To the editor:
Most patients with B-cell malignancies will die from their disease or are incurable. For this reason, innovative therapeutic approaches are direly needed. Patient T cells may be genetically modified to target antigens expressed on tumor cells through the expression of chimeric antigen receptors (CARs), which are antigen receptors designed to recognize cell surface antigens in a human leukocyte antigen–independent manner.1 CD19, which is expressed on most B-cell malignancies—including most non-Hodgkin's lymphomas, acute lymphoblastic leukemias, and chronic lymphocytic leukemias (CLLs)—is an attractive antigen for this approach.2 It is present on normal B-lineage cells from the early pre–B-cell stage until plasma cell differentiation. In a model of CD19+ acute lymphoblastic leukemia, we found that that a CAR termed 19-28z, which comprises the CD28 cytoplasmic domain in addition to that of the CD3 ζ-chain,3 induced better responses than a ζ-chain-based receptor.4 In preclinical in vitro studies, we demonstrated that human T cells that express CD19-specific CARs efficiently lyse human CD19+ tumor cell lines and that CLL patient–derived T cells effectively lyse autologous tumor cells.2,4 These results, and others,5 supported a phase I clinical trial treating refractory CLL patients with autologous T cells modified by retroviral gene transfer of the 19-28z CAR.
We have thus far enrolled six patients in this clinical trial. The cohort of subjects treated with modified T cells alone at the first dose level of T cells tolerated therapy well without dose-limiting toxicities. However, the first subject (subject 4) enrolled in the second cohort of patients, in whom cyclophosphamide lymphodepleting chemotherapy was administered before infusion of the same T-cell dose, developed a syndrome of hypotension, dyspnea, and renal failure following T-cell infusion. Subject 4 died 4 days after administration of cyclophosphamide and modified T cells. Herein we describe the chronology of his treatment and report the findings of an extensive postmortem analysis.
Clinical trial design
Subject 4 was treated in a phase I clinical trial (IRB no. 06-138, NIH-RAC no. 0507-721, NCT00466531) designed to assess the safety of infusing autologous T cells modified to express the CD19-targeted CAR 19-28z in subjects with relapsed or purine analog–refractory CLL. For 2–3 days following T-cell infusion, the subjects are closely monitored for tumor lysis and unforeseen adverse events. If stable, subjects are discharged and subsequently closely followed in the outpatient clinic setting.
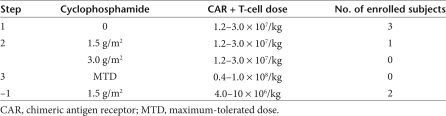
This phase I clinical trial has a three-step design (Table 1). In the first step, subjects are treated with dose level 1 of modified T cells (1.2–3.0 × 107 CAR+ T cells/kg) without prior lymphodepleting chemotherapy. The subject of the current report was enrolled in cohort 1 of step 2 and treated with 1.5 g/m2 of cyclophosphamide followed 2 days later by infusion of modified T cells at dose level 1. The enrollment thus far is summarized in Table 1.
Table 1.
Cyclophosphamide and T-cell doses in IRB protocol no. 06-138
Case report
Subject 4 was a 69-year-old man with refractory CLL who was enrolled in clinical trial IRB no. 06-138. At the time of enrollment, three previous subjects had been treated on this protocol without significant adverse events in the first planned cohort, receiving the lowest planned dose of modified T cells alone. Subject 4 was the first to receive lymphodepleting chemotherapy with cyclophosphamide (1.5 g/m2) followed 2 days later by infusion of modified T cells at the same dose tolerated earlier by the first three subjects enrolled in cohort 1 of this trial.
Subject 4's CLL treatment history. Subject 4 was initially diagnosed with CLL 8 years before treatment on this protocol, when he was noted to have an elevated lymphocyte count on a routine complete blood count in the context of lymphadenopathy. The subject had a significant past medical history of myocardial infarction, coronary artery disease, hypertension, and chronic renal insufficiency. Two years after diagnosis, because of progressive symptomatic abdominal lymphadenopathy and a rapidly doubling peripheral blood lymphocyte count, he was treated per Memorial Sloan–Kettering Cancer Center (MSKCC) IRB protocol no. 98-080 with sequential fludarabine (25 mg/m2) daily for 5 days every 4 weeks for six cycles, followed by high-dose cyclophosphamide (3 g/m2) given once every 3 weeks for three cycles, followed by rituximab (375 mg/m2) given weekly for 8 weeks. He achieved a durable partial response. Five years later, he developed evidence of progressive disease as shown by increasing lymphadenopathy, increasing peripheral blood lymphocyte counts, and cytopenias. The subject was enrolled in MSKCC IRB protocol no. 05-077 and treated with six monthly cycles of combination therapy with pentostatin (4 mg/m2), cyclophosphamide (600 mg/m2), rituximab (375 mg/m2 given only on cycles 2–6), and mitoxantrone (10 mg/m2). The subject once more achieved a partial response. Two years later, the subject presented with a rapidly increasing peripheral blood lymphocyte count, worsening cytopenias, and increasing lymphadenopathy and he was enrolled in IRB protocol no. 06-138 (NIH-RAC no. 0507-721, NCT00466531). The subject was assessed and met all criteria for enrollment in this trial.
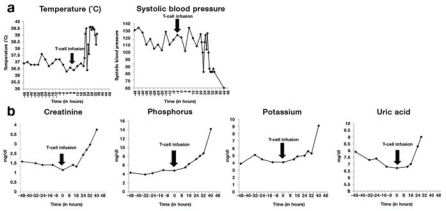
Treatment course. For IRB protocol no. 06-138, the subject underwent a leukapheresis procedure, and the product was processed and frozen. Subsequently, T cells were activated and retrovirally transduced with the 19-28z retroviral vector as described.6 The subject was then admitted and, per protocol, received tumor lysis prophylaxis with hydration and allopurinol followed by cyclophosphamide (1.5 g/m2) infusion. He tolerated therapy well, and twice-daily serum electrolyte studies revealed no evidence of tumor lysis. On the day of T-cell infusion, the subject's tumor lysis laboratory results were unremarkable, with the exception of a mildly elevated phosphorus level (4.8 mg/dl). His creatinine was 1.3 mg/dl. The T-cell infusion was completed over 3 hours without complication. Twenty hours after modified T-cell infusion, the subject developed a fever, a transient finding observed in all three subjects treated on step 1 of this protocol; however, in contrast to previously treated subjects, subject 4's fever persisted and was associated with concomitant hypotension (Figure 1a). At the same time, the subject developed respiratory distress despite a negative chest X-ray. After bacterial blood cultures had been obtained, the patient was started on broad-spectrum antibiotics (piperacillin/tazobactam and ciprofloxacin) with pressor support. He was subsequently transferred to the intensive care unit. Laboratory studies obtained at 24 hours after T-cell infusion demonstrated an elevated creatinine concentration (2.2 mg/dl) and rising phosphorus (7.4 mg/dl), potassium (5.0 mEq/L), and uric acid (8.3 mg/dl) concentrations.
Figure 1.
Clinical assessment of subject 4 on IRB no. 06-138, NIH-RAC no. 0507-721, NCT00466531. Assessment of patient from the time of admission to the hospital before cyclophosphamide chemotherapy (–48 hours), through time of modified T-cell infusion (0 hours), to time of death (44 hours). Clinical status was assessed by routine vital sign parameters, including (a) temperature and systolic blood pressure, as well as by laboratory chemistry measurements, including (b) renal function as measured by creatinine, potassium, phosphorus, and uric acid concentrations. Vital signs over time are consistent with a sepsis syndrome (fever with hypotension), whereas laboratory chemistry studies demonstrate an initial rise in creatinine coinciding with the patient's anuric state, followed by rising potassium, phosphorus, and uric acid at concentrations that are consistent with tumor lysis but confounded by the antecedent acute renal failure. The vertical arrow indicates the time of T-cell infusion.
The subject became anuric, consistent with acute renal failure. In the intensive care unit, his blood pressure responded to inotropic support, from which he was successfully and fully weaned over the course of the day. However, he remained anuric with increasing serum potassium and phosphorus concentrations (Figure 1b). By the early evening, he once more became hypotensive, and inotropic support was restarted. A worsening respiratory status led to intubation and mechanical ventilation. Supportive care was withdrawn shortly thereafter at the request of the subject's health-care proxy. The subject expired 44 hours after infusion of modified T cells. Laboratory studies just before his death demonstrated an increasing serum creatinine concentration at 3.7 mg/dl, as well as markedly elevated potassium and phosphorus concentrations at 9.1 mEq/L and 14.2 mg/dl, respectively (Figure 1b). The subject's peripheral blood lymphocytosis remained generally stable over time, beginning with initial chemotherapy and following modified T-cell infusion (data not shown).
Postmortem pathology report. Both gross and histologic postmortem analyses of tissues from subject 4 revealed extensive CLL with diffuse bulky adenopathy, including a large abdominal tumor (2.5 kg) and associated increased mesentery lymphadenopathy. Microscopic evaluation revealed diffuse CLL involvement in multiple organs, including the liver, pancreas, adrenal glands, and bone marrow, as well as the lymph nodes. Renal tissues were generally normal, other than scattered calcium crystals. Overall, these data fail to support a diagnosis of tumor lysis syndrome as the primary source of renal failure. Histology of the lung and cardiac tissues showed no significant pathology. Furthermore, initial blood cultures, as well as all subsequent cultures obtained after antibiotic administration, were negative. The infused cell product was sterile at the time of infusion, a result we reconfirmed after the occurrence of the serious adverse event (SAE).
Analysis of serum cytokines. As stipulated in the protocol, serial serum samples were routinely obtained from all subjects before and after each stage of the treatment regimen. Analyses of these serum samples revealed a significant increase in the concentrations of interleukin (IL)-2, IL-7, IL-15, and IL-12 cytokines following cyclophosphamide chemotherapy as compared with the pretreatment serum sample obtained 30 days earlier (Figure 2). An interpretation implicating the cyclophosphamide chemotherapy in this rise in serum cytokines is hampered by the 30-day time lag between obtaining the pre- and post-chemotherapy serum samples. Alternatively, the elevation of these cytokines may have been secondary to a prior subacute infectious process that was subsequently exacerbated by cyclophosphamide-mediated immune suppression, resulting in the sepsis syndrome seen in this subject. Significantly, serum tumor necrosis factor-α and interferon-γ were unchanged immediately before and after T-cell infusion (Figure 2).
Figure 2.
Serum cytokine concentrations measured in subject 4. Serum samples were obtained 30 days before cyclophosphamide (–30 d), 2 hours before T-cell infusion (–2 h), and 4 and 26 hours after T-cell infusion (+4 h, +26 h, respectively). The –2-h sample is therefore post-cyclophosphamide but pre-T-cell infusion. Pretreatment tumor necrosis factor-α (TNF-α) serum values were 200, 50, and 59 ng/ml in subjects 1, 2, and 3, respectively. IFN-γ, interferon-γ IL, interleukin.
Conclusion
Etiology of SAE. Subject 4, a 69-year-old patient with bulky CLL, was the first to receive T cells following prior lymphodepleting chemotherapy (IRB protocol no. 06-138, step 2, cohort 1). In the first cohort (step 1), in which three subjects were treated with the lowest planned modified T-cell dose alone, all experienced transient fevers following T-cell infusion but otherwise tolerated therapy well. These subjects had no evidence of hypotension, tumor lysis, or acute renal failure. In contrast to subjects treated in the first cohort, subject 4 developed persistent fevers following T-cell infusion, became hypotensive, and developed acute renal failure, all consistent with a clinical picture of sepsis. Significantly, acute renal failure developed in the absence of any clinical evidence suggestive of tumor lysis syndrome. A later rise in serum potassium, phosphorus, and uric acid concentrations may be indicative of a subsequent incipient tumor lysis. This sequence of events thus suggests that the patient developed renal failure due primarily to hypotension as a consequence of sepsis-like syndrome, a conclusion supported by the postmortem examination of renal tissues.
Although consistent with an infectious etiology, the subject's blood cultures as well as postmortem cultures failed to detect any bacterial growth, noting that the latter may have been compromised by broad-spectrum antibiotic therapy.
Serum cytokine analysis revealed markedly elevated levels of the proinflammatory and homeostatic cytokines IL-2, IL-7, IL-15, and IL-12 following cyclophosphamide chemotherapy and preceding the T-cell infusion. The etiology of this elevated cytokine profile remains unclear, because the pretreatment serum sample was obtained 30 days before chemotherapy. Nevertheless, regardless of the etiology, this cytokine milieu, highly favorable to T-cell persistence, activation, and proliferation, may account for the possible incipient tumor lysis seen in subject 4 but not in subjects 1–3.
Combining this biological evidence with the unremarkable lung, heart, and kidney pathology, this SAE, despite negative blood cultures, is consistent with sepsis due to infection, leading to hypotension, leading to acute renal failure and, ultimately, death. This scenario is also consistent with infection as a prevalent and leading cause of morbidity and mortality in patients with advanced CLL.
Consequent modification of clinical trial protocol. Our findings fail to directly attribute the SAE in subject 4 (IRB protocol no. 06-138) to the modified T cells. Nevertheless, because of the temporal relationship of the autologous T-cell infusion to this SAE, we conservatively attributed infusion of modified T cells as a “possible” source contributing to this SAE. As stipulated in the protocol, we reduced the CAR+ T-cell dose in the next cohort of patients to the –1 dose (0.4–1 × 107 modified T cells/kg, Table 1). As a further precaution to enhance patient safety, we modified the protocol by administering T cells as a split infusion, infusing one-third of the dose on day 2 following cyclophosphamide therapy and, in the absence of evidence of tumor lysis, hypotension, or renal failure, administering the remaining two-thirds of the planned T-cell dose on day 3 following cyclophosphamide therapy. Significantly, the subsequent subject treated on this trial under these modified conditions did not exhibit evidence of a “cytokine storm” following cyclophosphamide chemotherapy and further tolerated infusion of modified T cells without any notable toxicities. We will continue to focus on analyses of serum cytokine studies before and following both cyclophosphamide chemotherapy and modified T-cell infusion, with special attention to the conditioning-induced cytokine response and its relationship to post-T-cell infusion cytokine concentrations.
Note
Since the submission of this letter, we have treated a sixth subject at the −1 treatment dose (see Table 1). This subject exhibited a transient hypotensive episode 24 hours after T-cell infusion that responded to increased intravenous hydration, accompanied by a mild, transient fever. This episode rapidly resolved with no evidence of infectious etiology or renal compromise. Cytokine serum analyses were unremarkable.
REFERENCES
- Sadelain M, Rivière I., and , Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C.et al.(2003Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15 Nat Med 9279–286. [DOI] [PubMed] [Google Scholar]
- Maher J, Brentjens R, Gunset G, Rivière I., and , Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- Brentjens RJ, Santos E, Nikhamin Y, Yeh R, Matsushita M, La Perle K.et al. (2007Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts Clin Cancer Res 135426–5435. [DOI] [PubMed] [Google Scholar]
- Sadelain M, Brentjens R., and , Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyman D, Stefanski J, Przybylowski M, Bartido S, Borquez-Ojeda O, Taylor C.et al. (2009Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy J Immunother 32169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]