Abstract
Neurons in higher cortical areas appear to become active during action observation, either by mirroring observed actions (termed mirror neurons) or by eliciting mental rehearsal of observed motor acts. We report the existence of neurons in primary motor cortex (MI) responding to viewed actions, an area generally considered to initiate and guide movement performance. Multielectrode recordings in monkeys performing or observing a well-learned step tracking task showed that approximately half of MI neurons, active when monkeys performed the task, were also active when they observed the action being performed by a human. These ‘view’ neurons were spatially intermingled with ‘do’ neurons, active only during movement performance. Simultaneously recorded, ‘view’ neurons comprised two groups: ∼38% retained the same preferred direction (PD) and timing during performance and viewing, while the remainder (62%) changed their PDs and time lag during viewing compared with performance. Nevertheless, population activity during viewing was sufficient to predict the direction and trajectory of viewed movements as action unfolded, although less accurately than during performance. ‘View’ neurons became less active and contained poorer representations of action when viewing only sub-components of the task. MI ‘view’ neurons thus appear to reflect the aspects of a learned movement when observed in others and form part of a broadly engaged set of cortical areas routinely responding to learned behaviors. These findings suggest that viewing a learned action elicits replay of aspects of MI activity needed to perform the observed action and could additionally reflect processing related to understanding, learning or mentally rehearsing action.
Keywords: action observation, decoding, MI neurons, primates, timing
Introduction
During motor skill learning, observation and practice presumably engage neural mechanisms to create internal motor representations, which then provide the ability to accurately reproduce those voluntary actions. Mirror neurons, which are active both when an action is performed and when viewing that same action performed by another, have been proposed as one possible basis of action knowledge acquisition (Grafton et al., 1997; Rizzolatti et al., 2001; Umilta et al., 2001; Rizzolatti & Craighero 2004; Buccino et al., 2004; Rizzolatti, 2005). Mirror neurons, according to the above definition, have been identified in ventral premotor (PMv) and inferior parietal cortex (Rizzolatti et al., 1996a; Fogassi et al., 2005) in monkeys. Indirect methods in humans suggest that mirror responses may occur in homologous areas in the human inferior frontal gyrus as well (Decety et al., 1997; Buccino et al., 2004; Iacoboni et al., 2005; Molnar-Szakacs et al., 2006; Aziz-Zadeh et al., 2006). Because the mirror neuron system responds to the viewing of natural actions and not abstractions such as static video images (Craighero et al., 2007), it has been linked to action recognition and understanding (Rizzolatti et al., 2001). Further, a class of veridical mirror neurons predicts hidden goals to join nonidentical observed and executed actions that serve a common goal (Gallese et al., 1996; Newman-Norlund et al., 2007). Veridical neurons are also influenced by task complexity (Iacoboni et al., 1999; Buccino et al., 2004) and motivation or effector orientation (Maeda et al., 2002; Cheng et al., 2007). These findings demonstrate that motor learning through observation leads to widespread activation of parietofrontal circuits.
A large body of data has shown that the primary motor cortex (MI) is directly engaged in action generation. MI neurons become active before a movement is performed and this activity correlates with performed actions. MI activity is necessary for the initiation and control of voluntary movement, and is presumably downstream of cortical areas containing mirror neurons that may eventually engage MI to enact movements. MI has not been generally held to be part of the mirror neuron system (Gallese et al., 1996; Iacoboni et al., 1999; Fogassi et al., 2001). However, a body of indirect and conflicting evidence from functional imaging, electroencephalography, magnetoencephalography, metabolic labeling, and transcranial stimulation suggests that MI may be engaged during action observation (Fadiga et al., 1995; Hari et al., 1998; Cochin et al., 1998; Nishitani & Hari, 2000; Baldissera et al., 2001; Montagna et al., 2005; Raos et al., 2004, 2007; Caetano et al., 2007). Conversely, PET scans have failed to find MI labeling in mirror tasks (Rizzolatti et al., 1996b; Decety et al., 1997). The fact that some single neuron studies had also failed to find MI mirror neurons (Gallese et al., 1996; Fogassi et al., 2001) led to a conclusion that indirect methods may be detecting field potential activity related to mirror input to MI and not spiking in response to action viewing (Hari et al., 1998). However, transcranial magnetic stimulation above the precentral gyrus produces a larger response in muscles that are used in a task when the subject views another doing that task (Fadiga et al., 1995), suggesting that M1 neurons are engaged during viewing.
Neurons engaged in dorsal premotor (PMd) (Cisek & Kalaska, 2004) and primary motor cortex (Wahnoun et al., 2006; Tkach et al., 2007) associated with action viewing have been observed, but this activity has been interpreted as mental rehearsal of a learned motor action and not processes related to action recognition/understanding that are the hallmark of mirror activity. Cisek and Kalaska (2004) carefully outlined the evidence for neurons in PMd fitting best with mental rehearsal. In their study, neurons fired in anticipation of an abstraction of action instead of responding to it; both eye movements and licking suggested that the activity was elicited by an impending action that led to a reward. They concluded that the similar forms of single neuron discharge in these two conditions reflected the rehearsal of the motor activity that would generate the reward if performed by the monkey. Wahnoun et al. (2006) and Tkach et al. (2007) found similar neurons in primary motor cortex (MI), suggesting that neural activity observed in this area by viewing only cursor motion is also mental rehearsal of action. More recently it has been shown that MI neurons are engaged in humans with paralysis who are explicitly rehearsing actions in the absence of any movement (Hochberg et al., 2006; Truccolo et al., 2008). Whether these neurons would be engaged by viewing only was not tested and testing their activity during movement performance in these people was not possible. In these studies, activity was not tested during viewing a human producing the action, one feature of mirror neurons. Here we demonstrate in three monkeys the existence of MI ‘view’ neurons that were engaged during the performance of a visuomotor task and also when viewing a human agent performing the same task. Further, these ‘view’ neurons were spatially distributed amongst a set of simultaneously recorded neurons that were only active during movement. Based on differential timing, spatial distribution, and directional tuning features of these neurons while movement was performed or viewed we suggest that viewing an action elicits an internal or mental rehearsal in populations of MI neurons, but that this population also reflects properties of mirror neurons at the same time.
Materials and Methods
Recordings
Three female macaque monkeys weighing 4.5 - 6 kg (RN, CL, and LA) were studied in these experiments. Surgical multielectrode array implantation procedures and recording methods followed those previously reported (Paninski et al., 2004; Suner et al., 2005). Animals were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care, National Institutes of Health (AAALAC, NIH) approved animal care and use committee (IACUC) of Brown University. All surgeries were performed using standard sterile procedures in an approved animal surgical facility. Analgesics and antibiotics were administered postoperatively as needed, using established protocols and veterinary supervision and have been reported elsewhere (Suner et al., 2005). Briefly, the animal was sedated with ketamine HCl (15 mg/kg) before surgery, and received antibiotic (Claforan 50 mg/kg), steroid (dexamethasone 0.5 mg/kg) and analgesic (buprenorphine 0.01 mg/kg). The animal's head was shaved and placed in a stereotaxic headholder. During the surgical procedure, deep and stable anesthesia was maintained with 1%-2.5% isoflurane. A warm lactated Ringer's solution was administered at a rate of 5-10 cc/kg/h. The surgery was carried out under aseptic conditions, with continuous monitoring of the following parameters: heart rate, respiration rate, expired CO2, arterial O2 saturation and body temperature to maintain a stable and deep plane of anesthesia. After surgery, the animal was observed until it was spontaneously moving and holding its head upright. Buprenorphine (0.01 mg/kg) for analgesia was administered intramuscularly 8-12 h after the procedure and was continued on subsequent days together with antibiotic therapy (Claforan 50 mg/kg), under direct supervision of a facility veterinarian who is highly experienced with non-human primate clinical care. After a suitable recovery period as specified by the veterinarian, neurons were recorded simultaneously from a 96-microelectrode array chronically implanted in the MI arm area. Electrodes were arranged in a 10× 10 array (4× 4 mm base), each spaced by 400 μm and 1 mm in length (Blackrock Microsystems, Salt Lake City, UT). Electrode impedance ranged between 100-750 kΩ, at 1 kHz. Signals were recorded during sessions lasting up to 3 h while the monkey either performed or viewed visually guided movement tasks. Waveforms were stored and spike sorted using Offline Sorter (Plexon, Dallas, TX). Principal component clusters, autocorrelation functions, inter-spike interval distributions, and signal-to-noise ratio were used offline to classify each recorded waveform as a neuron, using the same criteria across all tasks. Signal-to-noise ratio (S/N) was defined as the difference in mean peak-to-peak voltage divided by twice the mean standard deviation of waveforms at each of 50 sample time points over all acquired spikes and then averaging (Suner et al., 2005). All cells with S/N≥3 were included for analysis. This comprised more than one cell from some electrodes.
Electrode array location
Array insertion in the MI arm representation was guided by surface landmarks identified intraoperatively. The array was placed as far posteriorly as possible to be immediately anterior to the precentral gyrus and medial to a line reflected posteriorly from the genu of the arcuate sulcus at the level of the principal sulcus (Suner et al., 2005). This location reliably provides recordings of neurons related to arm actions.
Tasks
We evaluated the same neuron population during performance of a well-learned point to point arm reaching (do condition) and then during observation of that same task as it was performed by a human (view condition). During sessions, monkeys were seated in a primate chair with head fixed. Monkeys were trained to make step tracking arm movements that were instructed by visual targets displayed on a vertically oriented computer monitor placed ∼57 cm in front of the monkey. The monkey held a low friction/low inertia two-link manipulandum that allowed horizontal two dimensional arm motions across a planar surface (Fig. S1 A, B). Hand position was determined from a sensor in the manipulandum handle that moved across a 30-cm digitizing tablet (Wacom Technology, Vancouver, WA). Position was sampled at 167 Hz with an accuracy of 0.25 mm and recorded to disk. The hand position on the tablet was represented by a cursor of 0.6° (1.5 cm tablet radius) displayed on a monitor. The task was based on a standard center out format that required movement from a center start position, after a fixed hold, to one of eight targets placed equidistant in a circle (Fig. S1D). On a particular trial a randomly selected target was displayed, which also served as a go cue. The monkey was required to begin movement to the target within 200 ms, and after 300 ms (CL) or 500 ms (RN, LA) target hold, the monkey returned to the start position (Fig. S1 A, B). The short duration (<500 ms) hold period was used to reduce movement preparation and to circumvent movement of the eyes to the goal before the hand. Following four to six movements (randomly determined) to a set of targets, a juice reward was delivered. If a target was not acquired, the trial aborted and a target reappeared at a new position to begin the next trial. For the do condition the monkey's hand motion controlled the cursor, while in view condition, the experimenter stood alongside the monkey to the side of the tablet, ipsilateral to the monkey's ‘moving’ arm and moved the manipulandum to perform the identical task. In one session for monkey CL, the experimenter's moving hand was contralateral to the monkey's ‘moving’ arm. All actions accomplished by the experimenter involved the same apparatus and recording room, immediately following or preceding the do task. In the view task the monkeys received rewards (1 per 4-6 trials) while watching a human perform the task. In one experiment, in addition to the standard view condition, the monkey observed parts of the view task either with the monitor occluded (view device task), or while the hand performing the task was hidden from view (view screen task).
Frame of reference for muscle activity
The monkey's arm was always positioned in abducted posture for the do task. It was adducted during the view task in two monkeys (CL, LA), while in one (RN) it remained in the abducted posture for both tasks as a control for postural effects between both conditions. In order to restrict arm motion during viewing, monkeys were required to hold one or two lever arm hand switches in the closed position using a sustained finger flexion motion. During the view task, CL held switches in both hands, LA closed a switch with the ‘moving’ hand, and RN simply rested the ‘moving’ hand. In the latter case, a barrier blocked the access to the manipulandum.
Task learning
Training and practice on the do condition task spanned more than 1 year (typically 5 sessions/week). During a 1-2 month period prior to data collection, each monkey was exposed to four view sessions and performed the task in that same session. Data reported here was taken after approximately one additional month of task exposure and data collection, during a single session for each monkey in which 111 (RN), 120 (CL), and 72 (LA) neurons were recorded simultaneously. Each single recording session (do and view tasks) contained 400 (CL, LA) and 900 (RN) trials.
Preferred Direction Analysis
To evaluate the similarity of neural activity in do and view tasks, we compared features of neural activity on a single cell and population basis. We analyzed only those trials that fell within one standard deviation of mean movement time. We first identified all neurons that showed a significant change in firing rate associated with cued movement and then identified all neurons of this set that were directionally tuned. We measured differences in firing rate during movement and similarity of preferred direction (PD) on a cell by cell basis. We also compared the relative timing of firing with respect to movement onset in the two conditions. Movement onset and end were defined using a hand speed threshold-crossing criterion of 0.2 cm/s (200-360 ms). The modulation of movement-related activity in each condition was determined by comparison of the firing rate around the time of real or observed movement (Kruskal-Wallis [KW] test, P < 0.05, mean firing rate comparison for intervals before and after movement onset). The firing rates between do and view conditions were compared using a nonparametric test (KW test, α < 0.05).
We determined the PD of each neuron based on a cosine fit to the peak firing rate during the movement or viewing period across the eight directions. We applied a nonparametric bootstrap test (1000 samples) to define the 95% confidence for the cosine fit model (Amirikian & Georgopolous, 2000), and to assess the statistical significance of cosine tuning (Cisek et al., 2003). To identify a significant change in PD across conditions we used a nonparametric bootstrap statistic (KW test, P < 0.001) to compare the PD in the view condition against a distribution derived from multiple recalculations of PD from subsets of trials in the do condition (Cisek & Kalaska, 2004). Shifts of more than ±25° around the mean PD were usually significantly different (Amirikian & Georgopolous, 2003). We defined cells tuned only in the do task as ‘do’ or movement-related cells, those directionally tuned in both the do and view tasks as ‘view’ neurons. The population of ‘view’ cells with PDs that were not significantly different in view and do conditions (bootstrap procedure, P > 0.001), were termed similar PD neurons (sPD). ‘View’ cells for which PDs changed between do and view conditions (P < 0.001) were called different PD neurons (dPD).
Analysis of firing rate timing changes
The use of simultaneous multielectrode recording allowed direct within-session comparison of each neuron's timing and activation with respect to others in the population during identical conditions of motivation, attention, intention and other general factors. Cross correlations between do and view conditions were performed separately for the reaction (RT) and movement (MT) intervals, allowing both the strength of similarity (correlation coefficient, cc) and timing relations (lag of the cc peak) to be evaluated. The RT interval began at the go cue and ended when the center of the moving cursor left the go cue target border, while the MT was defined as the interval from when the hand speed exceeded 0.2 cm/sec to when it dropped below that speed at the goal location (see Data Analyses). These timing relationships were used to provide an index of task involvement, where selective lags suggest that a population is more dominated by sensory feedback while leads suggest a stronger central or predictive drive. Differences in the peak activation time during RT and MT behavioral epochs were used to identify shifts between do and view conditions. By these criteria, the RT and MT behavioral intervals did not overlap in time. Changes in the timing of firing were determined by the peak of the firing rate cross correlogram of each neuron between the do and view condition, computed for each interval. Timing differences were represented as a lead or lag in the peak time, calculated in 2 ms bins of the instantaneous firing rate during the MT and RT intervals respectively. A lead (positive time values) indicated that MI neurons fired earlier in the do condition than in the view condition, whereas a lag (negative time values) indicated that the cells fired earlier in the view condition. We evaluated timing separately for sPD and dPD neurons to test whether these groups had systematic and different timing shifts during viewing. For dPD cells, we performed correlations using their PDs. We compared the firing latency during viewing of human movements to the target closest to the new PD (i.e. the direction of maximum firing during viewing) with the firing latency during the monkey's movement to the PD target during the do condition.
Classifier procedure
Standard state classification methods were used to identify population direction information and to compare it during do and view conditions. A probabilistic Bayesian classifier (Shenoy et al., 2003) was applied to predict target location α (α= 1, 2 …8) using the mean firing rate fi(α) for each neuron with the number of spikes ni (i=1…N, for N neurons) during a time interval t in each movement direction. The conditional probability for the number of spikes n to reach direction α is P(α∣n)=C(t,n)P(α)(ΠN i=1fi(α)n)iexp(-tΣNi=1 fi(α)). Spike rate was assumed to have Poisson distribution. The spike distribution was normalized with factor C (t, n) so that the sum of the probabilities was equal to one. P(α) is the prior probability for each direction. We used the highest probability to identify the direction α from the multidimensional distributions of probabilities. Randomly selected subsets of trials were used for the different test data and each set was cross-validated for each target location. The chance level of the classifier performance is the minimal statistically significant movement prediction, defined from the 8 possible movement directions (12.5%). Classification methods were also used to compare the similarity of models do(do) (i.e. how well does the do condition model predict the do direction of movement) and view(view) of action present during view and do tasks. Thus, a classifier created from data obtained when the task is performed should classify trials from do or view periods equally well if the same population model operates in both conditions. We used a Bayesian classifier (BC) with cross-validation to compare direction information available in the population during do and view tasks by means of models do(view) and view(do). When the cells in the view period were used to classify the performed movement, this was a ‘do prediction based on view model’, termed do(view). Similarly, a classifier created from do activity predicting direction in the view task was called view(do). Although the term ‘direction’ was used here, it is important to note for this analysis that goal and movement direction were indistinguishable. Prediction of the direction of the instructed movement goal was made using i) all cells, and two sub-samples- ii) all sPD neurons and iii) all dPD neurons.
We also evaluated trajectory information available in individual cell firing using correlation methods. The ability to reconstruct movement trajectory from view related population activity was evaluated by calculating the cross-correlation between the firing rate of each neuron and the observed kinematic trajectory, as determined from cursor motion while a human performed the task in view of the monkey. This analysis was done only for those cells that retained the same PD (sPD neurons) in the do and view tasks. We calculated the single trial correlations as a Pearson correlation c[t] between the viewed movement trajectory X[i]={x[1], x[2], …, x[n-t]} and the rate histogram of each tuning cell y[i]={y[t+1], y[t+2], …, y[n]}. (i=1…n, for n sampling points in the MT interval). Bins were set equal to the A/D sampling interval (6 ms) of the reference variable coordinates of the trajectory Xt, Yt. Correlation values were calculated as the mean of all cross-correlation values for each direction-cell pairing.
Eye movements
We measured eye movements to determine their possible contribution to neural activity during the view task. Eye movements during task observation were recorded during one session in monkey CL, using an infrared eye-tracking system (ISCAN model-200) with a spatial resolution of 0.06° and a temporal resolution 240 Hz, with a sampling rate of 500 Hz (Cerebus, Cyberkinetics Neurotechnology Systems, Inc). We evaluated 51 directionally tuned neurons to test the hypothesis that the directional tuning of the neurons was a result of directionally tuned eye movement during the view task.
First, we examined eye movements during an approximate 500 ms time interval from the go cue to the time when the next target was reached by the hand motion. We selected trials having a short fixation on the target during the reaction interval and where the instantaneous eye movement speed (differentiation of the oculomotor signals) did not exceed 10 times the maximum of its amplitude, to avoid artifacts (blinking or closed eyes). The first 100 ms after the go cue was excluded to avoid potential confounds of perisaccadic activity (Cisek & Kalaska, 2002). In all other ways, the oculomotor behavior of the monkey was unconstrained (Fig. S1D). We calculated the correlation between the rate histogram of each cell y[i], (i=1,‥,n) and the reference variable x[i] as eye movement parameters: xte, yte coordinates of the trajectory; vxe, vye coordinates of the velocity; vme magnitude of the speed; and vτe tangential velocity of the eye movement.
Results
Behavioral performance
Task performance by the monkeys and the experimenter were similar. Monkeys completed ≥88% of trials correctly [98% (RN), 90% (CL), and 88% (LA)] and showed <50° deviation from a straight trajectory at any time during the movement. Human completed correct trials were also ≥90%. Peak speed during the trails was not significantly different for the monkey (9.97 ± 4.65 cm/s) and the experimenter (11.15 ± 8.76 cm/s; Wilcoxon rank sum test, P = 0.06). Mean movement time for the do task was 266 ± 62 ms (pooled for three monkeys) and 285 ± 78 ms for human in the view task (not significantly different; Kolmogorov-Smirnov test, P = 0.91; Fig. S1C).
Neural activity patterns
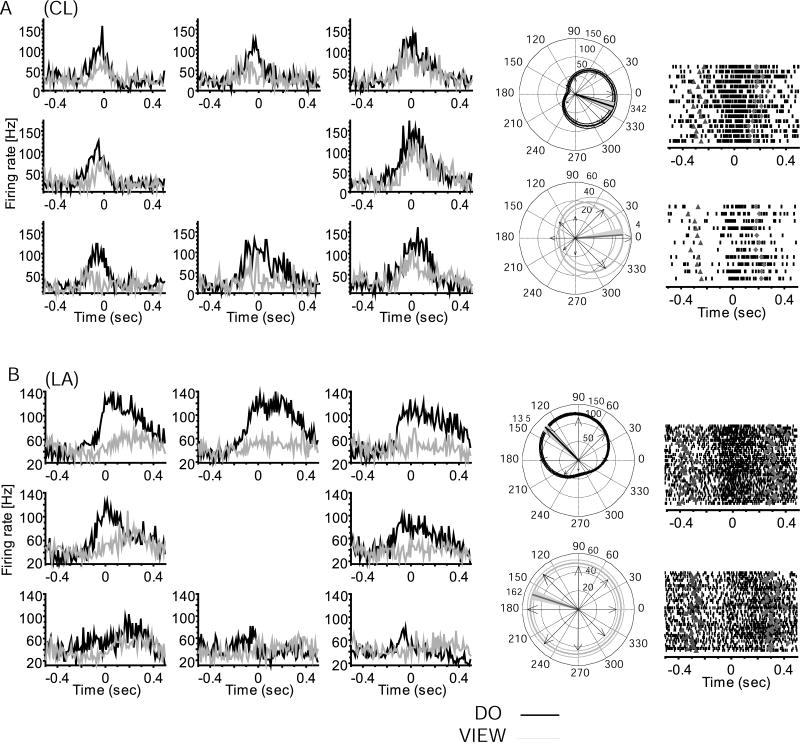
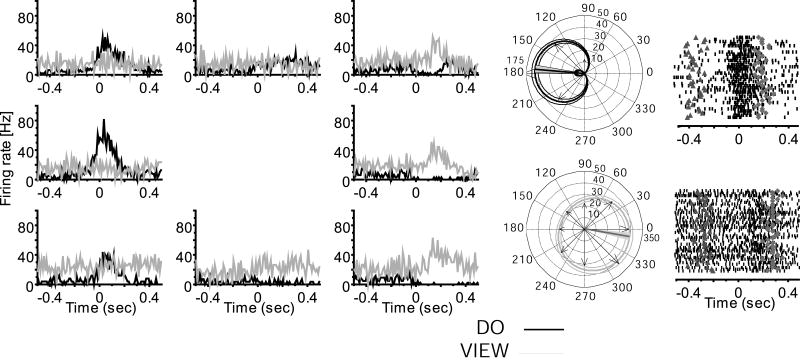
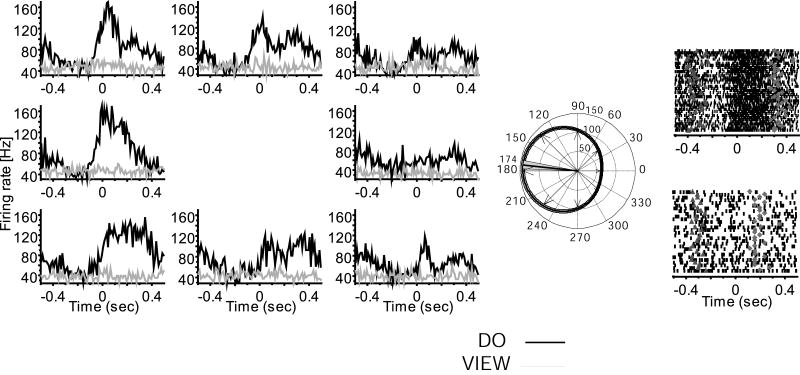
As typically encountered in MI, many neurons recorded across the chronically implanted multielectrode array were active in association with limb movement during the center out task. More than one unit was identified per electrode for 33% of the cells in RN, 28% in CL, and 17% in LA within a recording session (Fig. S2). Based on shape and waveform signal to noise ratio, the same neurons appeared to be recorded within a session across all tasks (Suner et al., 2005; Fig. S3). A subpopulation of movement related neurons remained active when viewing the task performed by a human (Figures 1, 3, 5). Of 303 neurons recorded in the three monkeys a total of 227 (mean 75%; 80 of 111 in RN, 76 of 120 in CL, 71 of 72 in LA) showed significant modulation around the time of movement (KW test, H = 3.7, P = 0.03; mean firing rate comparison for intervals before and after movement onset) and were directionally tuned when the monkey performed the center out task. Of this do task-engaged population, 54% (122/227; bootstrap, H > 9.7, P < 0.001) showed no significant modulation during the view task (KW test, H = 2.3, P = 0.06; Fig. 2). However, the remaining 46% (105/227; bootstrap, H > 11.5, P < 0.001) of the do task-engaged neurons also modulated and were directionally tuned during the view task. Neurons directionally tuned in both do and view tasks were defined as view cells (Figures 1, 3, S4). All view neurons changed firing rates and retained directional tuning in association with viewed action, although firing rates were significantly lower when only viewing, as can be appreciated both in trial rasters and histograms shown in Figure 1. Mean firing rate during viewing across the population was significantly decreased to about half (46%; KW test, H = 4.2, P = 0.02) of that found in the do condition. Preferred direction changes and timing differences between conditions separated two apparent sub-classes of view neurons, as summarized in Table 1. A minority of view neurons maintained their preferred direction (same preferred direction, or sPD cells) between do and view, while most shifted their PDs (different preferred direction, or dPD cells). Overall, 38% (40/105) of view cells retained a similar PD (sPD, Fig. 1) in both conditions (bootstrap, H < 1.6, P > 0.2) and 62% of view neurons (65/105; bootstrap, H > 15.3, P < 0.001) had different PDs (dPD) in the view and do conditions (Figures 3, S4). The locations of these two populations were mostly non-overlapping; the incidence of both types of cells detected at one and the same location comprised only 7% (7/105) of view cells. A previous study using the same 100-multielectrode array and a similar task showed that the somewhat randomly sampled population of MI arm area neurons had a roughly uniform distribution of PDs (Maynard et al., 1999). In agreement with that study, we found that the sPD population had a uniform distribution of PDs in both do and view tasks (Table 1). However, the PDs were not uniformly distributed in either task for the dPD population (circular test, P < 0.01; Fisher, 1993). In addition, dPD neurons showed a significant shift in their preferred direction (Kuiper test, P < 0.01; Fisher, 1993), in which PDs on average flipped approximately to the opposite direction (mean shift 187 ± 84 degrees) between do and view conditions (Fig. S4B). Because arm postural shifts from shoulder abduction to adduction made between do and view tasks in two monkeys, rather than viewing alone, might have generated PD rotations seen in dPD cells (Fig. 3), we compared PD shifts for one monkey in which the arm maintained the same posture in both do and view conditions. The amount and direction of PD shift showed a similar distribution of direction change whether or not a postural shift was made (Fig. S5), suggesting that the selective rotation of preferred direction of dPD cells during viewing was not the result of shoulder angle changes.
Figure 1.
Comparison of neural activity during performance and viewed action. Neurons in A, B show view task-related firing and directional tuning during action observation PD(view)=4°±8° (bootstrap, p<0.001), PD(view)=162°±6° (p<0.001). Left, perievent histograms of average firing across all trials aligned on the start of movement (time 0) showing do (black) and view (gray) task-related activity for example MI neurons in monkey A) CL, and B) LA. Histograms are placed at the respective target locations. Do and view task related firing for each of 8 directions, where a rightward movement is towards 0° (middle-right histogram), and an upward movement is towards 90° (top-center histogram). Center: circular plots showing directional tuning in do (black, above) and view (gray, below). Arrows plot observed peak firing for each direction. The firing rate scale is reported near the 90° line; the circular plot shows the best fit cosine function with (95% confidence limit) for the best fit model; the thick straight line marks the PD and the gray shadow indicates the 95% confidence interval. For (A) PD(do)=342°±7° (bootstrap, p<0.001), (B) PD(do)=135°±5° (p<0.001). Right, rasters showing firing rate for trials in each neuron's PD(do) (up) and PD(view) (down), aligned on the start of movement (0); the earlier triangles mark go cue; diamonds mark the end of movement. Note that firing in view task is reduced and more variable than in do task, but these neurons retain tuning and movement relationship across the two conditions.
Figure 3.
Example of a view neuron with changed preferred direction between do and view conditions (same format as Fig. 1, monkey LA).
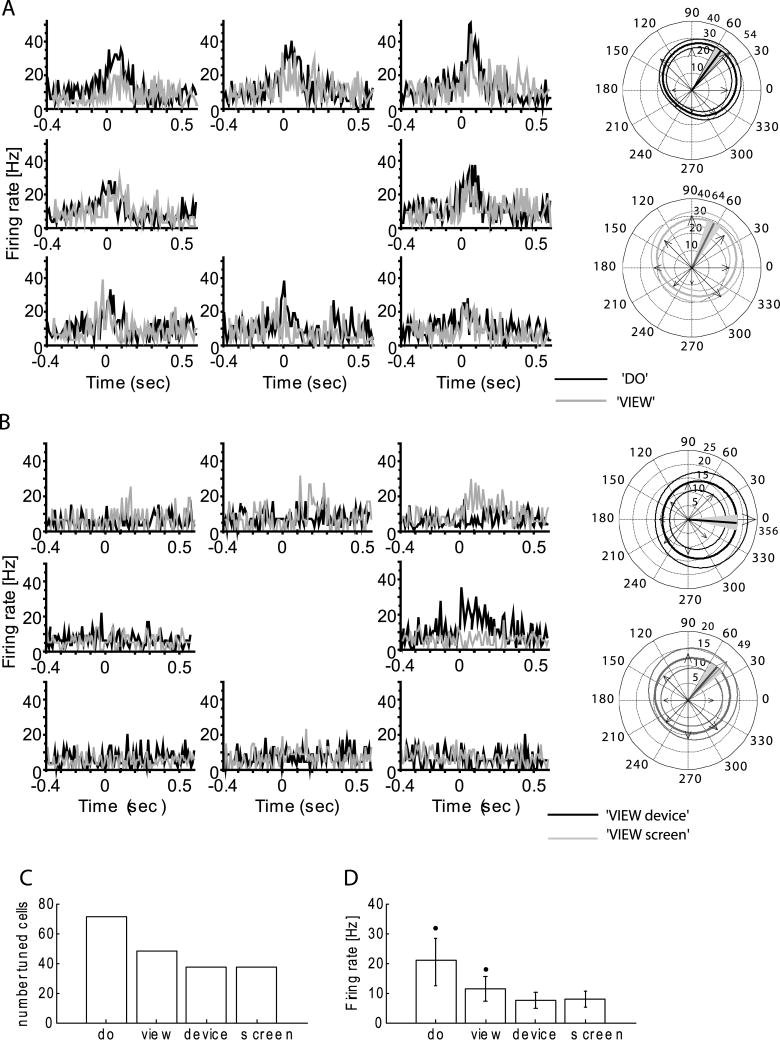
Figure 5.
MI activity for full and partial task conditions: A, B) histograms for a single neuron during (A) do task (black) compared to full view task (gray) (B) viewing only hand actions termed view device (black), or viewing only the screen, termed view screen (gray). View activity is markedly reduced and highly variable when only subcomponents of the task are present. The circular plots are the same format as Fig. 1. PD for each condition, marked with thick line: (A) do, sPD(do)=54°±7°; full view, sPD(view)=64°±8° (B); view device, PD(view device)=356°±9°; view screen PD(view screen)=49°±9°; (C) Number of tuned cells; (D) mean firing rate in full and partial task conditions1.
1 * =a significant difference in the firing rate during ‘do’ from that during the other three conditions, as well as view from do, view screen, view device (KW test, p<0.05).
Figure 2.
Example of a MI neuron only active during movement (same format as Fig. 1): Note that this neuron is not modulated in the view condition (monkey LA).
Table 1.
Comparison of view neurons that retain (sPD) and change (dPD) their preferred direction.
| sPD | dPD | |
|---|---|---|
| % of view population | 38% | 62% |
| (number of units) | (40/105) | (65/105) |
| PD distribution (do, view) | uniform | not uniform |
| CC (RT) | 0.77 ± 0.01 | 0.69 ± 0.02* |
| CC (MT) | 0.72 ± 0.02 | 0.66 ± 0.01* |
| L (RT) | -16 ± 8.4 | -10.3 ± 7.2 |
| L—(MT) | -4.8 ± 6.2 | +2.5 ± 3.7** |
Note: PD distribution for each condition separately; CC – correlation of view firing rate with those in do task, L (ms) – leads/lags during view with respect to do task; RT – reaction time; MT – movement time;
p<0.05 dPD compared to sPD;
p<0.05 Lag in reaction compared to movement period for dPD neurons.
The regular 10× 10 arrangement of electrodes in the recording array made it possible to evaluate whether there was an underlying spatial organization of view neurons. The maps of the array location and distribution of cell features show that in all three monkeys there was no specific grouping of view neurons across this 4× 4 mm patch (Fig S4A). This demonstrates that neurons active with viewing and action were intermingled with action-selective neurons, at least within this part of the MI arm area.
Timing and Firing Pattern Relationships
Firing rates of view neurons in the do task were significantly correlated with that present during view trials, although these correlations were lower for those cells that changed their preferred direction (Table 1, Fig. S6A). View neurons also had similar firing patterns in the do and view tasks, although those neurons that retained their preferred direction were significantly better correlated with direction than those that changed their PD, during both the movement period (KW test, H = 6.2, P = 0.005) and the go cue interval (KW test, H = 3.7, P = 0.04).
Both classes of view neurons (sPD and dPD) showed a range of changes in peak firing time between conditions (Table 1, Fig. S6 B) but as a population, the time of peak discharge with respect to the go cue or to movement onset was comparable whether the monkey was performing or viewing the action, suggesting that view related activity was predicting upcoming action. The onset of activity for the population of sPD neurons during viewed actions was not different from that during performed movement across the three monkeys. During the view task sPD neurons became active (16.3 ± 8.4 ms, mean ± SE) earlier in the RT interval and the MT interval (4.8 ± 6.2 ms) as compared with the do task, but neither shift was significant (KW test, H = 1.3, P = 0.3). Further, dPD neurons reached their peak firing rate slightly, but not significantly, later (2.5 ± 3.7 ms) during the MT interval, and earlier (-10.3 ± 7.2 ms) during the RT interval. Although timing shifts for behavioral intervals RT and MT were not different across do and view conditions within a class, sPD and dPD populations showed different timing shifts in the RT interval as compared with the MT interval (Kolmogorov-Smirnov test, P < 0.001; Fig. S6 C). A test of the effect of trial interval on sPD and dPD cell activity indicated that there was a significant difference in timing shift pattern between RT and MT period for dPD (KW test, H = 4.8, P = 0.008; Table 1; Fig. S6 B), but not for sPD cells (H = 2.5, P = 0.06). This greater spread in activation time only for dPD neurons, suggests that those neurons that shifted their directional tuning also had changes in the firing pattern elicited by viewing, compared to their pattern during movement. The temporal correlation of firing with viewed movement further supports a close relationship between movement and view activity.
Direction information in view active neurons
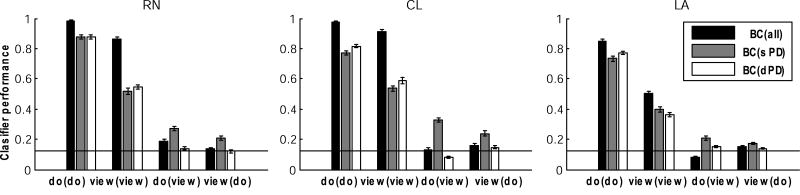
Classification methods were used here to test how well the performed or viewed movement direction could be predicted from the population activity on individual trials (see Methods). Goal direction for movements was correctly predicted in 97%, 98% and 85% of trials when movement was actually performed (RN, CL, LA, Fig. 4, BC(all)do(do) - do model, tested on do condition), consistent with previous studies (e.g., Maynard et al., 1999). Using a classifier model restricted only to sPD cell activity in the do condition, classifier success was moderately lower (BC(sPD)do(do) = 88%, 77%, 74%; Fig. 5) than when all cells were used, perhaps because of the smaller data set. However, the direction classification for this sub-population BC(sPD)do(do) was significantly higher (KW test, H = 15.8, P = 0.0001) than for the dPD cells (BC(dPD)do(do)), except for monkey RN, where classification success was not different (H = 0.84, P = 0.5). These results demonstrate that the recorded population contained information about direction, or a correlate of direction, when the movement was performed.
Figure 4.
Prediction of movement direction from MI activity in view and do conditions. Each bar shows the predicted direction of movement based on the entire population (all, black), those that retain the same preferred direction (sPD; gray), and those that change their (dPD white) in view and do tasks for each of the three monkey (RN, CL, LA). The Bayesian classifier (BC) has relative values in the range [0, 1], where 1 = perfect classification. Groupings show the results of different classifier models. The do(do) model predicts movement from a classifier built from data in the do task using a new do task trial; the view(view) model predicts viewed direction from view related activity; do(view) predicts movement in do task from view related activity; view(do) predicts viewed direction using a model created from the do task activity. Horizontal line=chance level (12.5%).
The ability to predict observed direction was next evaluated using activity present during viewing (termed a ‘view (view)’ comparison). When the view task model was applied to view trials classification success was reduced, but remained well above the 12.5% chance level in all three monkeys (BC(all)view(view) = 85%, 91% and 50% for RN, CL, LA; Fig. 4). Classification using the entire population was significantly higher (KW test, H = 10.3, P < 0.0005) than achieved by either sPD or dPD sub-populations in each monkey. When only those cells that retained the same PD across view conditions (sPD cells) were considered, classification success was also reduced, but remained above chance (BC(sPD)view(view) = 52%, 54%, 40%). Direction classification for dPD cells exceeded that for sPD in monkeys RN and CL, but not for monkey LA (view(view); KW test, H = 11.9, P < 0.0003). Thus, these results demonstrate that activity patterns during viewing contained substantial information about viewed actions, although less than predicted by activity of neurons in the same region when the monkeys performed these actions.
Classification methods were next used to evaluate whether activity during viewing was the same as that during performance. If the same model operated in both conditions, a classifier created from data obtained when the task was performed should classify trials from do or view periods equally well. This hypothesis was not supported. In general, classification success when predicting the direction of performed movement based on activity during viewed actions, termed do(view), was at chance levels either when all cells in the view period were used to classify the performed movement or when only dPD cells were used in the classifier (Fig. 4, BC(all)do(view), BC(dPD)do(view)). This suggests that MI was operating differently during viewing and performing the task. View neurons preserved some features of movement during viewing but changed others, suggesting that these neurons were operating in a different mode in each of the two conditions. However, a classifier model from view cell activity incorporating only sPD cells was about twice chance in predicting direction (BC(sPD)do(view) = 27%, 33%, 21%), suggesting that this sub-populations of neurons retained a similar model in viewing and performing the task. The classifier BC(sPD), created from do task activity, predicted direction in the view task about 1.7 × chance (BC(sPD)view(do) = 21%, 24%, 17%; KW test, H = 9.2, P < 0.0009). Although the models built and tested on the same task were successful, these results indicated that MI view sPD neurons transformed viewed action into a partial realization of the activity necessary to produce that movement, because the sPD models varied with that whether the goals or the movements played a role in the information preprocessing during viewing and task performance.
In this task, activity related to goal or direction was not specifically separated. To attempt to disambiguate direction and goal related activity, we computed the correlation of firing rate to trajectory for 15 sPD cells in monkey CL. Firing rates of view neurons were significantly correlated with observed arm and cursor trajectory during viewing cm= 0.38 ± 0.17 (mean ± SD), with single cell correlations ranging from 0.8 to 0.03 across the cells. This suggests that view related activity carried information related to the details of the viewed movement trajectory and not just its goal.
Task Contributions
Contribution of view task components
During the view condition the monkey could observe at once the task evolving on the screen, which reveals a goal and an abstraction of the arm's action (i.e. cursor motion), its own non-moving arm holding a stable position, and the experimenter's moving hand and manipulandum. In monkey CL, (Fig. 5) we evaluated how screen and hand components of the task separately influenced view neuron activity. We compared the activity of cells during (a) full view of do or view tasks, (b) separate observation of a human performing the task with the monitor occluded (view device), and (c) with the hand and manipulandum hidden, and only the monitor visible (view screen). Compared to the full view condition, MI neurons showed significantly less firing, but retained weak directional tuning when viewing only task components (view device and view screen, Fig. 5). In this test, 40% of recorded neurons (48/120) were directionally tuned when observing the entire task (Fig. 5A, C). The number was reduced when only part of the task was evident (31%, 37/120 for view device and the same number for view screen, Fig. 5C). In addition, overall mean population firing rate was about 1/3 lower when only components of the task were present (7.8 ± 5.2 Hz for view device, and 7.6 ± 5.2 Hz for view screen) compared to the full view (11.4 ± 9.1 Hz; KW test, H = 5.1, P = 0.006; Fig. 5D). Firing rates in the view device and view screen conditions were not significantly different (KW test, H = 2.4, P = 0.06). These data indicate that viewing any component of the task continued to activate some MI neurons weakly, but that viewing the entire task produced significantly greater activation in a larger population of cells.
Contribution of other behavioral variables
The task used here involved other associated behaviors that might influence neuronal activity, including gaze shifts, reward contingencies, and limb actions. Although not described for the MI arm region, the ability for the eyes to move freely would allow gaze shifts to contribute to view related activity (Cisek & Kalaska, 2002). To test this hypothesis, we compared eye position, recorded with an infrared eye tracker, and firing during the view task in monkey CL. While viewing the task the monkey's gaze was directed variously at the screen, the moving hand, or other locations (Fig. S1D). The mean correlation cm for all cells between the eye movement trajectory and rate histograms of the tuned cells was weak and not significant (mean ± s.d.: xte=0.015±0.0086; yte=0.0154±0.059). Likewise, correlation with eye movement velocity (x and y), eye speed, and tangential velocity was not significant. The highest cm for any single cell did not exceed 0.018. From these data, we concluded that eye movements in this task were not directly correlated with neural activity (Fig. S1D, see Methods), and could not account for the view task-related modulation in the MI arm area.
We next addressed whether view activation was sensitive to the laterality of the viewed arm. View neurons were found in both hemispheres contralateral to the arm used in the do task, which was the right hemisphere in one monkey (CL, 31% of all PD neurons were view neurons, 37/120 based on one recording session) and the left hemisphere in the other two (RN, 32%, 35/111; LA, 46%, 33/71), where the monkey viewed the experimenter's arm performing the task alongside the same arm as used by the monkey in do condition. In one monkey (CL), view activity existed whether the experimenter's moving hand was located contralateral (40%, 48/120) or ipsilateral (31%, 37/120) to the arm used by the monkey to perform the task. Despite the limited sample here, this finding suggests that both hemispheres are engaged during action observation and that the precise view of the agent may not be essential to evoke this activity.
View related firing could also have been influenced by the expectation of rewards. Our task design made it possible to evaluate the influence of reward expectancy during view trials since reward was never delivered on the first three trials and was randomly delivered on subsequent 4th-6th trials. This design has increasing probability of reward across trials 4-6 (33, 66, and 100%). Firing rates during unrewarded trials (the first three trials 1-3) were significantly greater than during the rewarded trials 4, 5 or 6th (KW test, H = 4.9, P = 0.007), indicating that the view influence on cell activity diminished as reward expectancy increased. Thus, reward was not a correlate of increased firing.
Finally, uncontrolled hand motions during task viewing could account for view related activity. We controlled for covert hand movements by requiring that the monkey maintain a hand switch in a closed position using finger flexion with one (LA) or both hands (CL) during viewing, thus preventing both overt mimicry of the viewed action and the active engagement of the same limb in another action during viewing. Review of the hand video in RN, in which there was no required hold, showed virtually no hand motion during task trials and none of the rare movements were systematically related to viewed action. In this case, the monkey simply held a plastic plate that was part of the chair. Thus, it is unlikely that activity was the result of systematic hand motion during viewing.
Discussion
These experiments reveal that a substantial subpopulation of MI neurons is actively engaged both when performing a well-learned skilled action and when viewing a human perform that same action. Using simultaneous multielectrode recording methods, we demonstrate that view neurons are interspersed within a larger population of movement-related neurons that are only active when action is performed. Firing is more variable and of lower intensity during viewing, but is generally similar to activity observed during performance in that: view neurons (a) modulate around the time of movement, (b) retain directional tuning, and (c) contain information about movement trajectory. Thus, activity during viewing resembles that generally observed during performed actions. However, differences among view neurons suggest that there may be two subgroups, which we identified as those that retain (sPD) and those that change (dPD) their preferred directions across the two conditions. View related firing in MI for this well-learned task best represents action when the entire task, including the agent and the abstraction of the task, is viewed. These properties suggest that MI, an area closely linked to movement production, is also involved in movement rehearsal or a related prospective activity when observing action performed by another agent.
We found that a substantial number, nearly half (46%) of all directionally tuned MI neurons recorded were active during both viewing and movement. Previous studies found that ∼70-90% of PMd (Cisek & Kalaska, 2004) and 70% of MI neurons (Wahnoun et al., 2006; Tkach et al., 2007) were engaged during viewing the motion of cursors that were an abstraction of a learned movement, without the monkey seeing either its own arm or the agent performing the viewed task. Thus, it appears that a smaller percentage of directionally tuned neurons may be engaged when viewing an agent and an abstract representation of that task, compared to viewing the abstraction of a task alone. The lower percentage of tuned engaged neurons is not likely to be related to sampling biases because similar non-selective array methods were used by Tkach et al. (2007) and Wahnoun et al. (2006). Task, training, or reward contingencies might also contribute to variability in these responses. The body of studies so far shows that many MI neurons are actively spiking when viewing learned actions, which helps to explain earlier stimulation and imaging studies that suggested MI activation during various forms of observation using indirect methods (Hari et al., 1998; Cochin et al., 1998; Nishitani & Hari, 2000; Baldissera et al., 2001; Montagna et al., 2005; Raos et al., 2004, 2007; Caetano et al., 2007).
Our results suggest that dPD and sPD groups may form two classes of view neurons in MI not previously recognized. Compared to their activity during movement, dPD and sPD neurons differed in their tendency to shift preferred direction and in their pattern of shift in peak firing time between do and view conditions. We found that 38% of MI view neurons retained the same directional tuning present during movement (sPD cells), but a majority (62%) showed marked tuning shifts (dPD). In our experiments, when monkeys viewed the task, dPD neurons as a population showed significant change in their PD. These shifts occurred while other neurons in the same, simultaneously recorded population did not change. This selective effect on a subset of neurons rules out the possibility of a simple underlying global mechanism and suggests one that is more selective with respect to these two conditions. The PD shift was not due to a postural change because both types of neurons were also observed when the posture was held constant across view and do conditions. Timing shifts also differentiated d/sPD groups. Neurons that shifted their PD peaked later with respect to movement onset, compared to neurons that retained same PDs (Table 1) across do and view conditions. Interestingly, dPD neurons did not have a uniform distribution of their PDs during either performance or viewing, while sPD neurons retained a regular distribution of PDs, even within our small sample of neurons. This collection of distinguishing features suggests that part of the view selective population more closely mimics the learned action, because they retain the same properties across conditions, while another set is engaged in a different manner during viewing. However, the mechanism that leads to this segregation is not clear.
The sPD/dPD subpopulation hypothesis is further supported by the superior direction decoding of the monkey's performed movement using view period activity of the sPD neurons compared to the dPD cells. That is, sPD activity while viewing appeared to be a closer match to the actual activity produced when movement is performed. The dPD neurons in our data compare with the set of cells described by Wahnoun et al. (2006) that appeared to have different tuning in viewed and performed movements. By contrast, Tkach et al. (2007) found only a small subset of MI neurons that shifted their PD between acting and viewing. These investigators encountered a greater percentage of view neurons than in our study and had a larger sample than Wahnoun et al. (2006) suggesting that they likely did not miss view cells. We cannot readily attribute these differences to the task, since Wahnoun et al. (2006) and Tkach et al. (2007) both showed only an abstraction of action, while in our experiment monkeys viewed both the agent, a physical device that created abstract cursor motion, as well as a visual representation of that task on a monitor. Eye movements are not likely to have accounted for the differences between do and view conditions because our monkeys looked freely at various aspects of the task. By contrast, in Cisek et al. (2004) and Tkach et al. (2007), monkeys appeared to track the target cursor with eye movements, perhaps because other distractors, such as the experimenter, manipulandum, and the screen, were not viewed in these other studies.
Finally, our monkeys were actively engaged in a secondary task, either voluntarily gripping of a plastic plate in one case, or mandatory holding of a switch closed with the hand that would ordinarily have produced the observed movement. Monkeys were restrained in the other studies, a condition which may not preclude movements in the same way as in our task. In sum, these differences in attention, motivation, performance, visual tracking and the complexity of scenes with regard to the agents and abstractions of the task across studies suggest that a range of variables might influence the way the motor cortex is engaged by viewing. These factors may also account for the much more pronounced trial to trial firing variability evident during viewing, as can be seen by inspection of spiking rasters shown for this and other studies. None of the experiments to date can adequately rule out any of this assortment of features as potential sources of variance.
View neurons are active both when a movement is performed and when that same action is observed, one hallmark of mirror neurons that have been identified other cortical areas (Rizzolatti & Craighero, 2004). Neurons engaged in the premotor cortex when viewing action appear to form subcategories of neurons: those in PMv responding to viewing actions, labeled as mirror neurons (Rizzolatti & Craighero, 2004), and others found in PMd that are related to the rehearsal of motor actions (Cisek & Kalaska, 2004). MI view neurons appear to have features resembling both mental rehearsal and mirror neurons and therefore cannot be easily categorized into either group of neurons.
Mirror and rehearsal ‘classes’ have been distinguished by their timing with respect to viewed action, how they respond to natural actions or abstractions, and contextual and reward sensitivity (Rizzolatti & Craighero, 2004; Cisek & Kalaska, 2004). Classically defined mirror neurons have features suggesting that they link cognitive aspects of transforming sensation to action (di Pellegrino et al., 1992; Gallese et al., 1996; Fadiga et al., 2000; Rizzolatti et al., 2001; Craighero et al., 2007). Mirror neurons labeled as ‘congruent’ respond in the same way to action observation and execution when the movement and the observed action coincide in terms of goal and how the goal is achieved. They reflect cognitive aspects (e.g. goal, strategy) of the observed action. The subset of ‘broadly congruent’ mirror neurons appears to further generalize the goal of the observed action across many instances of the goal (Rizzolatti & Craighero, 2004). Similar to mirror neurons, mental rehearsal neurons exhibit activity during action performance and observation, but they become active earlier, appearing to reflect a prospective mental rehearsal of an upcoming learned action (Cisek & Kalaska, 2004). Mental rehearsal is considered a replay of the internal movement plan in which neurons reenact their movement activity as if the learned action itself was being performed, but in a weaker way (Cisek & Kalaska, 2004). Consistent with the rehearsal hypothesis, Tkach et al. (2007) showed MI neurons fire during an exact replay of learned cursor-tracking motion that the monkey had made earlier, much like our sPD neurons. The early activity of sPD view neurons with respect to movement in our task is consistent with a role in mental rehearsal. However, dPD cells appear to have a new PD during viewing, a trait not consistent with a simple prospective function in which motor action is replayed. The slight, but not significant tendency for dPD cells to shift to later times during the movement interval and their timing differences compared to sPD neurons could suggest that the dPD subset is has been modified by different processing systems, resembling the cognitive role attributed to mirror neurons. The evidence suggesting that these broadly tuned neurons both generalize the goal and show delayed activation (compared to simultaneoulsy recorded sPD neurons) could link this subset of MI view neurons to action comprehension. Such response patterns can be explained with reference to the monkey's learning history. Overtraining made it possible for the monkeys to be able to reliably predict the movement's trajectory and goal based on the ongoing motion of the cursor and the experimenter's arm (Catmur et al., 2008).
We found that view neurons became less active, more variable and contained poorer representations of action when viewing reduced versions of the task (e.g. viewing only the agent performing the task or an abstraction of the task). Tkach et al. (2007) also noted that neurons were engaged in MI when only an abstraction of action, e.g. the motion of a cursor alone, was viewed. They also found that the activity diminished when pieces of the task were removed. These results suggest that view activity could be evoking a rehearsal of the action that is dependent on viewing the entire scene of the task as learned in its original context and that degradation of the task introduces greater uncertainty about the consequences of the viewed action, as reflected in reduced and more variable activity. This property is unlike the greater reliability of responses that appears to be evident for broadly congruent mirror neurons (Umilta et al., 2001) and thus makes MI view neurons less like mirror neurons.
Mental rehearsal neurons are sensitive to whether a trial is rewarded, which is presumably a driving force to rehearse the action. By contrast, mirror neurons respond to natural actions and their associated cues (Kohler et al., 2002), with an interaction between a biological agent and some object, without the same reward sensitivity. In our experiments MI view neurons were influenced by reward expectancy, a property unlike mirror neurons but more like neurons engaged in mental rehearsal (Cisek & Kalaska, 2004). These investigators found that PMd neurons active during task viewing ceased firing as motivation and attention diminished, which was also noted in MI view neurons by Tkach et al. (2007). We identified a change in view activity related to reward expectancy in that cells fired less as reward became more likely. This is the opposite of what might be expected; these cells more closely follow their movement activity for actions that are not rewarded. However, in our task the monkey could be certain that the first three center-out actions in a block of six would not be rewarded (by task design), but they were part of an attention capturing sequence that would lead to an eventual reward. Alternatively, firing may have been related to the certainty of outcomes, which was high at the beginning of a block (no probability of reward) and then was variable on the last few trials. Thus while the apparent sign of change varied, the coupling of firing rate to reward expectancy further suggests that these neurons are more like rehearsal neurons than classically defined mirror neurons. In summary, neurons engaged by action observation share features of mirror and mental rehearsal neurons that is consistent with the fact that MI is a target of dorsal and ventral premotor areas, as well as parietal cortex, and therefore may reflect properties of each of these inputs.
The concept that MI view activity only reflects mental rehearsal is challenged by our observations using decoding methods. The decoding classifier created from the monkey's movement activity accurately predicted direction on single trials when the monkey performed the task. Similarly, view activity was also reasonably good at predicting direction of viewed action. Using the term ‘representation’ operationally, we can say that the activity during movement and viewing both contains a directional ‘representation’. However, when these classifiers were tested on their respective conditions (that is, view model on do trials or the converse) they performed poorly, indicating that the patterns of activity in the two conditions, though somewhat predictive, are not the same. A similar result was reported for MI by Wahnoun et al. (2006). Thus, this analysis directly tests the hypothesis that MI is simply unfolding the same learned motor pattern for mental rehearsal. The differences in these two classifier models suggest that dynamic changes occur in the functional organization of MI between performing and viewing, and that neurons during viewing are influenced in ways beyond those that are active during self-performance. Although only a speculation, this could be one way to attribute agency (i.e., who is the actor?) for viewed and self-performed movements.
Recently, it has been possible to examine activity in MI in humans with tetraplegia during viewing and attempted performance of cursor motion (Hochberg et al., 2006). Here, humans were explicitly asked to mentally rehearse movement while watching motion of cursor produced covertly by a human or by a computer and this activity was then used to control a cursor in behavioral tasks. Neurons during this rehearsal of an abstract action were directionally tuned and led the cursor motion, demonstrating that rehearsal engages MI neurons (Trucollo et al., 2008), although the effect of viewing alone was not examined. Information in this model was directly demonstrated by showing that direction of a target cursor could be predicted offline from this activity (Trucollo et al., 2008) and that the human could use this model to control a cursor in a center-out task (Hochberg et al., 2006). These results further support the conclusion that MI activity while viewing abstract action reflects mental rehearsal as well as knowledge of an abstract task.
In conclusion, view activity in MI during arm movement observation has many features indicative of mental rehearsal of known actions. However, this explanation does not fully explain timing, PD shifts, sensitivity to task changes, or ensemble tuning properties. It is possible that these features reflect influences from more classical mirror neurons in PMv and from other parts of the mirror system that project to MI (Matelli et al., 1986) as well as rehearsal of the viewed action (Cisek & Kalaska, 2004; Hochberg et al., 2006). Our results further confirm that MI is part of a large network of areas engaged in action processing whether or not movement is produced. These observations have practical value for human neural prosthesis applications because they point out differences in the nature of actual and viewed motor tasks and suggest that subgroups of MI neurons may respond to viewing in different ways.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health (grant NS25074). The authors thank Dr. J. Simeral for his assistance of data preprocessing, L. Reiss and A. Rydberg for technical support, B. Travers for animal assistance. Conflict of Interest: None declared.
Abbreviations
- MI
motor cortex
- PD
prefer direction
- sPD
similar prefer direction view neurons
- dPD
different prefer direction view neurons
- BC
probabilistic Bayesian classifier
Footnotes
Supporting Information: Additional Supporting Information may be found in the online version of this article.
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Amirikian B, Georgopoulos AP. Directional tuning profiles of motor cortical neurons. Neurosci Res. 2000;36:73–79. doi: 10.1016/s0168-0102(99)00112-1. [DOI] [PubMed] [Google Scholar]
- Amirikian B, Georgopoulos AP. Modular organization of directionally tuned cells in the motor cortex: is there a short-range order? Proc Natl Acad Sci USA. 2003;100:12474–12479. doi: 10.1073/pnas.2037719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M. Lateralization of the human mirror neuron system. J Neurosci. 2006;26:2964–2970. doi: 10.1523/JNEUROSCI.2921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Cavallari P, Craighero L, Fadiga L. Modulation of spinal excitability during observation of hand actions in humans. Eur J Neurosci. 2001;13:190–194. doi: 10.1046/j.0953-816x.2000.01368.x. [DOI] [PubMed] [Google Scholar]
- Buccino G, Vogt S, Ritzl A, Fink GR, Zilles K, Freund HJ, Rizzolatti G. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron. 2004;42:323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- Caetano G, Jousmäki V, Hari R. Actor's and observer's primary motor cortices stabilize similarly after seen or heard motor actions. Proc Natl Acad Sci USA. 2007;104:9058–9062. doi: 10.1073/pnas.0702453104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catmur C, Gillmeister H, Bird G, Liepelt R, Brass M, Heyes C. Through the looking glass: counter-mirror activation following incompatible sensorimotor learning. Eur J Neurosci. 2008;28:1208–1215. doi: 10.1111/j.1460-9568.2008.06419.x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Meltzoff AN, Decety J. Motivation modulates the activity of the human mirror-neuron system. Cereb Cortex. 2007;17:1979–1986. doi: 10.1093/cercor/bhl107. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Modest gaze-related discharge modulation in monkey dorsal premotor cortex during a reaching task performed with free fixation. J Neurophysiol. 2002;88:1064–1072. doi: 10.1152/jn.00995.2001. [DOI] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol. 2003;89:922–942. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- Cisek P, Kalaska JF. Neural correlates of mental rehearsal in dorsal premotor cortex. Nature. 2004;431:993–996. doi: 10.1038/nature03005. [DOI] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J. Perception of motion and qEEG activity in human adults. Electroencephalogr Clin Neurophysiol. 1998;107:287–295. doi: 10.1016/s0013-4694(98)00071-6. [DOI] [PubMed] [Google Scholar]
- Craighero L, Metta G, Sandini G, Fadiga L. The mirror-neurons system: data and models. Prog Brain Res. 2007;164:39–59. doi: 10.1016/S0079-6123(07)64003-5. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J, Costes N, Perani D, Jeannerod M, Procyk E, Grassi F, Fazio F. Brain activity during observation of actions. Influence of action content and subject's strategy. Brain. 1997;120:1763–1777. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 1995;73:2608–2611. doi: 10.1152/jn.1995.73.6.2608. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Visuomotor neurons: ambiguity of the discharge or ‘motor’ perception? Int J Psychophysiol. 2000;35:165–177. doi: 10.1016/s0167-8760(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Fisher NI. Statistical Analysis of Circular Data. Cambridge University Press; Cambridge: 1993. p. 277. [Google Scholar]
- Fogassi L, Gallese V, Buccino G, Craighero L, Fadiga L, Rizzolatti G. Cortical mechanism for the visual guidance of hand grasping movements in the monkey: A reversible inactivation study. Brain. 2001;124:571–586. doi: 10.1093/brain/124.3.571. [DOI] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308:662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Gallese V, Fadiga F, Fogassi L, Rizzolatti G. Action recognition in the premotor cortex. Brain. 1996;119:593–609. doi: 10.1093/brain/119.2.593. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Fadiga L, Arbib MA, Rizzolatti G. Premotor cortex activation during observation and naming of familiar tools. NeuroImage. 1997;6:231–236. doi: 10.1006/nimg.1997.0293. [DOI] [PubMed] [Google Scholar]
- Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc Natl Acad Sci USA. 1998;95:15061–15065. doi: 10.1073/pnas.95.25.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006;442:164–171. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–2528. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 2005;3:529–535. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda F, Kleiner-Fisman G, Pascual-Leone A. Motor facilitation while observing hand actions: specificity of the effect and role of observer's orientation. J Neurophysiol. 2002;87:1329–1335. doi: 10.1152/jn.00773.2000. [DOI] [PubMed] [Google Scholar]
- Matelli M, Camarda R, Glickstein M, Rizzolatti G. Afferent and efferent projections of the inferior area 6 in the macaque monkey. J Comp Neurol. 1986;251:281–298. doi: 10.1002/cne.902510302. [DOI] [PubMed] [Google Scholar]
- Maynard EM, Hatsopoulos NG, Ojakangas CL, Acuna BD, Sanes JN, Normann RA, Donoghue JP. Neuronal interactions improve cortical population coding of movement direction. J Neurosci. 1999;19:8083–8093. doi: 10.1523/JNEUROSCI.19-18-08083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Kaplan J, Greenfield PM, Iacoboni M. Observing complex action sequences: the role of the fronto-parietal mirror neuron system. NeuroImage. 2006;33:923–935. doi: 10.1016/j.neuroimage.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Montagna M, Cerri G, Borroni P, Baldissera F. Excitability changes in human corticospinal projections to muscles moving hand and fingers while viewing a reaching and grasping action. Eur J Neurosci. 2005;22:1513–1520. doi: 10.1111/j.1460-9568.2005.04336.x. [DOI] [PubMed] [Google Scholar]
- Newman-Norlund RD, van Schie HT, van Zuijlen AM, Bekkering H. The mirror neuron system is more active during complementary compared with imitative action. Nat Neurosci. 2007;10:817–818. doi: 10.1038/nn1911. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R. Temporal dynamics of cortical representation for action. Proc Natl Acad Sci USA. 2000;97:913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paninski L, Fellows MR, Hatsopoulos NG, Donoghue JP. Spatiotemporal tuning of motor cortical neurons for hand position and velocity. J Neurophysiol. 2004;91:515–532. doi: 10.1152/jn.00587.2002. [DOI] [PubMed] [Google Scholar]
- di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. Understanding motor events: a neurophysiological study. Exp Brain Res. 1992;91:176–180. doi: 10.1007/BF00230027. [DOI] [PubMed] [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE. Observation of action: grasping with the mind's hand. NeuroImage. 2004;23:193–201. doi: 10.1016/j.neuroimage.2004.04.024. [DOI] [PubMed] [Google Scholar]
- Raos V, Evangeliou MN, Savaki HE. Mental simulation of action in the service of action perception. J Neurosci. 2007;27:12675–12683. doi: 10.1523/JNEUROSCI.2988-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. Premotor cortex and the recognition of motor actions. Brain Res Cogn Brain Res. 1996a;3:131–141. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Matelli M, Bettinardi V, Paulesu E, Perani D, Fazio F. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp Brain Res. 1996b;111:246–252. doi: 10.1007/BF00227301. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G. The mirror neuron system and its function in humans. Anat Embryol (Berl) 2005;210:419–421. doi: 10.1007/s00429-005-0039-z. [DOI] [PubMed] [Google Scholar]
- Shenoy KV, Meeker D, Cao S, Kureshi SA, Pesaran B, Buneo CA, Batista AP, Mitra PP, Burdick JW, Andersen RA. Neural prosthetic control signals from plan activity. Neuroreport. 2003;14:591–596. doi: 10.1097/00001756-200303240-00013. [DOI] [PubMed] [Google Scholar]
- Suner S, Fellows MR, Vargas-Irwin C, Nakata GK, Donoghue JP. Reliability of signals from a chronically implanted, silicon-based electrode array in non-human primate primary motor cortex. IEEE Trans Neural Syst Rehabil Eng. 2005;13:524–541. doi: 10.1109/TNSRE.2005.857687. [DOI] [PubMed] [Google Scholar]
- Tkach D, Reimer J, Hatsopoulos NG. Congruent activity during action and action observation in motor cortex. J Neurosci. 2007;27:13241–13250. doi: 10.1523/JNEUROSCI.2895-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truccolo W, Friehs GM, Donoghue JP, Hochberg LR. Primary motor cortex tuning to intended movement kinematics in humans with tetraplegia. J Neurosci. 2008;28:1163–1178. doi: 10.1523/JNEUROSCI.4415-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umilta MA, Kohler E, Gallese V, Fogassi L, Fadiga L, Keysers C, Rizzolatti G. I know what you are doing. A neurophysiological study. Neuron. 2001;31:155–165. doi: 10.1016/s0896-6273(01)00337-3. [DOI] [PubMed] [Google Scholar]
- Wahnoun R, He J, Helms Tillery SI. Selection and parameterization of cortical neurons for neuroprosthetic control. J Neural Eng. 2006;3:162–171. doi: 10.1088/1741-2560/3/2/010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.