Abstract
Short-interfering RNAs (siRNAs) have engendered much enthusiasm for their ability to silence the expression of specific genes. However, it is now well established that siRNAs, depending on their sequence, can be variably sensed by the innate immune system through recruitment of toll-like receptors 7 and 8 (TLR7/8). Here, we aimed to identify sequence-based modifications allowing for the design of bifunctional siRNAs with both proinflammatory and specific silencing activities, and with potentially increased therapeutic benefits. We found that the introduction of a micro-RNA (miRNA)-like nonpairing uridine-bulge in the passenger strand robustly increased immunostimulatory activity on human immune cells. This sequence modification had no effect on the silencing efficiency of the siRNA. Increased immunostimulation with the uridine-bulge design was specific to human cells, and conserved silencing efficiency required a Dicer-substrate scaffold. The increased cytokine production with the uridine-bulge design resulted in enhanced protection against Semliki Forest virus (SFV) infection, in viral assays. Thus, we characterize a design scaffold applicable to any given siRNA sequence, that results in increased innate immune activation without affecting gene silencing. Our data suggest that this sequence modification coupled with structural modification differentially recruits human TLR8 over TLR7, and could have potential application in antiviral therapies.
Introduction
RNA interference (RNAi) is an evolutionarily conserved antiviral mechanism that relies on short molecules of double-stranded RNA of 21–23 base pairs (bp), known as short-interfering RNAs (siRNAs). Among species, the hallmark of RNAi is its specificity, affecting only the expression of the target gene sharing perfect homology with the siRNA sequence. Several siRNAs targeting disease-causing genes are currently in clinical trials, and the therapeutic potential of these double-stranded oligoribonucleotides is extensive.1 However, we and others have shown that siRNAs can be sensed by the mammalian immune system, compromising the specificity of silencing.2,3,4 In addition to the recognition of low-molecular-mass synthetic agonists such as imidazoquinolines, toll-like receptors 7 and 8 (TLR7/8) can sense single-stranded RNAs (ssRNAs) and siRNAs in a sequence-specific manner.2,3,5 Recent publications indicate that short RNAs can be differentially sensed by TLR7 and TLR8, according to their sequence.6,7 The expression of these receptors is restricted to certain blood immune cell subtypes, including human plasmacytoid dendritic cells, which express the highest levels of TLR7, and human monocytes/macrophages that express the highest levels of TLR8 (ref. 8). Systemic delivery of siRNA in vivo is intrinsically related to a potential recruitment of these immune cells, as both TLR7 and TLR8 are located in the endosomal compartment and can sense endocytosed ssRNA/double-stranded RNA.9 TLR7 activation in plasmacytoid dendritic cells preferentially induces interferon-α (IFN-α) production, whereas TLR8 activation in monocytes/macrophages results in production of tumor necrosis factor-α (TNF-α) and interleukin-12(p70) (IL-12(p70)) (ref. 10).
Although immune activation caused by siRNAs was unanticipated and is a cause of concern,11 backbone modifications have been developed that dampen activation of an immune response.12,13,14 However, we have previously proposed that it might be therapeutically beneficial to design siRNAs that provoke an enhanced immune response and enhance siRNA-mediated antiviral and antitumor therapy.11 Indeed, proof of principle of a “bifunctional” siRNA approach combining gene silencing and immunostimulation demonstrating antitumoral synergy between innate immune recruitment and gene-specific targeting has recently been published.15 However, this study relied on a 5′-triphosphate modification of siRNA and activation of innate immunity via retinoic acid–inducible gene I (RIG-I).15
To date, there are several reports that certain motifs in siRNA duplexes specifically induce TLR7/8 (refs. 2,3), and detailed studies of 19–21 nucleotide (nt) ssRNAs have identified different motifs that promote TLR7/8 recruitment (ref. 6, European patents EP1764107 and EP1764108). Although useful, determination of immunostimulatory motifs in each individual strand of an siRNA is a poor predictor of the immunostimulatory potential of the resulting duplex (refs. 4,16 and M.P. Gantier and B.R.G. Williams, unpublished results). Importantly, rational design of efficient siRNAs remains predictive, and in most cases only a few siRNAs can be confirmed to promote strong silencing. Selection of siRNAs based on criteria of both RNAi efficiency and sequenced-based immunostimulatory potential may compromise one or other parameter. To our knowledge, there is currently no modification described that can be applied to a given siRNA duplex to increase its ability to recruit TLR7/8 without impacting on its gene-silencing efficiency. Here, we characterize a micro-RNA (miRNA)-like sequence modification that robustly increases immunostimulation of three independent Dicer-substrate siRNAs (D-siRNAs). This modification did not impact on silencing efficiency of the duplexes, and likely involves specific TLR8 recruitment.
Results
Structural/sequence requirements of short RNAs for TLR7/8 activation
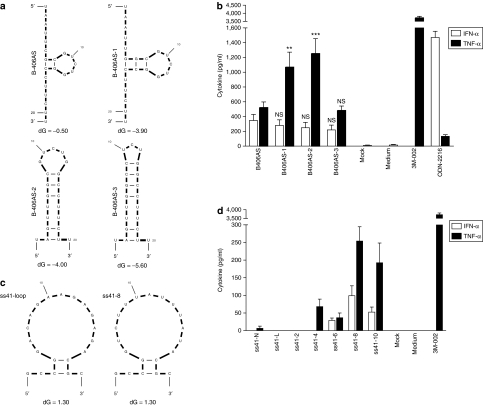
We and others have previously demonstrated that TLR7/8 sensing of short RNAs is uridine dependent.5,7,17 However, our previous findings also indicated that the position of the uridine residues within the secondary structure of an ssRNA could impact on immunostimulation.7 To further characterize the impact of secondary structure of ssRNAs on TLR7/8 recruitment, we used a rational approach to increase the predicted self-complementarity of an ssRNA previously shown to activate both IFN-α and TNF-α in human peripheral blood mononuclear cells (PBMCs), without affecting its uridine content (B-406AS; ref. 7) (Figure 1a and Table 1). Increasing the secondary structure did not significantly affect IFN-α, but it strongly impacted on the TNF-α induction profile in human PBMCs (more than twofold increase for B-406AS-1 and B-406AS-2 compared to B-406AS; Figure 1a,b). Surprisingly, restriction of the 5-nt loop of B-406AS-2 to a 3-nt loop (see B-406AS-3) ablated the TNF-α induction seen in B-406AS-2. In parallel, we also assessed the net impact of uridine content in an ssRNA with low self-secondary structure, independent of structure variation (Figure 1c and Table 1). In accord with uridine-dependent TLR7/8 sensing, both IFN-α and TNF-α were induced in a dose-dependent manner with increasing uridines (Figure 1d, compare ss41-N and ss41-4/6/8/10). At the doses of ssRNA used (90 nmol/l), a minimum of four uridines was required to detect immunostimulation (see ss41-4, for TNF-α), consistent with our previous observations (see SC and SB ssRNAs7). However, more than eight uridines did not result in any further increase in cytokine production (compare ss41-8 and ss41-10, for IFN-α and TNF-α Figure 1d). Collectively, these results support a role for both uridine content and secondary structure in TLR7/8 sensing of ssRNAs.
Figure 1.
Relative immunostimulation of ssRNAs with sequence or structural alterations. (a,b) mFOLD-predicted structure of ssRNAs at 37 °C (ref. 44). All ss41-RNAs have a similar predicted secondary structure, and only ss41-loop and ss41-8 are presented here. The amount of uridines in the ss41 series is indicated by the x in the ss41-x name. Predicted free-energy of the loops is indicated with dG value. ss41-Native does not have a predicted secondary structure. All sequences are shown in Table 1. (c,d) Immunostimulation of ssRNAs in human peripheral blood mononuclear cells treated with 90 nmol/l of indicated ssRNA complexed with N-[1-(2,3-dioleoyloxy)propyl]-N,N, Ntrimethylammonium methylsulfate and incubated for 16 hours at 37 °C. Cytokine production was measured by specific enzyme-linked immunosorbent assay as described in Materials and Methods. The data are averaged from (c) three independent experiments in biological triplicate from four blood donors and (d) two independent experiments in biological triplicate from three blood donors. (c) Unpaired two-tailed t-test and nonparametric two-tailed Mann–Whitney test were used for IFN-α and TNF-α statistical comparison compared with B-406AS, respectively. IFN, interferon; NS, nonsignificant; ssRNA, single-stranded RNA; TNF, tumor necrosis factor.
Table 1.
Sequences of ssRNAs used in the study
Rational design of immunostimulatory siRNAs
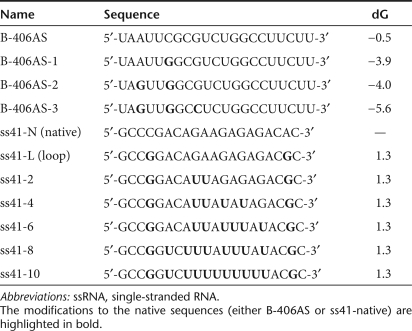
To study the impact of uridine content and secondary structure of siRNA duplexes on both immunostimulation and silencing efficiency, we used a rational approach affecting the sequence of siRNA duplexes. Although siRNAs are conventionally designed as 21–23 bp duplexes to mimic Dicer products, it has been shown that double-stranded RNAs that are long enough to be treated as Dicer-substrates (D-siRNAs) can also promote RNAi in mammalian cells.18,19 Further, using an asymmetric D-siRNA of 25/27 nt with both blunt and 3′ overhang ends helps to mimic the natural substrates of mammalian Dicer (i.e., pre-miRNA hairpins) and allows for increased directional processing and selection of the guide strand of the siRNA.18,19 The 3′-end of an asymmetric D-siRNA (using the passenger strand as a reference) is preferentially cleaved by Dicer and should have little impact on sequence-specific silencing.18 Accordingly, a set of three D-siRNAs was synthesized with increased uridines in the region predicted to be cleaved by Dicer (siLam-1, siLam-2, siLam-3; Figure 2a). Noting that TLR8 may be sensitive to loose secondary structures embedded within double-stranded structures (Fig. 1), we designed a Dicer-substrate with an miR-29a-like uridine-bulge on the passenger strand (position 9-12), predicting that this modification would not alter Dicer-processing and strand selection (siLam-4; Figure 2a). These siRNAs were derived from a previously validated D-siRNA sequence targeting the human LaminA/C (LMNA) mRNA (M.A. Behlke, unpublished results). Unexpectedly, when assayed for immunostimulation in human PBMCs, a decrease in IFN-α and/or TNF-α levels with siLam-1 and siLam-2 was observed when compared to the native siLam control (Figure 2b). Nevertheless, both siLam-3 and siLam-4 promoted a significant increase in IFN-α and/or TNF-α production. In HEK 293T cells, siLam-2 and siLam-4 both silenced LaminA/C mRNA expression with equal efficacy to the control sequence, but the other approaches were less effective (Figure 2c). siLam-4 silencing efficacy was confirmed by measuring LaminA/C mRNA silencing by siLam-4 compared to control siLam-N in dose–response experiments (at 10, 2, and 0.4 nmol/l), where no significant differences were observed (data not shown). Silencing was sequence-specific and mutation of two bases in the guide strand of the siRNA-ablated RNAi (Supplementary Figure S1, compare siLam-4 and siLam-4-MIS; sequence detailed in Figure 4a). These results indicated that the addition of an miRNA-like uridine-bulge to a D-siRNA scaffold resulted in increased immunostimulation without affecting RNAi activity.
Figure 2.
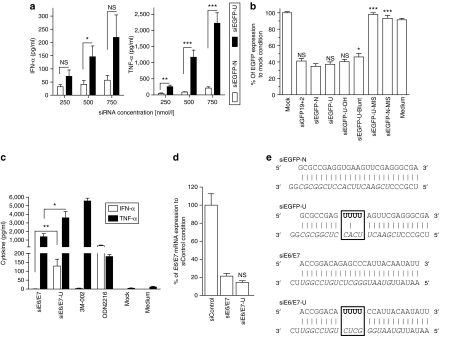
Rational design of immunostimulatory siRNA scaffold. (a) Overview of design approach. siLam-N is the original siRNA sequence targeting the LaminA/C mRNA. Watson–Crick base-pairing is shown with a “і” symbol; G:U wobble pairing is indicated with a dot. The target-specific sequence of the guide strand is in italics, and the “GU” immunostimulatory motifs added to the native sequence are in bold. (b) Immunostimulation of siRNAs in human peripheral blood mononuclear cells treated with 600 nmol/l of siRNA complexed with N-[1-(2,3-dioleoyloxy)propyl]-N,N, Ntrimethylammonium methylsulfate and incubated for 16 hours at 37 °C. Cytokine production was measured by specific enzyme-linked immunosorbent assay. The data are averaged from three independent experiments in three different blood donors. Unpaired two-tailed nonparametric Mann–Whitney tests are versus siLam-N. (c) Knockdown efficiency of siRNAs transfected in HEK 293 cells at a final concentration of 10 nmol/l. After 24 hours at 37 °C, total RNA was extracted and reverse-transcribed as described in Materials and Methods. LaminA/C mRNA expression was assessed by quantitative real-time PCR, and reported to that of GAPDH. The data are expressed as percentage of fold expression of siControl, and are averaged from two independent experiments in biological triplicate. Unpaired two-tailed t-test is presented using siLam-N as a reference. IFN, interferon; siRNA, short-interfering RNA; TNF, tumor necrosis factor.
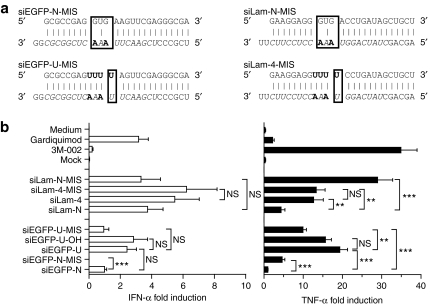
Figure 4.
Relative contribution of uridine motif and bulge structural modification in increased immunostimulation. (a) Details of siEGFP and siLam mutated designs. Watson–Crick base-pairing is shown with a “і” symbol, the 19-nt target-specific sequence of the guide strand is in italics with the exception of the introduced mutations in bold, and the “U” bulge immunostimulatory motifs added to the native sequences are in bold. The regions with structural distortions are boxed. (b) Human peripheral blood mononuclear cells were treated with 600 nmol/l of specified siRNA complexed with N-[1-(2,3-dioleoyloxy)propyl]-N,N, Ntrimethylammonium methylsulfate and incubated for 16 hours at 37 °C. Cytokine production was measured by specific enzyme-linked immunosorbent assay. The data are averaged from two independent experiments in three blood donors in biological triplicate. The results for each treatment were reported to the siEGFP-N condition, and are presented as fold induction of cytokine production to account for variations in cytokine levels between blood donors. Unpaired two-tailed nonparametric Mann–Whitney tests are shown. IFN, interferon; siRNA, short-interfering RNA; TNF, tumor necrosis factor.
Validation of the uridine-bulge siRNA design
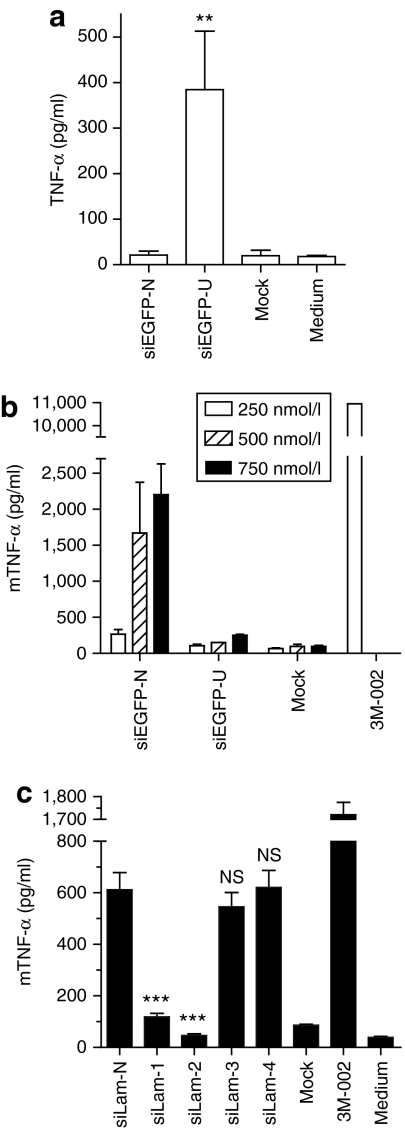
In order to validate that the previous observations made with the siLam-4 design could be reproduced independently of the sequence of the duplex/target chosen, a D-siRNA targeting the enhanced green fluorescent protein (EGFP) was selected using the siRNA design software BIOPREDsi.20 From the five best hits suggested by BIOPREDsi, the duplex with the least number of uridine residues was selected to minimize the basal immunostimulatory activity of the siRNA, and 6-nt complementary to the EGFP target added to make it a D-siRNA (Figure 3e). When comparing this EGFP D-siRNA with (siEGFP-U) or without (siEGFP) the bulge modification in immunostimulatory assays, a strong increase in TNF-α induction (>10 fold at 500 and 750 nmol/l) and a significantly higher induction of IFN-α (at 500 nmol/l) with the uridine-bulge modification was observed (Figure 3a). In addition, the uridine-bulge did not affect the ability of the EGFP D-siRNA to trigger RNAi in HEK 293T cells stably expressing EGFP (Figure 3b, compare siEGFP-N and siEGFP-U). Similarly to what was shown for the LaminA/C siRNAs, introduction of two mismatches in the guide strand of the siRNA ablated the downregulation of EGFP (siEGFP-U-MIS and siEGFP-N-MIS; sequences detailed in Figure 4a). Similar results were seen with another D-siRNA target, the E6/E7 oncogene of the human papilloma virus 16 (Figure 3c–e, compare siE6/E7 and siE6/E7-U). Collectively, these results support the findings that independent of the D-siRNA sequence, the uridine-bulge design robustly increases TNF-α and IFN-α induction in human PBMCs, without altering the ability of the D-siRNA to enter the RNAi pathway.
Figure 3.
Validation of uridine-bulge siRNA design scaffold. Human peripheral blood mononuclear cells were treated with indicated concentration of (a) specified siRNA or (c) 750 nmol/l complexed with N-[1-(2,3-dioleoyloxy)propyl]-N,N, Ntrimethylammonium methylsulfate and incubated for 16 hours at 37 °C. Cytokine production was measured by specific enzyme-linked immunosorbent assay. (a) The data are averaged from four independent experiments in four blood donors. Unpaired two-tailed nonparametric Mann–Whitney tests are shown. (b) RNA interference efficiency of enhanced green fluorescent protein (EGFP) siRNAs. HEK 293 cells stably expressing EGFP were transfected with 10 nmol/l of indicated siRNA for ~48 hours. EGFP production was assayed in fluorescent plate-reader as described in Materials and Methods. The data are presented as the EGFP protein concentration relative to that of the mock-transfected control, and are averaged from three independent experiments in biological triplicate. siGFP19+2 is a 21-bp siRNA targeting EGFP,4 siEGFP-U-MIS and siEGFP-N-MIS bear two mismatches with the target sequence (see Figure 4a), and siEGFP-U-Blunt and siEGFP-U-OH have altered termini (Supplementary Table S1). Unpaired two-tailed t-test is presented using siEGFP-U as a reference. (c) The data are averaged from two blood donors in biological triplicate and are representative of two independent experiments. Unpaired two-tailed nonparametric Mann–Whitney tests are shown. (d) Knockdown efficiency of siRNAs transfected in Murine TC-1 cells at a final concentration of 10 nmol/l. After 24 hours at 37 °C, total RNA was extracted and reverse-transcribed as described in Materials and Methods. The E6/E7 mRNA was assessed by quantitative real-time PCR, and presented relative to that of mouse GAPDH. The data are expressed as percentage fold expression of siControl, and are averaged from two independent experiments in biological triplicate. Unpaired two-tailed t-test comparing siE6/E7 and siE6/E7-U is shown using siE6/E7 as a reference. (e) Details of siEGFP and siE6/E7 designs. Watson–Crick base-pairing is shown with a “і” symbol, the 19-nt target-specific sequence of the guide strand is in italics, and the “U” bulge immunostimulatory motifs added to the native sequences are in bold. IFN, interferon; siRNA, short-interfering RNA; TNF, tumor necrosis factor.
Cooperative action of the uridine residues and the bulge structural modification increases immunostimulation
Introduction of the uridine-bulge modification combines both an increase in the overall amount of uridines in the siRNA duplexes, and a structural distortion of the central pairing of the two strands. Having observed that an increase in the amount of uridines did not necessarily correlate with enhanced immunostimulation of an siRNA duplex (Figure 2b, see siLam-1 and siLam-2), we next addressed the contribution of the structural distortion imposed by the uridine modification, in the increased immunostimulation. Mutation of the guide strand restoring partial pairing between the uridine motif of the sense strand (Figure 4a) significantly impaired TNF-α production (approximately twofold) for siEGFP-U-MIS compared to siEGFP-U (Figure 4b). However, siEGFP-U-MIS remained more immunostimulatory than the native siEGFP-N duplex (indicating a role for the uridines in the increased immunostimulation of siEGFP-U). Nevertheless, the same modification had no significant impact on siLam-4-MIS immunostimulatory potential (Figure 4b, compare siLam-4 and siLam-4-MIS), suggesting that the uridine motif is the prevalent modification inducing immunostimulation for this duplex. In addition, we generated duplexes with a central structural distortion but no uridine motif using the native sense strand of our EGFP and Lamin siRNAs (Figure 4a, siEGFP-N-MIS and siLam-N-MIS compared to siEGFP-N and siLam-N). For both siRNA species, the addition of central mismatches in the D-siRNA duplex resulted in a significant increase of TNF-α production (more than sixfold), although the IFN-α levels were not significantly upregulated (Figure 4a, siEGFP-N-MIS and siLam-N-MIS). Taken together, these results indicated that increased cytokine production with the uridine-bulge modification results from a cooperative effect of both increased uridine content and structural distortion.
Uridine-bulge sensing is not conserved between human and mouse
The preferential induction of TNF-α over IFN-α with the uridine-bulge modification of all D-siRNAs suggested a predominant role for monocytes in sensing the bulge. To better define the immune cell subtype involved in the sensing of the modification, the ability of isolated CD14+ monocytes to respond to the uridine-bulge of siEGFP-U was assessed (Figure 5a). As anticipated, a significantly increased production of TNF-α was observed with the uridine-bulge D-siRNA (siEGFP-U) (>15-fold) in these cells. We have recently reported that human monocytic cells sense endosomal short RNAs through both TLR7 and TLR8 recruitment,7 and we found that the N-[1-(2,3-dioleoyloxy)propyl]-N,N, Ntrimethylammonium methylsulfate (DOTAP)–mediated delivery of fluorescently labeled siRNAs to adherent primary human monocytes localizes to the endosomal compartment (data not shown). To further characterize the respective involvement of both receptors in the sensing of the uridine-bulge modification, we used mouse bone-marrow-derived macrophages (BMMs), in which sensing of DOTAP-transfected ssRNA/siRNA exclusively relies on TLR7 due to the lack of mouse TLR8 response to RNA agonists (Supplementary Figure S2).2,5,7 Unexpectedly, the uridine-bulge modification inhibited rather than promoted the immunostimulatory effect of the siEGFP D-siRNA, as measured by TNF-α production (Figure 5b). A similar observation was made in a mouse macrophage cell line (RAW-ELAM) with the LaminA/C series of siRNAs (Figure 5c). Although the mouse macrophage cells responded to siLam-N and siLam-1/2 in a similar fashion to that seen in human PBMCs, there was no significant increase of TNF-α production with either siLam-3 or the uridine-bulge variant siLam-4, highlighting species-specific differences in sensing of these siRNAs. Although implicating human TLR8 in sensing of the uridine-bulge modification, it precludes using murine-based disease models to measure potential therapeutic benefits of the uridine-bulge modification.
Figure 5.
Species-specific response to the uridine-bulge. (a) Fluorescence-activated cell-sorting-purified CD14+ human monocytes and (b) mouse bone-marrow-derived macrophages were stimulated with (a) 500 nmol/l or (b) indicated concentration of specified siRNA complexed with N-[1-(2,3-dioleoyloxy)propyl]-N,N, Ntrimethylammonium methylsulfate (DOTAP) and incubated for 16 hours at 37 °C. Cytokine production was measured by mouse TNF-α enzyme-linked immunosorbent assay (ELISA). (a) The data are averaged from two independent experiments in biological triplicate in two blood donors. Unpaired two-tailed nonparametric Mann–Whitney test comparing siEGFP and siEGFP-U is shown. (b) The data are representative of two independent experiments in biological triplicate. (c) RAW-ELAM mouse macrophages were treated with 750 nmol/l of indicated DOTAP-complexed siRNA and incubated for 16 hours at 37 °C. Cytokine production was measured by mouse TNF-α ELISA. The data are averaged from three independent experiments in biological triplicate. Unpaired two-tailed nonparametric Mann–Whitney tests comparing to siLam-N are shown. IFN, interferon; mTNF, murine tumor necrosis factor; siRNA, short-interfering RNA; TNF, tumor necrosis factor.
Proinflammatory/antiviral effects of uridine-bulge modification
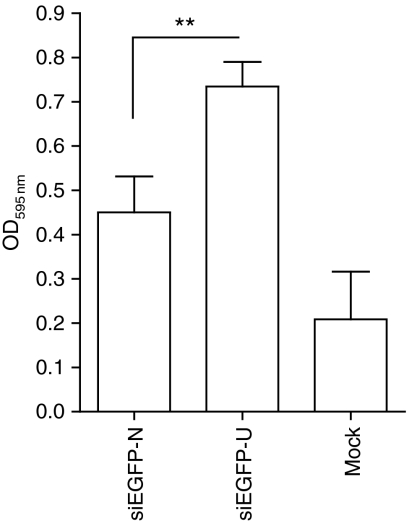
We next looked to elucidate the cytokine profile induced by the uridine-bulge modification of the three D-siRNAs (siLam-4, siEGFP-U, and siE6/E7-U) in human PBMCs. Multi-cytokine protein array analysis of 17 cytokines confirmed significant upregulation of the proinflammatory cytokines TNF-α, IFN-γ, IL-1β, and IL-12(p70) (Supplementary Figure S3). In addition, IL-4, IL-5, IL-7, IL-10, IL-17, G-CSF, and GM-CSF were also significantly induced in at least two of the three D-siRNAs analyzed (data not shown). Notably, both the IL-6 and IL-8 values were outside the linear range of the standard curve and were not accurately assessed. As there was increased induction of type I and II IFNs by the uridine-bulge modification, the antiviral effects of the modified siEGFP D-siRNAs were assessed. Conditioned media from PBMCs treated overnight with the EGFP D-siRNAs was used to treat HeLa cells before infection with Semliki Forest virus (SFV) and HeLa cell survival was subsequently assayed (see Materials and Methods). As expected from the higher IFN-α production (Figure 3), the results showed that the uridine modification of siEGFP-U conferred significant protection to viral infection compared to the native siEGFP D-siRNA (Figure 6).
Figure 6.
Antiviral effects of the uridine-bulge-driven increased immunostimulation. Human peripheral blood mononuclear cells were treated overnight with N-[1-(2,3-dioleoyloxy)propyl]-N,N, Ntrimethylammonium methylsulfate–complexed siEGFP and its uridine-bulge variant (at 750 nmol/l), and the supernatants collected. The 100% confluent HeLa cells were pretreated with the resulting conditioned media for 4 hours, before infection with Semliki Forest virus, as described in Materials and Methods. Following 36 hours of incubation at 37 °C, the cells were fixed, stained with crystal violet, and relative absorbance measured. Cell survival inversely correlates with viral activity, thereby resulting in increased absorbance readings when the antiviral effect is greater. The data are averaged from four independent viral assays, from two blood donors. Unpaired two-tailed t-test is presented comparing siEGFP and siEGFP-U conditions. OD, optical density.
Use of uridine-bulge modification with 21-bp siRNA scaffold
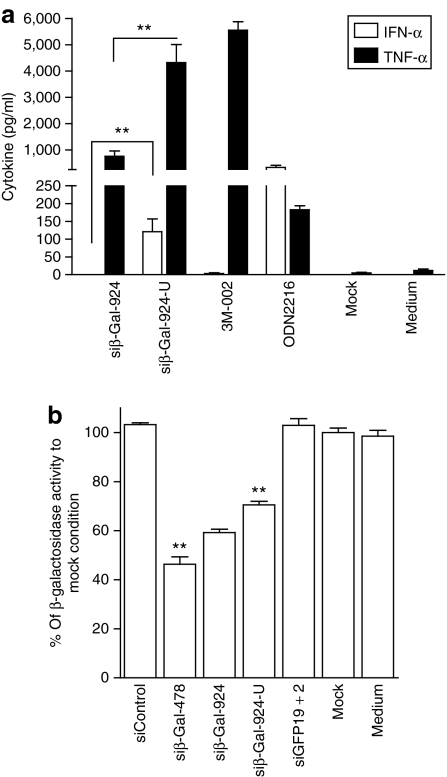
siβ-Gal-924 is a 21-bp siRNA duplex targeting the β-galactosidase mRNA, demonstrating silencing efficiency but low immunostimulatory potential in human PBMCs.3 To define whether the uridine-bulge modification could also be applied independently of the Dicer-substrate scaffold, we synthesized siβ-Gal-924 and its uridine-bulge variant (siβ-Gal-924-U, Supplementary Table S1) and tested for immunostimulation and downregulation efficiency. In agreement with our previous results, the uridine-bulge modification of siβ-Gal-924 promoted significant induction of TNF-α and IFN-α in human PBMCs, compared to its native variant (Figure 7a). Nevertheless, in HEK 293T cells transiently expressing β-galactosidase, the downregulation efficiency of siβ-Gal-924 was reduced (~10%) by the uridine-bulge modification, indicating an adverse effect of the modification on RNAi recruitment (Figure 7b). Of note, siβ-Gal-478 promoted stronger downregulation of β-galactosidase than siβ-Gal-924, as previously reported.3
Figure 7.
Conserved RNAi efficiency, but not increased immunostimulation, depends on the siRNA scaffold. (a) Human peripheral blood mononuclear cells were treated with 750 nmol/l of specified siRNA complexed with N-[1-(2,3-dioleoyloxy)propyl]-N,N, Ntrimethylammonium methylsulfate and incubated for 16 hours at 37 °C. Cytokine production was measured by specific enzyme-linked immunosorbent assay. The data are averaged from two blood donors in biological triplicate and are representative of two independent experiments. (b) HEK 293T cells were transfected with pLenti4/TO/V5-GW/lacZ for 16 hours at 37 °C before transfection with 10 nmol/l of indicated siRNA. At 24 hours post-siRNA transfection, the cells were lysed and assayed for β-galactosidase activity as described in Materials and Methods. The data are averaged from two independent experiments in biological triplicate and expressed as percentage of the β-galactosidase activity of the mock condition. Unpaired two-tailed t-test is presented compared to siβ-Gal-924. IFN, interferon; siRNA, short-interfering RNA; TNF, tumor necrosis factor.
Discussion
The innate immune system is the first line of defense against infection with viruses or pathogens. Sensing of specific pathogen-associated molecular patterns by innate immune receptors such as the TLRs, is followed by the induction of a targeted response limiting the infection. As key mediators of this response, IFNs coordinate the expression of hundreds of antiviral genes.21 For this reason, IFNs are commonly used in the treatment of hepatitis C or chronic hepatitis B virus infection.22,23 Recruitment of TLR7 or both TLR7 and TLR8 by synthetic agonists (such as isatoribine and resiquimod) potently induces IFNs, and reduces hepatitis C virus plasma virus concentration.24
The use of siRNAs or RNAi-based therapies in the treatment of chronic viral infections such as immunodeficiency virus type 1, hepatitis C virus, and hepatitis B virus is currently under intense scrutiny and promises to overcome the limitations of currently available treatments.25,26,27 Given that IFNs are antiviral, it is reasonable to assume that in certain viral infections a strategy aimed at recruiting TLR7/8 together with specific gene silencing could have a therapeutic effect on viral clearance.27 In support of this, two groups have recently demonstrated the antiviral effects promoted by sequence-specific siRNA recruitment of TLR7 in murine models of Influenza infection.28,29 In addition, dual recruitment of antiviral RNAi and TLR7/8 by siRNAs can be achieved in vivo, as shown by Morrissey et al.30 This group demonstrated RNAi-driven specific reduction of hepatitis B virus replication, together with a TLR7-driven antiviral response with the same siRNA-lipid delivery system.30
In this work, we sought to develop siRNAs with enhanced immunostimulatory potential via TLR7/8 activation. It has previously been suggested that TLR7/8 sensing of single-stranded short RNAs is related to the propensity of an ssRNA to form inter- and intramolecular uridine-containing stem–loop structures at 37 °C (ref. 31). The finding that TNF-α but not IFN-α was strongly induced when modulating the intramolecular self-secondary structure of an ssRNA in human PBMCs (Figure 1b), led us to speculate that beyond recognition of putative respective specific motifs,6,7 TLR7 (measured through IFN-α production) and TLR8 (measured through TNF-α production) sensing of short RNAs is also governed by a different sensing of secondary structures. Because TNF-α levels but not IFN-α levels were affected by these sequence modifications, it can be ruled out that this is the result of an increased protection of the oligoribonucleotides from nuclease degradation through stronger secondary structure. A recent study suggested that TLR8 sensing of short RNAs strictly requires single-stranded conformation, which appears to contradict our findings that increased intramolecular secondary structure of B-406AS-1 and B-406AS-2 increased TLR8 sensing.10 However, we found that restriction of the 5-nt intramolecular stem–loop structure to a 3-nt loop inhibits TNF-α production, suggesting a direct role for the loose loop structure of this ssRNA in TLR8 sensing (Figure 1b, compare B-406AS-2 and B-406AS-3). We note that Ablasser et al. only looked at the ability of ssRNAs to form predicted intermolecular self-dimers following Watson–Crick pairing in their interpretation, and did not take into account intramolecular self-secondary structure.10 In fact, mFOLD modeling of the sequences used in that study reveals that all the sequences form intramolecular stem–loops with various motifs and energy stability.10 In addition, the sequence modifications used did not keep a constant amount of uridine residues, rendering the analysis of the results particularly challenging.10 In agreement with our previous findings indicating a role for the intramolecular secondary structure of ssRNA in TLR8 sensing of uridine residues,7 we propose that TLR8 preferentially senses regions of loose secondary structures but that this sensing is impacted on by neighboring regions with double-stranded structures (hence affecting the stability of the loose secondary structures).
In accordance with this hypothesis, introduction of mismatches creating a structural distortion of low base-pairing in the middle of two perfectly complementary siRNAs also conferred increased specific TNF-α production in human PBMCs, independent of increased IFN-α levels (Figure 4, siEGFP-N-MIS and siLam-N-MIS, compared to siEGFP-N and siLam-N). However, production of both cytokines (with a predominance of TNF-α) was further induced when a four-uridine motif was used to create the mismatch structural distortion in the middle of siRNA duplexes in human PBMCs and monocytes, indicating an additional contribution of the uridine residues to the immunostimulatory effects (Figures 3 and 5a). We found that irrespective of the double-stranded nature of the short RNAs, immunostimulation induced by DOTAP-mediated delivery of ssRNAs and siRNAs to mouse macrophages is entirely dependent on endosomal TLR7 (Supplementary Figure S2 and ref. 7). The contribution of endosomal TLR3 or cytosolic RIG-I is negligible with this delivery system, as seen with the ablation of signaling to siLam-N-MIS in TLR7−/− BMMs (Supplementary Figure S2 and ref. 7), although these cells are responsive to TLR3 or RIG-I ligands (Supplementary Figure S2). Similarly, DOTAP-mediated ssRNA/siRNA delivery in human adherent monocytes is targeted to the endosome compartment (data not shown and ref. 32). Given that the increased immunostimulation following DOTAP-transfection of siRNAs with the uridine-bulge modification was only seen in human monocytes (Figure 5a) but not in mouse macrophages, we speculate that human TLR8 contributes to the sensing of the uridine-bulge modification. Nonetheless, further investigations will be required to better characterize the respective roles of human TLR7 and TLR8 in sensing structural variations in the secondary structure of ssRNAs and siRNA duplexes.
Unexpectedly, the introduction of additional uridines to the 3′-end of a D-siRNA (using the passenger strand as reference) decreased immunostimulation when the Watson–Crick base-pairing was conserved, in both human PBMCs and mouse macrophages (Figures 2 and 5c, siLam-1 and siLam-2). Nevertheless, the loose secondary structure of this 3′-end region with G:U wobble pairing resulted in increased TNF-α, independent of IFN-α (Figure 2, siLam-3). Although confirming a role for uridines in TLR7/8 sensing (Figure 1), our results emphasize that the immunostimulatory potential of an siRNA duplex cannot be accurately predicted through sequence analysis. In support of our findings, a screen of 207 siRNAs for TLR7/8 stimulation in human PBMCs recently suggested that in addition to uridine content, decreased strength of hybridization between the two complementary strands of an siRNA duplex positively correlated with increased immunostimulatory activity.33 The results from our three distinct siRNA duplexes are unequivocal: addition of the uridine-bulge promotes increased TNF-α, IFN-γ, IL-1β, and the Th1 polarizing IL-12(p70) (Supplementary Figure S3). However, we also observed possible saturation in the immunostimulation conferred by the uridine bulge with one siRNA sequence exhibiting high basal immunostimulatory potential (Supplementary Figure S4—compare siEGFP-U 500 nmol/l and siBcl2l12-N 250 nmol/l). Based on these results, we propose that the addition of the uridine-bulge modification will increase proinflammatory activation of any given siRNA sequence that is not already strongly immunostimulatory.
The measurement of both mRNA and protein levels of targeted genes indicated that although the uridine-bulge did not impact on the silencing efficiency of any of the D-siRNAs studied (Figures 2c and 3b,d), it significantly inhibited RNAi when used in the context of a 21-bp siRNA scaffold (Figure 7b). Relying on the consistent observation that the bulge did not alter silencing on three different D-siRNAs, it is unlikely that the difference observed with the shorter β-galactosidase uridine-siRNA is simply related to this particular sequence. Because the two scaffold designs compared here differ only from one another by their position in the Dicer-processing step (i.e., upstream or downstream of Dicer), it can be speculated that Dicer-processing and direct recruitment of the bulge-siRNA is required for correct unwinding and RNA-induced silencing complex loading of the imperfect miRNA-like siRNA duplex. However, it could also be argued that the lower affinity of the two strands destabilizes the 21-bp bulge β-galactosidase siRNA duplex and renders it more prone to degradation, whereas the longer conformation of the D-siRNAs would confer protection. Our observation that the bulge modification of the D-siRNAs did not affect RNAi efficiency when compared to a perfectly pairing duplex was unexpected. It is currently thought that perfectly pairing duplexes undergo Ago2 processing of the passenger strand,34 although miRNA-like imperfect duplexes would be unwound through an elusive helicase.35 The finding that similar RNAi efficiency can be achieved regardless of the affinity of the two strands of a D-siRNA highlights the concept that Ago2–RNA-induced silencing complex cleavage efficiency of the complementary target is not affected by the nature of the prior degradation or unwinding of the passenger strand of the duplex, when Dicer is involved in active RNA-induced silencing complex formation. Further in-depth investigations of the role of Dicer in the recruitment of RNAi by bulge-modified siRNAs with the two scaffold designs should help to better characterize the previous findings, and could be informative in the design of better miRNA mimics. It is worth mentioning that the design of D-siRNAs used in these experiments did not incorporate the 2-nt DNA residues in the 3′ of the sense strand previously shown to limit the heterogenous Dicer cleavage processing pattern.19 However, this did not have any significant adverse effect on the silencing efficiency of the siEGFP siRNA (compare siEGFP-U and siEGFP-U-OH; Figure 3b and Supplementary Table S1), and did not impact on the immunostimulatory efficacy of the duplex (Figure 4b). Conversely, a 27-mer double blunt-end duplex significantly affected RNAi recruitment, in accordance with previous findings (compare siEGFP-N, siEGFP-U and siEGFP-U-Blunt; Figure 3b and Supplementary Table S1).19
SFV is a single-stranded positive-sense alphavirus that induces apoptosis of continuously cultured cells 24–48 hours after infection.36 SFV is particularly sensitive to IFN pretreatment of cells before infection.37 Here, we found that conditioned media from human PBMCs treated with a uridine-bulge D-siRNA conferred increased protection to SFV infection of HeLa cells, when compared to a perfect D-siRNA duplex (Figure 6). This can be attributed to IFN-α production by the PBMCs and demonstrates that the increased immunostimulatory potential conferred by the uridine-bulge modification can have potent antiviral benefits. However, because the increased immunostimulatory effects of the uridine-bulge were not reproduced in mouse due to mouse TLR8 unresponsiveness to ss/siRNAs5, we were unable to further characterize the benefits of the uridine-bulge modification in vivo.
In conclusion, we provide here the first rational design of a bifunctional D-siRNA scaffold, which can be applied to any given siRNA sequence. We demonstrate that the addition of an miRNA-like uridine-bulge to a Dicer-substrate siRNA duplex confers increased immunostimulation in human PBMCs, with potential protective effect in viral infection. The bulge modification does not compromise RNAi recruitment and target-specific downregulation, when used in a D-siRNA scaffold. Our data are indicative of a TLR8-preferential recruitment of short RNAs with loose secondary structure, including D-siRNAs with the uridine-bulge modification. Because it relies on a sequence modification, the uridine-bulge strategy presented in this work could be immediately amenable to large-scale industrial production of oligoribonucleotides. Combination of this sequence modification together with a recently reported CpG-siRNA delivery strategy could allow for further modulation of immune cell activation and have therapeutic benefits in the treatment of viral infections and other diseases, including cancer.38,39
Materials and Methods
Cell isolation and culture. Fresh blood from healthy male donors was collected in heparin-treated tubes and PBMCs separated by Ficoll-paque plus (cat. no. 17-1440-02; GE Healthcare, Rydalmere, Australia) gradient purification, and plated in a 96-well plate at 2 × 105 cells per well in RPMI 1640+L-glutamine medium (cat. no. 11875085; Invitrogen, Carlsbad, CA) complemented with 1× antibiotic/antimycotic (cat. no. 15240062; Invitrogen) and 10% fetal bovine serum (FBS; cat. no. FBS-500; ICPBio, Auckland, New Zealand) (referred to as complete RPMI), as previously reported.7 For the purification of CD14+ monocytes, flow cytometric sorting of PBMCs was carried out using anti-CD14-PE (cat. no. 555398; BD Biosciences, San Jose, CA) as previously reported.40 HEK 293T cells and their GFP stable variant (gift from A. Sadler, Monash Institute of Medical Research, Melbourne, Australia) were cultured in complete Dulbecco's modified Eagle's medium (DMEM) (cat. no. 11965-092; Invitrogen) supplemented with 10% sterile FBS and 1× antibiotic/antimycotic. TC-1 cells, a murine fibroblast cell line expressing the human papilloma virus 16, were grown in complete DMEM. RAW-ELAM cells were grown as previously described.7 Stable TLR7−/− BMMs and TLR2/4 double knockout BMMs were generated using the J2 retrovirus encoding v-raf and v-myc, as previously reported41,42 and grown in complete DMEM.
Isolation of bone marrow macrophages. Mice were housed at the Monash animal facility (Monash University, Clayton campus, Australia) and approved under MMCA2007/07. Femurs were collected and flushed with RPMI, and cells were plated in complete RPMI supplemented with 20% L929-cell-conditioned medium on 10-cm bacteriological plastic plates for 7 days at 37 °C in a 5% CO2 atmosphere.
Cell stimulation. All siRNA duplexes were synthesized as ssRNAs by Integrated DNA Technologies with high-performance liquid chromatography purification, and resuspended in duplex buffer (100 mmol/l potassium acetate, 30 mmol/l HEPES, pH 7.5, DNase–RNase free H2O) to a concentration of 80 µmol/l. ssRNAs were annealed to form siRNA duplexes at 92 °C for 2 minutes and left for 30 minutes at room temperature before aliquoting (giving a final concentration of 40 µmol/l). 3M-002 (human TLR8 agonist and mouse TLR7 agonist,7 cat. no. tlrl-c75), Gardiquimod (human TLR7 agonist, cat. no. tlrl-gdq), Pam3CSK4 (TLR2/1 agonist, cat. no. tlrl-pms), synthetic monophosphoryl lipid A (TLR4 agonist, cat. no. tlrl-mpls), Poly(I:C) (TLR3 agonist when used untransfected, cat. no. tlrl-pic-5), and ODN2216 (TLR9 agonist, cat. no. tlrl-hodna) were purchased from Invivogen (San Diego, CA) and used at a final concentration of 1 µg/ml (3M-002 and Gardiquimod), 100 ng/ml (Pam3CSK4 and lipid A), 10 µg/ml (Poly(I:C)), and 3 µmol/l (ODN2216). Sendai virus (SeV, RIG-I ligand43) was used at a final 300 hemagglutination units/ml (gift from A. Mansell, Monash Institute of Medical Research, Melbourne, Australia). For immunostimulation assays in PBMCs, CD14+ cells, BMMs, immortalized BMMs, and RAW-ELAM cells, ss/siRNAs were transfected with DOTAP (cat. no. 1811177; Roche, Nutley, NJ), as previously reported.7 Transfections were carried out in biological triplicate in all experiments. The ratios of DOTAP to ss/siRNA were as follows: 5.3 µg/µl of 80 µmol/l ssRNA (Figure 1a,b) and 1.87 µg/µl of 40 µmol/l siRNA (Figures 2–5 and 7, Supplementary Figures S2–S4). When dose–response experiments were carried out, the ratio of siRNA to DOTAP was kept constant.
RNAi by reverse transfection. For Figures 3b and 7b, 1.35 µl Lipofectamine 2000 (cat. no. 11668; Invitrogen) was diluted in 150 µl of Opti-MEM (cat. no. 51985-034; Invitrogen), and 1.5 µl of siRNA molecules (diluted to 4 µmol/l in duplex buffer or freshly in Opti-MEM) was added such that the final concentration of siRNA in each well was 10 nmol/l. After 20 minutes of incubation, 50 µl of the Lipofectamine 2000/siRNA/Opti-MEM mixture was added directly into each well of a 96-well plate (in triplicate). Approximately 15,000 HEK 293T-GFP cells suspended in 150 µl of antibiotic-free DMEM (supplemented with 10% FBS) was added to each well, giving a final volume of 200 µl per well. For Figures 2c and 3d and Supplementary Figure S1, 4.5 µl of Lipofectamine 2000 was diluted in 300 µl of Opti-MEM and 4.5 µl of siRNA molecules (diluted to 4 µmol/l in duplex buffer or freshly in Opti-MEM) was added such that the final concentration of siRNA in each well was 10 nmol/l. After 20 minutes of incubation, 100 µl of the mix was added directly into each well of a 24-well plate (in triplicate). A total of 100,000 HEK 293T cells or TC-1 cells resuspended in 500 µl of antibiotic-free DMEM (supplemented with 10% FBS) was added to each well giving a final volume of 600 µl per well. For the mock condition, no siRNA was added to the Lipofectamine mix, whereas for the medium condition, Opti-MEM only was added to the wells (i.e., no Lipofectamine or siRNA was added). siControl is a nontargeting siRNA control (cat. no. 4635; Ambion, Austin, TX), and siGFP19+2 is a published 21-bp siRNA molecule previously shown to downregulate EGFP expression.4
Real-time reverse transcriptase-PCR. Complementary DNA was synthesized from column-purified RNA (NucleoSpin RNAII columns, cat. no. 740955; Macherey-Nagel, Bethlehem, PA) using the SuperScript III First-Strand Kit (cat. no. 18080-051; Invitrogen), with random hexamer priming and following the manufacturer's instructions. Real-time PCR was carried out with the SYBR GreenER qPCR SuperMix for iCycler instrument (cat. no. 11761-500; Invitrogen). hGAPDH (NM_002046) and mGAPDH (NM_008084) were used as reference and were amplified with the following primer pairs, respectively (5′–3′): hGAPDH-FWD: CATCTTCCAGGAGCGAGATCCC; hGAPDH-REV: TTCACACCCATGACGAACAT; mGAPDH-FWD: TTCACCACCATGGAGAAGGC; mGAPDH-REV: GGCATGGACTGTGGTCATGA. hLaminA/C (NM_17 0707) and E6/E7 of HPV16 (FJ610149) were amplified with the following primer pairs, respectively: Lamin-FWD: AGCAAAGTGCGTGAGGAGTT; Lamin-REV: GAGTTCAGCAGAGCCTCCAG; E6-FWD: TTGCTTTTCGGGATTTATGC; E6-REV: CAGGACACAGTGGCTTTTGA. Each amplicon was sequence verified and used to generate a standard curve for the quantification of gene expression.
Detection of cytokines. Human IFN-α in culture supernatants was quantified by sandwich enzyme-linked immunosorbent assay using mouse monoclonal (0.5 µg/ml, cat. no. 21112-1; PBL Biomedical, Piscataway, NJ) and rabbit polyclonal antibodies (0.5 µg/ml, cat. no. 31130-1; PBL Biomedical). A goat anti-rabbit horseradish peroxidase–conjugated antibody (0.8 µg/ml, cat. no. 31460; Pierce, Rockford, IL) was used for detection. Human and mouse TNF-α were measured using the BD OptEIA ELISA sets (cat. no. 555212 and cat. no. 558874; respectively, BD Biosciences) and mouse RANTES was quantified using the R&D Systems DuoSet (cat. no. DY478; R&D Systems, Minneapolis, MN). In both IFN-α and TNF-α enzyme-linked immunosorbent assays, TMB substrate (cat. no. T0440; Sigma Aldrich, St Louis, MO) was used for quantification of the cytokines on a Fluostar OPTIMA (BMG LABTECH, Offenburg, Germany) plate-reader. For the Bio-Plex human cytokine 17-plex panel (cat. no. 171-A11171; Bio-Rad laboratories, Hercules, CA), the assays were performed following the manufacturer's guidelines. A standard curve was generated for each cytokine using a five-parameter logistic regression curve fit and analyzed concentrations were determined automatically using Bio-Plex Manager software (v4.01; Bio-Rad Laboratories). All results below the detectable limit were recorded as zero, and those above the standard curve were assigned a value equal to the top standard.
Fluorescent-based measure of EGFP knockdown. EGFP expression (and downregulation) in HEK 293T-GFP cells was measured using a Fluostar OPTIMA plate-reader. RNAi experiments were performed in 96-well black plates with clear bottoms (cat. no. 353948; BD Biosciences Falcon). At 48 hours after siRNA treatment, the supernatants were discarded and 50 µl of phosphate-buffered saline was added to the cells. A standard curve was generated by serially diluting a recombinant EGFP-fusion protein (gift from D. Wang, Monash Institute of Medical Research, Melbourne, Australia) to cover a range from 150–4.68 ng/ml in 50 µl phosphate-buffered saline. The equivalent EGFP concentration in each well was then inferred from the fluorescence at ex485/em520 correlated to the standard curve.
β-Galactosidase enzymatic assay. A total of 300,000 HEK 293T cells were reverse transfected with 300 ng of pLenti4/TO/V5-GW/lacZ (Invitrogen) complexed to 1 µl of Lipofectamine 2000 in 100 µl of Opti-MEM, in a well of a 6-well plate. At 16 hours post-transfection, the cells were collected with 300 µl TrypLE Express Stable Trypsin (cat. no. 12604-021; Invitrogen) and 1.5 ml of antibiotic-free DMEM was added. The siRNA mixes were prepared as indicated in “RNAi by reverse transfection” section, and 150 µl of cells were added per well, giving a final volume of 200 µl at 10 nmol/l. The cells were further incubated for 24 hours before being lysed for 20 minutes in 40 µl of 1× Reporter Lysis Buffer (cat. no. E2000; Promega, Madison, WI) per well. A volume of 40 µl of the 2× assay buffer was added per well, and the plate was incubated for 30–45 minutes at 37 °C. The enzymatic reaction was stopped with the addition of 40 µl sodium carbonate (1 mol/l), and the plate was read at 440 nm on a Fluostar OPTIMA plate-reader.
SFV viral assay. HeLa cells were seeded into 96-well plates and incubated overnight at 37 °C to reach ~100% confluency. The cells were treated with 100 µl of supernatant from siRNA-treated PBMCs. A one-fourth serial dilution of the conditioned media was performed for each of the siRNA treatments. Following a 4-hour pretreatment with conditioned media, the cells were washed and infected with 100 µl of DMEM (10% FBS) containing 200 plaque-forming units/ml of SFV. The cells were incubated with the virus for 36 hours before being rinsed with phosphate-buffered saline and fixed with 10% formalin for 30 minutes. The cells were further stained for 30 minutes with 0.05% crystal violet in 20% ethanol, before thorough H2O washes and reading at 590 nm on a Fluostar OPTIMA plate-reader.
Statistical analyses. Statistical analyses were carried out using GraphPad Instat version 3.05. (GraphPad Software, La Jolla, CA). Two-tailed unpaired t-tests or nonparametric Mann–Whitney tests were used according to the standard deviation of the compared populations. Error bars on each figure represent the SEM. Symbols used represent *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.0001, and NS is nonsignificant.
SUPPLEMENTARY MATERIALFigure S1. Target-specific silencing with LaminA/C siRNAs.Figure S2. TLR7-dependent sensing of siRNA in mouse macrophages.Figure S3. Immunostimulatory profile of uridine-bulge modification.Figure S4. Immunostimulation of siRNA targeting Bcl2L12.Table S1. Sequences of dsRNAs used in the study.
Supplementary Material
Target-specific silencing with LaminA/C siRNAs.
TLR7-dependent sensing of siRNA in mouse macrophages.
Immunostimulatory profile of uridine-bulge modification.
Immunostimulation of siRNA targeting Bcl2L12.
Sequences of dsRNAs used in the study.
Acknowledgments
We are grateful to Scott Rose (Integrated DNA Technologies) for his help in the production of the RNA oligonucleotides, Paul Cameron and Vanessa Evans (Monash University, Department of Medicine, Alfred Campus, Melbourne, Australia) for their help in the purification of human monocytes, Die Wang (Monash Institute of Medical Research) for the recombinant GFP, Anthony Sadler (Monash Institute of Medical Research) for the HEK 293T-GFP cells and Ashley Mansell (Monash Institute of Medical Research) for his assistance with SeV infections. This work is supported by funding from the Australian NHMRC 491106, The Arthur Wilson Fellowship from The RANZCOG Research Foundation, and the Victorian Government's Operational Infrastructure Support Program. S.T. is supported by an NHMRC Career Development Award (490970). M.A.B. is employed by Integrated DNA Technologies, which offers oligonucleotides for sale similar to some of the compounds described in the article. Integrated DNA Technologies (IDT) is, however, not a publicly traded company, and he does not own any shares or hold equity in IDT. M.P.G., S.T., and B.R.G.W. are the inventors of the Monash University international (PCT) patent application PCT/AU2009/000175, entitled Immunostimulatory siRNA Molecules.
REFERENCES
- Castanotto D., and , Rossi JJ. The promises and pitfalls of RNA-interference-based therapeutics. Nature. 2009;457:426–433. doi: 10.1038/nature07758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Guenthner-Biller M, Bourquin C, Ablasser A, Schlee M, Uematsu S, et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nat Med. 2005;11:263–270. doi: 10.1038/nm1191. [DOI] [PubMed] [Google Scholar]
- Judge AD, Sood V, Shaw JR, Fang D, McClintock K., and , MacLachlan I. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol. 2005;23:457–462. doi: 10.1038/nbt1081. [DOI] [PubMed] [Google Scholar]
- Zamanian-Daryoush M, Marques JT, Gantier MP, Behlke MA, John M, Rayman P, et al. Determinants of cytokine induction by small interfering RNA in human peripheral blood mononuclear cells. J Interferon Cytokine Res. 2008;28:221–233. doi: 10.1089/jir.2007.0090. [DOI] [PubMed] [Google Scholar]
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- Forsbach A, Nemorin JG, Montino C, Müller C, Samulowitz U, Vicari AP, et al. Identification of RNA sequence motifs stimulating sequence-specific TLR8-dependent immune responses. J Immunol. 2008;180:3729–3738. doi: 10.4049/jimmunol.180.6.3729. [DOI] [PubMed] [Google Scholar]
- Gantier MP, Tong S, Behlke MA, Xu D, Phipps S, Foster PS, et al. TLR7 is involved in sequence-specific sensing of single-stranded RNAs in human macrophages. J Immunol. 2008;180:2117–2124. doi: 10.4049/jimmunol.180.4.2117. [DOI] [PubMed] [Google Scholar]
- Gantier MP., and , Williams BR. The response of mammalian cells to double-stranded RNA. Cytokine Growth Factor Rev. 2007;18:363–371. doi: 10.1016/j.cytogfr.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlee M, Hornung V., and , Hartmann G. siRNA and isRNA: two edges of one sword. Mol Ther. 2006;14:463–470. doi: 10.1016/j.ymthe.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Ablasser A, Poeck H, Anz D, Berger M, Schlee M, Kim S, et al. Selection of molecular structure and delivery of RNA oligonucleotides to activate TLR7 versus TLR8 and to induce high amounts of IL-12p70 in primary human monocytes. J Immunol. 2009;182:6824–6833. doi: 10.4049/jimmunol.0803001. [DOI] [PubMed] [Google Scholar]
- Marques JT., and , Williams BR. Activation of the mammalian immune system by siRNAs. Nat Biotechnol. 2005;23:1399–1405. doi: 10.1038/nbt1161. [DOI] [PubMed] [Google Scholar]
- Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18:305–319. doi: 10.1089/oli.2008.0164. [DOI] [PubMed] [Google Scholar]
- Robbins M, Judge A, Liang L, McClintock K, Yaworski E., and , MacLachlan I. 2′-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther. 2007;15:1663–1669. doi: 10.1038/sj.mt.6300240. [DOI] [PubMed] [Google Scholar]
- Judge AD, Bola G, Lee AC., and , MacLachlan I. Design of noninflammatory synthetic siRNA mediating potent gene silencing in vivo. Mol Ther. 2006;13:494–505. doi: 10.1016/j.ymthe.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Poeck H, Besch R, Maihoefer C, Renn M, Tormo D, Morskaya SS, et al. 5′-Triphosphate-siRNA: turning gene silencing and Rig-I activation against melanoma. Nat Med. 2008;14:1256–1263. doi: 10.1038/nm.1887. [DOI] [PubMed] [Google Scholar]
- Sioud M. Single-stranded small interfering RNA are more immunostimulatory than their double-stranded counterparts: a central role for 2′-hydroxyl uridines in immune responses. Eur J Immunol. 2006;36:1222–1230. doi: 10.1002/eji.200535708. [DOI] [PubMed] [Google Scholar]
- Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y., and , Reis e Sousa C. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol. 2006;36:3256–3267. doi: 10.1002/eji.200636617. [DOI] [PubMed] [Google Scholar]
- Amarzguioui M, Lundberg P, Cantin E, Hagstrom J, Behlke MA., and , Rossi JJ. Rational design and in vitro and in vivo delivery of Dicer substrate siRNA. Nat Protoc. 2006;1:508–517. doi: 10.1038/nprot.2006.72. [DOI] [PubMed] [Google Scholar]
- Rose SD, Kim DH, Amarzguioui M, Heidel JD, Collingwood MA, Davis ME, et al. Functional polarity is introduced by Dicer processing of short substrate RNAs. Nucleic Acids Res. 2005;33:4140–4156. doi: 10.1093/nar/gki732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huesken D, Lange J, Mickanin C, Weiler J, Asselbergs F, Warner J, et al. Design of a genome-wide siRNA library using an artificial neural network. Nat Biotechnol. 2005;23:995–1001. doi: 10.1038/nbt1118. [DOI] [PubMed] [Google Scholar]
- Sadler AJ., and , Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco V., and , Craxì A. Chronic hepatitis B: who to treat and which choice of treatment. Expert Rev Anti Infect Ther. 2009;7:281–291. doi: 10.1586/eri.09.4. [DOI] [PubMed] [Google Scholar]
- Webster DP, Klenerman P, Collier J., and , Jeffery KJ. Development of novel treatments for hepatitis C. Lancet Infect Dis. 2009;9:108–117. doi: 10.1016/S1473-3099(09)70020-9. [DOI] [PubMed] [Google Scholar]
- Averett DR, Fletcher SP, Li W, Webber SE., and , Appleman JR. The pharmacology of endosomal TLR agonists in viral disease. Biochem Soc Trans. 2007;35:1468–1472. doi: 10.1042/BST0351468. [DOI] [PubMed] [Google Scholar]
- Arbuthnot P, Longshaw V, Naidoo T., and , Weinberg MS. Opportunities for treating chronic hepatitis B and C virus infection using RNA interference. J Viral Hepat. 2007;14:447–459. doi: 10.1111/j.1365-2893.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- Berkhout B., and , ter Brake O. Towards a durable RNAi gene therapy for HIV-AIDS. Expert Opin Biol Ther. 2009;9:161–170. doi: 10.1517/14712590802653619. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Umehara T., and , Kohara M. Therapeutic application of RNA interference for hepatitis C virus. Adv Drug Deliv Rev. 2007;59:1263–1276. doi: 10.1016/j.addr.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Robbins M, Judge A, Ambegia E, Choi C, Yaworski E, Palmer L, et al. Misinterpreting the therapeutic effects of small interfering RNA caused by immune stimulation. Hum Gene Ther. 2008;19:991–999. doi: 10.1089/hum.2008.131. [DOI] [PubMed] [Google Scholar]
- Nguyen DN, Chen SC, Lu J, Goldberg M, Kim P, Sprague A, et al. Drug delivery-mediated control of RNA immunostimulation. Mol Ther. 2009;17:1555–1562. doi: 10.1038/mt.2009.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey DV, Lockridge JA, Shaw L, Blanchard K, Jensen K, Breen W, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007. doi: 10.1038/nbt1122. [DOI] [PubMed] [Google Scholar]
- Eberle F, Giessler K, Deck C, Heeg K, Peter M, Richert C, et al. Modifications in small interfering RNA that separate immunostimulation from RNA interference. J Immunol. 2008;180:3229–3237. doi: 10.4049/jimmunol.180.5.3229. [DOI] [PubMed] [Google Scholar]
- Sioud M. Induction of inflammatory cytokines and interferon responses by double-stranded and single-stranded siRNAs is sequence-dependent and requires endosomal localization. J Mol Biol. 2005;348:1079–1090. doi: 10.1016/j.jmb.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Goodchild A, Nopper N, King A, Doan T, Tanudji M, Arndt GM, et al. Sequence determinants of innate immune activation by short interfering RNAs. BMC Immunol. 2009;10:40. doi: 10.1186/1471-2172-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matranga C, Tomari Y, Shin C, Bartel DP., and , Zamore PD. Passenger-strand cleavage facilitates assembly of siRNA into Ago2-containing RNAi enzyme complexes. Cell. 2005;123:607–620. doi: 10.1016/j.cell.2005.08.044. [DOI] [PubMed] [Google Scholar]
- Winter J, Jung S, Keller S, Gregory RI., and , Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- Glasgow GM, McGee MM, Sheahan BJ., and , Atkins GJ. Death mechanisms in cultured cells infected by Semliki Forest virus. J Gen Virol. 1997;78:1559–1563. doi: 10.1099/0022-1317-78-7-1559. [DOI] [PubMed] [Google Scholar]
- Deuber SA., and , Pavlovic J. Virulence of a mouse-adapted Semliki Forest virus strain is associated with reduced susceptibility to interferon. J Gen Virol. 2007;88:1952–1959. doi: 10.1099/vir.0.82264-0. [DOI] [PubMed] [Google Scholar]
- Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, et al. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantier MP., and , Williams BR. siRNA delivery not Toll-free. Nat Biotechnol. 2009;27:911–912. doi: 10.1038/nbt1009-911. [DOI] [PubMed] [Google Scholar]
- Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Roberson SM., and , Walker WS. Immortalization of cloned mouse splenic macrophages with a retrovirus containing the v-raf/mil and v-myc oncogenes. Cell Immunol. 1988;116:341–351. doi: 10.1016/0008-8749(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Target-specific silencing with LaminA/C siRNAs.
TLR7-dependent sensing of siRNA in mouse macrophages.
Immunostimulatory profile of uridine-bulge modification.
Immunostimulation of siRNA targeting Bcl2L12.
Sequences of dsRNAs used in the study.