Abstract
The purpose of this study is to assess the accuracy of spatiotemporal source analysis of magnetoencephalography (MEG) and scalp electroencephalography (EEG) for representing the propagation of frontotemporal spikes in patients with partial epilepsy. This study focuses on frontotemporal spikes, which are typically characterized by a preceding anterior temporal peak followed by an ipsilateral inferior frontal peak. Ten patients with frontotemporal spikes on MEG/EEG were studied. We analyzed the propagation of temporal to frontal epileptic spikes on both MEG and EEG independently by using a cortically-constrained minimum norm estimate (MNE). Spatiotemporal source distribution of each spike was obtained on the cortical surface derived from the patient’s MRI. All patients underwent an extraoperative intracranial EEG (IEEG) recording covering temporal and frontal lobes after presurgical evaluation. We extracted source waveforms of MEG and EEG from the source distribution of interictal spikes at the sites corresponding to the location of intracranial electrodes. The time differences of the ipsilateral temporal and frontal peaks as obtained by MEG, EEG and IEEG were statistically compared in each patient. In all patients, MEG and IEEG showed similar time differences between temporal and frontal peaks. The time differences of EEG spikes were significantly smaller than those of IEEG in nine of ten patients. Spatiotemporal analysis of MEG spikes models the time course of frontotemporal spikes as observed on IEEG more adequately than EEG in our patients. Spatiotemporal source analysis may be useful for planning epilepsy surgery, by predicting the pattern of IEEG spikes.
Keywords: epilepsy, magnetoencephalography, electroencephalography, propagation, spatiotemporal source analysis, minimum norm estimate
Introduction
Investigating the propagation of epileptic spikes is important for understanding the pathophysiology of epilepsy. Spike propagation may reflect neural networks associated with epilepsy (Spencer, 2002). Several studies have reported that the propagation patterns of interictal spikes are related to the outcome of epilepsy surgery (Alarcon et al., 1997; Hufnagel et al., 2000; Schulz et al., 2000). In addition, the correlation of localization between spike involvement and eloquent cortex should be investigated in presurgical evaluation for estimating the risk of functional deficits caused by epilepsy surgery (Rosenow and Lüders, 2001). Thus, accurate information of spike propagation is needed for appropriate clinical decision making in the treatment of epilepsy.
These propagation patterns can be determined by using intracranial electroencephalography (IEEG). Previous IEEG studies have revealed that interictal spikes arising from the unilateral temporal lobe can propagate widely, such as to the ipsilateral inferior frontal, parietal, and contralateral temporal lobes, likely through the white matter tracts such as uncinate fasciculus, external capsule and corpus callosum (Lieb et al., 1991; Alarcon et al., 1994; Alarcon et al., 1997; Lantz et al., 2003a). A limitation of the IEEG method is that investigating long distance propagation may require widespread employment of larger intracranial electrode grids, which may increase the risk of complication.
Spike propagation can also be studied non-invasively with magnetoencephalography (MEG) and scalp electroencephalography (EEG). Previous studies have mostly described the propagation in terms of changes in the magnetic field patterns during interictal spiking on MEG (Sutherling et al., 1989) and time differences between different electrode sites on EEG (Emerson et al., 1995). However, analyses of the surface MEG/EEG signals without considering anatomical information of the brain may provide only limited information about spike propagation.
Spatiotemporal distributed source analysis of MEG and EEG (Dale and Sereno, 1993; Hämäläinen and Ilmoniemi, 1994) incorporates anatomical information for each individual, and calculates the time-course of source distribution constrained to the cortex. This technique is well suited for investigating the origin of epileptic spikes, and several studies have demonstrated that it can provide a better localization than more conventional source analyses, such as single dipole modeling, in the evaluation of epilepsy (Shiraishi et al., 2005a,b; Tanaka et al., 2009).
The possibility of spatiotemporal source analysis for examining spike propagation has been described by Shiraishi et al. (2005a,b) in studies of widespread interictal spikes. However, it remains unclear how well it represents the pattern of propagation as compared with intracranial electroencephalography (IEEG).
The purpose of this study is to assess the usefulness of spatiotemporal source analysis of MEG and EEG for evaluating the spike propagation. If the non-invasive MEG and EEG procedures used in this study are sufficiently accurate compared with IEEG, they may be applicable and useful for planning epilepsy surgery by providing information of the spike propagation that is related with surgical outcomes. For this purpose, the present study focuses on frontotemporal spikes, which are typically characterized by a preceding anterior temporal peak followed by an ipsilateral inferior frontal peak. We extracted source waveforms of MEG and EEG from source distribution of interictal spikes, and compared the time difference of temporal and frontal peaks between these source waveforms and IEEG in ten patients with partial epilepsy.
Patients and Methods
Patients
We studied ten patients with intractable partial epilepsy who had unilateral frontotemporal spikes on interictal MEG/EEG. One of the patients (Patient 7) showed independent left- and right-sided spikes on IEEG, MEG and EEG; we analyzed the right-sided spikes, which showed more frequent frontotemporal involvement than the left. The MEG/EEG recording was performed as a part of presurgical evaluation at Athinoula A. Martinos Center for Biomedical Imaging. Written informed consent was obtained from each patient and/or the guardian for research, which was approved by the institutional review board. All patients underwent an extraoperative IEEG recording at the Children’s Hospital Boston or the Brigham and Women’s Hospital. Table 1 gives an overview of the clinical profiles of all patients.
Table 1.
Patient profile
| Patient | Age/Sex/Age of onset | Diagnosis | Seizure type | MRI |
|---|---|---|---|---|
| 1 | 15/F/10 | R-TLE | SPS with abnormal feeling in the abdomen, CPS with staring | R hippocampal atrophy |
| 2 | 15/M/9 | L-TLE | CPS with staring, lip smacking, flexion of the right arm | L hippocampal atrophy |
| 3 | 13/M/11 | L-TLE | SPS with pain in the stomach, sensation of twitching of the right arm, pGTC |
Normal |
| 4 | 9/F/1 | L-TLE | SPS with staring, aphasia | L temporal cortical dysplasia |
| 5 | 19/F/16 | R-TLE | SPS with abnormal sensation the stomach, salivation | Normal |
| 6 | 20/M/early infancy | L-TLE | CPS with head turning to the right | Normal |
| 7 | 14/F/8 | multifocal | CPS with inability to follow commands, inappropriate verbal responses |
Normal |
| 8 | 9/M/5 | R-TLE | SPS with left facial pain, CPS with lip smacking | R temporal gliosis |
| 9 | 9/F/4 | L-TLE | CPS with staring, hand fumbling | Normal |
| 10 | 17/F/13 | L-TLE | SPS with hearing sounds muffled, CPS with head turning to the right |
Normal |
F: female, M: male, R: right, L: left, TLE: temporal lobe epilepsy, SPS: simple partial seizure, CPS: complex partial seizure, pGTC: partial-onset generalized tonic-clonic seizure
Simultaneous MEG and EEG recordings
MEG was recorded with a 306-channel (204 planar gradiometers and 102 magnetometers), whole-head MEG system (Elekta-Neuromag, Helsinki, Finland) in a magnetically shielded chamber. EEG was simultaneously recorded with a non-magnetic 70-channel electrode cap, based on the 10-10 electrode placement system. The sampling rate was 600Hz with anti-aliasing filter bandpass of 0.1-200Hz, for the purpose of obtaining a high temporal resolution for our clinical studies. For the source analysis, the MEG and EEG data were low-pass filtered at 40 Hz to reduce the contamination of high-frequency artifacts. The details of the MEG/EEG recording have previously been described (Knake et al., 2006).
MRI
In all patients, high-resolution 3T anatomical MRI data were acquired with magnetization-prepared rapid acquisition gradient-echo (MPRAGE; TE 3.37ms, TR 2000ms, voxel size 1×1×1 mm) and multi-echo fast low-angle shot pulse sequences (FLASH) (Fischl et al., 2004) (TIM TRIO, Siemens AG, Erlangen, Germany).
IEEG
All patients underwent an extraoperative IEEG recording by employing grid and strips covering temporal and frontal lobes, based on the results of presurgical evaluation. IEEG was recorded with a digital EEG system (Ceegraph Vision; Biologic Systems Corp, Mundelein, USA, or XLTEK EEG system; Excel-Tech Ltd, Oakville, Canada). The sampling rate was 512Hz, and the data was low-pass filtered at 30 Hz for the analysis.
Spatiotemporal source analysis of MEG and EEG data
Spatiotemporal source distribution was calculated by using a minimum norm estimate (MNE) (Hämäläinen and Ilmoniemi, 1994; Dale and Sereno, 1993). MEG and EEG were analyzed independently. In this procedure, each patient’s cortical surface was reconstructed from the MPRAGE MRI data (Dale et al., 1999; Fischl et al., 1999) (Fig. 1a). A three-layer head model was calculated by a boundary elemental method (BEM), considering the boundaries of inner skull, outer skull and outer skin (Hamäläinen et al., 1989; Oostendorp and Van Oosterom, 1989). These boundaries were obtained from reconstructed surfaces and FLASH sequences (Fig. 1b). The surface was tessellated with 5120 triangles, providing adequate numerical accuracy (Crouzeix et al., 1999; Fuchs et al., 2001; Tarkiainen et al., 2003; de Jongh et al., 2005). The forward solution, which models the signal pattern generated by a unit dipole at each location on the cortical surface, was calculated by using a BEM. The inverse solution was calculated from the forward solution for MEG and EEG, independently. Thus, the activation at each cortical location was estimated at each time point of the activity, by using the inverse solution (Hämäläinen and Ilmoniemi, 1994; Dale and Sereno, 1993). The activation pattern of MEG and EEG was mapped onto geometrical representations of cortical surfaces (Fig. 1c).
Fig. 1.
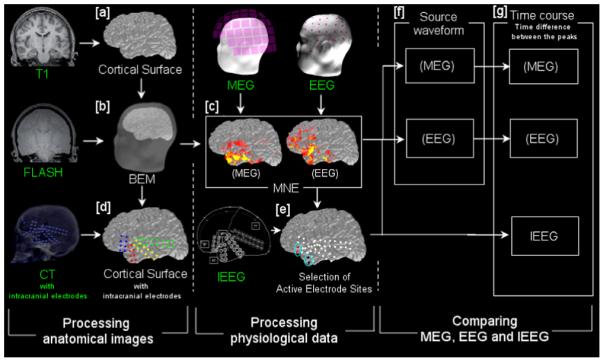
Illustrated procedure of the analysis
The steps of our procedure. The steps are classified into three major groups: (i) processing of anatomical images; (ii) physiological analysis of MEG, EEG and IEEG, and (iii) comparing MEG, EEG and IEEG time courses. Data shown with a green label is obtained directly from the patients. White labels show the data generated from the green-labeled or other processed (white-labeled) data. [a] Cortical surface reconstruction. [b] A head model is obtained from the cortical surface and multi-echo fast low-angle shot pulse sequences (FLASH) by using a boundary elemental method (BEM). [c] Spatiotemporal source distribution of MEG and EEG spikes is obtained by using minimum norm estimate (MNE). The map is shown on the cortical surface, with red and yellow indicating the cortical activation. [d] The location of intracranial electrodes is determined by co-registering the patient’s post-implantation computed tomography (CT) images onto the cortical surface. [e] Two frequently spiking sites of intracranial electrodes are selected each of anterior temporal and inferior frontal lobes. [f] Source waveforms are extracted from the MNE-derived spatiotemporal source distribution of each MEG and EEG spike, at the sites selected in [e]. [g] Time differences between the temporal and frontal peaks are obtained for each of MEG- and EEG-source waveforms and IEEG spikes.
Determination of the location of intracranial electrodes on cortical surfaces
All patients underwent computed tomography (CT) after implantation of intracranial electrodes. We co-registered the CT images onto the cortical surface derived from each patient’s MRI and determined the location of the electrodes on the cortical surface (Fig. 1d).
Spike analysis
The MEG, EEG and IEEG waveforms in all sensors were visually examined, and epileptic spikes were identified independently in each set of data. We selected spikes appearing simultaneously on MEG and EEG. For IEEG, frontotemporal spikes, in which an anterior temporal peak preceded an inferior frontal peak, were analyzed. The grids involved at least 12 intracranial electrodes with 10mm distance between adjacent electrodes in both temporal and frontal lobes. Two active, frequently spiking intracranial electrodes in each area (temporal and frontal) were selected in each individual patient (Fig. 1e). The largest time difference between the temporal and frontal peaks at these electrodes was obtained from each spike (Fig. 1g). Similarly, frontotemporal spikes with a temporal peak followed by a frontal peak were analyzed on MEG and EEG. Source waveforms of MEG and EEG were extracted from the spatiotemporal source distribution obtained from MNE, at the cortical sites corresponding to the intracranial electrodes used in the IEEG analysis (Fig. 1f). The time difference between the temporal and frontal peaks was obtained for each spike, in the same manner as IEEG (Fig. 1g). In addition to the source waveform analysis, we examined the timing of the peak signals in selected MEG and EEG sensors in one patient, without applying any inverse modeling.
The whole process of the analysis described above is illustrated in Fig. 1.
Statistics
We compared the propagation times (differences in the peak time of frontal and temporal spikes) in each patient, by using an analysis of variance (ANOVA). We adjusted for multiple comparisons with a Bonferroni correction. The time difference was also compared for MEG-IEEG and EEG-IEEG independently, when ANOVA showed statistical significance. P value below 0.05 was considered significant in all statistical procedures.
Results
Table 2 gives an overview of the results for all patients. In each patient, 15-50, 15-50 and 26-50 spikes were analyzed on EEG, MEG and IEEG, respectively. The time difference between temporal and frontal peaks of these spikes ranged from 4-86ms on MEG, 4-71ms on EEG, 1-97ms on IEEG and in overall patients. The mean value of time difference of each individual patient ranged from 24-46ms on MEG, 12-25ms on EEG and 29-42ms on IEEG. Within each individual, MEG and IEEG showed quite similar time differences without any statistically significant difference in all patients (p<0.05). The time differences of EEG spikes were significantly smaller than those of IEEG in nine of ten patients (Patient 1-4, 6-9, p<0.05).
Table 2.
Results of MEG/EEG/IEEG
| Patient | No. of MEG spikes |
No. of EEG spikes |
No. of IEEG spikes |
Time difference of MEG spikes (ms) |
Time difference of EEG spikes (ms) |
Time difference of IEEG spikes (ms) |
|---|---|---|---|---|---|---|
| 1 | 27 | 27 | 50 | 8-85 (Mean: 41) |
8-71 (Mean: 20*) |
2-97 (Mean: 36) |
| 2 | 50 | 50 | 50 | 8-75 (Mean: 33.8) |
7-63 (Mean: 12*) |
2-89 (Mean: 41) |
| 3 | 50 | 50 | 50 | 8-78 (Mean: 24) |
8-31 (Mean: 13*) |
2-75 (Mean: 32) |
| 4 | 46 | 46 | 50 | 8-86 (Mean: 34) |
4-47 (Mean: 12*) |
2-86 (Mean: 42) |
| 5 | 28 | 28 | 50 | 8-66 (Mean: 34) |
6-63 (Mean: 26) |
2-82 (Mean: 29) |
| 6 | 50 | 50 | 50 | 8-86 (Mean: 37) |
8-47 (Mean: 23*) |
2-93 (Mean: 34) |
| 7 | 50 | 50 | 26 | 8-78 (Mean: 33) |
8-47 (Mean: 21*) |
6-73 (Mean: 30) |
| 8 | 40 | 40 | 50 | 8-78 (Mean: 31) |
8-63 (Mean: 19*) |
3-88 (Mean: 36) |
| 9 | 15 | 15 | 50 | 8-86 (Mean: 42) |
8-47 (Mean: 21*) |
1-84 (Mean: 37) |
| 10 | 50 | 50 | 50 | 8-94 (Mean: 39) |
8-63 (Mean: 22*) |
2-93 (Mean: 44) |
IEEG: intracranial EEG, R: right, L: left
significant at P<0.05
Illustrative case
Patient 1 (Fig. 2)
Fig. 2.
A typical spike in Patient 1.
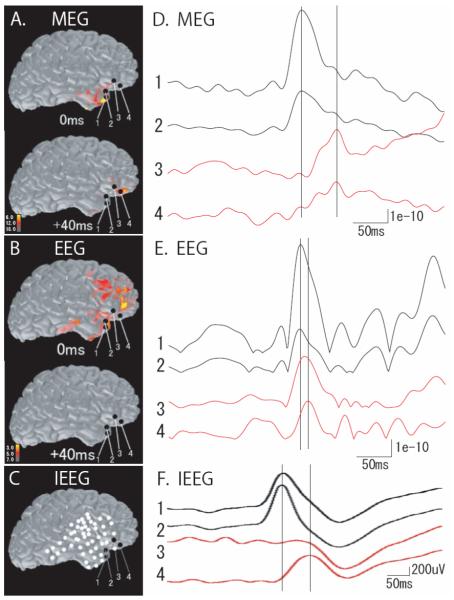
The MNE-derived source distribution map of a spike obtained through (A) MEG; (B) EEG. Cortical activation is shown with red and yellow colors. Black circles show the sites shown in (C). The map of the MEG spike demonstrates activation in the right anterior temporal lobe at the temporal peak (0ms), and in the right inferior frontal area (+40ms). The EEG spike map demonstrates activation in both temporal and frontal lobes at mostly the same time (0ms), and disappears quickly (+40ms). (C) The location of intracranial electrodes determined on the cortical surface by using post-implantation CT images. Black circles numbered 1-4 show the frequently spiking sites of electrodes in the anterior temporal and inferior frontal lobes. Source waveforms of (D) MEG and (E) EEG extracted from the sites 1-4, shown on the left column. The temporal peak (black lines) precedes the frontal peak (red lines) approximately by 50ms in the MEG spike, and by 10ms in the EEG spike. (F) Waveforms of a typical IEEG spike obtained from the sites 1-4, shown on the left column. The temporal peak (black lines) precedes the frontal peak (red lines) approximately by 50ms. The time difference is similar in IEEG and MEG, whereas EEG shows a smaller difference.
Patient 1 was a 15-year-old girl with frequent partial seizures since age 10. Her seizures consisted of abnormal feeling of the abdomen, staring and unresponsiveness. MRI showed right hippocampal atrophy.
Frequent right frontotemporal spikes were seen on both MEG and EEG. We analyzed 27 spikes by using MNE. Spatiotemporal source distribution maps were calculated for the MEG and EEG spikes, independently. The maps of MEG spikes showed anterior temporal activation, followed by inferior frontal activation. A map of a typical spike is shown in Fig. 2A. EEG spike maps showed almost simultaneous activation in the right temporal and frontal lobes, and the activation typically quickly disappeared as shown in Fig. 2B.
The patient underwent an extraoperative IEEG recording covering the right temporal and frontal lobes (Fig. 2C). Frequent interictal spikes were seen in the right anterior temporal and inferior frontal cortex, and two active sites were selected in each of these areas (shown as 1-4 in Fig. 2C). The waveforms of a typical IEEG spike are shown in Fig. 2F. The time difference between the temporal and frontal peaks at these sites was obtained from 50 IEEG spikes. The source waveforms of MEG and EEG, which were extracted at the same sites from MNE-based source distribution of spikes, showed a temporal peak followed by a frontal peak, as well as IEEG spikes. The source waveforms of a typical MEG and EEG spike are shown in Fig. 2D and 2E, respectively. The time difference on MEG spikes between these peaks was similar to that on IEEG spikes. On the other hand, EEG spikes demonstrated significantly smaller time difference than IEEG spikes (p<0.05). The mean value of the time differences on MEG, EEG and IEEG was 46.2ms, 18.2ms and 35.5ms, respectively.
In Patient 1, we also examined the peak times as obtained directly from the recorded signals, without applying inverse modeling. We identified the MEG and EEG sensors that were nearest to the selected IEEG electrodes of the temporal and frontal lobes, and obtained the time difference between the peak signals in these sensors (MEG: sensors 144 and 122; EEG: electrodes FT10 and AF8). The time difference ranged from 12-91ms (mean: 41ms) on MEG and 2-55ms (mean: 18ms) on EEG, being similar to the results of the source waveform analysis.
This patient underwent a right anterior temporal lobectomy, and has been seizure free for 2 years after surgery.
Discussion
In the present study, we examined spatiotemporal source analysis of MEG and EEG for representing spike propagation. The source waveforms on the MRI-derived cortical surface were obtained from the estimated spatiotemporal source distributions. This approach enables the comparison of the time course of spikes between MEG, EEG and IEEG at corresponding sites, by determining the location of implanted intracranial electrodes on the surface. The process of analyzing MEG and EEG a non-invasive method except for correlation with IEEG, i.e., the source waveforms of MEG and EEG can be non-invasively obtained at any cortical sites. As a result, statistical analysis of time difference between the temporal and frontal peaks demonstrated that MEG and IEEG had a similar time course of spikes, whereas the time difference of EEG spikes was significantly smaller than that of IEEG in nine of ten patients.
MEG/EEG and IEEG were obtained from the same patient group, but were recorded separately. The exact correspondence of each spike between MEG/EEG and IEEG was not obtained in this study. Previous studies also compared them by using separately recorded data, due to technical difficulties of simultaneous recording (Sato et al., 1985; Sutherling et al., 1988; Minassian et al., 1999). Most of their observations, including lower sensitivity of MEG for epileptic spikes in the deep region, have been confirmed by recent studies of simultaneous recording of iEEG and MEG (Mikuni et al., 1997; Sutherling et al., 2001; Oishi et al., 2002). Thus, our results can be an important step to encouraging further studies of simultaneous recording of iEEG and MEG/EEG, despite the limitation of the study. Considering the variability of individual spikes, we used a statistical approach to compare these types of data. We analyzed spikes appearing on both MEG and EEG at the same time for obtaining smaller variability. The minimum amount of spiking cortex observed on IEEG has been estimated to be about 4 cm^2 and 6 cm^2 in order to cause detectable spikes in MEG and EEG, respectively (Cooper et al., 1965; Mikuni et al., 1997; Oishi et al., 2002). The IEEG spikes analyzed in this study involved at least 12 electrodes, and they may be included in the types of spikes that can cause the corresponding spikes on MEG and EEG.
Previous studies have suggested that the time difference between the appearance of a temporal peak and the subsequent peak obtained from a temporal spike reflects a spike propagation (Alarcon et al., 1994; Emerson et al., 1995; Alarcon et al., 1997). The time difference observed in propagation of temporal spikes has been reported to be typically <100 or <50ms on IEEG (Alarcon et al., 1994; Alarcon et al., 1997). In this study, frontotemporal spike distribution and the time difference between the peaks on IEEG were consistent with these previous reports.
Our results suggest that spatiotemporal analysis of MEG spikes may best represent the spike propagation as observed on IEEG. On the other hand, EEG spike analysis did not show good agreement with the time course that was seen on IEEG spikes. Previous studies have demonstrated the small time difference between the temporal and frontal peaks at the scalp electrodes (Emerson et al., 1995) and current density analysis of EEG spikes (Huppertz et al., 2001). These observations suggest that the time difference of EEG source waveforms may reflect that of scalp EEG. To our knowledge, there are no previous studies describing these time differences of MEG spike propagation. In this study, Patient 1 showed similar time differences between source and sensor waveforms. The result may suggest that the source waveforms reflect the time course at the sensor level as well as EEG.
There are several possible explanations of the different results of MEG and EEG. One is the difference of the numbers of measurement sites between MEG and EEG. In this study, MEG had 102 sites (306 channels) of measurements, whereas EEG had 70 measurement sites. Previous studies have demonstrated the usefulness of high-density EEG for analyzing epileptic spikes (Lanz et al., 2003a; Michael et al., 2004), and the number of EEG electrodes affects the reliability of source analysis (Lantz et al., 2003b). Although our EEG setting has more electrodes than the standard clinical EEG of the international 10-20 method, higher density EEG, such as 128-channel EEG, may provide better performance for representing the time difference associated with spike propagation. This study focused on investigating the performance of the routine clinical settings, and further detailed basic research is needed for testing the various settings of MEG/EEG. Another one is the sensitivity difference of MEG and EEG. Previous studies have demonstrated that MEG has a higher signal-to-noise ratio than EEG in the lateral temporal and frontal cortex (de Jongh et al., 2005; Goldenholz et al., 2009). The cortically-constrained MNE used in this study assumes that the sources are located on the cortical surface (Dale and Sereno, 1993; Hämäläinen and Ilmoniemi, 1994). MNE may enhance a superficial bias when calculating the source distribution of MEG signals. The difference in the time course of the source waveforms may be related with the higher sensitivity of MEG than EEG in these cortical areas.
In this study, the temporal and frontal peaks of EEG source waveforms were close in their latency. Therefore, we should be careful with the risk of overlapped waveforms from the frontal and temporal lobes. It is known that a spherical head model can cause a localization error of EEG sources, and previous studies have demonstrated that a realistic three-layer head model can improve the accuracy of source localization, especially in the “Z” direction (Cuffin, 1996; Roth et al., 1997; Fuchs et al., 2002). They suggested the realistic head model reduced the erroneous source localization of temporal spikes to the frontal lobe. Therefore, we consider the EEG source waveform can reflect the different components at frontal and temporal surfaces, even though they may be overlapped at the sensor level.
Several researchers have suggested that source analysis of EEG spikes may also accurately represent the spike propagation in some cases (Scherg et al., 1999; Waberski et al., 2000; Zumsteg et al., 2006). These studies mainly showed the propagation between medial and lateral parts of the temporal lobe, a different anatomic area which may be more sensitive to EEG than MEG. Our study suggests that MEG and EEG analysis can also be applied to the evaluation of extratemporal propagation of epileptic spikes.
Two pathways have been mainly described for explaining frontotemporal spike propagation (Lieb et al., 1991; Emerson et al., 1995). One pathway is a projection from amygdala to prefrontal region, partly via thalamus. The other is mediated by uncinate fasciculus, which connects anterior temporal lobe and inferior frontal lobe (Makris and Pandya, 2009). Our selected regions of the spike peaks may correspond to the latter pathway, whose anatomical basis has recently been described by a diffusion tensor imaging study (Lin et al., 2008).
In conclusion, spatiotemporal analysis of MEG spikes may provide more accurate information of spike propagation in our patients than EEG. It may be clinically useful in the presurgical evaluation of epilepsy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alarcon G, Guy CN, Binnie CD, Walker SR, Elwes RD, Polkey CE. Intracerebral propagation of interictal activity in partial epilepsy: implications for source localisation. J Neurol Neurosurg Psychiatry. 1994;57:435–449. doi: 10.1136/jnnp.57.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon G, Garcia Seoane JJ, Binnie CD, Martin Miguel MC, Juler J, Polkey CE, Elwes RD, Ortiz Blasco JM. Origin and propagation of interictal discharges in the acute electrocorticogram. Implications for pathophysiology and surgical treatment of temporal lobe epilepsy. Brain. 1997;120:2259–2282. doi: 10.1093/brain/120.12.2259. [DOI] [PubMed] [Google Scholar]
- Cooper R, Winter AL, Crow HJ, Walter WG. Comparison of subcortical, cortical and scalp activity using chronically indwelling electrodes in man. Electroencephalogt Clin Neurophysiol. 1965;18:217–228. doi: 10.1016/0013-4694(65)90088-x. [DOI] [PubMed] [Google Scholar]
- Crouzeix A, Yvert B, Bertrand O, Pernier J. An evaluation of dipole reconstruction accuracy with spherical head model and realistic head models in MEG. Clin Neurophysiol. 1999;110:2176–2188. doi: 10.1016/s1388-2457(99)00174-1. [DOI] [PubMed] [Google Scholar]
- Cuffin B,N. EEG localization accuracy improvements using realistically shaped head models. IEEE Trans Biomed Eng. 1996;43:299–303. doi: 10.1109/10.486287. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- de Jongh A, de Munck JC, Gonçalves SI, Ossenblok P. Differences in MEG/EEG epileptic spike yields explained by regional differences in signal-to-noise ratios. J Clin Neurophysiol. 2005;22:153–158. doi: 10.1097/01.wnp.0000158947.68733.51. [DOI] [PubMed] [Google Scholar]
- Emerson RG, Turner CA, Pedley TA, Walczak TS, Forgione M. Propagation patterns of temporal spikes. Electroencephalogr Clin Neurophysiol. 1995;94:338–348. doi: 10.1016/0013-4694(94)00316-d. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis, II: inflation, flattening, a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Kastner J, Wagner M, Hawes S, Ebersole J,S. A standardized boundary element method volume conductor model. Clin Neurophysiol. 2002;113:702–712. doi: 10.1016/s1388-2457(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Fuchs M, Wagner M, Kastner J. Boundary element method volume conductor models for EEG source reconstruction. Clin Neurophysiol. 2001;112:1400–1407. doi: 10.1016/s1388-2457(01)00589-2. [DOI] [PubMed] [Google Scholar]
- Goldenholz DM, Ahlfors SP, Hämäläinen MS, Sharon D, Ishitobi M, Vaina LM, Stufflebeam SM. Mapping the signal-to-noise-ratios of cortical sources in magnetoencephalography and electroencephalography. Hum Brain Map. 2009;30:1077–1086. doi: 10.1002/hbm.20571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamäläinen M, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Huppertz HJ, Hoegg S, Sick C, L·cling CH, Zentner J, Schulze-Bonhage A, Kristeva-Feige R. Cortical current density reconstruction of interictal epileptiform activity in temporal lobe epilepsy. Clin Neurophysiol. 2001;112:1761–1772. doi: 10.1016/s1388-2457(01)00588-0. [DOI] [PubMed] [Google Scholar]
- Hufnagel A, Düpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41:467–478. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Knake S, Halgren E, Shiraishi H, Hara K, Hamer HM, Grant PE, Carr VA, Foxe D, Camposano S, Busa E, Witzel T, Hamäläinen MS, Ahlfors SP, Bromfield EB, Black PM, Bourgeois BF, Cole AJ, Cosgrove GR, Dworetzky BA, Madsen JR, Larsson PG, Schomer DL, Thiele EA, Dale AM, Rosen BR, Stufflebeam SM. The value of multichannel MEG and EEG in the presurgical evaluation of 70 epilepsy patients. Epilepsy Res. 2006;69:80–86. doi: 10.1016/j.eplepsyres.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Lantz G, Spinelli L, Seeck M, de Peralta Menendez RG, Sottas CC, Michael CM. Propagation of interictal epileptiform activity can lead to erroneous source localizations: A 128-channel EEG mapping study. J Clin Neurophysiol. 2003a;20:311–319. doi: 10.1097/00004691-200309000-00003. [DOI] [PubMed] [Google Scholar]
- Lantz G, Grave de Peralta R, Spinelli L, Seeck M, Michel CM. Epileptic source localization with high density EEG: how many electrodes are needed? Clin Neurophysiol. 2003b;114:63–69. doi: 10.1016/s1388-2457(02)00337-1. [DOI] [PubMed] [Google Scholar]
- Lieb JP, Dasheiff RM, Engel J., Jr. Role of the frontal lobes in the propagation of mesial temporal lobe seizures. Epilepsia. 1991;32:822–837. doi: 10.1111/j.1528-1157.1991.tb05539.x. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 2008;82:162–170. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Makris N, PandyaD N. The extreme capsule in humans and rethinking of the language circuitry. Brain Struct Funct. 2009;213:343–358. doi: 10.1007/s00429-008-0199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel CM, Lantz G, Spinelli L, De Peralta RG, Landis T, Seeck M. 128-channel EEG source imaging in epilepsy: clinical yield and localization precision. J Clin Neurophysiol. 2004;21:71–83. doi: 10.1097/00004691-200403000-00001. [DOI] [PubMed] [Google Scholar]
- Mikuni N, Nagamine T, Ikeda A, Terada K, Taki W, Kimura J, Kikuchi H, Shibsaki H. Simultaneous recording of epileptiform discharges by MEG and subdural electrodes in temporal lobe epilepsy. Neuroimage. 1997;5:298–306. doi: 10.1006/nimg.1997.0272. [DOI] [PubMed] [Google Scholar]
- Minassian BA, Otsubo H, Weiss S, Elliott I, Rutka JT, Snead OC., 3rd Magnetoencephalographic localization in pediatric epilepsy surgery: comparison with invasive intracranial electroencephalography. Ann Neurol. 1999;46:627–633. doi: 10.1002/1531-8249(199910)46:4<627::aid-ana11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Oishi M, Otsubo H, Kameyama S, Morota N, Masuda H, Kitayama M, Tanaka R. Epileptic spikes: Magnetoencephalography versus simultaneous electrocorticography. Epilepsia. 2002;43:1390–1395. doi: 10.1046/j.1528-1157.2002.10702.x. [DOI] [PubMed] [Google Scholar]
- Oostendorp TF, Van Oosterom A. Source parameter estimation in inhomogeneous volume conductors of arbitrary shape. IEEE Trans Biomed Eng. 1989;36:382–391. doi: 10.1109/10.19859. [DOI] [PubMed] [Google Scholar]
- Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124 doi: 10.1093/brain/124.9.1683. 168301700. [DOI] [PubMed] [Google Scholar]
- Roth B,J, Ko D, von Albertini-Carletti I,R, Scaffidi D, Sato S. Dipole localization in patients with epilepsy using the realistically shaped head model. Electroencephalogr Clin Neurophysiol. 1997;102:159–166. doi: 10.1016/s0013-4694(96)95111-5. [DOI] [PubMed] [Google Scholar]
- Sato S, Sheridan P, Smith P. Comparison of EEG, MEG, and ECoG in epileptic patients. In: Weinberg H, Stroink G, Katila T, editors. Biomagnetism: Applications and Theory. Pergamon; New York: 1985. pp. 311–5. [Google Scholar]
- Scherg M, Bast T, Berg P. Multiple source analysis of interictal spikes: Goals, requirements, and clinical value. J Clin Neurophysiol. 1999;16:214–224. doi: 10.1097/00004691-199905000-00003. [DOI] [PubMed] [Google Scholar]
- Schulz R, Lüders HO, Hoppe M, Tuxhorn I, May T, Ebner A. Interictal EEG and ictal scalp EEG propagation are highly predictive of surgical outcome in mesial temporal lobe epilepsy. Epilepsia. 2000;41:564–570. doi: 10.1111/j.1528-1157.2000.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Ahlfors SP, Stufflebeam SM, Takano K, Okajima M, Knake S, Hatanaka K, Kohsaka S, Saitoh S, Dale AM, Halgren E. Application of magnetoencephalography in epilepsy patients with widespread spike or slow-wave activity. Epilepsia. 2005a;46:1264–1272. doi: 10.1111/j.1528-1167.2005.65504.x. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Stufflebeam SM, Knake S, Ahlfors SP, Sudo A, Asahina N, Egawa K, Hatanaka K, Kohsaka S, Saitoh S, Grant E, Dale AM, Halgren E. Dynamic statistical parametric mapping for analyzing the magnetoencephalographic epileptiform activity in patients with epilepsy. J Child Neurol. 2005b;20:363–369. doi: 10.1177/08830738050200041601. [DOI] [PubMed] [Google Scholar]
- Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Sutherling WW, Crandall PH, Cahan LD, Barth DS. The magnetic field of epileptic spikes agrees with intracranial localization in complex partial epilepsy. Neurology. 1988;38:778–786. doi: 10.1212/wnl.38.5.778. [DOI] [PubMed] [Google Scholar]
- Sutherling WW, Barth DS. Neocortical propagation in temporal lobe spike foci on magnetoencephalography and electroencephalography. Ann Neurol. 1989;25:373–381. doi: 10.1002/ana.410250409. [DOI] [PubMed] [Google Scholar]
- Sutherling WW, Akhtari M, Mamelak AN, Mosher J, Arthur D, Sands S, Weiss P, Lopez N, DiMauro M, Flynn E, Leah R. Dipole localization of human induced focal afterdischarge seizure in simultaneous magnetoencephalography and electrocorticography. Brain Topogr. 2001;14:101–116. doi: 10.1023/a:1012940812742. [DOI] [PubMed] [Google Scholar]
- Tanaka N, Cole AJ, von Pechmann D, Wakeman DG, Hämäläinen MS, Liu H, Madsen JR, Bourgeois BF, Stufflebeam SM. Dynamic statistical parametric mapping for analyzing ictal magnetoencephalographic spikes in patients with intractable frontal lobe epilepsy. Epilepsy Res. 2009 doi: 10.1016/j.eplepsyres.2009.03.023. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkiainen A, Liljestrom M, Seppa M, Salmelin R. The 3D topography of MEG source localization accuracy: effects of conductor model and noise. Clin Neurophysiol. 2003;114:1977–1992. doi: 10.1016/s1388-2457(03)00195-0. [DOI] [PubMed] [Google Scholar]
- Waberski TD, Gobbelé R, Herrendorf G, Steinhoff BJ, Kolle R, Fuchs M, Paulus W, Buchner H. Source reconstruction of mesial-temporal epileptiform activity: Comparison of inverse techniques. Epilepsia. 2000;41:1574–1583. doi: 10.1111/j.1499-1654.2000.001574.x. [DOI] [PubMed] [Google Scholar]
- Zumsteg D, Friedman A, Wieser HG, Wennberg RA. Propagation of interictal discharges in temporal lobe epilepsy: correlation of spatiotemporal mapping with intracranial foramen ovale electrode recordings. Clin Neurophysiol. 2006;117:2615–2626. doi: 10.1016/j.clinph.2006.07.319. [DOI] [PubMed] [Google Scholar]