Abstract
In an attempt to treat cancer patients with ERBB2 overexpressing tumors, we developed a chimeric antigen receptor (CAR) based on the widely used humanized monoclonal antibody (mAb) Trastuzumab (Herceptin). An optimized CAR vector containing CD28, 4-1BB, and CD3ζ signaling moieties was assembled in a γ-retroviral vector and used to transduce autologous peripheral blood lymphocytes (PBLs) from a patient with colon cancer metastatic to the lungs and liver, refractory to multiple standard treatments. The gene transfer efficiency into autologous T cells was 79% CAR+ in CD3+ cells and these cells demonstrated high-specific reactivity in in vitro coculture assays. Following completion of nonmyeloablative conditioning, the patient received 1010 cells intravenously. Within 15 minutes after cell infusion the patient experienced respiratory distress, and displayed a dramatic pulmonary infiltrate on chest X-ray. She was intubated and despite intensive medical intervention the patient died 5 days after treatment. Serum samples after cell infusion showed marked increases in interferon-γ (IFN-γ), granulocyte macrophage-colony stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and IL-10, consistent with a cytokine storm. We speculate that the large number of administered cells localized to the lung immediately following infusion and were triggered to release cytokine by the recognition of low levels of ERBB2 on lung epithelial cells.
Introduction
ERBB2 (HER-2/neu) is a member of the epidermal growth factor receptor family. Epidermal growth factor receptor–ligand interaction induces the heterodimerization of receptors, which in turn results in the activation of intracellular tyrosine kinase domain signaling cascades that mediate cell growth, differentiation, and survival.1,2,3 Overexpression of ERBB2 can induce dimerization of ERBB2 and initiates signal transduction activities without ligand binding. ERBB2 overexpression/amplification occurs in ~15–25% of human breast cancer patients, and is associated with more aggressive disease.4 A proportion of other human cancers are also associated with ERBB2 gene amplification and protein overexpression; including cancers of the colon, ovary, stomach, kidney, melanoma, and others.5,6,7 Investigation of agents that target the ERBB2 protein led to the development of Trastuzumab (Herceptin), a humanized monoclonal antibody (mAb) that binds to the extracellular domain of the receptor.8 Trastuzumab has been shown to be of clinical benefit for metastatic breast cancer patients with ERBB2 overexpression/amplification, either alone or in combination with chemotherapy regimens.9,10 ERBB2 has also been the target of several cancer vaccine trials,11,12,13 as well as, adoptive cell therapy using anti-ERBB2 cytotoxic T lymphocyte lines.14
Adoptive cell therapy has emerged as the most effective treatment for patients with metastatic melanoma. Adoptive cell therapy using tumor-reactive autologous tumor infiltrating lymphocytes (TIL) in combination with nonmyeloablative but lymphodepleting conditioning resulted in 50% objective clinical regression in melanoma patients.15 Intensifying the lymphodepletion by adding total-body irradiation to the chemotherapy conditioning regimen improved the objective response rate to 72%.16 This potent therapy, however, has been limited by the requisite surgery to procure tumor-reactive TIL, by ex vivo identification and expansion of these cells, and by the failure to reproducibly isolate similar cells from common epithelial tumors.
The transfer of genes into primary human lymphocytes permits the introduction of tumor antigen receptor molecules that can endow the engineered cell with antitumor specificity.17,18,19 We reported the first clinical trials using autologous peripheral blood lymphocytes (PBLs) modified to express a tumor antigen-reactive T-cell receptor in the treatment of patients with metastatic melanoma that resulted in objective tumor regressions.20,21 These strategies, however, have a lower response rate than TIL, and only a minority of patients are eligible for current protocols, as they must express human leukocyte antigen-A*0201 in order to be recognized by the T-cell receptor-engineered cells.
An alternative to T-cell receptor gene therapy is the use of a chimeric antigen receptor (CAR) that is capable of relaying excitatory signals to T cells in a non-Major histocompatibility complex-restricted manner. These hybrid proteins, composed of an extracellular antigen recognition domain fused to an intracellular T-cell activation domain,22,23 may therefore be used in patients regardless of their human leukocyte antigen genotype. The absence of human leukocyte antigen-restricted antigen recognition is achieved by harnessing the antigen-binding properties of mAb; this recognition is also independent of antigen processing, thus bypassing a potential mechanism by which tumor cells can evade the immune system in vivo. Several clinical trials using CAR-transduced T cells have been reported.24,25,26,27 ERBB2-based CARs reported thus far are composed of single-chain Fv fragment from murine mAb, which have been shown to induce anti-CAR immune responses in humans.25,26 The anti-ERBB2 CAR used in this case report was a next generation CAR containing both the humanized Herceptin single-chain Fv fragment and optimized costimulatory signaling domains designed for increased cytokine secretion, lytic activity, and shown to display robust in vivo antitumor activity in a human breast cancer xenograft model.28
Results
In vitro characteristics of the ErbB2-CAR transduced T cells for patient treatment
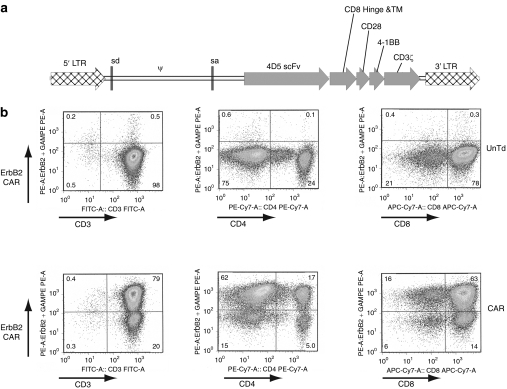
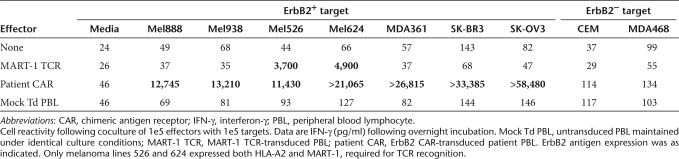
Leukophoresis was performed to obtain patient peripheral blood mononuclear cells (PBMCs), which were stimulated with an anti-CD3 mAb and interleukin-2 (IL-2) to initiate T-cell expansion followed by transduction with the 4D5-CD8-28BBZ ERBB2-CAR vector as described in Materials and Methods section. At 4 days before infusion, cells were analyzed for the expression of the ERBB2 CAR using an ERBB2-Fc fusion protein as previously described.28 As shown in Figure 1, 79% of CD3+ T cells expressed the CAR with gene transfer into both CD4+ (17%) and CD8+ (63%) T-cell subsets. To determine functional activity, transduced T cells were cocultured with ERBB2+ melanoma, breast cancer, and ovarian cancer cell lines, or ERBB2− breast cancer and T lymphoblastoid cell lines. ERBB2-specific reactivity was demonstrated by production of effector cytokine interferon-γ (IFN-γ) only in cell lines expressing ERBB2 (Table 1). Background cytokine production of ERBB2-CAR transduced cells, when cocultured with ERBB2-targets, was similar to untransduced control cells. To complete the certificate of analysis for patient treatment, transduced cells were also tested to be negative for the presence of replication competent retrovirus by PCR and passed sterility testing (data not shown). Retrospective testing using an amplification-based S+/L− assay was also negative for replication competent retrovirus.
Figure 1.
Expression of the ERBB2 CAR. Diagram of the ERBB2-CAR vector (MSGV1-4D5-CD8-28BBZ) used in this trial is as shown on the top of the figure. As described in Materials and Methods section, patient PBMC were stimulated to induced T-cell division and then transduced with the CAR vector. Four days before cell infusion samples were removed for analysis of ERBB2-CAR gene expression by FACS (along with the CD3, CD4, or CD8 T-cell markers). CAR, chimeric antigen receptor; LTR, long terminal repeats; PBMC, peripheral blood mononuclear cell; scFv, single-chain Fv fragment.
Table 1.
ErbB2-CAR certificate of analysis
Clinical course
The patient was a 39-year-old female who 3 years earlier had undergone a sigmoid resection for colon cancer that on pathologic analysis exhibited lymphatic invasion and vascular involvement, with spread to 6 of 21 lymph nodes and the presence of synchronous liver metastases. She was treated with a chemotherapy regimen consisting of 5-fluorourocil, leucovorin and oxaliplatin plus the antivascular endothelial growth factor mAb, bevacizumab. The tumor progressed and the patient was then treated with an alternate chemotherapy regimen in which irinotecan was substituted for oxaliplatin (FOLFIRI). The patient again progressed and after desensitization to oxaliplatin for an allergic reaction, she received a third chemotherapy regimen consisting of capecitabine, oxaliplatin, and bevacizumab. The tumors in the lung and liver continued to progress and the patient was referred to the Surgery Branch, National Cancer Institute (NCI; Bethesda, MD) and signed an informed consent for our protocol. The protocol was reviewed and approved by the National Institutes of Health Institutional Biosafety Committee, the NCI Institutional Review Board, the National Institutes of Health Office of Biotechnology Activities, and the Food and Drug Administration (all Bethesda, MD). Patient inclusion criteria included metastatic cancer that expressed ERBB2 (Her-2/neu) at ≥2+ as assessed by immunohistochemistry.
To facilitate homeostatic expansion of the transduced cells, the patient received a lymphodepleting regimen (60 mg/kg cyclophosphamide daily for 2 days followed by flurodarabine 25 mg/m2 for the next 5 days). On the day following the last chemotherapy dose the patient received an intravenous infusion of 1010 cells transduced with the ERBB2 CAR in 125 ml over 30 minutes. This was the largest number of cells permitted in the first dose-escalation cohort. Within 15 minutes after completing the infusion, the patient developed respiratory distress with decreased blood oxygen saturation that worsened over the next hour. Chest X-ray obtained 40 minutes after completion of the infusion showed pulmonary edema, which appeared worse on chest X-rays repeated at 2 and 4 hours after the infusion. Because of decreasing respiratory function the patient was transferred to the intensive care unit and was intubated about 1 hour after the cell infusion. The patient then developed severe hypotension requiring vasopressors. Dexamethasone, 8 mg every 6 hours, was administered starting at about 5 hours after the cell infusion (it was continued for 2 days, after which the dose was tapered). The patient experienced two cardiac arrests in the next 12 hours after cell infusion both requiring cardiopulmonary resuscitation. She was maximally supported with vasopressors and ventilatory support. She remained severely ill with maximum intensive care unit support for the next 5 days at which time progressive hypotension and bradycardia as well as gastrointestinal bleeding resulted in cardiac arrest from which she could not be resuscitated.
Postmortem analysis
At autopsy, multiple organs exhibited signs of systemic ischemia and hemorrhagic microangiopathic injury. The lungs also showed diffuse alveolar damage consistent with the clinical findings of acute respiratory distress syndrome. Autopsy also revealed a generalized rhabdomyolysis. Copious blood in the small intestine indicated that the patient succumbed to hemorrhage in the setting of multiple organ failure secondary to systematic microangiopathic injury. The autopsy findings appeared to be a combination of the initial pulmonary injury followed by the sequela of several days of hypotension and organ ischemia.
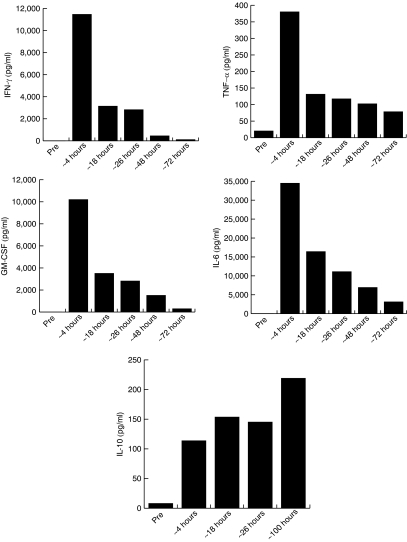
Beginning at about 4 hours after cell infusion serum samples were obtained and stored for analysis. Compared to pretreatment samples, the patient's serum displayed a rapid and marked increase in the levels of five cytokines; IFN-γ, granulocyte macrophage-colony stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), IL-6, and IL-10 (Figure 2). At 4 hours after infusion, four of the five cytokines displayed peak serum levels: IFN-γ,11, 456 pg/ml; TNF-α, 380 pg/ml; GM-CSF, 10,191 pg/ml; and IL-6, 34,467 pg/ml. Preinfusion levels for IFN-γ, GM-CSF, and IL-6 were undetectable, whereas TNF-α values varied from 0 to 51 pg/ml. The levels of these cytokines decreased over the next 3 days but remained above baseline values. The levels of seven additional cytokines (IL-1β, IL-2, IL-4, IL-7, IL-10, IL-12, and TRAIL) were determined by cytokine array (SearchLight assay) with only IL-10 showing increased levels after infusion, which unlike the other cytokines, was sustained through the study period (8 pg/ml preinfusion, 219 pg/ml at about 100 hours after infusion, Figure 2). An increased amount of IL-2 was observed at 4 hours only and was likely associated with the administration of the cell product, which was given in saline with 300 IU/ml IL-2 (the patient received no other IL-2). The possible involvement of an anaphylactic response to the infused cells was deemed unlikely because we measured only a modest twofold increase in serum tryptase levels at the 4 hours time point (from 7–9 pg/ml preinfusion to 15 pg/ml at 4 hours).
Figure 2.
Serum cytokine levels. Serum samples obtained at the approximate times indicated after cell infusion were assayed for cytokine expression using commercial ELISA kits for cytokines IL-6, TNF-α, GM-CSF, and IFN-γ. The levels of cytokine IL-10 were independently determined by cytokine array (SearchLight) assay. All samples were diluted as necessary as to be in the linear range of the assay. ELISA, enzyme-linked immunosorbent assay; GM-CSF, granulocyte macrophage-colony stimulating factor; IFN-γ, interferon-γ IL, interleukin; TNF-α, tumor necrosis factor-α.
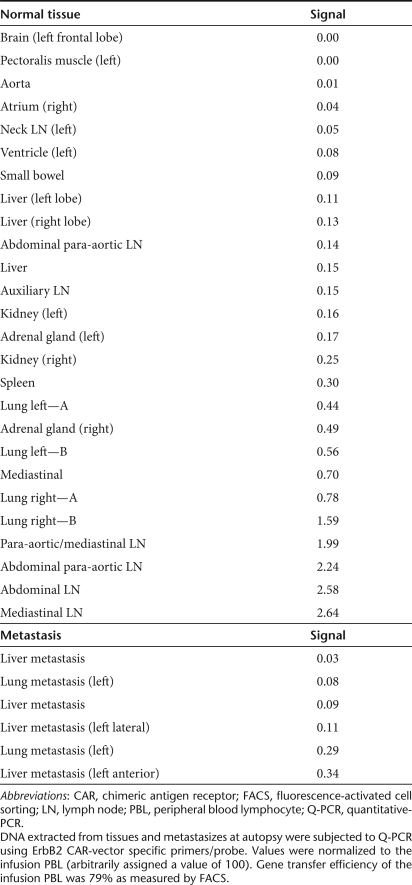
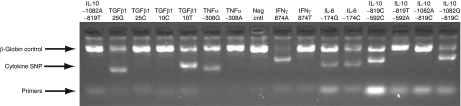
To determine the relative tissue distribution of vector-containing cells, DNA was isolated from samples obtained at autopsy and subjected to quantitative-PCR using vector-specific primers and probe. As a reference for this analysis, DNA was extracted from the infusion sample (79% ERBB2-CAR+) and arbitrarily assigned a value of 100 for comparison to tissue samples. There was a wide variation in the presence of vector-containing cells found in multiple tissues, though the highest levels were seen in the lung and abdominal/mediastinal lymph nodes (Table 2). There did not appear to be a preferential accumulation of vector-containing cells in metastatic deposits in the liver or lungs. DNA from the pretreatment PBMC was also subjected to analysis of single-nucleotide polymorphisms associated with the activity/function of five cytokine genes (IL-6, IL-10, IFN-γ, TGF-β1, and TNF-α). PCR using sequence-specific primers (Figure 3) indicated that the patients' genotype was: IL-6, heterozygous −741G/C; IL-10, homozygous −1082G, −819C, −592C; IFN-γ, homozygous 874A; transforming growth factor-β1, homozygous 10T, 25G; and TNF-α homozygous −308G. This genotype is consistent with a phenotype of increased synthesis of transforming growth factor-β, IL-10, and IL-6, but lower production of TNF-α and IFN-γ.
Table 2.
Vector tissue distribution
Figure 3.
Cytokine genotype. DNA extracted for patient PBMC was subject to PCR with sequence-specific primers (PCR-SSP) as described in Materials and Methods section. Primer pairs are designed to have perfect matches only with a single allele or group of alleles. Matched primer pairs result in the amplification of target sequences (i.e., a positive amplification band), whereas mismatched primer pairs do not result in amplification (i.e., a negative result). The specific genotypes detected by the SSP are shown above each lane (Neg cntl, reaction without cytokine primers). Shown is one of duplicate determinations. IFN-γ, interferon-γ IL, interleukin; SNP, single-nucleotide polymorphism; TNF-α, tumor necrosis factor-α.
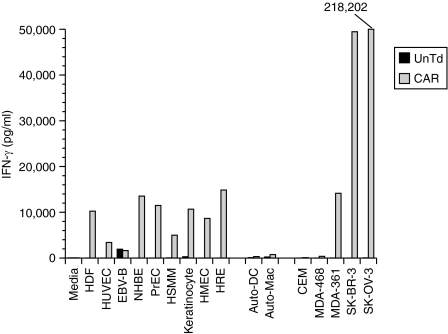
Analysis of the ERBB2-CAR transduced T cells before infusion demonstrated specific recognition of ERBB2-expressing tumor cells (Table 1). To confirm and extend this analysis, an aliquot of the cryopreserved infusion sample was thawed and retested. In addition to cell lines used for the certificate of analysis, the patient's PBMC were used to derive autologous dendritic cell and macrophage cultures and several cultures of allogeneic primary cells adapted for growth in culture by a commercial supplier were obtained. Coculture results presented in Figure 4 confirm the data obtained in the certificate of analysis. There was no reactivity seen to the dendritic cell and macrophage autologous cell cultures. Cytokine release was observed in several cocultures using allogeneic primary cells adapted for growth in culture.
Figure 4.
In vitro cytokine production. An aliquot of the ErbB2-CAR transduced (CAR) T lymphocytes infused into the patient or untransduced control T cells (UnTd) where assayed for IFN-γ cytokine production following overnight coculture with the indicated cell lines. ErbB2+ target cells were SK-OV3, SK-BR3, and MDA361. ErbB2− tumor lines were MDA468 and CCRF-CEM (CEM). Primary cells adapted for growth in culture were HDF, human diploid fibroblast, HUVEC, human umbilical vein endothelial cells; EBV-B-EBV transformed B cell line, NHBE, normal human bronchial/tracheal epithelial cells; PrEC, human prostate epithelial cells, HSMM, human skeletal muscle myoblasts, keratinocytes-human keratinocytes, HMEC, human mammary epithelial cells, and HRE, human renal epithelial cells. Autologous patient cells were auto-DC-patient 6-day dendritic cell culture, and auto-mac-patient 6-day macrophage culture. All samples were diluted as necessary as to be in the linear range of the assay (sample SK-OV3 was off-scale in this assay and the value from a repeat determination was as indicated). CAR, chimeric antigen receptor; IFN-γ, interferon-γ.
Discussion
The ERBB2 gene has been extensively studied as a target for both chemotherapy and immunotherapy. In breast cancer, overexpression of ERBB2 is correlated with a poor clinical outcome and at the same time, is a positive predictive factor for those women who are most likely to respond to therapy with the anti-ERBB2 antibody, trastuzumab.10 In randomized clinical trials, trastuzumab in combination with chemotherapy lead to increased disease-free and overall survival in breast cancer patients.9,29,30 The mechanism of action of trastuzumab appears to be multifactorial, but there are several studies that indicated the involvement of cell-mediated immunity (natural killer cell–based antibody-dependent cellular cytotoxicity) in patient responses.31,32,33 Furthermore, patient immune responses (antibody-dependent cellular cytotoxicity) can be increased when trastuzumab is combined with concomitant cytokine (IL-2, IL-12) administration,34,35 or in vaccine trials where anti-ERBB2 T cells responses have been reported.11,13
Safety considerations that preceded our clinical trial included the use of trastuzumab in thousands of cancer patients, the lack of toxicity seen in multiple studies immunizing against epitopes of ERBB2, and the lack of toxicity seen in a report of the adoptive transfer of autologous anti-ERBB2 cytotoxic T lymphocyte clones in the setting of breast cancer.14 In this report, three different cytotoxic T lymphocyte clones were administered in five transfers given 2 weeks apart. A total of 2.65 × 109 total cells were administered along with low-dose IL-2. With the exception of low-grade fever and chills following the third and fourth infusions, no side effects were noted. The therapy was associated with a decrease in tumor cells within the patient's bone marrow, but larger metastatic sites (such as liver) were not impacted. Radioimaging was performed using 111In-labeled cytotoxic T lymphocyte that demonstrated an immediate accumulation of cells in the lung that decreased over 72 hours, whereas uptake to the liver and spleen increased over the first 24 hours then remained stable for the 72-hour study period. We have demonstrated similar uptake of 111In-labeled TIL36 and have observed that lung uptake of TIL happens at the first pass (at the 90% retention level) after which the TIL then leak out of the lungs to the liver and other organs (ref. 36 and J.C. Yang, unpublished results).
The γ-retroviral vector construct used in this cancer gene therapy trial was designed for optimal ERBB2-CAR gene expression and anti-ERBB2 reactivity. It was demonstrated to be highly specific, was able to recognize a wide range of tumor histologies, and was able to significantly prevent the growth of human breast cancer cells orthotopically implanted into the mammary fat pad of severe combined immunodeficiency mice.28 Part of the process of optimizing this anti-ERBB2-CAR vector was the inclusion of two T-cell costimulatory domains from CD28 and 4-1BB (CD137) that were linked to the CD3z signaling element. 4-1BB is essential for the optimal activity of CD8+ T cells37,38 and inclusion of 4-1BB signaling domains was shown to enhance the in vivo antitumor activity of CARs in tumor xenograft models.28,39,40,41 To date, the reported clinical application of CAR-engineered T cells has been limited to constructs containing CD3z alone.24,25,26,27,42 In one report targeting carbonic anhydrase IX, on-target toxicity was observed most likely due to recognition of antigen expression on biliary epithelium. The results with the carbonic anhydrase IX directed CAR suggest that on-target toxicity may be antigen dependant and does not require the presence of costimulatory signals (such as CD28 and 4-1BB) in the CAR construct.
In our initial report we observed, in some transductions, T-cell recognition of the ERBB2−-tumor line MDA468 (this line is negative for ERBB2 expression by fluorescence-activated cell sorting, but ERBB2 mRNA can be detected by PCR). Transduction of the current patient's T cells with the identical ERBB2-CAR vector did not result in recognition of the MDA468 cell line (Table 1, Figure 4). The significance of the recognition of allogenic primary cells adapted for growth in culture (Figure 4) is not clear, as it is not known how the adaptation of these cells for growth in ex vivo culture influences the expression of ERBB2. For example, it was reported that while normal rat liver hepatocytes do not express ERBB2, that upon ex vivo culture, ERBB2 expression was rapidly induced.43
The most compelling finding in this case was the rapid rise in serum cytokine levels that has been associated with a multiple organ dysfunction syndrome.44,45,46 The administration of biologics resulting in cytokine release syndrome was first reported to be associated with the administration of anti-CD3 mAb OTK3, which was administered as a systemic immunosuppressive agent during organ transplantation.47,48 Within 1–4 hours after OKT3 injection, serum levels of proinflammatory cytokines such as TNF-α, IFN-γ, and IL-6 were markedly elevated. Most recently a cytokine storm was reported in six of six patients that were treated with anti-CD28 mAb TGN1412.48,49,50 In that report,50 TNF-α levels peaked within 1 hour after infusion and IL-2, IL-6, IL-10, and IFN-γ reached maximum levels at the next time point, 4 hours after infusion (elevation in other cytokines included IL-4, IL-8, IL-12, and IL-1β). All six patients in that study required supportive care in an intensive care unit and two of the six required extensive intensive care unit stays of 11 and 21 days. Similar to patients suffering serious adverse events from other mAb infusions, these patients showed signs of cardiovascular instability and disseminated intravascular coagulation, but unlike other treatments, these patients manifested additional sequelae including early and acute lung injury. Of interest was the lack of toxicity seen with this agent in preclinical tests of the anti-CD28 mAb TGN1412 in nonhuman primate studies.48,49
Perhaps the most relevant to our patient, were results observed in a phase I trial of a bispecific antibody that targeted both ERBB2 and FcγRIII.51 In this study, attempting to target FcγRIII-expressing cells (e.g., natural killer cells) to ERBB2-expressing tumors, some patients experienced side effects including dyspnea with arterial oxygen desaturation and hypotension. Analysis of serum cytokine levels demonstrated an increase in TNF-α within 30 minutes that peaked at 2.3 hours before declining over the next 24 hours with slightly delayed increases in IL-6, IL-8, IL-2, IL-1β, GM-CSF, and IFN-γ. A major difference between therapeutic antibody administration and CAR-engineered T cells, is that while antibodies are subject to clearance by the body (e.g., the bispecific mAb used in the cited trial had a t1/2 of 20 hours), T cells can continuously produce effector cytokines and can expand in cell numbers following antigen stimulation. The severity of this patients' response lead us to investigate her cytokine genotype (Figure 3), as polymorphisms in cytokine genes are associated with both levels of cytokine production and immunological responses, such as organ graft rejection.52,53,54 Although her genotype for IFN-γ (−874A) and TNF-α (−308G) are associated with lower levels of cytokine production, her IL-6, IL-10, and transforming growth factor-β1 genotypes are associated with higher levels of cytokine production, and the specific IL-6 (−174G/C) and the IL-10 (−1082G) genotypes are associated with shock in patients with sepsis55 and increased mortality in severe sepsis,56 respectively.
Since 2004, the Surgery Branch, NCI has conducted clinical trials involving the transfer of T-cell receptor genes into autologous lymphocytes (either PBL or TIL) using the identical nonmyeloablative conditioning regimen used in the present study (in some cases total body irradiation was added to further reduce endogenous lymphocytes). These treatments were undertaken in a variety of cancers (melanoma, synovial cell sarcoma, and cancers of the breast, colon, and kidney) and there was no indication that this conditioning regime was associated with the tumor lysis syndrome most frequently observed in cancers of hematopoietic origin.57 Similar numbers of cells were administered in these trials as well as the present trial, and review of the certificates of analysis of these products demonstrated similar levels of background cytokine production. A total of 143 patients were treated during this period without any treatment related mortality. These trials, included 11 patients treated with PBL engineered to target the wild-type p53 tumor suppressor protein,58,59 and no significant treatment related toxicity was seen (data not shown). In our recent report describing the use of high-avidity T-cell receptors targeting melanocyte differentiation antigens, on-target toxicity was observed in the skin, ears, and eyes, with serum IFN-γ levels peaking at days 3–6 after infusion at cytokine levels over tenfold less than observed in this patient.21 ERBB2 is known to be expressed at low levels in a variety of normal tissues including the lung.60 Histological examination of tissues obtained at autopsy confirmed ERBB2 overexpression in both liver metastases (3+ positivity, >50%), and lung metastases (2+ positivity, >50%), but also noted much lower levels of ERBB2 expression in normal lung parenchyma, normal liver tissue, and normal breast lobules consistent with the known tissue distribution of ERBB2 expression.
We postulate that the death of this patient was the result of the transfer highly active anti-ERBB2 directed T cells that upon first-pass clearance in the lung, recognized ERBB2 expressed by normal lung cells and released inflammatory cytokines (including TNF-α and IFN-γ) that caused pulmonary toxicity and edema followed by a cascading cytokine storm resulting in multiorgan failure similar to multiple organ dysfunction syndrome caused by a variety of acute physiological insults (e.g., trauma or severe infections). It is likely that the lack of toxicity seen in multiple vaccine trials that induced the generation of anti-ERBB2 lymphocytes was due to the very low levels of reactivity of these cells following immunization with self-antigens.
In initiating any new effort in patients with terminal cancer, there is a tension between administering a sufficient dose to provide clinical benefit while paying strict attention to patient safety. Our recommendation for future first-in-man trials of similar regents would be to conduct a more restricted dose-escalation trial starting at low doses that are unlikely to cause serious toxicity.
Materials and Methods
Clinical protocol. The clinical trial under which this patient was treated was: NCI-09-C-0041 entitled Phase I/II Study of Metastatic Cancer that Expresses Her-2 Using Lymphodepleting Conditioning Followed by Infusion of Anti-Her-2 Gene Engineered Lymphocytes. Patient inclusion criteria included metastatic cancer that expressed ErbB2 (Her-2/neu) at ≥2+ as assessed by immunohistochemistry in a Clinical Laboratory Improvement Amendments approved laboratory. Patients must have previously received systemic standard care (or effective salvage chemotherapy regimens) for metastatic disease, if known to be effective for that disease, and have been either nonresponders (progressive disease) or have recurred. Patients must be ≥18 years of age. Declaration of Helsinki protocols were followed and patients gave their written informed consent. Before receiving treatment with transduced PBLs, patients were transiently lymphoablated using a nonmyeloablative lymphodepleting regimen as previously described, by intravenous administration of cyclophosphamide 60 mg/kg for 2 days followed by fludarabine 25 mg/m2 for 5 days. One day after completion of their lymphodepleting regimen, patients were to receive transduced lymphocytes infused intravenously followed by high-dose (720,000 U/kg) IL-2 (Aldesleukin; Chiron, Emeryville, CA) every 8 hours to tolerance, although the patient reported here did not receive additional IL-2. The protocol was designed as a cell dose escalation in cohorts of three patients each. The lowest cell dose cohort was ≤1010 cells/infusion.
Gene transfer procedure. A detailed description of the generation of γ-retroviral vector construct designed to express the ERBB2-specific single-chain Fv fragment (4D5-CD8-28BBZ) from Herceptin mAb was recently published.28 In brief, the single-chain Fv fragment from mAb 4D5 was linked the CD8α-chain hinge and transmembrane region with CD28, 4-1BB, and CD3z intracellular signaling domains and this cassette inserted into the MSGV-1 γ-retroviral vector. The complete sequence of the vector is presented in Supplementary Figure S1. A high-titer PG13 cell-based producer cell line was selected and current good manufacturing practice grade retroviral vector supernatant produced by the Indiana University Vector Production Facility (Indianapolis, IN). The vector supernatant was tested and passed all currently required US Food and Drug Administration guidelines for the production of recombinant γ-retroviral vectors for clinical application.
The transduction procedure was initiated by stimulating PBMCs with anti-CD3 mAb OKT3 (Ortho Diagnostic Systems, Raritan, NJ) at a final concentration of 50 ng/ml with recombinant human IL-2 at a final concentration of 300 IU/ml in AIM-V medium (Invitrogen, Carlsbad, CA) containing 5% human serum (Surgery Branch, NCI). Cells were harvested for retroviral transduction on day 2 and resuspended in the same medium without OKT3. Retroviral vector supernatant was thawed and diluted with two parts of medium before being loaded onto RetroNectin (CH-296; Takara Bio, Ohtsu, Japan) coated (coated using 10 mg/ml of CH-296) non-tissue culture treated six-well plates. Vector supernatant was “spun loaded” onto coated plates by centrifugation at 2,000 g for 2 hours at <32 °C. Retroviral vector was aspirated from the wells and 1–2 × 106 activated PBMC were added pre-well followed by centrifugation at 1,000g for 10 minutes. Plates are incubated at 37 °C overnight and the next day all wells are harvested, pooled, and the transduction procedure repeated. Following the second transduction, cells are collected and maintained in medium at 0.5–2.0 × 106 cells/ml for a total of 10 days after stimulation. At day 10 after stimulation, cells were subject to a rapid expansion procedure for an additional 14 days using 6,000 IU/ml IL-2 with 50 ng/ml anti-CD3 mAb OKT3 and 100-fold excess 5 Gy irradiated allogeneic PBL feeder cells. Treatment cells were washed in saline before infusion and resuspended in 125 ml containing 300 IU/ml IL-2 then administered to the patient intravenously.
In vitro assays. Two to four days before infusion, CAR-transduced PBLs were evaluated for ERBB2-specific CAR expression using an ERBB2-Fc fusion protein, or as control, VEGFR2-Fc (R&D Systems, Minneapolis, MN) followed by phycoerythrin-conjugated antihuman IgG Fc antibody (eBioscience, San Diego, CA). Immunofluorescence was analyzed as the relative log fluorescence of live cells, was measured using a FACSCalibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ). Cell function was evaluated by overnight coculture with ERBB2-expressing and nonexpressing target cells (1 × 105 target plus 1 × 105 effector T cells) followed by enzyme-linked immunosorbent assay measurement (Pierce Endogen, Rockford, IL) of IFN-γ. ERBB2+ target cell were melanoma cell lines 526, 624, 888, 938 (generated at the Surgery Branch, NCI) and tumor lines, SK-OV3, SK-BR3, MDA361 (American Type Culture Collection, Rockville, MD), and ERBB2− tumor lines MDA468 and CCRF-CEM (CEM) obtained from ATCC. All tumor cell lines were cultured in media consisting of RPMI-1640 supplemented with 10% heat inactivated fetal bovine serum (Biofluids, Rockville, MD), 100 U/ml penicillin, 100 µg/ml streptomycin (Invitrogen). Nontransformed human cell cultures were purchased from Lonza (Walkersville, MD), and maintained in media recommended by the supplier. Autologous dendritic cells and macrophage cultures were obtained from patient PBMC as previously described.61
Genomic DNA was isolated from flash-frozen tissue samples using Maxwell 16 Tissue DNA Purification kit (Promega, Madison, WI) according to the manufacture's instruction. One hundred nanogram of each DNA was used for the Real-time quantitative-PCR assay (TaqMan; Applied Biosystems, Foster City, CA). All PCR were performed using an ABI 7500 Fast Real-time PCR System instrument (Applied Biosystems). The TaqMan gene-specific assay was designed by ABI Assays-by-Designs software (Applied Biosystems). Primers and probe used for detection of the ERBB2-CAR vector were: 4D5BBCD3Z-F TGCCGATTTCCAGAAGAAGAAGAAG, 4D5BBCD3Z-R TGCGCTCCTGCTGAACT, 4D5BBCD3Z-M FAM probe CACTCTCAGTTCACATCCT. The reference standard curve was established using the DNA extracted from the cells infused into the patient, with undiluted infusion DNA being given a value of 100 as reference. TaqMan β-actin control reagents kit (Applied Biosystems) was used to normalize reactions to input DNA amounts. Cytokine genotype was determined using a commercially available PCR-sequence specific primer kit (Cytokine Genotype Tray; One Lambda, Canoga Park, CA) as directed by the supplier.
Serum cytokine levels were assayed for using commercially available enzyme-linked immunosorbent assay kits [IFN-γ, TNF-α, GM-CSF, and IL-6 (Endogen, Cambridge, MA)] or SearchLight cytokine array (Aushon Biosystems, Billerica, MA). Cytokine secretion was measured in samples diluted to be in the linear range of the assay.
SUPPLEMENTARY MATERIALFigure S1. Nucleotide sequence of ERBB2-CAR vector. Listed are the features of the plasmid DNA containing the gamma-retroviral vector MSGV1-4D5-CD8-28BBZ followed by the nucleotide sequence.
Supplementary Material
Nucleotide sequence of ERBB2-CAR vector. Listed are the features of the plasmid DNA containing the gamma-retroviral vector MSGV1-4D5-CD8-28BBZ followed by the nucleotide sequence.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
REFERENCES
- Rubin I., and , Yarden Y.2001The basic biology of HER2 Ann Oncol 12(suppl. 1): S3–S8. [DOI] [PubMed] [Google Scholar]
- Yarden Y., and , Shilo BZ. SnapShot: EGFR signaling pathway. Cell. 2007;131:1018. doi: 10.1016/j.cell.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Yarden Y., and , Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Ross JS., and , McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554–568. doi: 10.1081/cnv-100103852. [DOI] [PubMed] [Google Scholar]
- Lassus H, Sihto H, Leminen A, Joensuu H, Isola J, Nupponen NN, et al. Gene amplification, mutation, and protein expression of EGFR and mutations of ERBB2 in serous ovarian carcinoma. J Mol Med. 2006;84:671–681. doi: 10.1007/s00109-006-0054-4. [DOI] [PubMed] [Google Scholar]
- Kameda T, Yasui W, Yoshida K, Tsujino T, Nakayama H, Ito M, et al. Expression of ERBB2 in human gastric carcinomas: relationship between p185ERBB2 expression and the gene amplification. Cancer Res. 1990;50:8002–8009. [PubMed] [Google Scholar]
- Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. 1992;89:4285–4289. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzdar AU, Ibrahim NK, Francis D, Booser DJ, Thomas ES, Theriault RL, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. doi: 10.1200/JCO.2005.07.032. [DOI] [PubMed] [Google Scholar]
- Hudis CA. Trastuzumab–mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- Peoples GE, Gurney JM, Hueman MT, Woll MM, Ryan GB, Storrer CE, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Morse MA, Hobeika A, Osada T, Niedzwiecki D, Marcom PK, Blackwell KL, et al. Long term disease-free survival and T cell and antibody responses in women with high-risk Her2+ breast cancer following vaccination against Her2. J Transl Med. 2007;5:42. doi: 10.1186/1479-5876-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nährig J, et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera JF, Brenner MK., and , Dotti G. Immunotherapy of human cancers using gene modified T lymphocytes. Curr Gene Ther. 2009;9:396–408. doi: 10.2174/156652309789753338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain, MRI., and , Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- Murphy A, Westwood JA, Teng MW, Moeller M, Darcy PK., and , Kershaw MH. Gene modification strategies to induce tumor immunity. Immunity. 2005;22:403–414. doi: 10.1016/j.immuni.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshhar Z. The T-body approach: redirecting T cells with antibody specificity. Handb Exp Pharmacol. 2008. pp. 329–342. [DOI] [PubMed]
- Eshhar Z, Waks T, Gross G., and , Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the γ or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci USA. 1993;90:720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, et al. 2006A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer Clin Cancer Res 12(20 Pt 1): 6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol. 2009;183:5563–5574. doi: 10.4049/jimmunol.0900447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- Beano A, Signorino E, Evangelista A, Brusa D, Mistrangelo M, Polimeni MA, et al. Correlation between NK function and response to trastuzumab in metastatic breast cancer patients. J Transl Med. 2008;6:25. doi: 10.1186/1479-5876-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari R, Menard S, Fagnoni F, Ponchio L, Scelsi M, Tagliabue E, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin Cancer Res. 2004;10:5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- Varchetta S, Gibelli N, Oliviero B, Nardini E, Gennari R, Gatti G, et al. Elements related to heterogeneity of antibody-dependent cell cytotoxicity in patients under trastuzumab therapy for primary operable breast cancer overexpressing Her2. Cancer Res. 2007;67:11991–11999. doi: 10.1158/0008-5472.CAN-07-2068. [DOI] [PubMed] [Google Scholar]
- Mani A, Roda J, Young D, Caligiuri MA, Fleming GF, Kaufman P, et al. A phase II trial of trastuzumab in combination with low-dose interleukin-2 (IL-2) in patients (PTS) with metastatic breast cancer (MBC) who have previously failed trastuzumab. Breast Cancer Res Treat. 2009;117:83–89. doi: 10.1007/s10549-008-0251-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parihar R, Nadella P, Lewis A, Jensen R, De Hoff C, Dierksheide JE, et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon γ production in a subset of patients. Clin Cancer Res. 2004;10:5027–5037. doi: 10.1158/1078-0432.CCR-04-0265. [DOI] [PubMed] [Google Scholar]
- Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, et al. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- Taraban VY, Rowley TF, O'Brien L, Chan HT, Haswell LE, Green MH, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32:3617–3627. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Wang C, Lin GH, McPherson AJ., and , Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- Zhong XS, Matsushita M, Plotkin J, Riviere I., and , Sadelain M.2009Chimeric Antigen Receptors Combining 4-1BB and CD28 Signaling Domains Augment PI(3)kinase/AKT/Bcl-X(L) Activation and CD8(+) T Cell-mediated Tumor Eradication Mol Ther(epub ahead of print). [DOI] [PMC free article] [PubMed]
- Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–2271. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheving LA, Zhang L, Stevenson MC, Kwak ES., and , Russell WE. The emergence of ERBB2 expression in cultured rat hepatocytes correlates with enhanced and diversified EGF-mediated signaling. Am J Physiol Gastrointest Liver Physiol. 2006;291:G16–G25. doi: 10.1152/ajpgi.00328.2005. [DOI] [PubMed] [Google Scholar]
- Lenz A, Franklin GA., and , Cheadle WG. Systemic inflammation after trauma. Injury. 2007;38:1336–1345. doi: 10.1016/j.injury.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Wang H., and , Ma S. The cytokine storm and factors determining the sequence and severity of organ dysfunction in multiple organ dysfunction syndrome. Am J Emerg Med. 2008;26:711–715. doi: 10.1016/j.ajem.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Ferran C, Legendre C, Thouard I, Merite S, Reuter A, et al. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation. 1990;49:697–702. doi: 10.1097/00007890-199004000-00009. [DOI] [PubMed] [Google Scholar]
- Sgro C. Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: bibliographic review. Toxicology. 1995;105:23–29. doi: 10.1016/0300-483x(95)03123-w. [DOI] [PubMed] [Google Scholar]
- Stebbings R, Findlay L, Edwards C, Eastwood D, Bird C, North D, et al. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. J Immunol. 2007;179:3325–3331. doi: 10.4049/jimmunol.179.5.3325. [DOI] [PubMed] [Google Scholar]
- Stebbings R, Poole S., and , Thorpe R. Safety of biologics, lessons learnt from TGN1412. Curr Opin Biotechnol. 2009;20:673–677. doi: 10.1016/j.copbio.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- Weiner LM, Clark JI, Davey M, Li WS, Garcia de Palazzo I, Ring DB, et al. Phase I trial of 2B1, a bispecific monoclonal antibody targeting c-erbB-2 and Fcγ RIII. Cancer Res. 1995;55:4586–4593. [PubMed] [Google Scholar]
- Akalin E., and , Murphy B. Gene polymorphisms and transplantation. Curr Opin Immunol. 2001;13:572–576. doi: 10.1016/s0952-7915(00)00261-2. [DOI] [PubMed] [Google Scholar]
- Girnita DM, Webber SA., and , Zeevi A. Clinical impact of cytokine and growth factor genetic polymorphisms in thoracic organ transplantation. Clin Lab Med. 2008;28:423–40, vi. doi: 10.1016/j.cll.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Marshall SE., and , Welsh KI. The role of cytokine polymorphisms in rejection after solid organ transplantation. Genes Immun. 2001;2:297–303. doi: 10.1038/sj.gene.6363795. [DOI] [PubMed] [Google Scholar]
- Tischendorf JJ, Yagmur E, Scholten D, Vidacek D, Koch A, Winograd R, et al. The interleukin-6 (IL6)-174 G/C promoter genotype is associated with the presence of septic shock and the ex vivo secretion of IL6. Int J Immunogenet. 2007;34:413–418. doi: 10.1111/j.1744-313X.2007.00712.x. [DOI] [PubMed] [Google Scholar]
- Stanilova SA, Miteva LD, Karakolev ZT., and , Stefanov CS. Interleukin-10-1082 promoter polymorphism in association with cytokine production and sepsis susceptibility. Intensive Care Med. 2006;32:260–266. doi: 10.1007/s00134-005-0022-4. [DOI] [PubMed] [Google Scholar]
- Cope D. Tumor lysis syndrome. Clin J Oncol Nurs. 2004;8:415–416. doi: 10.1188/04.CJON.415-416. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Zheng Z, Bray R, Zhao Y, Sherman LA, Rosenberg SA, et al. Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR. J Immunol. 2005;175:5799–5808. doi: 10.4049/jimmunol.175.9.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoret MR, Cohen CJ, Nahvi AV, Ngo LT, Suri KB, Powell DJ, Jr, et al. Relationship of p53 overexpression on cancers and recognition by anti-p53 T cell receptor-transduced T cells. Hum Gene Ther. 2008;19:1219–1232. doi: 10.1089/hum.2008.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press MF, Cordon-Cardo C., and , Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene. 1990;5:953–962. [PubMed] [Google Scholar]
- Lotem M, Zhao Y, Riley J, Hwu P, Morgan RA, Rosenberg SA, et al. Presentation of tumor antigens by dendritic cells genetically modified with viral and nonviral vectors. J Immunother. 2006;29:616–627. doi: 10.1097/01.cji.0000211312.36363.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Nucleotide sequence of ERBB2-CAR vector. Listed are the features of the plasmid DNA containing the gamma-retroviral vector MSGV1-4D5-CD8-28BBZ followed by the nucleotide sequence.