Small interfering RNA (siRNA) has generated enormous interest among both basic and clinical researchers despite the relatively young age of this technology. Theoretically any gene could serve as a target for RNA interference, not just the “druggable” targets against which small-molecule compounds are directed. To develop therapeutics based on siRNA, effective delivery to target tissues and cells is required. However, siRNA cannot cross the cell membrane easily owing to its relatively large size and high negative charge. In addition, systemically delivered siRNAs face a series of barriers in vivo: aggregation with serum proteins, uptake by the reticuloendothelial system, kidney filtration, and degradation by endogenous nucleases. Therefore, lack of an efficient delivery system is currently the primary bottleneck for clinical application of siRNA. Although siRNA has entered human clinical trials for the treatment of various diseases, most of these approaches have been limited to local delivery such as by direct injection into the eye or skin.1,2 Recently, two groups (Semple et al.3 and Love et al.4) independently reported promising results of delivery of siRNA to the liver using cationic lipids, bringing us one step closer to the goal of realizing systemic siRNA application to treat human disease.
Cationic lipids have been widely used in nonviral delivery systems for oligonucleotides since Felgner et al. first demonstrated successful transfection in vitro.5 Most of these systems involve formulations of nanoparticles comprising cationic lipids and neutral lipids, such as cholesterol. The role of cationic lipid is to encapsulate the negatively charged siRNA by electrostatic interaction and to facilitate nanoparticle interaction with cell membranes, so as to aid internalization (typically by endocytosis). Furthermore, cationic lipids can form ion pairs with anionic phospholipids within the endosomal membrane and can thus destabilize the membrane by adopting an inverted micelle or hexagonal (HII) phase, a characteristic expected to trigger endosomal escape and improve cytoplasmic localization of the cargo siRNA.6,7 However, simple ionic complexes of siRNA and cationic lipid are not suitable for systemic administration because of their poor serum stability and tolerability. Therefore, formulation strategies have to be applied. Typically, polyethylene glycol (PEG) is used to shield the positive charge from the cationic lipid.
To expand the diversity of cationic lipids available for in vivo siRNA delivery, Semple et al. used a rational-design approach based on structure–activity relationship. 1,2-dilinoleyloxy-3-dimethylaminopropane (DLinDMA) was selected as the lead compound. DLinDMA was used as the key component of the previously reported “stable nucleic acid lipid particles” (SNALP) formulation, developed by the same group for systemic administration.8 A cationic lipid was separated into three functional moieties: the alkyl chain, a linker, and an amine-based head group. It was found that a linoleyl lipid containing two double bonds per hydrocarbon chain (DLinDMA) was optimal for the delivery activity. Therefore, further optimization was focused on the linker and the head group.
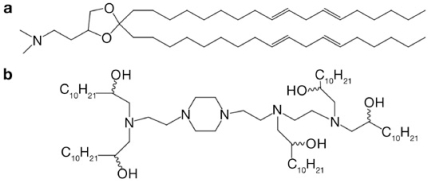
Chemical or enzymatic stability and hydrophilicity are important criteria for linker design. In the new work, Semple et al. found that the introduction of a ketal ring linker significantly increased activity in vivo, whereas ester-containing lipids were less favorable, probably because they are more susceptible to hydrolysis. The most important parameters for this study are the pKa of the ionizable head group and the distance of the charge presented to the lipid bilayer interface.3 The amine head group should maintain a neutral or low cationic surface charge density at pH 7.4, providing longer half-life in the circulation and reducing nonspecific cytotoxicity. Once reaching the acidic environment of the endosome, the amine should be protonated so as to induce an endosomolytic HII (inverted micelle) phase structure. The best-performing lipid, DLin-KC2-DMA (Figure 1a), decreased the half-maximal effective dose (ED50) of the siRNA in rodents from ~0.1 mg/kg for the unoptimized SNALP to ~0.02 mg/kg for the SNALP formulated with this lipid. This same formulation also showed an exciting and very promising silencing effect in nonhuman primates. The mechanism-guided design enabled the authors to better control the serum stability and intracellular trafficking, resulting in increased potency.
Figure 1.
The best-performing lipids in two recent studies. (a) DLin-KC2-DMA, generated by Semple et al.3 (b) C12-200, generated by Love et al.
By contrast, Love et al. used a library approach to identify novel lipids.4 They synthesized a library of combinatorial epoxide-derived, oligoamine-containing, lipidlike materials termed “lipidoids.” This one-step synthetic scheme greatly increased the scale and variability of the delivery lipids. One of the major advantages of this strategy is that it is amenable to coupling with high-throughput screening approaches because the reactions can be completed rapidly and the products can be used in cell experiments without purification. In this study, the resultant materials were made to form complexes with siRNA directly at different weight ratios for in vitro screening. Twelve of the top-performing lipidoids were formulated with neutral lipids as well as PEG and then evaluated in vivo in a mouse hepatocyte-specific delivery model. The best-performing lipidoid, C12-200 (Figure 1b), was able to achieve siRNA-mediated gene silencing at a dose of 0.01 mg/kg in mice and 0.03 mg/kg in nonhuman primates. It is worth noting that the in vitro and in vivo delivery effects were not strongly correlated. Therefore, a high-throughput in vivo screening might be more helpful in this library-based research to identify the best lipidoid.
The structures of the best-performing compounds identified by these two groups are shown in Figure 1. DLin-KC2-DMA, designed by Semple et al., contains a tertiary amine head group. The pKa of this amine on the nanoparticle surface should be close to 7 because of crowding with the neighboring groups. Yet, in the acidic endosome, it should become protonated and positively charged so as to be available for ion pairing with the negatively charged endosomal lipids. C12-200, designed by Love et al., is almost a macromolecule. It contains multiple tertiary amines, the pKa of which should also be close to but no greater than 7. As with DLin-KC2-DMA, C12-200 should be protonated in the acidic endosome to form ion pairs with the negatively charged endosome lipids. The molecule also contains five alkyl chains. As a whole, C12-200 contains bulky hydrophobic tails. Whether it has the tendency to form the inverted micelle, or HII phase, as does DLin-KC2-DMA, will need to be tested. Thus, the following design criteria may be useful for creating future delivery agents: (i) one or more tertiary amines with relatively low pKa to generate a weakly cationic head group in which the positive charge density is highly pH dependent, (ii) more than one alkyl tail so as to form a bulky hydrophobic corona, and (iii) an ideal length of alkyl tail that may lie within the range of 12–18 carbons.
The unprecedented low-dose delivery achieved by these two studies is of great importance. Not only was the delivery efficiency improved, the carrier material was well tolerated by the treated animals. Because silencing can be induced at a low dose, the amount of excipients required is also greatly reduced. In fact, dose-dependent toxicity and pulmonary inflammation of cationic lipid limits its effectiveness. It has been demonstrated that cationic lipids can modify cellular signaling pathways and stimulate specific immune or anti-inflammatory responses.9 Tekmira Pharmaceuticals (British Columbia, Canada) recently terminated a clinical trial of liposomal siRNA for hypercholesterolemia because of “potential for immune stimulation to interfere with further dose escalation” (http://clinicaltrials.gov/ct2/show/NCT00927459?term=siRNA&rank=9). Although the underlying reasons have not been clearly identified, the immunostimulatory activity of cationic lipid should be considered carefully. The lipid or lipidoid presented by these two studies may help reduce or even eliminate such adverse effects. Orders-of-magnitude decreases in the required dose will further decrease the potential for toxicity.
Cationic lipids hold great promise for systemic delivery of siRNA. However, they are not the only solution. The fate of a siRNA formulation in vivo is affected by various factors such as particle size, morphology, and surface chemistry. Sophisticated structures of the particles and preparation methods also influence the in vivo effect considerably. Other formulation strategies, such as attaching a targeting moiety to the nanoparticle, could further enhance the delivery efficiency. We believe that rationally designed delivery systems formulated with promising novel delivery materials will facilitate the path to the development of the full potential of siRNA-based therapeutics.
REFERENCES
- Castanotto D., and , Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leachman SA, Hickerson RP, Schwartz ME, Bullough EE, Hutcherson SL, Boucher KM, et al. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol Ther. 2010;18:442–446. doi: 10.1038/mt.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG, Whitehead KA, Querbes W, Dorkin JR, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107:1864–1869. doi: 10.1073/pnas.0910603106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., and , Szoka FC., Jr Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- Hafez IM, Maurer N., and , Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- Lonez C, Vandenbranden M., and , Ruysschaert JM. Cationic liposomal lipids: from gene carriers to cell signaling. Prog Lipid Res. 2008;47:340–347. doi: 10.1016/j.plipres.2008.03.002. [DOI] [PubMed] [Google Scholar]