulated this in vivo cell infiltration by seeding cells onto the virus-loaded collagen gel. Lenti-luc was loaded in the collagen gel for the determination of the cell transduction activity, with lentivirus in the medium alone used as a control. Two cell lines were investigated that had been classified as either noninvasive NIH/3T3 cells17 or invasive C6 glioma cells.18 For the noninvasive NIH/3T3 cells, the transduction activity was <10% of control in 0.15 and 0.3% collagen gel. In contrast, the transduction activity in the 0.05% collagen gel was relatively high (16.6%), which likely results from a greater release of virus from the gel due to the low concentration of collagen. For the invasive C6 glioma cells, transduction activity within all collagen gel conditions was ~80% of the control (Figure 2b). These results suggested that the enhanced migration of the invasive cells through the gel results in substantial transduction by virus retained within the gel.
Figure 2.
In vitro cell transduction in virus-loaded collagen gel. Lentiviruses encoding luciferase were mixed with collagen during gelation. (a) NIH3T3 cells (noninvasive cell) and (b) C6 cells (invasive cells) were seeded on the top of the virus-loaded collagen gel. Virus in 10% FBS-DMEM was used as control. At 3 days after cell seeding, the gels were solubilized with collagenase, and the luciferase activity was measured using a luminometer. Amount of the active virus was presented as the percentage of control. DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum.
Increase of transduction efficiency as cells migrate into the gel
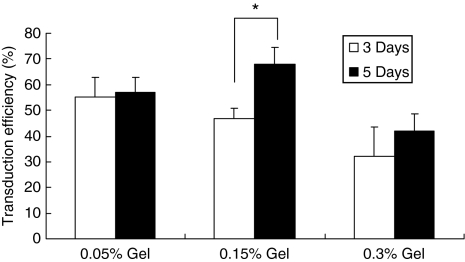
We subsequently quantified the percentage of transduced cells within the collagen gel, which would be expected to increase if infiltrating cells were being transduced. Transduction efficiencies at multiple time points were determined by entrapping a lentivirus encoding green fluorescent protein (GFP), with subsequent quantification of GFP+ and total cells (Figure 3). In 0.05% collagen gel, the transduction efficiencies were similar between days 3 and 5 after cell seeding. The C6 cells migrate completely throughout the 0.05% gel within 3 days. For the 0.15% collagen gel, a significant increase in transduction efficiency was observed between days 3 and 5 (P < 0.05). This increase in transduction efficiency indicated that cells were likely transduced after approximately day 2, which assumes ~24 hours between virus entry and sufficient GFP production for visualization. Additionally, the increase suggested that active virus was present after 2 days, and that cell migration through the gel increased exposure of the cells to the virus. For the 0.3% hydrogel, the mean transduction efficiency also increased between days 3 and 5. However, the increase was substantially reduced, which likely results from a decrease in the rate of cell migration at the increased collagen density.19
Figure 3.
Increase of transduction efficiency as cells migrate into the gel. Invasive C6 cells were seeded on the lentivirus encoding GFP-loaded collagen gel. At 3 and 5 days after cell seeding, transduction efficiency in collagen gel was measured. Collagen gel was digested with collagenase, and diluted cells were seeded on the plates. Transduction efficiency was calculated by counting GFP+ and total cells (at least a total of 500 cells). Values are mean ± SD. *Statistical significance relative to control group at P < 0.05. GFP, green fluorescent protein.
Protection of virus activity in HA–lentivirus complex
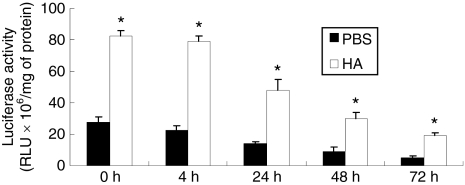
We hypothesized that the localization of lentivirus within the collagen gel and the transduction efficiency can be enhanced through binding of the lentivirus to HA nanoparticles. HA is biocompatible, naturally found in the body, binds several proteins, and has previously been employed to bind adenovirus via ionic interactions.13 HA is also insoluble in aqueous solution, and thus, the nanoparticles can be entrapped and retained within the hydrogel. The binding of lentivirus to HA was investigated by incubation of lentivirus encoding for luciferase with HA in phosphate-buffered saline (PBS), or PBS alone, and subsequent transfer of the virus or HA/virus complex to cells at designated incubation time points (Figure 4). HA/virus complex had 2.9 times higher viral activity than the virus in PBS (P < 0.01), which was consistent with a previous study using adenovirus and may have resulted from a more efficient delivery to the cell surface for HA–virus complex compared to the typical bolus infection.13 Virus activity decreased with increasing time for both HA and PBS conditions. However, the virus activity in HA–virus complex had a slower decline relative to PBS alone (Figure 4), which was observed by the increasing ratio of luciferase from HA relative to PBS. Assuming an exponential decay, a thermal half-life of the virus was determined to be 29 hours, consistent with previous reports,16 and 34 hours for virus alone and HA–virus complexes, an increase of ~17%. These results indicated that the nanoparticle bound lentivirus and maintained its activity for longer times.
Figure 4.
Protection of virus activity in hydroxylapatite–lentivirus complex. Lentiviruses encoding luciferase (1 × 106 lentivirus particles) were mixed with PBS or hydroxylapatite (0.1 mg), and incubated at room temperature. At each time point, 293T cells were mixed with the mixtures and seeded on the 24-well plate. At 2 days after cell seeding, the luciferase activities from the cell lysates were measured by luminometer. Values are mean ± SD. *Statistical significance relative to control group at P < 0.01. HA, hydroxylapatite; PBS, phosphate-buffered saline; RLU, relative light unit.
In vitro cell transduction in collagen and HA/collagen gel
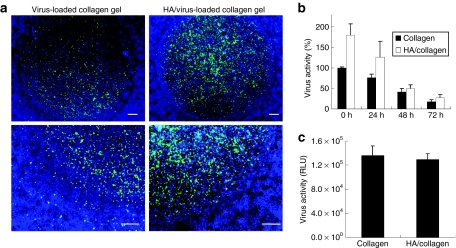
We subsequently investigated the localization of transduction by delivery of lentivirus from collagen gels, with and without HA. Virus- or HA/virus-loaded 0.15% collagen gels were transferred to 24-well plate. The gel did not occupy the entire surface; thus, seeding of C6 cells into the well resulted in cells both on the collagen gel and on the tissue culture polystyrene. GFP-expressing cells were localized primarily to the collagen gel for both virus and HA/virus complex (Figure 5a). Low numbers of GFP+ cells were observed outside of the virus-loaded collagen gel, which likely occurs due to the virus released from the gel. However, for the HA/virus-loaded gel, the number of cells transduced on the polystyrene were substantially reduced. The amount of active virus retained within HA/virus-loaded collagen gel was greater than that retained with virus-loaded collagen gel at all time points (Figure 5b). Transgene expression was similar between virus- and HA/virus-loaded collagen gel, indicating that the virus released from the collagen gel alone produced activity similar to the virus retained by the nanoparticles (Figure 5c). If hydrogels were incubated in media prior to cell seeding in order to remove unbound virus, transgene expression was greater for the HA/collagen hydrogels relative to collagen alone (data not shown).
Figure 5.
Localization of lentivirus in collagen gel. (a) HA (hydroxylapatite) nanoparticles were complexed with lentivirus encoding green fluorescent protein for 10 minutes, and the complex was mixed with collagen solution. After gelation, the virus-loaded collagen gel was transferred to the 24-well plates. C6 cells were then seeded on the virus-loaded hydrogel. At 3 days after cell seeding, cells were stained with Hoechst and observed under the fluorescence microscope. Bars indicate 150 µm. (b) Active viruses within gels were measured after solubilizing the collagen gel with collagenase. The solubilized solution was added to 293 cells to assess the virus activity. Values are mean ± SD. (c) Lentivirus encoding luciferase was complexed with HA and mixed with collagen solution. C6 cells were seeded on the virus- or HA/virus- loaded collagen gel, and the luciferase activities were measured 3 days after cell seeding. RLU, relative light unit.
In vivo cell transduction in collagen and HA/collagen gel
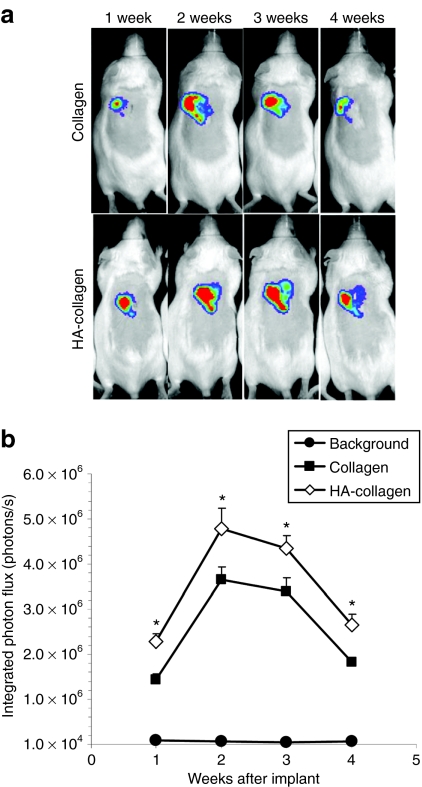
Lenti-luc-loaded collagen gels were subsequently implanted to mice subcutaneously to investigate the ability to promote long-term and localized expression in vivo. Transgene expression was localized to the implantation site for all time points and persisted for at least 4 weeks in vivo and was consistent for all animals implanted (Figure 6). Transgene expression was not observed for imaging performed at 6 weeks of implantation or later. For the initial 4 weeks of implantation, transgene expression in mice implanted with HA/virus-loaded collagen gel was greater at all time points relative to mice implanted with virus-loaded collagen gel (P < 0.05). This increased activity was consistent with the in vitro observation and suggested that virus activity was maintained in HA/virus-loaded collagen gel. Interestingly, maximal transgene expression was observed 2 weeks after implantation and declined through 4 weeks. Maximal expression at 2 weeks may result from time required for cells to infiltrate the hydrogel. Taken together, these results indicated that lentivirus-loaded collagen gel may be a valuable tool to promote localized and transient gene expression in vivo.
Figure 6.
In vivo cell transduction by lentivirus in collagen gels. (a) Imaging and quantification of firefly luciferase expression for 4 weeks following subcutaneous implantation of lentivirus or hydroxylapatite (HA)/lentivirus-loaded collagen gels. Lentivirus encoding luciferase (Lenti-luc, 3 × 108 lentivirus particles) was loaded during gelation. (b) Integrated light flux (photons/second) as measured using constant-size regions of interest over the implant site (n = 4 for experimental and background data). Collagen gels loaded with lentivirus encoding luciferase (closed squares), HA/lentivirus (open diamonds), and background (closed circles). Values are mean ± SEM. *Statistical significance relative to control group at P < 0.05.
Discussion
In this article, we report the in vitro and in vivo delivery of lentiviral vectors from collagen gels, and demonstrate that retaining and stabilizing the vector by immobilization to HA nanoparticles enhanced and localized expression within the hydrogel. Biomaterial scaffolds serve a central role in many regenerative medicine strategies by supporting cell adhesion and migration, and providing a structure that creates and maintains a space for tissue formation. Hydrogels are increasingly being developed as they have physical properties similar to soft tissues and can potentially be delivered in a minimally invasive manner. Although these features are desirable, they are typically insufficient to promote regeneration, and thus, a variety of strategies are being employed to increase the bioactivity of the hydrogels. Delivery of gene therapy vectors is potentially a powerful and versatile tool for regenerative medicine applications, or as a research tool to molecularly dissect tissue formation. However, achieving efficient and localized gene delivery is challenging due to vector release and inactivation. We thus investigated an approach based on retaining, rather than releasing, the vectors within the hydrogel in order to promote localized, efficient delivery. Importantly, retention of vectors within the hydrogel will likely require cell infiltration for gene delivery, and thus, the vectors must be stabilized to maintain their activity until cell infiltration occurs.
The collagen hydrogel provided a foundation for lentivirus encapsulation and served as the primary structure for localized delivery. Collagen is a natural biomaterial that is available in several forms, including solutions, sponges, fibers, and hydrogels at physiological temperatures. Collagen was the first tissue engineering scaffold used as a gene delivery vehicle,20 and has been employed with a range of nonviral and viral vectors. Vectors encapsulated within the hydrogel alone are released, with release occurring by a combination of diffusion and matrix degradation. The release kinetics and delivery efficiencies depend on the vector. For example, polyplexes and lipoplexes have a slower release than plasmid alone,21 though modification of the nonviral vector with PEG can enhance release. For the studies herein, the release of lentivirus incorporated directly into collagen hydrogels was dependent on the collagen content. Increasing the solid content for hydrogels is a common mechanism for slowing release.22 Previous reports have indicated that lentivirus does not bind directly to collagen I;23 thus, release was likely restricted by the physical properties of the hydrogel, such as the pore size. For viral vectors, delivery from collagen gels has enabled persistent localized expression.24,25,26 These hydrogel systems have been applied to promote tissue formation, and have induced transgene expression in vivo at a variety of sites and promoted physiological improvements in applications such as bone regeneration,1,20 wound healing,24,25,27 muscle repair,11 and optic nerve repair.28
Specific binding of lentivirus to HA nanoparticles for encapsulation within the hydrogel enhanced retention and activity of the virus relative to hydrogel alone, which increased and localized transgene expression. We report that HA-loaded collagen hydrogels implanted subcutaneously had significantly increased expression relative to the collagen hydrogels alone. Previous approaches for retention of viruses have been performed with adenovirus. An avidin-modified surface was employed to bind a biotinylated antiadenovirus antibody, which functioned to immobilize the vector.12 Although these approaches have been effective, the modification of biomaterials with large proteins (e.g., antibodies) can be challenging due to the large size of the proteins. We investigated an approach based on the inclusion of HA nanoparticles that provide binding sites that can retain and stabilize the vector. HA contains calcium, phosphate, and hydroxyl groups, which provides a complex combination of cationic and anionic residues for interaction with the lentivirus. Lentiviruses interact with the anionic head group on phosphatidylserine,29 which may suggest a potential interaction with the negatively charged phosphate groups. HA has been employed for the association of adenoviral vectors,13 and motivated the application to the lentiviral vectors. The incorporation of these particles into the hydrogel is relatively simple and may translate to other hydrogel systems. Additionally, HA nanoparticles may also be applicable to other vectors, as HA has been reported to increase activity of other vectors.13 The inclusion of HA does impact the hydrogel properties, which must be factored into the hydrogel design. A greater quantity of HA in the gel can more effectively bind entrapped lentivirus, yet can produce more rigid gels. In vitro, a 10 mg/ml dose of HA in the collagen gel had slightly lower transduction compared to a dose of 1 mg/ml HA, suggesting that the interaction between collagen and HA that may influence the physical properties of the hydrogel and the subsequent cellular response (e.g., cell infiltration).
In vivo strategies based on retaining the vector within the hydrogel must maintain the vector activity until cells infiltrate the hydrogel. Our in vitro studies suggest a connection between the rate of cell migration and the extent of transgene expression. The solid content impacts cell migration through multiple mechanisms. The rate of cell migration represents a balance of adhesion and traction forces, with matrix stiffness also playing a role.30,31 Motility through hydrogels with a mesh size smaller than cellular dimensions is also dependent on proteolytic activity.31 Taken together, high densities of collagen can limit cell migration, and transgene expression was lowest at the greatest collagen density. Note that the solid content can also impact vector retention, with higher densities increasing the retention within the hydrogel,24,25 consistent with data reported herein. Additionally, we investigated gene delivery for two cell types that have varied migration within the collagen gel. Noninvasive cells remained on the hydrogel surface, whereas invasive cells infiltrate into the gel and had greater levels of expression. Additionally, the in vivo studies demonstrated increasing levels of expression initially after implantation. The increase in gene expression is hypothesized to be related to a combination of cell infiltration into the hydrogel and cell proliferation. The decline in gene expression after day 14 likely results from either clearance of the infected cells by the immune system or a turnover of the cells. The delivery of vectors from biomaterials results in transgene expression by macrophages, which are recruited as a component of the foreign body response and are transient at the implant site. Additionally, the decline in expression may be related to clearance of transduced cells.
In conclusion, we investigated the delivery of lentiviral vectors from collagen hydrogels to promote in vitro and in vivo gene delivery. Enhancing the retention and stability of the vector within the hydrogel promoted more localized and efficient gene transfer. The collagen gel contributed to the retention and stabilization of the vector; however, the immobilization of the vector to HA nanoparticles substantially increased the vector retention and activity, which led to efficient and localized expression. The stabilization of the vector is particularly significant, given the dependence of transgene expression on cell migration for this strategy of retaining the vector. Taken together, these studies demonstrate the ability to efficiently deliver lentiviral vectors from collagen hydrogels to yield both in vitro and in vivo transgene expression, which would have broad applicability to numerous applications in regenerative medicine and in model systems that investigate tissue formation.
Materials and Methods
Virus production. Lentivirus was prepared for the studies using established techniques. Lentivirus was produced in HEK-293T cells grown in Dulbecco's modified Eagle's medium plus 10% fetal bovine serum at 37 °C, and 5% CO2. The lentiviral packaging vectors (pMDL-GagPol, pRSV-Rev, and pIVS-VSV-G) were co-transfected along with plenti-CMV-GFP or plenti-CMV-luciferase into HEK-293T cells using Lipofectamine 2000 (Roche Bioscience, Palo Alto, CA). After 48 hours of transfection, the supernatant was collected and filtered (0.45 micron). Viruses were then concentrated using PEG-it (System Biosciences, Mountain, CA), with the precipitated lentiviruses resuspended with PBS. The virus titer [lentivirus particle (LP)] was determined by HIV-1 p24 Antigen ELISA Kit (ZeptoMetrix, Buffalo, NY). Infectious titer [infectious unit (IU)] of lenti-GFP was determined by counting the number of cell expression GFP at 2 days after incubation of serially diluted viruses with HEK-293T cells.
Preparation of lentivirus-loaded collagen gel. Virus-loaded collagen gels were prepared by mixing lentivirus encoding luciferase (lenti-luc, 107 LP) with acid-soluble rat tail collagen type I (BD Biosciences, San Jose, CA), followed by neutralization and incubation at 37 °C for 30 minutes. Final collagen gel concentration was set to 0.05, 0.15, or 0.3% (wt/vol).
Assessment of active viruses retained in and released from collagen gel. Lenti-luc-loaded collagen gels (100 µl) were prepared in 96-well plates. Dulbecco's modified Eagle's medium plus 10% fetal bovine serum (100 µl) were then added to the gel and incubated at 37 °C and 5% CO2. To measure the amount of entrapped active virus, gels were washed with PBS and treated with 1 mg/ml of collagenase (Sigma-Aldrich, St Louis, MO) for 30 minutes at 37 °C. Control studies were performed to determine the concentration of collagenase that had minimal influence on the virus activity. Virus without collagen gel was used as a control and incubated with media. Supernatant that contained the virus released from the collagen gel was collected at 1, 2, and 3 days after incubation. Control viruses and supernatant samples were treated with the collagenase (1 mg/ml). Active virus was assessed by adding 10 µl of each sample to HEK-293T cells growing in a monolayer. At 2 days after infection, cells were lysed with reporter lysis buffer (Promega, Madison, WI) and assayed for enzymatic activity. The luminometer was set for a 3-second delay with signal integration for 10 seconds. Luciferase activity was normalized to total cellular protein using the bicinchoninic acid (BCA) assay kit (Pierce, Rockford, IL). Virus activity was expressed as a percentage of control (i.e., no collagen gel).
In vitro cell transduction in the virus-loaded collagen gel. To examine the transduction of the cells growing on the virus-loaded collagen gel, invasive C6 cells and noninvasive NIH3T3 cells (105 cells) were seeded on virus encoding luciferase-loaded collagen gel, which were prepared in 96-well plate. At 3 days after cell seeding, the gels were washed with PBS and digested with 1 mg/ml of collagenase. Cells were collected after centrifugation of the solution and assayed for luciferase activity. To assess the change of transduction efficiency over time, virus encoding GFP (105 IU) was loaded on collagen gel in 96-well plate and 105 C6 cells were seeded on the gel at multiplicity of infection of 1. At 3 and 5 days after cell seeding, the cells in the gels were collected as described above. The cells were isolated by trypsin and then diluted (up to 1,000) and seeded on the 24-well plate. After attaching cells to the plate, the transduction efficiency was determined by counting GFP+ cells (at least 500 cells were counted per condition).
Complexation with HA nanoparticles. Hydoxylapatite nanoparticles (Sigma-Aldrich) were suspended in PBS, and lenti-luc (1 × 106 LP) was mixed with PBS (control) or HA-PBS (1 mg/ml of final concentration of HA) for 10 minutes and incubated at room temperature. At different time points of incubation, 105 HEK-293T cells were mixed with the virus or HA–virus complex, and seeded on the 24-well plate. At 2 days after cell seeding, the luciferase activity was assayed as described above.
Cell transduction in the collagen gel. Lentivirus encoding GFP (1 × 104 IU) alone or complexed with HA (1 mg/ml) was entrapped with 0.15% collagen gels (5 µl). After transferring the gels to 24-well plate, 5 × 105 C6 cells were seeded. At 3 days after cell seeding, cells were fixed with 4% paraformaldehyde and stained with Hoechst (blue). GFP-expressing cells were visualized using a fluorescence microscope. For the quantitative measurement of the transduction in the gel, lentivirus encoding luciferase (1 × 106 LP) alone or complexed with HA was used. At 3 days after cell-seeding gel, the luciferase activity was assayed as described above.
Bioluminescence imaging. Lenti-luc (3 × 108 LP) alone or complexed with HA (1 mg/ml) nanoparticles were loaded on collagen gel and the collagen gels were implanted subcutaneously into male CD1 mice (20–22 g). In vivo luciferase expression was monitored using an IVIS Imaging System (Xenogen, Alameda, CA), which includes a cooled CCD camera. For imaging, the animals were injected i.p. with D-luciferin (150 mg/kg body weight; Molecular Therapeutics, Ann Arbor, MI) using 28-gauge insulin syringes. The animals were placed in a light-tight chamber, and bioluminescence images were acquired (every 5 minutes for a total of 20 minutes) until the peak light emission was confirmed. Gray-scale and bioluminescence images were superimposed using the Living Image software (Xenogen). A constant-size region of interest was drawn over the implantation site. The signal intensity was reported as an integrated light flux (photons/second), which was determined by IGOR software (WaveMetrics, Tigard, OR). Background photon fluxes were obtained using the same procedures prior to the injection of D-luciferin. Care and use of the laboratory animals followed the guidelines established by the Northwestern University Institutional Animal Care and Use Committee.
Statistical analysis. The statistical significance of the differences between groups was analyzed by one-way analysis of variance followed by Tukey's post hoc test for multiple comparisons. A value of P < 0.05 was accepted as significant.
REFERENCES
- Bonadio J, Smiley E, Patil P., and , Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nat Med. 1999;5:753–759. doi: 10.1038/10473. [DOI] [PubMed] [Google Scholar]
- Sakiyama-Elbert SE, Panitch A., and , Hubbell JA. Development of growth factor fusion proteins for cell-triggered drug delivery. FASEB J. 2001;15:1300–1302. doi: 10.1096/fj.00-0564fje. [DOI] [PubMed] [Google Scholar]
- Stendahl JC, Wang LJ, Chow LW, Kaufman DB., and , Stupp SI. Growth factor delivery from self-assembling nanofibers to facilitate islet transplantation. Transplantation. 2008;86:478–481. doi: 10.1097/TP.0b013e3181806d9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Kreeger PK, Shea LD., and , Woodruff TK. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739–2746. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R., and , Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492. doi: 10.1038/nature02388. [DOI] [PubMed] [Google Scholar]
- Anseth KS, Bowman CN., and , Brannon-Peppas L. Mechanical properties of hydrogels and their experimental determination. Biomaterials. 1996;17:1647–1657. doi: 10.1016/0142-9612(96)87644-7. [DOI] [PubMed] [Google Scholar]
- Segura T, Anderson BC, Chung PH, Webber RE, Shull KR., and , Shea LD. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26:359–371. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- Hartgerink JD, Beniash E., and , Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA. 2002;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutolf MP, Tirelli N, Cerritelli S, Cavalli L., and , Hubbell JA. Systematic modulation of Michael-type reactivity of thiols through the use of charged amino acids. Bioconjug Chem. 2001;12:1051–1056. doi: 10.1021/bc015519e. [DOI] [PubMed] [Google Scholar]
- Salvay DM., and , Shea LD. Inductive tissue engineering with protein and DNA-releasing scaffolds. Mol Biosyst. 2006;2:36–48. doi: 10.1039/b514174p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas J, Blease K, Craig D, Ma C, Chandler LA, Sosnowski BA, et al. Delivery of FGF genes to wound repair cells enhances arteriogenesis and myogenesis in skeletal muscle. Mol Ther. 2002;5 5 Pt 1:517–527. doi: 10.1006/mthe.2002.0579. [DOI] [PubMed] [Google Scholar]
- Levy RJ, Song C, Tallapragada S, DeFelice S, Hinson JT, Vyavahare N, et al. Localized adenovirus gene delivery using antiviral IgG complexation. Gene Ther. 2001;8:659–667. doi: 10.1038/sj.gt.3301452. [DOI] [PubMed] [Google Scholar]
- Hu WW, Wang Z, Hollister SJ., and , Krebsbach PH. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14:891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- Croyle MA, Roessler BJ, Davidson BL, Hilfinger JM., and , Amidon GL. Factors that influence stability of recombinant adenoviral preparations for human gene therapy. Pharm Dev Technol. 1998;3:373–383. doi: 10.3109/10837459809009865. [DOI] [PubMed] [Google Scholar]
- Schek RM, Hollister SJ., and , Krebsbach PH. Delivery and protection of adenoviruses using biocompatible hydrogels for localized gene therapy. Mol Ther. 2004;9:130–138. doi: 10.1016/j.ymthe.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Higashikawa F., and , Chang L. Kinetic analyses of stability of simple and complex retroviral vectors. Virology. 2001;280:124–131. doi: 10.1006/viro.2000.0743. [DOI] [PubMed] [Google Scholar]
- Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Beliën AT, Paganetti PA., and , Schwab ME. Membrane-type 1 matrix metalloprotease (MT1-MMP) enables invasive migration of glioma cells in central nervous system white matter. J Cell Biol. 1999;144:373–384. doi: 10.1083/jcb.144.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Nakamura M, Okano H., and , Toyama Y. Establishment of three-dimensional culture of neural stem/progenitor cells in collagen Type-1 Gel. Restor Neurol Neurosci. 2007;25:109–117. [PubMed] [Google Scholar]
- Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci USA. 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer F, Schillinger U, Putz U, Stemberger A., and , Plank C. Nonviral vector loaded collagen sponges for sustained gene delivery in vitro and in vivo. J Gene Med. 2002;4:634–643. doi: 10.1002/jgm.298. [DOI] [PubMed] [Google Scholar]
- Premaraj S, Mundy BL, Morgan D, Winnard PL, Mooney MP., and , Moursi AM. Sustained delivery of bioactive cytokine using a dense collagen gel vehicle collagen gel delivery of bioactive cytokine. Arch Oral Biol. 2006;51:325–333. doi: 10.1016/j.archoralbio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Shin S, Salvay DM., and , Shea LD.2009Lentivirus delivery by adsorption to tissue engineering scaffolds J Biomed Mater Res Aepub ahead of print). [DOI] [PMC free article] [PubMed]
- Chandler LA, Doukas J, Gonzalez AM, Hoganson DK, Gu DL, Ma C, et al. FGF2-Targeted adenovirus encoding platelet-derived growth factor-B enhances de novo tissue formation. Mol Ther. 2000;2:153–160. doi: 10.1006/mthe.2000.0102. [DOI] [PubMed] [Google Scholar]
- Doukas J, Chandler LA, Gonzalez AM, Gu D, Hoganson DK, Ma C, et al. Matrix immobilization enhances the tissue repair activity of growth factor gene therapy vectors. Hum Gene Ther. 2001;12:783–798. doi: 10.1089/104303401750148720. [DOI] [PubMed] [Google Scholar]
- Ochiya T, Nagahara S, Sano A, Itoh H., and , Terada M. Biomaterials for gene delivery: atelocollagen-mediated controlled release of molecular medicines. Curr Gene Ther. 2001;1:31–52. doi: 10.2174/1566523013348887. [DOI] [PubMed] [Google Scholar]
- Tyrone JW, Marcus JR, Bonomo SR, Mogford JE, Xia Y., and , Mustoe TA. Transforming growth factor beta3 promotes fascial wound healing in a new animal model. Arch Surg. 2000;135:1154–1159. doi: 10.1001/archsurg.135.10.1154. [DOI] [PubMed] [Google Scholar]
- Berry M, Gonzalez AM, Clarke W, Greenlees L, Barrett L, Tsang W, et al. Sustained effects of gene-activated matrices after CNS injury. Mol Cell Neurosci. 2001;17:706–716. doi: 10.1006/mcne.2001.0975. [DOI] [PubMed] [Google Scholar]
- Schlegel R, Tralka TS, Willingham MC., and , Pastan I. Inhibition of VSV binding and infectivity by phosphatidylserine: is phosphatidylserine a VSV-binding site. Cell. 1983;32:639–646. doi: 10.1016/0092-8674(83)90483-x. [DOI] [PubMed] [Google Scholar]
- Zaman MH, Kamm RD, Matsudaira P., and , Lauffenburger DA. Computational model for cell migration in three-dimensional matrices. Biophys J. 2005;89:1389–1397. doi: 10.1529/biophysj.105.060723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman MH, Trapani LM, Sieminski AL, Siemeski A, Mackellar D, Gong H, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci USA. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]