Abstract
Multiple sclerosis (MS) is a demyelinating disease of the central nervous system (CNS) with an inflammatory and a neurodegenerative component. The neuropoietic cytokine leukemia inhibitory factor (LIF) is expressed in MS lesions, but its effect on lesion development is far from understood. LIF is an interesting candidate for MS therapy, as it has neuroprotective properties and may also promote the survival of myelinating oligodendrocytes (OLGs). However, therapeutic administration of LIF is complicated by its limited ability to cross the blood–brain barrier and its pleiotropic actions outside the CNS. In this study, lentiviral vectors (LVs) were used to achieve stable expression and secretion of LIF in the CNS of adult mice. CNS-targeted expression of LIF significantly reduced demyelination in a murine model of MS. In addition, local expression of LIF ameliorated clinical symptoms with enhanced efficacy compared to systemic treatment with recombinant protein. These findings demonstrate that gene therapeutic administration of LIF is a promising approach to limit lesion burden and clinical symptoms in neuroinflammatory disease.
Introduction
Multiple sclerosis (MS) is a chronic disabling disease of the central nervous system (CNS), characterized by focal areas of inflammation in which myelin and the myelin-producing cells, the oligodendrocytes (OLGs), are destroyed. Although the available anti-inflammatory therapies are effective in reducing the relapse rate, current therapeutics are unable to stop, let alone reverse, the physical and cognitive decline that MS patients inevitably face.1,2 Increasing evidence indicates demyelination and subsequent neurodegeneration as the major cause of this irreversible neurological disability. These findings emphasize the importance of neuroprotective and restorative strategies that aim to prevent or restore demyelination and neurodegeneration in MS.
Leukemia inhibitory factor (LIF) is an interesting therapeutic candidate to reduce the detrimental process of neurodegeneration in MS. LIF is expressed by infiltrating immune cells and activated astrocytes in MS lesions.3,4 Recent data suggest that activation of LIF receptor signaling participates in the endogenous neurobiological response that serves to limit the extent of immune-mediated injury in MS.5 LIF is a survival factor for neurons not only during development, but also after injury.6 Interestingly, LIF may also protect myelinating OLGs and influence ongoing inflammation in MS.7 However, data on the actions of LIF in neuroinflammatory responses are contradicting. Both pro- and anti-inflammatory effects have been reported, as well as a contribution to and inhibition of demyelination. In vitro studies show that LIF reduces the production of tumor necrosis factor-α and reactive oxygen species by macrophages,8 which may translate into an anti-inflammatory response in vivo. In contrast, LIF administration in the spinal cord has been reported to induce proliferation and activation of macrophages, leading to hindlimb motor dysfunction.9 Studies on the effects of LIF in murine models of MS, called experimental autoimmune encephalomyelitis (EAE), have also generated conflicting results. Systemic LIF treatment is described to ameliorate clinical symptoms in both chronic and relapsing–remitting EAE models.10 The favorable clinical effect of LIF was associated with increased OLG survival, although no significant effects on the immune response were detected. In contrast, a recent study revealed that depletion of LIF also reduces clinical symptoms and limits demyelination in EAE.11 Here, the observed effects were accompanied by reduced numbers of macrophage infiltrates.
In studies using systemic application of LIF protein or in LIF knock-out mice, the pleiotropic activities of LIF outside the CNS complicate assessment of its effects on CNS lesion development. For example, LIF stimulates megakaryocyte and platelet production,12 plays a role in embryonic implantation,13,14 induces expression of acute phase proteins in the liver,15 and enhances production of adrenocorticotropic hormone.16,17 This study was designed to elucidate the therapeutic potential of CNS-targeted LIF expression during neuroinflammatory conditions. The limited potential of LIF to cross the blood–brain barrier18 not only hampers its therapeutic potential, but also makes it difficult to evaluate the exact role of LIF on CNS lesion development. We hypothesized that a local production of LIF by means of lentiviral vectors (LVs) circumvents these problems and allows us to elucidate the role of LIF during neuroinflammation. Our study reveals that CNS-directed LIF expression has improved clinical efficacy compared to systemic treatment in limiting the detrimental effects of immune-mediated demyelination.
Results
Systemic LIF treatment does not significantly ameliorate EAE symptoms
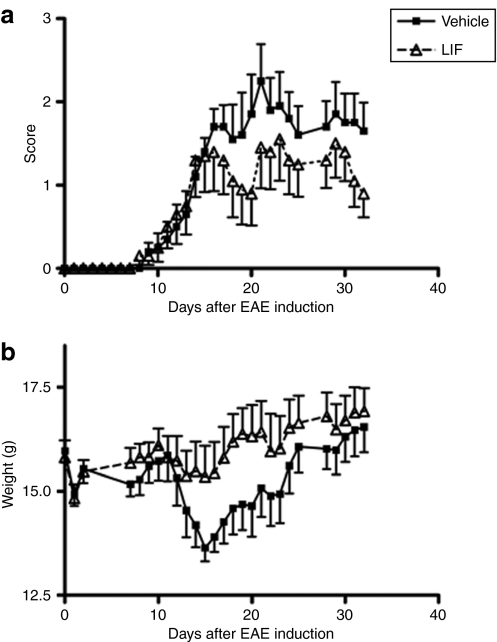
We first examined the therapeutic efficacy of systemic administration of recombinant mouse LIF in EAE. For this purpose, we used an EAE model induced by myelin OLG protein 35–55 peptide in C57Bl/6J mice. LIF treatment was started on day 13, when mean disease score was >0.5 (n = 10). A dose of 25 µg/kg/day LIF was given intraperitoneally over a 5-day period. Control animals received daily mouse serum albumin injections (n = 10). On day 15 postimmunization, LIF-treated animals displayed less weight loss than control EAE mice (P < 0.05). A trend toward clinical benefit was evident, but did not reach statistical significance (Figure 1).
Figure 1.
Systemic administration of LIF does not significantly alter EAE disease course. (a) Daily intraperitoneal injections with 25 µg/kg LIF (n = 10) from day 13 to 18 after EAE induction do not alter clinical symptoms compared to vehicle treated animals (n = 10). (b) On day 15 postimmunization, LIF-treated mice displayed less weight loss than control EAE mice. Data are expressed as the mean ± SEM. EAE, experimental autoimmune encephalomyelitis; LIF, leukemia inhibitory factor.
LVs mediate overexpression of LIF in the mouse CNS
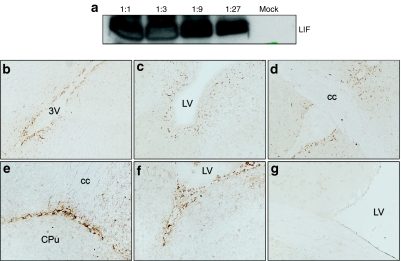
In order to deliver sufficient amounts of LIF to the site of inflammation, we administered LV encoding LIF (LV-LIF) to the brain ventricular system. Hereto, the complementary DNA encoding mouse LIF (sequence NM_008501) was cloned into a lentiviral transfer plasmid containing a central polypurine tract sequence, the SIN-18 deletion and the woodchuck hepatitis posttranscriptional regulatory element.19 After construction of LV plasmids encoding murine LIF, expression of the transgene was verified in cell culture. Western blot analysis of extracts from transduced 293T cells confirmed overexpression of the LIF protein (Figure 2a). Secretion of LIF into supernatant of cell cultures was confirmed by means of enzyme-linked immunosorbent assay. At 72 hours after transduction, 1,400 pg LIF/ml was detected, whereas LIF was undetectable in supernatant of mock-transduced 293T cells. Next, 6 µl of highly concentrated vector (p24: 5 × 107 pg/ml) were stereotactically injected into the mouse lateral ventricle. Control mice received the same LVs, encoding green fluorescent protein (GFP) instead of LIF. Coordinates used for injections into the right lateral ventricle were anteroposterior −0.02 cm, lateral −0.10, and dorsoventral −0.18 using bregma as reference. Injection in the lateral ventricle allows the vector to spread with the flow of cerebrospinal fluid through the entire mouse brain ventricular system. LIF expression is not detectable in untransduced healthy CNS. After LIF gene therapy, however, immunohistochemical staining revealed LIF expression in ependymal cells lining cerebrospinal fluid–filled spaces and in choroid plexus cells, although few cells in the adjacent parenchyma were also transduced (Figure 2b). Previous studies indicate that these cells are predominantly neuronal cells, although transduction of glial cells has been reported as well.
Figure 2.
Lentiviral mediated expression of LIF in vitro and in vivo. (a) Western blot analysis demonstrates transgene expression 72 hours post-transduction of 293T cells with vector solution at ratios of 1:1, 1:3, 1:9, and 1:27. Mock-transduced cells were used as control. (b–g) Immunohistochemical staining for (b–d) GFP or (e,f) LIF expression after lentivirus-mediated gene transfer in lateral ventricle. High expression of the transgene was found in ependymal cells lining cerebrospinal fluid–filled spaces and in choroid plexus cells, while few cells in the adjacent brain parenchyma were also transduced. (g) No expression was detected in untransduced central nervous system. cc, corpus callosum; CPu, caudate putamen; EAE, experimental autoimmune encephalomyelitis; LIF, leukemia inhibitory factor; LV, lateral ventricle; 3V, third ventricle.
Local LIF production significantly ameliorates EAE symptoms
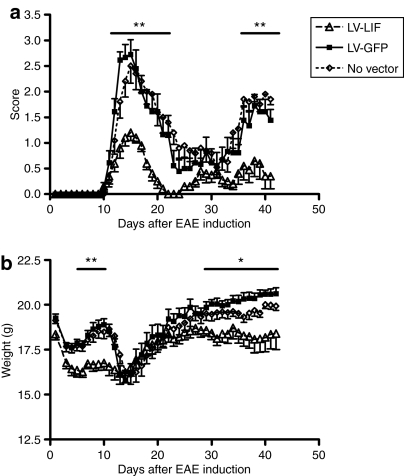
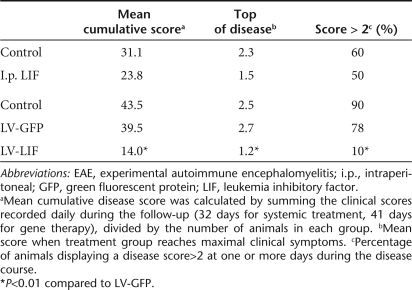
To examine the therapeutic potential of in situ LIF gene therapy during neuroinflammation, we stereotactically injected LV encoding LIF unilaterally into the lateral ventricle of adult C57Bl/6J mice (n = 10). Two weeks later, EAE was induced. At this point, mice overexpressing LIF appeared normal and did not show any signs of distress. LV-GFP-injected animals (n = 10) were used as control group. Importantly, intraventricular delivery of LV encoding GFP does not induce any changes in the clinical onset or course of EAE (Figure 3). Administration of LV-LIF in EAE animals on the other hand significantly decreased disease burden (P < 0.01), as measured by the average (Figure 3a) and cumulative clinical disease scores (Table 1). Amelioration of clinical symptoms was most prominent at the top of disease when control EAE mice suffered from hindlimb paralysis (score 2.7 ± 0.3), whereas mice overexpressing LIF only displayed a reduction in tail tone (score 1.2 ± 0.2) (Figure 3a, Table 1). Moreover, the beneficial effects of LIF gene therapy persisted during the relapse. Mice within the LV-LIF group displayed a reduction in body weight (Figure 3b), as has been previously described for central LIF application.20 This difference in weight disappeared at the onset of the first clinical attack, which was accompanied by a reduction in body weight in control and GFP-expressing EAE mice but not in LIF-treated animals.
Figure 3.
Lentiviral mediated expression of LIF in the central nervous system significantly reduces EAE severity. Lentivirus were injected in the lateral ventricle 2 weeks before induction of EAE. (a) Clinical scores of mice overexpressing LIF (n = 10) were significantly reduced during the first disease episode (day 12–22) as well as during the relapse (day 36–41) compared to control EAE mice expressing green fluorescent protein (GFP) (n = 10). (b) Central LIF expression reduced body weight compared to GFP-expressing mice. Data are expressed as the means ± SEM. *P < 0.05,**P < 0.01, as revealed by Mann–Whitney U-test. EAE, experimental autoimmune encephalomyelitis; LIF, leukemia inhibitory factor.
Table 1.
Clinical features of C57Bl/6J mice treated with lentiviral vectors encoding LIF or GFP 2 weeks before EAE induction
Local LIF production does not affect numbers of infiltrating T-cells and macrophages
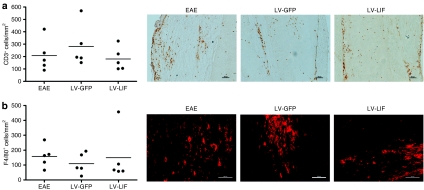
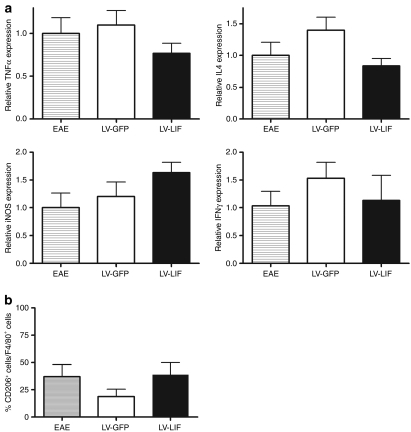
The extent of immune infiltration was analyzed in EAE mice that had been treated with either LV-GFP or LV-LIF. Animals were killed on day 41, during recovery from the second relapse. At this stage, mice overexpressing LIF showed significantly less severe clinical symptoms than GFP-expressing mice (P < 0.01). In this EAE model, clinical symptoms arise from inflammatory demyelinating lesions that are predominantly located in the spinal cord. Therefore, spinal cords of five mice per treatment group were studied for the presence of infiltrating T-cells and macrophages/microglia, as detected with CD3- or F4/80-positive staining, respectively. Local expression of LIF within the CNS did not significantly alter the number of macrophages/microglia or T cells infiltrating the spinal cord (Figure 4). To investigate whether LIF exerts its beneficial effects through immunomodulation, rather than inhibition of inflammation, we quantified CNS expression of tumor necrosis factor-α, interferon-γ, inducible NO synthase, and interleukin-4. No significant difference in pro- and anti-inflammatory cytokine profiles was detected between LIF-treated and control EAE groups. Furthermore, the amount of alternatively activated macrophages, as assessed by CD206 staining, did not differ significantly between the treatment groups (Figure 5).
Figure 4.
Immune infiltrates in the central nervous system (CNS) of LV-LIF and LV-GFP treated EAE mice. Five mice per group were studied in the immunohistological evaluation of spinal cord infiltrates. Local LIF expression did not significantly affect the amount of infiltrating T-cells and macrophages/microglia in the CNS of EAE mice compared to control EAE mice or LV-GFP treated EAE animals. Dots represent the mean number of (a) CD3- or (b) F4/80-positive cells detected in each animal at representative regions covering the entire spinal cord. Bars represent the means per treatment group. EAE, experimental autoimmune encephalomyelitis; LIF, leukemia inhibitory factor.
Figure 5.
Cytokine and mannose receptor expression in the central nervous system (CNS) of LV-LIF and LV-GFP treated EAE mice. (a) Real-time PCR revealed no significant differences in TNF-α, IL-4, iNOS, and IFN-γ expression levels in LIF-treated mice compared to untransduced or GFP-expressing EAE mice. (b) Local LIF expression did not significantly affect the amount of alternatively activated (CD206 positive) macrophages/microglia in the CNS of EAE mice, compared to control EAE mice or LV-GFP treated EAE animals. EAE, experimental autoimmune encephalomyelitis; GFP, green fluorescent protein; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; TNF, tumor necrosis factor; LIF, leukemia inhibitory factor.
Local LIF expression protects against auto immune-mediated demyelination
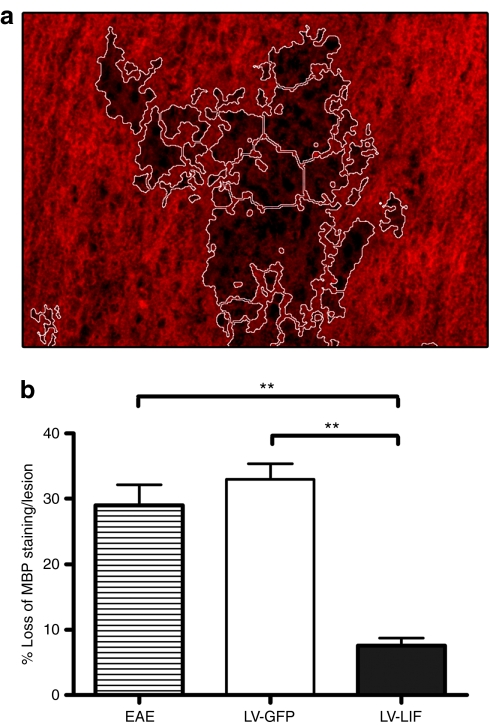
The effect of central LIF expression on immune-mediated demyelination was investigated by immunohistochemistry. Demyelination, revealed by a loss of myelin basic protein (MBP) staining, was analyzed in EAE lesions at day 41 postimmunization. We established that MBP expression was reduced by an average of 29.0 ± 3.1% in the affected regions of untreated EAE mice (Figure 6). Mice overexpressing GFP displayed a similar extent of demyelination, with an average of 32.9 ± 2.4%. Local production of LIF reduced the loss of myelin in CNS lesions to 7.6 ± 1.2%. Immune-mediated demyelination was significantly reduced in EAE mice overexpressing LIF compared to demyelination in control EAE mice or GFP-expressing EAE mice (P < 0.001).
Figure 6.
Local expression of LIF in the central nervous system significantly reduces immune-mediated demyelination in EAE. (a) Representative image of MBP staining in EAE spinal cord. Demyelinated area, indicated by loss of MBP staining, is marked using NIS-Elements software (outlined in white). (b) Demyelination is quantified and expressed as the means ± SEM. **P < 0.001. EAE, experimental autoimmune encephalomyelitis; LIF, leukemia inhibitory factor; MBP, myelin basic protein.
Discussion
This study was designed to evaluate the therapeutic potential of LIF during CNS inflammation, and to elucidate the effects of local LIF expression on immune-mediated demyelination. We investigated the effects of LIF in an EAE model induced by immunization with myelin OLG peptide in C57Bl/6J mice. Our study demonstrates that LIF has limited therapeutic efficacy after systemic administration, which was also described in other models of neuroinflammation. In experimental autoimmune neuritis, systemic LIF treatment also shows a slight but nonsignificant improvement in the clinical course.21 In an EAE model induced by proteolipid protein 139–151 peptide in SJL/J mice,10 systemic LIF delivery mediated a reduction of clinical symptoms, similar to the effect reported here. It is described that increasing the dose to 60 µg/kg/day does not lead to any additional benefit.10 This limited therapeutic efficacy may be explained by the fact that LIF enters the brain through a saturable transport system,18 making it hard to deliver sufficient amounts of LIF to the site of inflammation after systemic administration. Furthermore, because of the pleiotropic effects of LIF, systemic administration of higher doses may be accompanied by serious systemic side effects.22 This was demonstrated in phase I clinical trials with recombinant human LIF, also called AM424 or emfilermin (AMRAD Operations, Richmond, Australia). LIF was administered subcutaneously on a daily basis with doses ranging from 0.25 to 16 µg/kg body weight. Several patients developed side effects, such as autonomic dysfunction, in particular impotence and episodic hypotension. The dose-limiting toxicities were hypotension and rigors. In addition, the half-life of LIF is relatively short, ~1–5 hours, independent of the dose.23 This short half-life, together with the limited capacity of LIF to reach the CNS and its pleiotropic actions, complicate therapeutic use of LIF through systemic administration.
We demonstrate that local LIF production in the CNS by means of LVs is a successful strategy to circumvent this delivery problem. LVs are highly suitable and optimized for long-lasting expression of transgenes in the CNS.19 CNS expression of LIF was well tolerated, although a small reduction in body weight (1 g on average) was observed compared to GFP-expressing and control EAE mice. This is in agreement with studies describing that LIF can mediate leptin-like effects in reducing body weight by its central actions on the hypothalamus.20,24 Our study demonstrates that in contrast to systemic treatment, LV-mediated production of LIF in the CNS significantly reduces EAE severity. It has been suggested that systemic treatment of neurotrophic factors should be applicable during MS or EAE because of the blood–brain barrier disruption at sites of acute demyelination. However, our results demonstrate that a local production significantly enhances the therapeutic potential of LIF even in conditions where the blood–brain barrier is disrupted. Furthermore, CNS diseases with a chronic nature, such as MS, require a continuous supply of therapeutic agents to the brain and therefore benefit from long-lasting strategies, such as CNS-targeted gene therapy.
This study demonstrates that CNS-directed LIF expression is more effective than systemic administration, not only to achieve clinical benefit, but also to elucidate the local effects of LIF on CNS lesion development. We found that local expression of LIF has a beneficial effect on CNS lesion development as it significantly reduced the extent of demyelination. This is in line with previous reports describing reduction of demyelination by systemic LIF treatment after spinal cord injury25 and in LIF knock-out mice after cuprizone challenge.26 Although these studies suggest a protective effect of LIF against demyelination, they do not take into account the inflammatory component of MS. Studies in EAE models reflect both the demyelinating and inflammatory aspects of MS, but have generated conflicting results. Administration of neutralizing anti-LIF antibodies in EAE increases the extent of acute demyelination and doubles the OLG loss already induced by EAE.5 In contrast, a more recent study describes reduced autoimmune demyelination when EAE is induced in LIF knock-out mice,11 suggesting that LIF may play a detrimental role in neuroinflammation. Different findings in these studies may be explained by differences in experimental models used. Compensatory neurokine family members may be expressed in LIF knock-out mice that could be responsible for the observed effects. Our study demonstrates that local expression of LIF in the adult CNS significantly limits autoimmune-mediated demyelination.
Furthermore, our data show that local expression of LIF in the CNS does not suppress immune infiltration. However, LIF may modulate the immune response without altering the number of immune cells present at the lesion site. Indeed, we have previously reported that LIF mediates reduction of reactive oxygen species and tumor necrosis factor-α secretion by macrophages,8 which may also provide a mechanism for the beneficial effects of LIF in the CNS. Therefore, LIF may have additional beneficial effects during neuroinflammatory responses by limiting the production of these toxic mediators by macrophages. However, our in vivo findings show that there is no significant difference in proinflammatory cytokine expression upon LIF treatment. Furthermore, immunohistochemical analyses showed no difference in mannose receptor expression, a marker for alternatively activated macrophages. However, these data do not rule out the possibility that an immunomodulatory effect is effective at earlier stages in the disease.
Taken together, our study demonstrates that the local expression of LIF limits immune-mediated demyelination, without inhibiting the inflammatory response. The observed beneficial effect of LIF on demyelination may be a direct consequence of LIF-mediated enhanced survival of mature OLGs that has been described in EAE.10 In vitro studies demonstrate that LIF enhances the generation of OLG in cultures of dividing O-2A progenitors and promotes OLG maturation, as determined by expression of MBP.27 These in vitro effects are in agreement with our in vivo data, demonstrating that LIF gene therapy leads to a preservation of MBP expression during autoimmune demyelination. Furthermore, we have previously shown that LIF protects mature rat OLG cultures selectively against the combined insult of interferon-γ and tumor necrosis factor-α, important inflammatory mediators in MS. Although local expression of LIF may directly interfere with inflammatory cytokine signaling at the lesion site, LIF also induces subtle changes in oligodendroglial protein expression that shift the cellular machinery toward a prosurvival execution program. We demonstrated that the downstream mechanism involves activation of the Akt/PI3 kinase pathway and increased expression of 14-3-3 isotypes, known to control cell survival and apoptosis. Taken together, these data suggest that local expression of LIF in CNS lesions limits demyelination and oligodendroglial cell death induced by proinflammatory cytokines.
Although current MS therapeutics aim to inhibit the inflammatory response, complementary strategies that protect against demyelination could significantly improve disease outcome. The multifocal pathology of MS requires a widespread treatment covering the entire CNS. Our data demonstrate that lentiviral delivery of LIF in the cerebrospinal fluid mediates effective suppression of symptoms in an animal model of MS. Preclinical studies will have to elucidate whether LIF gene therapy is still effective when treatment is started after disease onset, which is a prerequisite for its applicability in MS patients. In addition, it would be interesting to investigate compatibility of LIF gene therapy with current immunomodulatory MS-treatments, such as interferons. Clinical application in the human CNS should permit adjustment and, if necessary, termination of expression in individual patients. In this regard, the introduction of expression cassettes with regulatable promoters that respond to exogenous drug administration may substantially enhance safety. In conclusion, although preclinical studies are necessary to further optimize dosing and timing of LIF gene therapy, as well as the ability to regulate gene expression, our study indicates that CNS delivery of LIF through LVs is a promising strategy in the treatment of MS.
Materials and Methods
LV construction and production. The complementary DNA encoding mouse LIF (sequence NM_008501) was cloned into a lentiviral transfer plasmid containing a central polypurine tract sequence, the SIN-18 deletion, and the woodchuck hepatitis posttranscriptional regulatory element.19 HIV-1 derived vector particles were produced by a triple transient transfection of 293T cells. Briefly, cells were transfected with a second-generation packaging plasmid, a plasmid encoding the glycoprotein G of vesicular stomatitis virus and a transfer plasmid encoding the neurokine gene under control of a cytomegalovirus promoter. Transient transfection of 293T cells was carried out in 10-cm dishes. For every plate, a DNA mixture containing 20 µg of transfer plasmid, 10 µg of packaging construct, and 5 µg of envelope plasmid in 700 µl of 150 mmol/l NaCl was prepared. A volume of 700 µl polyethyleneimine solution (1.42 µmol/l polyethyleneimine in 150 mmol/l NaCl) was added slowly. The mixture was incubated at room temperature for 15 minutes and then added dropwise to the 293T cells in OPTI-MEM (GIBCO, Invitrogen, Merelbeke, Belgium) without serum. The next morning, medium was replaced with serum free OPTI-MEM. Supernatants were collected and filtered at 48 and 72 hours post-transfection. The vector particles in the supernatant were concentrated using Vivaspin 15 columns (Vivascience, Hannover, Germany), aliquoted and stored at −80 °C. p24 antigen content was determined by HIV-1 p24 Core Profile ELISA (DuPont, Dreieich, Germany).
Analysis of in vitro transgene expression. One day before transduction, 75,000 293T cells were seeded in each well of a 24-well plate in Dulbecco's modified Eagle's medium containing 5% fetal calf serum. The next day, medium was replaced by Dulbecco's modified Eagle's medium containing 5% fetal calf serum and different dilutions (1:1, 1:3, 1:9, and 1:27) of an LV stock solution corresponding to 4.8 ± 2 × 107 pg of p24/ml. At 72 hours after transduction, supernatants were collected and the cells were lysed with 1% sodium dodecyl sulfate containing protease inhibitors (Complete Mini; Roche Diagnostics, Vilvoorde, Belgium) and boiled for 5 minutes. Protein content was determined using the BCA protein assay kit (Pierce, Erembodegem, Belgium). Twenty microgram of total protein extract was separated on a 12% sodium dodecyl sulfate–polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (Bio-Rad, Wattford, UK). Blots were blocked for 2 hours in phosphate-buffered saline (PBS) containing 5% nonfat dry milk and 0.5% Tween-20 and then probed overnight with an anti-LIF antibody (1:1,000) (R&D Systems, Oxon, UK). After washing with PBS containing 1% Tween-20, membranes were incubated with horseradish peroxidase conjugated to rabbit-anti-goat IgG, followed by detection with Enhanced Chemiluminescence (ECL Plus; GE Healthcare, Upsala, Sweden). LIF concentration in supernatant of transduced 293T cells was measured by means of an ELISA kit (R&D Systems Europe, Oxon, UK).
Stereotactic surgery. For local LIF administration, LV encoding murine LIF were stereotactically injected in the lateral ventricle 2 weeks before EAE induction. Adult C57Bl/6J mice were housed with free access to food and water under a 12:12-hour dark:light cycle. All surgical procedures were performed under ketamine (75 mg/kg) and medetomidine (1 mg/kg) anesthesia using aseptic procedures. Mice were placed in a stereotactic head frame (Stoelting, IL, USA) and after midline incision of the skin, a small hole was drilled in the skull. Injections were made using a 30-gauge needle and a 10-µl Hamilton syringe. Coordinates used for injections into the right lateral ventricle were anteroposterior −0.02 cm, lateral −0.10, and dorsoventral −0.18 using bregma as reference. Six microliters of highly concentrated vector (p24: LV-LIF = 4.8 × 107 pg/ml) supplemented with polybrene (4 µg/ml) was injected at a rate of 0.25 µl/minute. After the injection, the needle was left in place for an additional 5 minutes before being slowly withdrawn from the brain.
Induction of EAE. C57Bl/6J mice were purchased from Harlan. Mice were immunized with 200 µg myelin OLG 35–55 (MEVGWYRSPFS RVVHLYRNGK) (Ansynth, Berkel en Rodenrijs, the Netherlands) dissolved 100 µl PBS, to which 100 µl complete Freund's Adjuvant containing 5 mg/ml mycobacterium (Sigma, Nieuwegein, the Netherlands) was added. This mixture was injected subcutaneously into the flanks. Directly after immunization and 48 hours later, mice received an intraperitoneal injection of 200 ng pertussis toxin. Disease severity was graded using a standard 5-point scale with 0.5-point increments: 0, no symptoms; 1, decreased tail tone; 2, hindlimb paresis; 3, hindlimb paralysis; 4, quadraparesis; 5, death. Mice that reached grade 4.5 were killed in accordance with ethics committee requirements. All experiments were approved by the Hasselt University ethics committee.
LIF treatment. For systemic LIF treatment, cohorts were assembled after matching for disease severity and weight. Treatment started when mean disease scores were >0.5. Mice were treated from day 13 to 18 with daily intraperitoneal injections of recombinant murine LIF (R&D Systems, Abingdon, UK) at doses of 25 µg/kg, dissolved in 100 µl PBS containing 0.1% mouse serum albumin (Sigma). Control animals received daily injections of 100 µl PBS containing 0.1% mouse serum albumin.
Histology. At day 42 postimmunization (56 days after LV injection), brain and spinal cords were removed, snap frozen in liquid nitrogen and stored at −70 °C. Frozen sections (10-µm thick) were cut with a microtome (Leica Microsystems, Wetzelar, Germany). Immunohistochemistry was performed using antibodies raised against LIF (goat polyclonal, 1:100; R&D Systems Europe, Oxon, UK), enhanced GFP (rabbit polyclonal, 1:10,000, in-house28), or CD3 (rat, 1:100; Serotec, Düsseldorf, Germany). Sections were pretreated with 3% hydrogen peroxide, blocked for 20 minutes in blocking agent (Dako, Glostrup, Denmark) and incubated overnight with primary antibody diluted in 10% normal goat serum. Biotinylated secondary antibodies (Dako) were used in a 1:300 dilution, followed by incubation with streptavidin–biotin–horseradish peroxidase complex (ABC Kit; Dako). Immunoreactivity was visualized using 3,3-diaminobenzidine as a chromogen. For fluorescent staining, sections were blocked with blocking agent (Dako) for 20 minutes at room temperature and then incubated overnight with primary antibodies raised against MBP (rat, 1:100; Chemicon, Brussels, Belgium), F4/80 (rat, 1:100; Serotec), and CD206 (rat, 1:100; Serotec). Binding of primary antibody was visualized using goat-anti-rat Alexa 555 secondary antibodies (1:400; Molecular Probes, Invitrogen, Merelbeke, Belgium). Incubation with 4,6′-diamidino-2-phenylindole was performed for fluorescent counterstaining of cell nuclei. As a negative control, primary antibodies were omitted from the staining procedure.
Histological quantification. The number of infiltrating immune cells and the extent of demyelination were evaluated in spinal cords of five mice per treatment group. Mice chosen for histological analysis displayed disease scores most closely resembling the median of the respective treatment group. Every 300 µm, an entire spinal cord section was analyzed for immune infiltrates, with a total of three sections for each animal. Data are expressed as the mean number of CD3- or F4/80-positive cells detected in 10 pictures taken in each animal. Mannose receptor–positive macrophages are depicted as the percentage CD206 positive cells compared to the amount of F4/80-positive cells detected in these animals. Demyelinated area was assessed as loss of MBP staining in 10 random lesions at representative regions covering the entire spinal cord. Demyelinated area and the number of F4/80 and CD3 positive cells were quantified using an Eclipse 80i microscope with NIS-Elements Basic Research ver2.3 microscopy software (Nikon, Brussels, Belgium).
Real-time PCR. RNA samples were prepared from spinal cords of mice (n = 3 per group) by the RNeasy Lipid Tissue mini kit (Qiagen, Venlo, the Netherlands). After reverse transcription (Promega, Madison, USA), complementary DNAs were amplified using specific commercially available primers (Taqman Gene Expression assays) on an ABI PRISM 7500 sequence detection system (Applied Biosystems, Foster City, MA). A threshold cycle was calculated and relative quantification was obtained by comparison with the threshold cycle obtained by amplifying samples with glyceraldehyde-3-phosphate dehydrogenase- and 18S-specific primers.
Statistical analysis. Statistical analysis was performed using the Graphpad Prism4 software package. Results are expressed as means ± SE of the mean. Mann–Whitney U-test was used for statistical analyses of disease scores. Statistical significance level was set as follows: *P <0.05, **P <0.01.
Acknowledgments
We thank Wilfried Leyssens and Katrien Wauterickx for excellent technical assistance. We thank Zeger Debyser and Rik Gijsbers from the lab of Molecular Virology and Gene Therapy, Division of Molecular Medicine, Katholieke Universiteit Leuven in Belgium for help with the construction of the lentiviral vectors. This work was financially supported by the Flemish Fund for Scientific Research (FWO Vlaanderen) and the Belgian Charcot foundation. J.J.A.H. is a postdoctoral fellow of FWO Vlaanderen and H.S. holds a fellowship of the Belgian ‘Wetenschappelijk Onderzoek Multiple Sclerosis' (WOMS) Foundation.
REFERENCES
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group Randomised double-blind placebo-controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- Trapp BD., and , Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder. Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- Banner LR, Moayeri NN., and , Patterson PH. Leukemia inhibitory factor is expressed in astrocytes following cortical brain injury. Exp Neurol. 1997;147:1–9. doi: 10.1006/exnr.1997.6536. [DOI] [PubMed] [Google Scholar]
- Vanderlocht J, Hellings N, Hendriks JJ, Vandenabeele F, Moreels M, Buntinx M, et al. Leukemia inhibitory factor is produced by myelin-reactive T cells from multiple sclerosis patients and protects against tumor necrosis factor-α-induced oligodendrocyte apoptosis. J Neurosci Res. 2006;83:763–774. doi: 10.1002/jnr.20781. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Emery B, Cipriani T, Marriott MP., and , Kilpatrick TJ. Endogenous leukemia inhibitory factor production limits autoimmune demyelination and oligodendrocyte loss. Glia. 2006;53:696–703. doi: 10.1002/glia.20321. [DOI] [PubMed] [Google Scholar]
- Murphy M, Dutton R, Koblar S, Cheema S., and , Bartlett P. Cytokines which signal through the LIF receptor and their actions in the nervous system. Prog Neurobiol. 1997;52:355–378. doi: 10.1016/s0301-0082(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Slaets H, Dumont D, Vanderlocht J, Noben JP, Leprince P, Robben J, et al. Leukemia inhibitory factor induces an antiapoptotic response in oligodendrocytes through Akt-phosphorylation and up-regulation of 14-3-3. Proteomics. 2008;8:1237–1247. doi: 10.1002/pmic.200700641. [DOI] [PubMed] [Google Scholar]
- Hendriks JJ, Slaets H, Carmans S, de Vries HE, Dijkstra CD, Stinissen P, et al. Leukemia inhibitory factor modulates production of inflammatory mediators and myelin phagocytosis by macrophages. J Neuroimmunol. 2008;204:52–57. doi: 10.1016/j.jneuroim.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Kerr BJ., and , Patterson PH. Potent pro-inflammatory actions of leukemia inhibitory factor in the spinal cord of the adult mouse. Exp Neurol. 2004;188:391–407. doi: 10.1016/j.expneurol.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Zhang JG, Soilu-Hanninen M, Hochrein H, Chionh F, Shipham KA, et al. LIF receptor signaling limits immune-mediated demyelination by enhancing oligodendrocyte survival. Nat Med. 2002;8:613–619. doi: 10.1038/nm0602-613. [DOI] [PubMed] [Google Scholar]
- Linker RA, Kruse N, Israel S, Wei T, Seubert S, Hombach A, et al. Leukemia inhibitory factor deficiency modulates the immune response and limits autoimmune demyelination: a new role for neurotrophic cytokines in neuroinflammation. J Immunol. 2008;180:2204–2213. doi: 10.4049/jimmunol.180.4.2204. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Waring P., and , Nicola NA. Actions of leukaemia inhibitory factor on megakaryocyte and platelet formation. Ciba Found Symp. 1992;167:174–82; discussion 182. doi: 10.1002/9780470514269.ch11. [DOI] [PubMed] [Google Scholar]
- Ding T, Song H, Wang X, Khatua A., and , Paria BC. Leukemia inhibitory factor ligand-receptor signaling is important for uterine receptivity and implantation in golden hamsters (Mesocricetus auratus) Reproduction. 2008;135:41–53. doi: 10.1530/REP-07-0013. [DOI] [PubMed] [Google Scholar]
- Ni H, Ding NZ, Harper MJ., and , Yang ZM. Expression of leukemia inhibitory factor receptor and gp130 in mouse uterus during early pregnancy. Mol Reprod Dev. 2002;63:143–150. doi: 10.1002/mrd.10168. [DOI] [PubMed] [Google Scholar]
- Mayer P, Geissler K, Ward M., and , Metcalf D. Recombinant human leukemia inhibitory factor induces acute phase proteins and raises the blood platelet counts in nonhuman primates. Blood. 1993;81:3226–3233. [PubMed] [Google Scholar]
- Akita S, Conn PM., and , Melmed S. Leukemia inhibitory factor (LIF) induces acute adrenocorticotrophic hormone (ACTH) secretion in fetal rhesus macaque primates: a novel dynamic test of pituitary function. J Clin Endocrinol Metab. 1996;81:4170–4178. doi: 10.1210/jcem.81.11.8923879. [DOI] [PubMed] [Google Scholar]
- Chesnokova V., and , Melmed S. Leukemia inhibitory factor mediates the hypothalamic pituitary adrenal axis response to inflammation. Endocrinology. 2000;141:4032–4040. doi: 10.1210/endo.141.11.7778. [DOI] [PubMed] [Google Scholar]
- Pan W, Kastin AJ., and , Brennan JM. Saturable entry of leukemia inhibitory factor from blood to the central nervous system. J Neuroimmunol. 2000;106:172–180. doi: 10.1016/s0165-5728(00)00241-1. [DOI] [PubMed] [Google Scholar]
- Baekelandt V, Claeys A, Eggermont K, Lauwers E, De Strooper B, Nuttin B, et al. Characterization of lentiviral vector-mediated gene transfer in adult mouse brain. Hum Gene Ther. 2002;13:841–853. doi: 10.1089/10430340252899019. [DOI] [PubMed] [Google Scholar]
- Prima V, Tennant M, Gorbatyuk OS, Muzyczka N, Scarpace PJ., and , Zolotukhin S. Differential modulation of energy balance by leptin, ciliary neurotrophic factor, and leukemia inhibitory factor gene delivery: microarray deoxyribonucleic acid-chip analysis of gene expression. Endocrinology. 2004;145:2035–2045. doi: 10.1210/en.2003-1376. [DOI] [PubMed] [Google Scholar]
- Laurà M, Gregson NA, Curmi Y., and , Hughes RA. Efficacy of leukemia inhibitory factor in experimental autoimmune neuritis. J Neuroimmunol. 2002;133:56–59. doi: 10.1016/s0165-5728(02)00359-4. [DOI] [PubMed] [Google Scholar]
- Gunawardana DH, Basser RL, Davis ID, Cebon J, Mitchell P, Underhill C, et al. A phase I study of recombinant human leukemia inhibitory factor in patients with advanced cancer. Clin Cancer Res. 2003;9:2056–2065. [PubMed] [Google Scholar]
- Segrave AM, Mager DE, Charman SA, Edwards GA., and , Porter CJ. Pharmacokinetics of recombinant human leukemia inhibitory factor in sheep. J Pharmacol Exp Ther. 2004;309:1085–1092. doi: 10.1124/jpet.103.063289. [DOI] [PubMed] [Google Scholar]
- Beretta E, Dhillon H, Kalra PS., and , Kalra SP. Central LIF gene therapy suppresses food intake, body weight, serum leptin and insulin for extended periods. Peptides. 2002;23:975–984. doi: 10.1016/s0196-9781(02)00021-9. [DOI] [PubMed] [Google Scholar]
- Azari MF, Profyris C, Karnezis T, Bernard CC, Small DH, Cheema SS, et al. Leukemia inhibitory factor arrests oligodendrocyte death and demyelination in spinal cord injury. J Neuropathol Exp Neurol. 2006;65:914–929. doi: 10.1097/01.jnen.0000235855.77716.25. [DOI] [PubMed] [Google Scholar]
- Marriott MP, Emery B, Cate HS, Binder MD, Kemper D, Wu Q, et al. Leukemia inhibitory factor signaling modulates both central nervous system demyelination and myelin repair. Glia. 2008;56:686–698. doi: 10.1002/glia.20646. [DOI] [PubMed] [Google Scholar]
- Mayer M, Bhakoo K., and , Noble M. Ciliary neurotrophic factor and leukemia inhibitory factor promote the generation, maturation and survival of oligodendrocytes in vitro. Development. 1994;120:143–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- Baekelandt V, Eggermont K, Michiels M, Nuttin B., and , Debyser Z. Optimized lentiviral vector production and purification procedure prevents immune response after transduction of mouse brain. Gene Ther. 2003;10:1933–1940. doi: 10.1038/sj.gt.3302094. [DOI] [PubMed] [Google Scholar]