Abstract
We have designed a PEGylated LPD (liposome-polycation-DNA) nanoparticle for systemic, specific, and efficient delivery of small interfering RNA (siRNA) into solid tumors in mice by modification with NGR (aspargine–glycine–arginine) peptide, targeting aminopeptidase N (CD13) expressed in the tumor cells or tumor vascular endothelium. LPD-PEG-NGR efficiently delivered siRNA to the cytoplasm and downregulated the target gene in the HT-1080 cells but not CD13− HT-29 cells, whereas nanoparticles containing a control peptide, LPD-PEG-ARA, showed only little siRNA uptake and gene silencing activity. LPD-PEG-NGR efficiently delivered siRNA into the cytoplasm of HT-1080 xenograft tumor 4 hours after intravenous injection. Three daily injections (1.2 mg/kg) of c-myc siRNA formulated in the LPD-PEG-NGR effectively suppressed c-myc expression and triggered cellular apoptosis in the tumor, resulting in a partial tumor growth inhibition. When doxorubicin (DOX) and siRNA were co-formulated in LPD-PEG-NGR, an enhanced therapeutic effect was observed.
Introduction
c-myc oncogene is overexpressed and activated in various human tumors. It promotes cell growth, transformation, and angiogenesis that play important roles in the progression and metastasis of tumor.1 Downregulation of c-myc with antisense oligonucleotides inhibits tumor growth both in vitro and in vivo and sensitizes cancer cells to chemotherapy,2,3 possibly by induction of p53 and inhibition of Bcl-2 proteins that trigger cell apoptosis.3
Surgical resection is a common primary cancer treatment. Radiation and chemotherapeutic agents such as doxorubicin (DOX) may improve treatment response and survival. However, resistance to chemotherapy and radiation often occurs and leads to poor prognosis. Delivery of small interfering RNA (siRNA) against an oncogene or a drug-resistant gene is a new strategy to increase the therapeutic armament.4,5,6 DOX, one of the most effective anticancer agents, is efficacious against various neoplasms such as acute lymphoblastic and myeloblastic leukemia, malignant lymphoma, soft tissue and bone sarcoma, breast, ovarian, prostate, bladder, gastric, and bronchogenic carcinoma.7 However, the associated cardiotoxicity caused by free radicals generated by DOX has prompted the development of a targeted delivery vehicle to tumor cells.8
We have successfully developed a core/shell type of nanoparticle formulation, called liposome-polycation-DNA complex (LPD), to specifically deliver siRNA to tumor cells in vivo.9,10,11 DOX contains flat aromatic rings that intercalate into the DNA strands.12 Because dsDNA is a component of the LPD nanoparticle, we have decided to use the DNA as a carrier for DOX. We hypothesized that DOX may form a physical complex with the DNA inside the nanoparticles through noncovalent intercalation, which does not change the property of DOX or siRNA in the LPD nanoparticles. This novel system may provide a platform for efficient and specific co-delivery of siRNA and the DNA-binding chemotherapy drug for cancer treatment.
In this study, we have designed the LPD nanoparticles armed with NGR, a peptide motif targeting CD13 (ref. 13) which is upregulated in angiogenic tumor vasculature and various cancer cells such as HT-1080 human fibrosarcoma cells. CD13 is a multifunctional protein involved in cancer angiogenesis, invasion, and metastasis.14 NGR-containing peptides have been successfully used to deliver cytotoxic drugs such as DOX, apoptotic peptides, and cytokines such as tumor necrosis factor to the tumor or tumor vasculature, and enhance the antitumor activity of the cargo.13,15,16,17,18,19,20
In the present study, we have developed LPD nanoparticles modified with PEGylated NGR for targeted co-delivery of siRNA and DOX in vitro and in vivo. We have shown increasing cellular uptake of siRNA, profound downregulation of the target gene, enhanced apoptosis of the tumor cells and improved tumor growth inhibition effect triggered by LPD-PEG-NGR containing c-myc siRNA. These results indicate that the suppression of c-myc protein by NGR-targeted siRNA therapy and the co-delivery of c-myc siRNA and DOX could provide an efficient strategy for cancer treatment.
Results
Uptake of siRNA in vitro
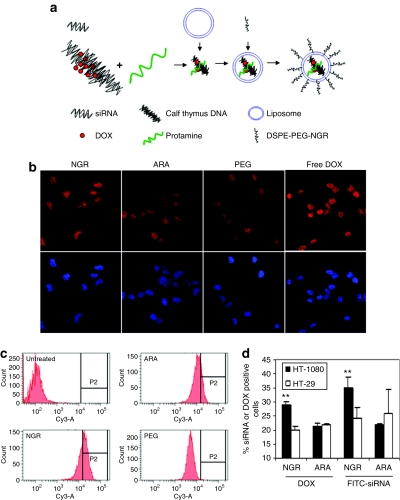
The average size of the LPD-PEG-NGR nanoparticles was 197 ± 1 nm, and the zeta potential was 30.5 ± 0.6 mV. As shown in Figure 1b, the uptake of fluorescently labeled siRNA was much greater in HT-1080 cells treated with LPD-PEG-NGR than cells treated with LPD-PEG-ARA. It indicates that NGR ligand increased the delivery efficiency of the nanoparticles for CD13 expressing cells, HT-1080. The CD13− cell line HT-29 (Figure 1b) did not show fluorescence, suggesting that uptake of siRNA is related to CD13 expression. To test the specificity of NGR-mediated uptake, competitive inhibition was performed with excess free NGR or ARA peptides. The presence of NGR, but not ARA, peptides reduces the siRNA uptake of HT-1080 cells treated with LPD-PEG-NGR (see Supplementary Materials and Supplementary Figure S1). Supplementary Figure S2 indicates that LPD-PEG-NGR can also efficiently deliver siRNA into HUVEC (human umbilical vein endothelial cells).
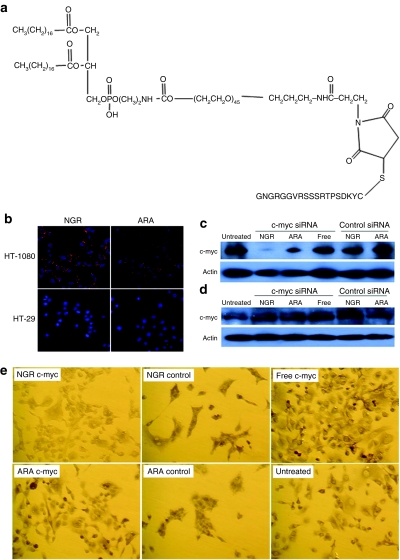
Figure 1.
Intracellular uptake of siRNA and c-myc expression inhibited by siRNA formulation in vitro. (a) The structure of DSPE-PEG-NGR. (b) Fluorescence photographs of HT-1080 and HT-29 cells after treatment with 5′-Cy3-labeled siRNA against an irrelevant target in LPD-PEG-NGR and LPD-PEG-ARA for 4 hours. Western blot analysis of c-myc and β-actin in (c) HT-1080 cells and (d) HT-29 after treatment with 250 nmol/l siRNA in different formulations for 24 hours. (e) Immunocytochemical staining of c-myc after treatment with 250 nmol/l siRNA in different formulations for 24 hours.
c-myc gene silencing in vitro
To further demonstrate the biological activity of the formulation, siRNA against human c-myc was delivered by either LPD-PEG-NGR or LPD-PEG-ARA. Its effect on c-myc levels was determined by quantitative RT-PCR (see Supplementary Materials and Methods), immunostaining, and western blot analysis (Figure 1c–e and Supplementary Figure S3). The c-myc mRNA and protein expression of HT-1080 cells treated with c-myc siRNA-containing LPD-PEG-NGR was significantly inhibited (Figure 1c,e and Supplementary Figure S3). However, anti-c-myc siRNA-containing LPD-PEG-ARA could only slightly downregulate c-myc. The CD13− cell line HT-29 (Figure 1d,e) did not show silencing activity. The data indicate that siRNA could effectively suppress c-myc expression, and the silencing activity was ligand dependent.
Uptake of siRNA in vivo
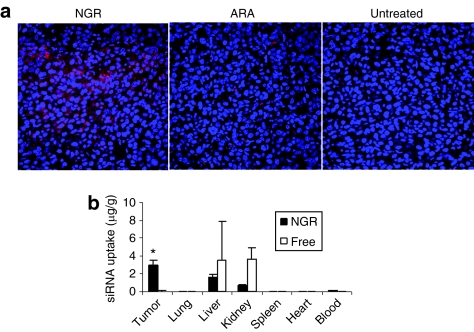
We studied the cy3-siRNA uptake of HT-1080 tumor tissue in the tumor-bearing mice 4 hours after IV injections using confocal microscopy. As shown in Figure 2a, the intracellular fluorescence signals were hardly detected in the tumor tissues collected from the mice treated with LPD-PEG-ARA. The LPD-PEG-NGR showed strong cytosolic delivery of cy3-siRNA in the tumor tissue. The distribution of cy3-siRNA in the tumor was heterogeneous. These results indicate that the LPD-PEG-NGR can efficiently deliver siRNA to the tumor tissue and the intracellular delivery is targeting peptide dependent. In other organs (Figure 2b), the liver and the kidney showed stronger uptake of free siRNA than siRNA formulated in the targeted nanoparticles, whereas the targeted nanoparticles showed stronger siRNA delivery in the tumor tissue than free siRNA. The uptake of siRNA formulated in the targeted nanoparticles was under the detection limit in the heart, the spleen, and the lung.
Figure 2.
Tumor uptake of siRNA in different formulations. (a) Fluorescence signal of cy3-labeled siRNA in HT-1080 tumor observed by confocal microscopy. (b) Tissue distribution of FITC-siRNA in different formulations. Data = mean ± SD, n = 3. *P < 0.05 compared with free siRNA. ARA: LPD-PEG-ARA; c-myc: c-myc siRNA; Control: control siRNA; NGR: LPD-PEG-NGR.
c-myc gene silencing and apoptosis induction
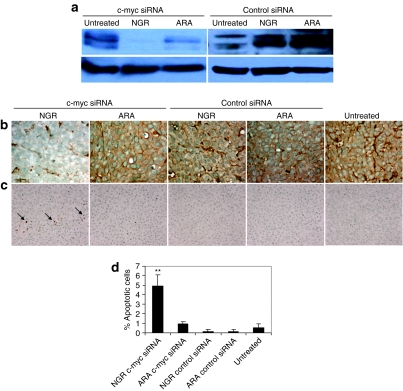
To examine the biological activities of siRNA in vivo, c-myc level in the tumor was assayed by western blot analysis and immunostaining (Figure 3a,b). c-myc expression in HT-1080 tumor was silenced by siRNA delivered with LPD-PEG-NGR. The LPD-PEG-ARA showed only a partial effect, whereas the control siRNA showed no effect. We also stained for the apoptotic markers in the HT-1080 tumor (Figure 3c). Figure 3c,d indicate that about 5% of HT-1080 cells treated with c-myc siRNA containing LPD-PEG-NGR underwent apoptosis as detected by the TUNEL staining. This value was significantly higher than the ones treated with c-myc siRNA formulated in LPD-PEG-ARA, or control siRNA formulated in either LPD-PEG-NGR or LPD-PEG-ARA. The results indicate that c-myc siRNA formulated with LPD-PEG-NGR can promote cell death in the HT-1080 tumor and the apoptosis effect was targeting peptide dependent.
Figure 3.
c-myc expression and apoptosis induction in HT-1080 xenograft tumor. (a) Western blot analysis of c-myc in the HT-1080 xenograft tumor after treatment with different formulations. (b) c-myc expression and (c) TUNEL staining in HT-1080 tumor cells after treated with siRNA with different formulation in vivo. (d) Quantitative analysis of TUNEL positive staining in the tumors treated with different formulations. N = 3~5. **P < 0.001.
Tumor growth inhibition by siRNA nanoparticles
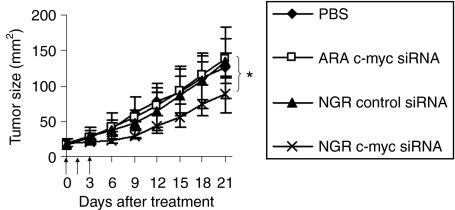
Three injections of c-myc siRNA in LPD-PEG-NGR showed a partial inhibition of tumor growth (P < 0.05 at day 21) (Figure 4). Other control groups treated with c-myc siRNA formulated in LPD-PEG-ARA, control siRNA formulated in LPD-PEG-NGR had no therapeutic effect. The results indicate that c-myc siRNA formulated with LPD-PEG-NGR can inhibit the growth of HT-1080 tumor and the tumor growth inhibition effect was targeting peptide dependent. The LPD-PEG-NGR nanoparticles efficiently delivered siRNA to the solid tumor and almost totally silenced the target gene, c-myc, throughout the entire HT-1080 tumor. Because only partial apoptosis and growth inhibition of the tumor was observed, we hypothesized that it was due to the existence of alternative mechanisms of proliferation or some antiapoptosis events in the tumor. To enhance the therapeutic activity of LPD-PEG-NGR nanoparticles containing c-myc siRNA, we co-delivered siRNA and DOX to the tumor cells. DOX has a unique property to bind with dsDNA by base intercalation.12 Because dsDNA is a component of the LPD nanoparticles, we have decided to use DNA as a carrier for DOX. We hypothesized that DOX will form a physical complex with DNA inside the LPD nanoparticles by noncovalent intercalation, which will not change the property of DOX or siRNA in the LPD nanoparticles.
Figure 4.
HT-1080 xenograft tumor growth inhibition by siRNA in different formulations. Solid arrows indicate the intravenous administrations of siRNA (1.2 mg/kg). Data = mean, n = 5–7. SD of the data points is not shown for clarity. *P < 0.05. PBS, phosphate-buffered saline.
Characterization of the nanoparticles containing siRNA and DOX
In this study, we have explored a novel strategy for specifically co-delivery of siRNA and DOX to the tumor by using DNA–DOX physical complex. The preparation of LPD-PEG-NGR containing DOX is shown in Figure 5a. Supplementary Figure S4 showed that the fluorescence of DOX was quenched upon its intercalation into DNA, whereas the fluorescence was enhanced when the cationic liposomes were added. The average size of the LPD-PEG-NGR nanoparticles containing DOX was 188 ± 29 nm and the zeta potential was 27.2 ± 1.0 mV. The resulting nanoparticles efficiently delivered DOX to the HT-1080 cells as much as the free DOX (Figure 5b). DOX was found in the nuclei of the cells. DOX uptake was further compared among different nanoparticle formulations by using flow cytometry. FITC-labeled siRNA was included in the nanoparticles for comparison. As can be seen in Figure 5d, both siRNA and DOX were taken up by CD13+ HT-1080 cells more than the CD13− HT-29 cells. However, the enhanced uptake was only seen with formulations targeted with NGR. Thus, peptide-targeted LPD nanoparticles showed potential to deliver both siRNA and DOX to tumor cells in a target-specific manner.
Figure 5.
Intracellular uptake of DOX in vitro. (a) Illustration of preparation of LPD-PEG-NGR containing siRNA and DOX. (b) Fluorescence photographs of HT-1080 and HT-29 cells after treatment with DOX in LPD-PEG-NGR (NGR), LPD-PEG-ARA (ARA), and LPD-PEG (PEG) for 1 hour. (c) Quantitative measurement of DOX uptake in HT-1080 cells by flow cytometry. (d) Uptake of siRNA and DOX by HT-1080 and HT-29 cells were compared. Cells were treated with different formulations containing DOX and FITC-siRNA for 1 hour and analyzed for fluorescence by flow cytometry. Data = mean ± SD, n = 3. **P < 0.001. DOX, doxorubicin.
Uptake of DOX in vivo
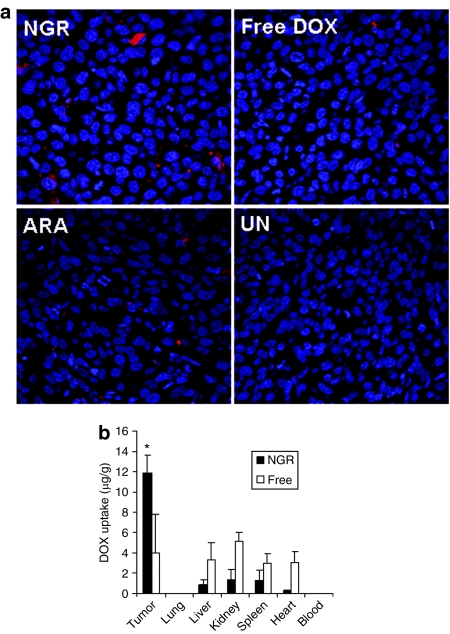
We studied the DOX uptake of HT-1080 tumor tissue in the tumor-bearing mice 4 hours after IV injection using confocal microscopy. As shown in Figure 6a, the LPD-PEG-NGR showed stronger cytosolic delivery of cy3-siRNA in the tumor tissue than LPD-PEG-ARA and free DOX. The distribution of DOX in the tumor was heterogeneous. These results indicate that the LPD-PEG-NGR can efficiently deliver DOX to the tumor tissue and the intracellular delivery is targeting peptide dependent. In the quantitative analysis (Figure 6b), liver, kidney, heart, and spleen showed stronger uptake of free DOX than DOX formulated in the targeted nanoparticles, whereas the targeted nanoparticles showed stronger DOX delivery in the tumor tissue than free DOX.
Figure 6.
Tumor uptake of DOX in different formulations. (a) Fluorescence signal of DOX in HT-1080 tumor observed by confocal microscopy. (b) Tissue distribution of DOX in different formulations. Data = mean ± SD, n = 3. *P < 0.05 compared with free DOX. DOX, doxorubicin.
Tumor growth inhibition by nanoparticles containing siRNA and DOX
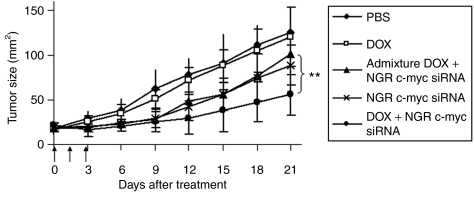
Three injections of c-myc siRNA in LPD-PEG-NGR showed a partial inhibition of tumor growth (P < 0.05 at day 21) (Figures 5 and 7). The control group treated with free DOX at a dose of 0.3 mg/kg had no therapeutic effect (Figure 7). The admixture of free DOX and c-myc siRNA in LPD-PEG-NGR induced similar tumor growth inhibition as c-myc siRNA in LPD-PEG-NGR. A significant improvement in tumor growth inhibition was observed when treated with DOX and c-myc siRNA coformulated in LPD-PEG-NGR. The results indicate that DOX and c-myc siRNA co-delivered by targeted nanoparticles can synergistically inhibit tumor growth and enhance the therapeutic effect.
Figure 7.
HT-1080 xenograft tumor growth inhibition by siRNA and DOX in different formulations. Solid arrows indicate the intravenous administrations of siRNA (1.2 mg/kg) and DOX (0.3 mg/kg). Data = mean, n = 5–7. SD of the data points is not shown for clarity. **P < 0.01. DOX, doxorubicin; PBS, phosphate-buffered saline.
Discussion
c-myc gene is a key in cancer onset and maintenance in various human tumors. The genetic alterations of c-myc are related to ~70,000 US cancer deaths per year. Overexpression and activation of c-myc induces various forms of cancer. However, expression of the c-myc is also essential for proliferation and regulation in normal mammalian cells. The aim of this study is to design a targeted nanoparticle formulation that can specifically deliver c-myc siRNA into the tumor site, downregulate c-myc expression in the tumor and achieve therapeutic cures. Our formulation can also help understand the function and mechanism of c-myc in tumor development.
Our results demonstrate that inhibition of c-myc protein expression by siRNA could induce apoptosis in HT-1080 tumors and significantly suppress tumor growth of HT-1080 cells in nude mice (Figures 3 and 4). Through co-delivery of DOX and siRNA in the LPD formulation, siRNA against c-myc sensitized HT-1080 cells to DOX chemotherapy (Figure 7). The combination strategy may be effective to overcome the drug resistance of cancer cells that overexpress c-myc gene. Therefore, the new formulation may serve as a new strategy for cancer therapy.
There are two major issues with siRNA-based approaches in cancer therapy: (i) poor selectivity in delivery, i.e., inadequate siRNA uptake by the tumor and nonspecific siRNA uptake by normal tissues, and (ii) unfavorable pharmacokinetics, i.e., rapid clearance of free siRNA in the blood. LPD-PEG-NGR is a PEGylated LPD containing siRNA or DOX, or both, coated with a tumor-targeting peptide containing the NGR motif. We have demonstrated that the nanoparticles can protect siRNA from degradation in the systemic circulation in our previous study.9 In this study, we have shown that it can deliver siRNA and DOX into the tumor site, increase the accumulation of siRNA and DOX in the tumor tissues (Figures 2 and 6), downregulate the target gene (Figure 3), and improve tumor growth inhibition effect (Figures 5 and 7). Furthermore, it has been reported that NGR motif can also target the tumor blood vessels.21 Our results also demonstrate that LPD-PEG-NGR can specifically and efficiently deliver the fluorescence-labeled siRNA into the HUVEC endothelial cells (Supplementary Figure S2). By using siRNA against angiogenesis, our delivery system could serve as a carrier to deliver therapeutic siRNA into tumor vascular endothelial cells and disrupt tumor vasculature. LPD-PEG-NGR containing therapeutic siRNA could serve as a novel anticancer agent for a wide variety of tumors.
In Figure 5d, NGR-targeted LPD nanoparticles deliver more siRNA and DOX to HT-1080 tumor cells than to HT-29 tumor cells. However, the nonspecific uptake of DOX (Figure 5c,d) may be due to the poor entrapment of DOX in the LPD nanoparticles. The data in Supplementary Figure S4 suggest that when the cationic liposomes interacted with the negatively charged DNA/protamine complex, there was a substantial leakage of DOX from the nanoparticle-associated DNA. Indeed, DOX encapsulation in the final LPD nanoparticles was only about 20%. It implies that addition of cationic liposome may decrease the entrapment efficiency. The positive charge of the C-3′ amine of DOX is required to stabilize the intercalation into DNA via charge interaction with the negative charge of the DNA phosphate backbone.22 Cationic lipid may directly compete with the DOX for interaction with calf thymus DNA and dislodge DOX from the DNA. However, the small amount of DOX entrapped in the LPD-PEG-NGR induced a significantly increased tumor uptake (Figure 6) in the HT-1080 tumor model. A significant improvement in therapeutic effect was also achieved by the small amount of DOX entrapped in the targeted nanoparticle (Figure 6). Free, unentrapped DOX was rapidly cleared from the blood circulation after IV administration and rarely uptaken in the tumor tissue. If the interaction of DNA with cationic liposomes, but not with protamine, was the cause of the DOX leakage, we will attempt to modify the nanoparticle formulation to enhance entrapment efficacy in our future study.
The tumor-homing peptide (NGR) modified nanoparticle provides an enhancement of drug potency and may potentially be a therapeutic agent against drug-resistant tumors when combined with siRNA against drug-resistant genes such as p-glycoprotein.
Materials and Methods
Materials. DOTAP and cholesterol were purchased from Avanti Polar Lipids (Alabaster, AL). Protamine sulfate (fraction X from salmon) and calf thymus DNA (for hybridization, phenol-chloroform extracted and ethanol precipitated) were purchased from Sigma-Aldrich (St Louis, MO). DOX was purchased from IFFECT Chemphar (Hong Kong, People's Republic of China). Synthetic 19-nt RNAs with 3′ dTdT overhangs on both sequences were purchased from Dharmacon (Lafayette, CO). For quantitative studies, cy3 was conjugated to 5′ sense sequence. 5′-cy3- and 5′-FITC-labeled siRNA sequence was also obtained from Dharmacon. The sequence of c-myc siRNA was 5′-AACGUUAGCUUCACCAACAUU-3′ and control siRNA with sequence 5′-AATTCTCCGAACGTGTCACGT-3′ was obtained from Dharmacon. DSPE-PEG-NGR (GNGRGGVRSSSRTPSDKYC), a peptide ligand conjugated to a PEG chain tethered to a phospholipid (Figure 1a), and DSPE-PEG-ARA (GARAGGVRSSSRTPSDKYC), a similar conjugate but containing the control peptide ARA, were supplied by Ambrilia Biopharma.23 These conjugates were used to modify the surface of the nanoparticles, as described,24 to obtain LPD-PEG-NGR and LPD-PEG-ARA containing siRNA.
Cell culture. HT-1080 and HT-29 cells were obtained from American Type Culture Collection, Manassas, VA. HT-1080 cells were maintained in MEM Alpha Media (Gibco-BRL, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen). HT-29 cells were maintained in McCoy's 5A Modified Medium (Cellgro, Manassas, VA) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen). To study the siRNA uptake, HT-1080 cells were chosen as the CD-13 receptor positive human tumor cell lines and HT-29 cells as receptor negative control cell lines.17
Preparation of PEGylated LPD formulations. LPD were prepared according to the previously method with slight modifications.24 Briefly, cationic liposomes composed of DOTAP and cholesterol (1:1 molar ratio) were prepared by thin film hydration followed by membrane extrusion to reduce the particle size. To prepare LPD, 18 µl of protamine (2 mg/ml), 140 µl of deionized water, and 24 µl of a mixture of siRNA and calf thymus DNA (2 mg/ml) were mixed and kept at room temperature for 10 minutes before adding 120 µl of cationic liposome (10 mmol/l). LPD stood at room temperature for 10 minutes before the addition of DSPE-PEG. LPD was then mixed with 37.8 µl of DSPE-PEG-NGR or DSPE-PEG-ARA (17 mg/ml) and kept at 50–60 °C for 10 minutes.
Cellular uptake study. Human HT-1080 and HT-29 cells, originally obtained from ATCC, (1 × 105 per well) were seeded in 12-well plates (Corning, Corning, NY) 12 hours before experiments. Cells were treated with different formulations at a concentration of 250 nmol/l for 5′-cy3-labeled siRNA or 1.5 µmol/l DOX in serum-containing medium at 37 °C for 4 hours. Cells were washed twice with phosphate-buffered saline (PBS), counterstained with DAPI and imaged using a Leica SP2 confocal microscope (Leica Microsystems, Bannockburn, IL). DOX uptake of HT-1080 and HT-29 cells was also measured by flow cytometry. Briefly, cells were treated with different formulations at a concentration of 1.5 µmol/l DOX in serum-containing medium at 37 °C for 1 hour. Cells were harvested and resuspended at a concentration of 1 × 106 cells/ml. Cells were washed with PBS and analyzed immediately by flow cytometry.
Gene silencing study. Sterile round cover slips (1 × 1 cm) were placed into each well in 24-well plates. HT-1080 cells (5 × 104 cells/0.5 ml/well) were then seeded into each well overnight. Cells were treated with different formulations at a concentration of 250 nmol/l for c-myc siRNA in 10% fetal bovine serum–containing medium at 37 °C for 24 hours. Cells were washed three times with PBS and fixed with cold acetone/methanol 1:1 for 10 minutes. Cells were incubated with anti-c-myc antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:50 dilution for 1 hour. After washing with PBS, immunostaining was carried out by using a kit (DakoCytomation Envision + Dual Link System-HRP (DAB+); DakoCytomation, Carpinteria, CA) following the vendor's protocol. For in vivo study, HT-1080 tumor-bearing mice (tumor size ~1 cm2) were IV injected with siRNA in different formulations (1.2 mg siRNA/kg, one injection per day for 3 days). A day after the third injection, tumors were collected, paraffin embedded and sectioned. Some sections were analyzed for TUNEL assay (see below). Sections of 7¼ µm thick were immunostained with primary antibodies and visualized by using kits from DakoCytomation. Samples were imaged by using a Nikon Microphot SA microscope (Nikon Instruments, Melville, NY).
Western blot analysis. Cells were lysed in lysis buffer CelLytic M Cell Lysis Reagent (Sigma-Aldrich) for 30 minutes on ice, and the supernatant was collected after centrifugation at 12,000 rpm. Cell lysates were separated on a 10% acrylamide gel and transferred to a PVDF membrane. Membranes were blocked for 1 hour in 5% skim milk and then incubated with polyclonal antibody against c-myc (Santa Cruz Biotechnology) overnight. Membranes were washed in PBST (PBS with 0.1% Tween-20) three times and then incubated for 1 hour with secondary antibody. Membranes were washed four times and then developed by an enhanced chemiluminescence system according to the manufacturer's instructions (PerkinElmer, Waltham, MA). For in vivo study, HT-1080 tumor-bearing mice (tumor size ~1 cm2) were IV injected with siRNA in different formulations (1.2 mg siRNA/kg, one injection per day for 3 days). A day after the third injection, mice were killed and tumor samples were collected. Total protein (40 µg) isolated from the tumors was loaded on a polyacrylamide gel, electrophoresed, blotted as described above.
Assessment of apoptosis by TUNEL staining. Paraffin sections of HT-1080 tumor were stained by using TACS TdT Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's recommendation. The apoptotic cells were counted in four randomly selected visual fields for each treatment. The apoptotic index was calculated as the ratio of apoptotic nuclei to total nuclei.
Tumor uptake study. Mice with tumor size of ~1 cm2 were IV injected with cy3-labeled siRNA (1.2 mg/kg) or DOX (1.2 mg/kg) in different formulations. After 4 hours, mice were killed and tissues were collected, fixed in 10% formalin and embedded in paraffin. Tumor tissues were sectioned (7¼ µm thick) and imaged using a Leica SP2 confocal microscope.
Tissue distribution study. Mice with tumor size of ~1 cm2 were IV injected with FITC-labeled siRNA or DOX in different formulations (1.2 mg/kg). After 4 hours, mice were killed, and tissues were collected and homogenized in lysis buffer and incubated at room temperature for 30 minutes. The supernatant was collected after centrifugation at 14,000 rpm for 10 minutes, and 50 µl supernatant was transferred to a black 96-well plate (Corning). The fluorescence intensity of the sample was measured by a plate reader (Bioscan, Washington, DC) at excitation wavelength of 485 nm and emission wavelength of 535 nm. siRNA and DOX concentration in each sample was calculated from a standard curve.
Tumor growth inhibition study. HT-1080 tumor-bearing mice (size 16–25 mm2) were IV injected with different formulations containing siRNA (1.2 mg/kg) or DOX (0.3 mg/kg) once per day for 3 days. Tumor size in the treated mice was measured after treatment.
Statistical analysis. All statistical analyses were performed by Student's t-test. Data were considered statistically significant when P value was <0.05.
SUPPLEMENTARY MATERIALFigure S1. Ligand competitive cellular uptake assay.Figure S2. Intracellular uptake of siRNA in HUVEC.Figure S3. Relative mRNA level in the HT1080 cells 24 h after treatment with siRNA in different formulations.Figure S4. DOX fluorescence intensity during the nanoparticle self-assembly.Materials and Methods.
Supplementary Material
Ligand competitive cellular uptake assay.
Intracellular uptake of siRNA in HUVEC.
Relative mRNA level in the HT1080 cells 24 h after treatment with siRNA in different formulations.
DOX fluorescence intensity during the nanoparticle self-assembly.
Acknowledgments
We thank the Michael Hooker Microscopy Facility of the UNC for the microscopy images. Technical assistance of Sheema Garde is acknowledged. This research was partially supported by Ambrilia Biopharma and by NIH grant CA129825. We appreciate Annie Mayo's (UNC) assistance in preparing the manuscript. The authors declare there is no conflict of interest.
REFERENCES
- Baudino TA, McKay C, Pendeville-Samain H, Nilsson JA, Maclean KH, White EL, et al. c-myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev. 2002;16:2530–2543. doi: 10.1101/gad.1024602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abaza MS, Al-Saffar A, Al-Sawan S., and , Al-Attiyah R. c-myc antisense oligonucleotides sensitize human colorectal cancer cells to chemotherapeutic drugs. Tumour Biol. 2008;29:287–303. doi: 10.1159/000156706. [DOI] [PubMed] [Google Scholar]
- Pastorino F, Brignole C, Marimpietri D, Pagnan G, Morando A, Ribatti D, et al. Targeted liposomal c-myc antisense oligodeoxynucleotides induce apoptosis and inhibit tumor growth and metastases in human melanoma models. Clin Cancer Res. 2003;9:4595–4605. [PubMed] [Google Scholar]
- Xia Z, Zhu Z, Zhang L, Royal C, Liu Z, Chen Q, et al. Specific reversal of MDR1/P-gp-dependent multidrug resistance by RNA interference in colon cancer cells. Oncol Rep. 2008;20:1433–1439. [PubMed] [Google Scholar]
- Yadav S, van Vlerken LE, Little SR., and , Amiji MM. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemother Pharmacol. 2009;63:711–722. doi: 10.1007/s00280-008-0790-y. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zhang Y, Sun M., and , He Y. Reversion of multidrug resistance in human glioma by RNA interference. Neurol Res. 2008;30:562–566. doi: 10.1179/174313208X297869. [DOI] [PubMed] [Google Scholar]
- Bagalkot V, Farokhzad OC, Langer R., and , Jon S. An aptamer-doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew Chem Int Ed Engl. 2006;45:8149–8152. doi: 10.1002/anie.200602251. [DOI] [PubMed] [Google Scholar]
- Minotti G, Menna P, Salvatorelli E, Cairo G., and , Gianni L. Anthracyclines: molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- Li SD, Chen YC, Hackett MJ., and , Huang L. Tumor-targeted delivery of siRNA by self-assembled nanoparticles. Mol Ther. 2008;16:163–169. doi: 10.1038/sj.mt.6300323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SD, Chono S., and , Huang L. Efficient oncogene silencing and metastasis inhibition via systemic delivery of siRNA. Mol Ther. 2008;16:942–946. doi: 10.1038/mt.2008.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Sen J, Bathula SR, Yang Q, Fittipaldi R., and , Huang L. Novel cationic lipid that delivers siRNA and enhances therapeutic effect in lung cancer cells. Mol Pharm. 2009;6:696–705. doi: 10.1021/mp800136v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel R, Ros R, Ros A, Wilking SD, Sewald N., and , Anselmetti D. Identification of binding mechanisms in single molecule-DNA complexes. Biophys J. 2003;85:1968–1973. doi: 10.1016/S0006-3495(03)74624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, et al. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000;60:722–727. [PMC free article] [PubMed] [Google Scholar]
- Mina-Osorio P. The moonlighting enzyme CD13: old and new functions to target. Trends Mol Med. 2008;14:361–371. doi: 10.1016/j.molmed.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arap W, Pasqualini R., and , Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- Curnis F, Sacchi A, Borgna L, Magni F, Gasparri A., and , Corti A. Enhancement of tumor necrosis factor alpha antitumor immunotherapeutic properties by targeted delivery to aminopeptidase N (CD13) Nat Biotechnol. 2000;18:1185–1190. doi: 10.1038/81183. [DOI] [PubMed] [Google Scholar]
- Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- Garde SV, Forté AJ, Ge M, Lepekhin EA, Panchal CJ, Rabbani SA, et al. Binding and internalization of NGR-peptide-targeted liposomal doxorubicin (TVT-DOX) in CD13-expressing cells and its antitumor effects. Anticancer Drugs. 2007;18:1189–1200. doi: 10.1097/CAD.0b013e3282a213ce. [DOI] [PubMed] [Google Scholar]
- Pastorino F, Brignole C, Di Paolo D, Nico B, Pezzolo A, Marimpietri D, et al. Targeting liposomal chemotherapy via both tumor cell-specific and tumor vasculature-specific ligands potentiates therapeutic efficacy. Cancer Res. 2006;66:10073–10082. doi: 10.1158/0008-5472.CAN-06-2117. [DOI] [PubMed] [Google Scholar]
- Pastorino F, Brignole C, Marimpietri D, Cilli M, Gambini C, Ribatti D, et al. Vascular damage and anti-angiogenic effects of tumor vessel-targeted liposomal chemotherapy. Cancer Res. 2003;63:7400–7409. [PubMed] [Google Scholar]
- Corti A, Curnis F, Arap W., and , Pasqualini R. The neovasculature homing motif NGR: more than meets the eye. Blood. 2008;112:2628–2635. doi: 10.1182/blood-2008-04-150862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe W, Van NT, Burke TG., and , Perez-Soler R. Removal of the basic center from doxorubicin partially overcomes multidrug resistance and decreases cardiotoxicity. Anticancer Drugs. 1993;4:37–48. doi: 10.1097/00001813-199302000-00005. [DOI] [PubMed] [Google Scholar]
- Corti A., and , Ponzoni M. Tumor vascular targeting with tumor necrosis factor alpha and chemotherapeutic drugs. Ann N Y Acad Sci. 2004;1028:104–112. doi: 10.1196/annals.1322.011. [DOI] [PubMed] [Google Scholar]
- Cui Z, Han SJ, Vangasseri DP., and , Huang L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol Pharm. 2005;2:22–28. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ligand competitive cellular uptake assay.
Intracellular uptake of siRNA in HUVEC.
Relative mRNA level in the HT1080 cells 24 h after treatment with siRNA in different formulations.
DOX fluorescence intensity during the nanoparticle self-assembly.