Summary
The brain of Drosophila melanogaster contains ca. 150 circadian neurons [1] functionally divided into Morning and Evening cells that control peaks in daily behavioral activity at dawn and dusk, respectively [2, 3]. The PIGMENT-DISPERSING FACTOR (PDF) positive small ventral Lateral Neurons (sLNvs) promote morning behavior, while the PDF negative sLNv and the dorsal Lateral Neurons (LNds) generate evening activity. Much less is known about the ca. 120 Dorsal Neurons (DN1, 2 and 3). Using a Clk-GAL4 driver that specifically targets a subset of DN1s, we generated mosaic per0 flies with clock function restored only in these neurons. We found that the Clk4.1M-GAL4 positive DN1s promote only morning activity under standard (high light intensity) light:dark cycles. Surprisingly however, these circadian neurons generate a robust evening peak of activity under a temperature cycle in constant darkness. Using different light intensities and ambient temperatures, we resolved this apparent paradox. The DN1 behavioral output is under both photic and thermal regulation. High light intensity suppresses DN1-generated evening activity. Low temperature inhibits morning behavior, but it promotes evening activity under high light intensity. Thus, the Clk4.1M-GAL4 positive DN1s, or the neurons they target, integrate light and temperature inputs to control locomotor rhythms. Our study therefore reveals a novel mechanism contributing to the plasticity of circadian behavior.
Results
A GAL4 driver specifically expressed in a subset of DN1 neurons
Several studies point to a role of the Dorsal Neurons (DNs) in the control of circadian behavioral rhythms. They have been proposed to drive circadian rhythms under constant illumination, and might participate in evening anticipatory behavior under a LD cycle [3-6]. However, determining their exact function has been difficult because no tools to specifically manipulate DNs were available.
CLOCK (CLK) plays a central role in the transcriptional feedback loop that generates circadian rhythms [7] and is expressed in all circadian neurons [8]. We therefore cloned fragments of the Clk promoter in front of the GAL4 cDNA to identify regulatory elements that drive restricted expression in the fly brain. This approach proved to be successful. We counted 8-10 GFP positive cells per brain hemisphere in flies carrying a GAL4 transgene controlled by the −2.0 to −0.5 kb fragment of the Clk promoter (Clk4.1M-GAL4) and a UAS-gfp reporter transgene (Figure 1A). 4-5 cells expressed high GFP levels, while staining was weaker in 4-5 additional cells. These GFP positive cells were localized in the dorsal region of the brain, where the DN1s are located. Weak GFP staining could also be detected in projections directed toward the dorsal protocerebrum, which is believed to play an important role in the control of locomotor activity [9, 10]. This pattern of projection is reminiscent of that of most DN1s [11, 12]. To better visualize the projections of the GAL4 positive neurons, we crossed flies carrying the Clk-GAL4 driver with UAS-cd8gfp. Staining in the dorsal projections was very intense. Interestingly, we could now also visualize projections that were directed ventrally (Figure S1A). This is consistent with reports demonstrating that a subset of DN1s sends projections ventrally toward the accessory medulla [12, 13]. We found these ventral projections to be in close proximity of the dorsal projections of the PIGMENT-DISPERSING FACTOR (PDF) positive sLNvs (Figure S1A).
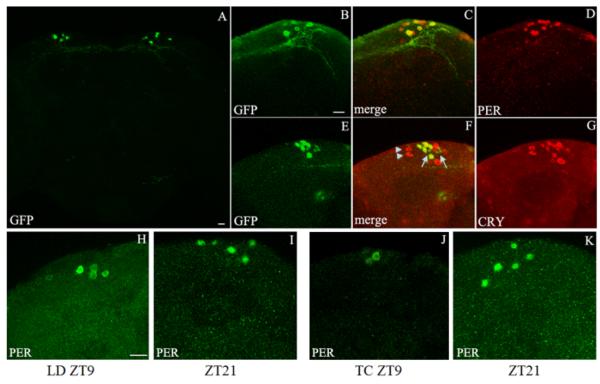
Figure 1. The Clk4.1M-GAL4 driver can rescue PER rhythms in a subset of DN1s in per0 flies under light:dark (LD) and thermophase:cryophase (TC) cycles.
(A) Brain of a UAS-gfp/+; Clk4.1M-GAL4/+ adult fly stained with anti-GFP (green). The Clk4.1MGAL4 driver is specifically expressed in 8-10 neurons/hemisphere in the dorsal region of the adult brain. (B-D) Brain of a UAS-cd8gfp/+; Clk4.1M-GAL4/+ adult fly entrained to a 500-lux LD cycle, dissected at ZT1 and coimmunolabeled with anti-GFP (green) and anti-PER (red). GFP staining was found exclusively in the DN1 group of circadian neurons. However, only a fraction of DN1s are GFP positive (ca. 50-60%). (E-G) Adult brain of a UAS-gfp/+; Clk4.1M-GAL4/+ fly dissected after three days of constant darkness and coimmunolabeled with anti-GFP (green) and anti-CRY (red). Most of the GFP-positive DN1s are CRY-positive, although we observed between 1-3 GFP positive neurons/brain hemisphere without detectable CRY expression (arrow). We also note that several DN1s with high CRY expression were GFP negative, including what appears to be the DN1as, based on their more anterior location (arrowheads). (H-K) Brains from per0 w;; Clk4.1M-GAL4/UAS-per 16 fly adults were synchronized by LD cycle (500 lux) or TC cycle (29-20°C) in constant darkness and dissected at ZT9 and ZT21 (ZT0 correspond to the lights-on or temperature-up transitions, ZT12 to the lights-off or temperature down transitions). Brains were then immunolabeled with anti-PER (green). (H, I) Under LD cycle, PER is detected in ~4-5 DN1s/brain hemisphere at both ZT9 and ZT21 (see also Figure S1C). However at ZT9, PER is mostly cytoplasmic, while at ZT21 it is mostly nuclear. (J-K) PER oscillations under TC cycles in constant darkness. PER abundance was much lower at ZT9 than at ZT21. The number of PER-positive DN1s per brain hemisphere varied from 0-3 at ZT9 to 4-6 at ZT21 (see also Figure S1C). All images are Z-stacks. Scale bars indicate 20 μm. See Figure S1 for quantifications and additional characterization of the Clk4.1M-GAL4 positive DN1s.
To verify that the GFP positive neurons are indeed DN1s, we coimmunostained brains with anti-GFP and either anti-PERIOD (PER) or anti-CRYPTOCHROME (CRY) antibodies (Figure 1B-1G). PER is an essential element of the molecular circadian pacemaker and is expressed in all circadian neurons [7]. CRY is Drosophila's primary circadian photoreceptor [14-16] and is expressed in many - but not all - clock neurons [12, 17]. We observed that all GFP positive neurons were PER positive and indeed correspond to DN1s. Most GFP positive neurons were also CRY positive. Several DN1s were GFP negative, and likely include the anterior DN1s (DN1as); a pair of large, strongly CRY positive, circadian neurons located in a more anterior position than the other DN1s (the posterior DN1s or DN1ps) [12, 13]. In summary, Clk4.1M-GAL4 is expressed exclusively in a subset of DN1s. We therefore used this driver to generate mosaic flies that express PER only in these DN1 cells to probe their function in the control of circadian behavior.
PER rhythms can be rescued in a subset of DN1s with Clk4.1M-GAL4
PER abundance and subcellular localization changes over the course of the day: night cycle. Its concentration reaches a peak after the middle of the night, when it becomes mostly nuclear [18-20]. We first determined whether we could restore PER molecular rhythms in the Clk4.1M-GAL4 positive DN1s. We entrained per0; Clk4.1MGAL4; UAS-per flies to a 12:12hr light:dark (LD) cycle or to a 12:12hr thermophase (29°C):cryophase (20°C) (TC) cycle under constant darkness (DD). Under LD, PER staining showed low amplitude cycling in intensity (Figure 1H, I): the number of PER positive neurons was actually constant, (~4 cells/brain hemisphere, Figure S1C), although signal quantification revealed that PER abundance peaked at ZT1 (ZT0 correspond to the lights-on or temperature-up transition) (Figure S1D). However, we detected a very clear change in PER subcellular localization: PER was cytoplasmic at ZT9, but nuclear at ZT21 (Figure 1H, I). Under a temperature cycle, we observed a more pronounced cycle in PER staining intensity. While ca. 5 cells/brain hemisphere were strongly PER positive at ZT21, with PER mostly localized in the nucleus, on average 2 cells were positive at ZT9, with weak cytoplasmic staining (Figure 1J, K and S1C). These observations could indicate that two separate subsets of Clk4.1MGAL4 positive DN1s respond specifically to TC or to LD cycles. This is not the case: the number of PER positive cells was similar at ZT1 (peak of PER expression) under LD, TC, or when LD and TC were combined (Figure S1E). Thus, the rescued DN1s appear to be more efficiently entrained by temperature input, even though most of them express CRY (Figure S1B). Interestingly, a subset of DN1s has been previously shown to be particularly sensitive to TC cycles [21].
The DN1s drive morning activity under high light intensity LD cycles
The DN1s are believed to be E cells [3-5]. To determine whether this is indeed the case, we measured the locomotor activity of DN1-rescued per0 flies under a LD cycle with a light intensity of 500 lux during the day (Figure 2A). Unexpectedly, we observed a robust anticipation of the light-on transition (morning peak), but evening anticipation was extremely weak and difficult to distinguish from an increase in locomotor activity also observed in control per0 flies at the end of the day (Figure 2A and S2A).
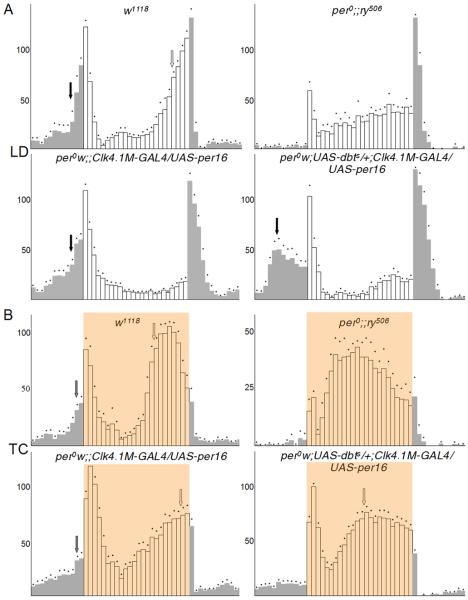
Figure 2. The Clk4.1M-GAL4 positive DN1s promote morning activity under LD cycles and evening activity under TC cycles.
(A) Locomotor activity of adult males (n=16), measured during 3 days of LD cycle (500 lux, 25°C) and averaged. The white bars represent activity levels during the light phase and black bars during the dark phase of the LD cycle. Dots above error bars correspond to Standard Errors of the Mean. (B) Locomotor activity of adult male flies measured during 3 daysunder a thermal cycle (29-20°C, 12:12hr) in DD and averaged. Orange shading indicates the thermophase of the TC cycle. Arrows indicate the morning (black) and evening anticipation (white). Dots above error bars correspond to Standard Errors of the Mean. Genotypes in (A) and (B): w1118 (upper left panels), per01;;ry506 (upper right panels), per01 w;; Clk4.1M-GAL4/UAS-per 16. (lower left panels) per01 w; UAS-dbts; Clk4.1M-GAL4/UAS-per 16 (lower right panels). See also Figure S2 for quantification of Evening behavior.
To verify that the morning anticipation is really controlled by the circadian clock in the DN1s, we co-expressed in these cells PER and DBTS, a mutant form of the DOUBLETIME (DBT) kinase, which controls the period of the circadian molecular oscillator [22, 23]. Expressing DBTS in circadian neurons speeds up the circadian clock by ca. 5 hours in constant conditions [24], and should thus advance morning anticipation. This is exactly what we observed: the morning anticipation generated by the DN1s was advanced by ca. 3 hours and is thus circadian. Taken together, these results demonstrate that the DN1s play an important role in circadian morning anticipatory behavior.
The DN1s drive evening activity under TC cycles (29/20°C)
When we tested circadian behavior under a 29°C/20°C TC cycle in DD, we were surprised to observe a clear rescue of the evening activity (Figure 2B). per0 flies show a temperature driven mid-day peak of activity that is due to the improper activity of PDF negative circadian neurons [25, 26]. In DN1-rescued flies, activity was shifted to the later part of the day. The phase of this evening behavior was advanced by coexpressing DBTS in the DN1s. This demonstrates that the rescued evening peak is indeed clock-driven. Although mid-day activity in DN1-rescued per0 flies was still somewhat elevated compared to wild-type flies, the mid-day activity peak seen in per0 flies was absent. Thus, the absence of a functional circadian pacemaker in Clk4.1M-GAL4 positive DN1s is largely responsible for the abnormal mid-day peak of activity observed in per0 flies under TC cycles [25, 26].
Light intensity modulates DN1 output
The presence of a robust evening peak under TC cycles, but not under LD cycles, could result from the DN1s' apparent greater sensitivity to TC cycles. However, this would not explain why a robust morning peak is observed under LD conditions. Interestingly, when flies expressing PER in Clk4.1M-GAL4 positive DN1s were tested during TC cycles in constant light (LL), no rescue of evening activity was seen (data not shown). Moreover, while a very weak circadian evening peak might be present under 500-lux LD cycles (Figure 2 and 3) since it appears to be sensitive to DBTS, it was undetectable at 2000 lux (Figure S2B). We therefore wondered whether light might actually repress evening activity. We exposed DN1-rescued flies to LD cycles with low light intensity during the day (50 lux). The result was very clear: at low light intensity, a robust circadian evening peak was observed (Figure 3B and S2A) that is shifted earlier by DBTS (Figure S2C). Since PER cycling was not affected by the decrease in light intensity (Figure S1D), it is the output from the DN1s that is modulated by photic input. Interestingly, the evening peak in wild-type flies does not strongly respond to light intensity (Figure 3) . Moreover, when we rescued PER expression with mai179-GAL4, which targets 3 LNds and all sLNvs [2], evening activity was not reduced (Figure S2E).
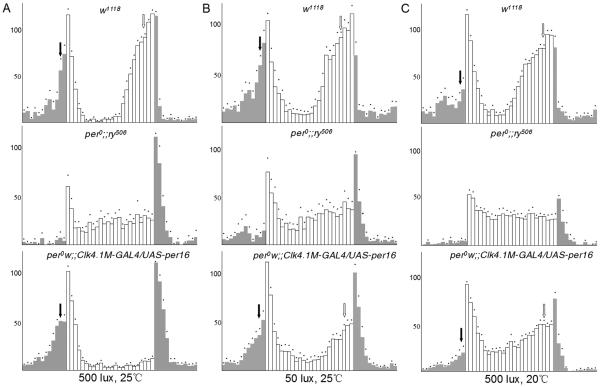
Figure 3. High light intensity suppresses DN1-generated evening locomotor activity and temperature modulates DN1-generated morning and evening behavior.
Locomotor activity of adult male flies measured during 3 days of LD cycle under different light intensities and temperatures and averaged. (A) 500 lux, 25°C. (B) 50 lux, 25°C (C). 500 lux, 20°C. Arrows indicate the morning anticipation (black) and evening anticipation (white). Dots above error bars correspond to Standard Errors of the Mean. Genotypes in (A), (B) and (C): w1118 (upper panels), per01;;ry506 (middle panels) per01 w;; Clk4.1M-GAL4/UAS-per 16. (lower panels). See also Figure S2 for additional controls and quantifications.
Combined, these results show that the contribution of the DN1s to circadian behavior is particularly sensitive to light intensities. The photic inhibition of DN1-driven locomotor behavior also indicates that the Clk4.1M-GAL4 positive DN1s are distinct from the subset of DN1s that can drive rhythms under constant illumination when CRY signaling is compromised [4]. As expected, per0; cryb flies [27] with PER expression rescued only in Clk4.1M-GAL4 positive DN1s were arrhythmic in LL (data not shown). In constant darkness, DN1-rescued per0 flies were also arrhythmic (data not shown), even during the 1st day of DD. However, these flies showed constantly elevated activity in DD, which could have masked residual DN1-driven behavior (data not shown).
The morning and evening peaks generated by the DN1s are differentially sensitive to ambient temperature
Late during the day, light intensity and temperature drop. Since evening activity generated by the DN1s is only observed when light intensity is low, we wondered whether low temperature might also promote evening activity. Thus, we compared LD behavior of DN1-rescued flies at 25°C and 20°C. Even though light intensity was kept at 500 lux, we observed a clear evening peak (Figure 3C and S2A), which was sensitive to DBTS (Figure S2D). Similar results were obtained at 2000 lux (Figure S2B). Thus both high light intensity and high temperature repress DN1-generated evening behavior. The evening peak observed when rescuing the LNds and the sLNvs of per0 flies with mai179-GAL4 was, like that of wild-type flies, insensitive to temperature (Figure S2E). The thermal regulation of evening activity is thus specific to the DN1s. Surprisingly, the DN1-generated morning peak behaved differently than the evening peak. The morning peak was severely suppressed at lower temperature in DN1 rescued flies and also in wild type flies.
Discussion
We have demonstrated that a group of cells belonging to the DN1 subset of circadian neurons plays an important role in the regulation of circadian behavior. Our results show that these DN1s can contribute to both morning and evening bouts of locomotor activity. Since the so-called E oscillators (3 LNds and the 5th sLNv) can also generate morning activity under light/moonlight cycles [28], the functional distinction of circadian neurons into M and E oscillators is probably an oversimplification. However, since the LNds and DN1s are a heterogeneous group of cells [12, 13, 29], we cannot exclude that some of these cells are dedicated to morning anticipation, while others control evening activity (see [28]). Importantly, the contribution of the Clk4.1M-GAL4 positive DN1s to circadian behavior is tightly regulated by the physical properties of the environment: light intensity and temperature modulate the DN1 output pathway (Figure 4). In most climates and latitudes, there is considerable variability in the physical properties of the day: night cycle. Photoperiod and overall temperature change with seasons, and daily weather patterns influence the temperature cycle and light intensities. Thermal and photic modulation of DN1 output thus provides a very simple mechanism for daily adaptation of Drosophila circadian behavior.
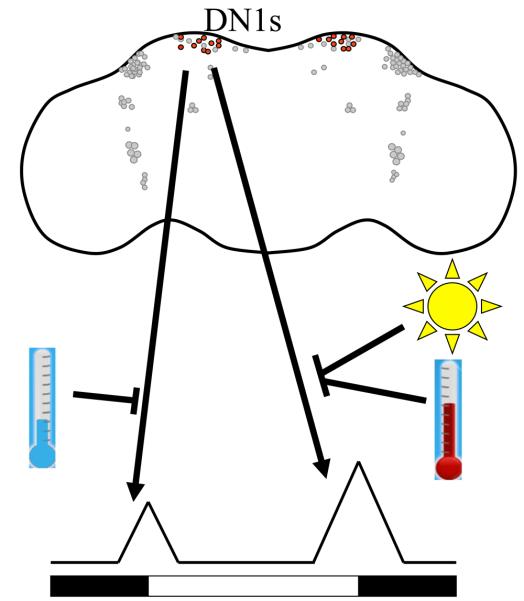
Figure 4. Model for the role of the DN1s in the control of circadian behavior, and their modulation.
The Clk4.1M-GAL4 positive DN1s (shown in red) have the potential of promoting both morning and evening activity. However, their contribution to circadian behavior is under tight environmental control. At high light intensity and high temperature, the DN1s or the neurons they target are unable to generate an evening peak. On the contrary, the morning peak is inhibited at low temperature. For simplicity, we represent in this model the effects of environmental input as direct repression on the DN1 output.
Interestingly, the evening peak of activity generated by the DN1s coherently responds positively to both low light intensities and reduced temperature - conditions found early and late during the day. Importantly, other evening activity-generating neurons seem unaffected by light and temperature, since the amplitude of the evening peak in wild-type flies and in mai179-GAL4 rescued flies only weakly respond to light intensities or temperature. This might be because the DN1s regulate a very specific type of behavior that requires locomotor activity. For example, exploratory behavior (for mate or for new sources of food) might need to be confined to the later part of the day, particularly in the summer, to avoid risks of dessication and predation. Feeding behavior - which is controlled by the circadian clock [30] - would usually happen at the surface or inside fruits and might thus not need to be as tightly regulated because the flies are in a relatively protected environment. Remarkably, the DN1-generated morning peak of activity responds completely differently to temperature than the evening peak. The morning peak is promoted under warmer temperature, and repressed when it is cooler. This suggests that flies might want to be active early during a warm morning before the day becomes too hot for them to be safely foraging, for example.
Recent results demonstrate that the E oscillator is synchronized with the LD cycle by both the intracellular circadian photoreceptor CRY and PDF signaling from the LNvs [31, 32]. The Clk4.1M-GAL4 positive DN1s are also probably synchronized by these two pathways, since most of them express CRY (Figure 1E-G and S1B) and are responsive to PDF signaling [33] (see also ref. [34, 35]). It has been proposed that PDF negative circadian neurons can drive sLNv output, because morning anticipation can be observed in the absence of a functional pacemaker in the PDF positive sLNvs [3] even though these cells are necessary and sufficient for morning anticipation [2, 3, 36, 37]. Interestingly, we rescued morning anticipation in per0 flies when PER expression was limited to the Clk4.1M-GAL4 positive DN1s. Moreover, the ventral projections of these circadian neurons are closely associated with the dorsal projections of the sLNvs (Figure S1A). Our results therefore suggest that the Clk4.1M-GAL4 positive DN1s are able to feedback on the sLNvs and modulate their output.
Highlights.
1) The circadian neurons called DN1s contribute to Drosophila's crepuscular behavior.
2) The DN1s can promote both morning (M) and evening (E) behavioral activity.
3) Whether the DN1s promote M or E activity depends on light intensity and temperature.
4) This photic and thermal regulation contributes to the plasticity of circadian behavior.
Supplementary Material
Acknowledgements
We would like to thank R. Allada for sharing data prior to publication. We are grateful to M. Rosbash, C. Helfrich-Förster, F. Rouyer and J. Price for antibodies and fly stocks, and S.H. Im and P. Taghert for advice on CRY immunostaining. We thank members of the Reppert, Weaver and Emery laboratories for helpful discussion. This work was supported by R01 NIH grants GM-079182 and GM-066777 to P.E. and NS-051280 to P.E.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 3.Stoleru D, Peng Y, Agusto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 4.Murad A, Emery-Le M, Emery P. A subset of dorsal neurons modulates circadian behavior and light responses in Drosophila. Neuron. 2007;53:689–701. doi: 10.1016/j.neuron.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoleru D, Nawathean P, Fernandez Mde L, Menet JS, Ceriani MF, Rosbash M. The Drosophila circadian network is a seasonal timer. Cell. 2007;129:207–219. doi: 10.1016/j.cell.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 6.Veleri S, Brandes C, Helfrich-Förster C, Hall JC, Stanewsky R. A self-sustaining, light-entrainable circadian oscillator in the Drosophila brain. Curr Biol. 2003;13:1758–1767. doi: 10.1016/j.cub.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 7.Hardin PE. The circadian timekeeping system of Drosophila. Curr. Biol. 2005;15:R714–722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Houl JH, Ng F, Taylor P, Hardin PE. CLOCK expression identifies developing circadian oscillator neurons in the brains of Drosophila embryos. BMC Neurosci. 2008;9:119. doi: 10.1186/1471-2202-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaneko M, Hall JC. Neuroanatomy of cells expressing clock genes in Drosophila: transgenic manipulation of the period and timeless genes to mark the perikarya of circadian pacemaker neurons and their projections. J. Comp. Neurol. 2000;422:66–94. doi: 10.1002/(sici)1096-9861(20000619)422:1<66::aid-cne5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Helfrich-Förster C. Neurobiology of the fruit fly's circadian clock. Genes Brain Behav. 2005;4:65–76. doi: 10.1111/j.1601-183X.2004.00092.x. [DOI] [PubMed] [Google Scholar]
- 11.Helfrich-Förster C, Yoshii T, Wulbeck C, Grieshaber E, Rieger D, Bachleitner W, Cusamano P, Rouyer F. The lateral and dorsal neurons of Drosophila melanogaster: new insights about their morphology and function. Cold Spring Harb Symp Quant Biol. 2007;72:517–525. doi: 10.1101/sqb.2007.72.063. [DOI] [PubMed] [Google Scholar]
- 12.Yoshii T, Todo T, Wulbeck C, Stanewsky R, Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila's clock neurons. J Comp Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 13.Shafer OT, Helfrich-Förster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95:669–679. doi: 10.1016/s0092-8674(00)81637-2. [DOI] [PubMed] [Google Scholar]
- 15.Stanewsky R, Kaneko M, Emery P, Beretta M, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- 16.Emery P, Stanewsky R, Hall JC, Rosbash M. A unique circadian-rhythm photoreceptor. Nature. 2000;404:456–457. doi: 10.1038/35006558. [DOI] [PubMed] [Google Scholar]
- 17.Benito J, Houl JH, Roman GW, Hardin PE. The blue-light photoreceptor CRYPTOCHROME is expressed in a subset of circadian oscillator neurons in the Drosophila CNS. J Biol Rhythms. 2008;23:296–307. doi: 10.1177/0748730408318588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtin K, Huang ZJ, Rosbash M. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron. 1995;14:365–372. doi: 10.1016/0896-6273(95)90292-9. [DOI] [PubMed] [Google Scholar]
- 19.Shafer OT, Rosbash M, Truman JW. Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J. Neurosci. 2002;22:5946–5954. doi: 10.1523/JNEUROSCI.22-14-05946.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J. Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyasako Y, Umezaki Y, Tomioka K. Separate sets of cerebral clock neurons are responsible for light and temperature entrainment of Drosophila circadian locomotor rhythms. J Biol Rhythms. 2007;22:115–126. doi: 10.1177/0748730407299344. [DOI] [PubMed] [Google Scholar]
- 22.Kloss B, Price JL, Saez L, Blau J, Rothenfluh-Hilfiker A, Wesley CS, Young MW. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iε. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 23.Price JL, Blau J, Rothenfluh-Hilfiker A, Abodeely M, Kloss B, Young MW. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 24.Preuss F, Fan JY, Kalive M, Bao S, Schuenemann E, Bjes ES, Price JL. Drosophila doubletime mutations which either shorten or lengthen the period of circadian rhythms decrease the protein kinase activity of casein kinase I. Mol Cell Biol. 2004;24:886–898. doi: 10.1128/MCB.24.2.886-898.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busza A, Murad A, Emery P. Interactions between circadian neurons control temperature synchronization of Drosophila behavior. J Neurosci. 2007;27:10722–10733. doi: 10.1523/JNEUROSCI.2479-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshii T, Sakamoto M, Tomioka K. A temperature-dependent timing mechanism is involved in the circadian system that drives locomotor rhythms in the fruit fly Drosophila melanogaster. Zoolog. Sci. 2002;19:841–850. doi: 10.2108/zsj.19.841. [DOI] [PubMed] [Google Scholar]
- 27.Picot M, Cusumano P, Klarsfeld A, Ueda R, Rouyer F. Light activates output from evening neurons and inhibits output from morning neurons in the Drosophila circadian clock. PLoS Biol. 2007;5:e315. doi: 10.1371/journal.pbio.0050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rieger D, Wulbeck C, Rouyer F, Helfrich-Förster C. Period gene expression in four neurons is sufficient for rhythmic activity of Drosophila melanogaster under dim light conditions. J Biol Rhythms. 2009;24:271–282. doi: 10.1177/0748730409338508. [DOI] [PubMed] [Google Scholar]
- 29.Johard HA, Yoishii T, Dircksen H, Cusumano P, Rouyer F, HelfrichFörster C, Nassel DR. Peptidergic clock neurons in Drosophila: ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J Comp Neurol. 2009;516:59–73. doi: 10.1002/cne.22099. [DOI] [PubMed] [Google Scholar]
- 30.Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cusumano P, Klarsfeld A, Chelot E, Picot M, Richier B, Rouyer F. PDF-modulated visual inputs and cryptochrome define diurnal behavior in Drosophila. Nat Neurosci. 2009;12:1431–1437. doi: 10.1038/nn.2429. [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Lear BC, Seluzicki A, Allada R. The CRYPTOCHROME photoreceptor gates PDF neuropeptide signaling to set circadian network hierarchy in Drosophila. Curr Biol. 2009;19:2050–2055. doi: 10.1016/j.cub.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Chung BY, Lear BC, Kilman VL, Liu Y, Mahesh G, Meissner RA, Hardin PE, Allada R. A subset of circadian neurons coordinates light and PDF signaling to produce robust daily behavior in Drosophila. Curr Biol. 2010 doi: 10.1016/j.cub.2010.02.056. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shafer OT, Kim DJ, Dunbar-Yaffe R, Nikolaev VO, Lohse MJ, Taghert PH. Widespread receptivity to neuropeptide PDF throughout the neuronal circadian clock network of Drosophila revealed by real-time cyclic AMP imaging. Neuron. 2008;58:223–237. doi: 10.1016/j.neuron.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi C, Fortin JP, McCarthy E, Oksman L, Kopin AS, Nitabach MN. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Curr Biol. 2009;19:1167–1175. doi: 10.1016/j.cub.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf Neuropeptide Gene Mutation and Ablation of PDF Neurons Each Cause Severe Abnormalities of Behavioral Circadian Rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 37.Shafer OT, Taghert PH. RNA-interference knockdown of Drosophila pigment dispersing factor in neuronal subsets: the anatomical basis of a neuropeptide's circadian functions. PLoS One. 2009;4:e8298. doi: 10.1371/journal.pone.0008298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.