Abstract
Introduction
The presence of erectile or ejaculatory dysfunction may indicate physical problems; however, individual perceptions (e.g., sexual satisfaction) may reflect the degree of concern about these changes. Long-term data showing how changes in multiple sexual function domains track together may be useful in understanding the importance of physical declines vs. sexual satisfaction.
Aim
The aim of this study was to describe changes in sexual function among a population-based sample of aging men.
Methods
A population-based cohort study using data from the Olmsted County Study of Urinary Symptoms and Health Status among Men. Sexual function was assessed biennially from 1996 to 2004 using a previously validated questionnaire in a random sample of 2,213 men.
Main Outcome Measures
Changes in erectile function, libido, ejaculatory function, sexual problems, and sexual satisfaction.
Results
Overall, we observed declines in all of the sexual function domains, ranging from an annual decrease of 0.03 point per year for sexual satisfaction to an annual decrease of 0.23 point per year in erectile function. Moderate correlations were observed among all longitudinal changes in sexual function (range in age-adjusted rs = 0.14–0.43); however, significantly smaller correlations between changes in the functional domains and changes in sexual satisfaction and problem assessment were observed among older men (range in age-adjusted rs = 0.03–0.29).
Conclusion
Overall, these results demonstrate that longitudinal changes in five sexual function domains change together over time in our community-based cohort. Erectile function, ejaculatory function, and sexual drive decrease over time with greater rates of decline for older men. However, older men may be less likely to perceive these declines as a problem and be dissatisfied. These data may prove helpful to patients and clinicians in understanding and discussing changes in multiple aspects of sexual function.
Keywords: Sexual Function, Longitudinal Changes, Sexual Satisfaction
Introduction
Erectile dysfunction (ED) is a common problem associated with aging [1–3], and it is estimated that there will be 322 million men worldwide with ED by 2025 [4]. The focus of much of the research into male sexuality has been on ED, especially with the introduction of phosphodiesterase-5 (PDE-5) inhibitors [5–8]. However, sexual function encompasses multiple domains including erectile function, sexual drive, ejaculatory function, and overall concern about sexual function and sexual satisfaction. In order to obtain a more complete picture of sexual function, all of these domains must be examined [9,10].
Despite the interest in male sexual function, little is known about the natural history of male sexual function in the community [11]. Cross-sectional studies in community cohorts show a decrease in sexual function as age increases [10,12–14]. There are several possible reasons for this decline, including the development of lower urinary tract symptoms [15–19]; urogenital pain [20]; decreases in androgen levels [21,22]; and development of comorbid conditions, such as diabetes [23], cardiovascular disease [24,25], and obesity [26], as well as lifestyle factors, such as smoking [27]. These cross-sectional declines in sexual function suggest longitudinal declines as well; unfortunately, there are limited longitudinal data.
The earliest longitudinal studies (Duke longitudinal studies) [28,29] of sexual function in aging men implied cross-sectional age trends may be confounded by variation in cohorts [30]. The potential limitations of these initial longitudinal studies have previously been discussed [30]. Briefly, the potential limitations include: (i) the relevance of data to modern trends in sexual behavior; (ii) the restrictive sample size used in the data analysis; and (iii) lack of data on all the domains encompassed by sexual function [30]. More recently, the Massachusetts male aging study (MMAS) has observed an increased incidence of ED in aging men [2], longitudinal declines in sexual function [30], and a natural progression and remission of ED [31]. In this cohort, the overall risk of ED was calculated at 26 cases per 1,000 men annually, increasing with age, lower education, and comorbidities, including diabetes, heart disease, and hypertension. With regard to sexual function, their study found significant longitudinal declines in sexual intercourse, erection frequency, sexual desire, satisfaction with sex, and increasing difficulty with orgasm. Their study also documented the natural remission and progression of erectile function in a large number of men, which was associated with body mass index (BMI), as well as smoking and general health status. This information has contributed substantially to our continued understanding of sexual function, particularly ED; however, it has been limited to only two measurements per man. The Krimpin study [32] has examined predictors of ejaculatory dysfunction in a community-based Dutch population. They observed a cumulative incidence of ejaculatory dysfunction of 33.1% after 6.5 years of follow-up, which was associated with age, social impairment, and ED.
While these studies have observed declines in sexual function associated with aging, they have not assessed how changes in the sexual function domains track together over time. The presence of ED or ejaculatory dysfunction may indicate physical declines; however, individual perception (e.g., personal assessment of sexual satisfaction) may reflect the concern about these changes. Therefore, we took advantage of the substantial number of years of follow-up, with up to five measurements per man, to determine longitudinal changes in five sexual function domains and to assess the associations among changes in these measures over time using data from the cohort of men participating in the Olmsted County Study of Urinary Symptoms and Health Status among Men.
Methods
Study Population
Olmsted County Study of Urinary Symptoms and Health Status among Men is an ongoing cohort study of urological conditions in a community population of men. Details of the study have been previously published [33,34]. Briefly, a cohort of Caucasian men 40–79 years old was randomly selected from an enumeration of the 1990 Olmsted County, MN population [35]. Men who had a history of prostate or bladder surgery, urethral surgery or stricture, or medical or other neurological conditions that could affect normal urinary function were excluded. After excluding men with these preexisting conditions, 3,874 men were asked to join the study, and 2,115 (55%) agreed to participate and completed a previously validated questionnaire.
The cohort was actively followed on a biennial basis for 14 years using a protocol similar to the initial examination. During the second and third round of visits, men who did not participate in the follow-up were replaced by men randomly selected from the community after being screened for the exclusion criteria used at baseline (total cohort N = 332). Since that time, the study has been maintained as a closed cohort. This study received approval from the Mayo Clinic and Olmsted Medical Center Institutional Review Boards.
Sexual Function Questionnaire
The brief male sexual function inventory (BMSFI) was incorporated into the follow-up questionnaire in 1996 and biennially thereafter (Figure 1) [9,10]. This previously validated questionnaire consists of 11 items related to five sexual function domains: sexual drive (two questions), erectile function (three questions), ejaculatory function (two questions), sexual problem assessment (three questions), and overall sexual satisfaction (one question). All questions were scored on a scale from 0 to 4 with domain scores equaling the sum of the individual questions comprising the domain. The domain scores range from 0 to 4 for overall sexual satisfaction, 0–8 for sexual drive and ejaculatory function, and 0–12 for erectile function and sexual problem assessment. For categorical analysis, the following cut-points were used to define sexual dysfunction: low libido if the sexual drive domain ≤2, ED if the erectile function domain ≤3, ejaculatory dysfunction if the ejaculatory domain ≤2, perceived sexual problems if the problem assessment domain ≤3, and low sexual satisfaction if the sexual satisfaction domain ≤1 [20,23].
Figure 1.
Questionnaire.
Other Patient Characteristics
Additional baseline characteristics were also assessed, including demographics, presence of comorbidities, and the availability of a regular sexual partner. Demographic measures assessed in the baseline questionnaire included education level and employment status. Medical comorbidities included self-report of diabetes, hypertension, and smoking status, and electronic ascertainment of coronary heart disease from the Mayo Clinic Medical Index [36]. Information on the use of PDE-5 inhibitors, medical and surgical BPH treatments, and prostate cancer diagnoses was obtained through self-report and through passive follow-up of the community medical records.
Statistical Analyses
To assess the natural history of sexual function, observations after treatment for ED or BPH, and diagnosis of prostate cancer were censored. Incidence rates for each sexual function domain were estimated by dividing the number of events by person–time of follow-up [37]. Follow-up was from the date of the baseline questionnaire assessing sexual function to the first occurrence of meeting the criteria for sexual dysfunction or the date of the last follow-up questionnaire. Rates are expressed as the incidence per 1,000 person-years, and 95% confidence intervals were computed assuming a Poisson error distribution. The cumulative incidence for each sexual dysfunction was plotted by age at diagnosis.
Linear mixed-effects regression models were used to estimate annual longitudinal changes in each sexual function domain by regressing each measure on the time from initial sexual function questionnaire and 10-year age groups. An interaction term was included to allow for different slopes across age groups. Additional models for each baseline characteristic (regular sexual partner, ED, education level, smoking status, and presence of comorbidities) included the baseline characteristic and an interaction with time from the initial sexual function questionnaire term to allow for comparison of slopes across the levels of the baseline characteristic. An overall annual change for each man was determined by combining the average longitudinal changes (fixed effects) with the individual changes (random effects) [38,39]. The maximum likelihood methodology used in mixed model analysis implicitly averages over the predictive distribution for missing data; therefore, no further imputing was performed.
Spearman rank correlation coefficients were used to describe the relationship between annual changes in sexual function domains. Partial correlations were calculated controlling for the confounding effects of age on these relationships. Age-stratified correlations were also calculated with men categorized 40–59 and ≥60 years of age.
Results
Of the 2,213 men who participated in the study during or after 1996, 2,087 (94%) responded to the sexual function questions at least once. After censoring, 1,827 (83% of 2,213) men remained available for analyses (mean age at baseline = 58.39 years; standard deviation = 10.21). The baseline characteristics of the study population are shown in Table 1. The person-years of follow-up ranged from 8,357 years for the sexual satisfaction domain to 9,815 years for the sexual problem assessment domain (Table 2). The incidence of ED increased nearly 20-fold from 6/1,000 person-years for men in their 40s to 118/1,000 person-years for men in their 70s (Table 2). Similar increases were observed for low libido and ejaculatory dysfunction. The incidence of perceived sexual problems showed less increase with age (Figure 2), with an approximately sixfold increase from 7/1,000 person-years for men in their 40s to a rate of 41/1,000 person-years for men in their 70s (Table 2). The incidence of low sexual satisfaction was greater for men in their 40s and 50s (rate of 23/1,000 person-years and 31/1,000 person-years, respectively) than the incidence of the functional sexual domains (among 40 year olds, 6/1,000 reported ED and 4/1,000 reported ejaculatory dysfunction) (Figure 2).
Table 1.
Selected baseline (1996–2004) characteristics of participants in the Olmsted County Study of Urinary Symptoms and Health Status among Men
| Characteristic | Analysis sample (N = 1,827) N (%)* |
|---|---|
| Age | |
| 40–49 | 493 (27.0) |
| 50–59 | 634 (34.7) |
| 60–69 | 402 (22.0) |
| 70+ | 298 (16.3) |
| Regular sexual partner | |
| Yes | 1,534 (84.0) |
| No | 283 (15.5) |
| Education | |
| <High school | 126 (6.9) |
| High school graduate | 341 (18.7) |
| >High school | 1,346 (73.7) |
| Smoking status | |
| Never | 643 (35.2) |
| Current | 204 (11.2) |
| Former | 935 (51.2) |
| Diabetes | |
| Yes | 70 (3.8) |
| No | 1,757 (96.2) |
| Hypertension | |
| Yes | 331 (18.1) |
| No | 1,489 (81.5) |
| Coronary heart disease | |
| Yes | 227 (12.4) |
| No | 1,600 (87.6) |
Because of missing data, some measures were not available for all 1,827 men.
Table 2.
Sexual function incidence (1996–2004) without prevalent cases for men in the Olmsted County Study of Urinary Symptoms and Health Status among Men
| Age | Erectile dysfunction | |||
|---|---|---|---|---|
| Incident cases | Person-years | Incidence/1,000 | 95% CI | |
| 40–49 | 8 | 1,328 | 6 | 3, 12 |
| 50–59 | 54 | 4,406 | 12 | 9, 16 |
| 60–69 | 88 | 2,346 | 38 | 30, 46 |
| 70+ | 102 | 866 | 118 | 96, 143 |
| 40+ | 252 | 8,946 | 28 | 25, 32 |
| Age | Low libido | |||
| Incident cases | Person-years | Incidence/1,000 | 95% CI | |
| 40–49 | 13 | 1,333 | 10 | 5, 17 |
| 50–59 | 94 | 4,211 | 22 | 18, 27 |
| 60–69 | 98 | 2,269 | 43 | 35, 53 |
| 70+ | 96 | 813 | 118 | 96, 144 |
| 40+ | 301 | 8,626 | 35 | 31, 39 |
| Age | Ejaculatory dysfunction | |||
| Incident cases | Person-years | Incidence/1,000 | 95% CI | |
| 40–49 | 5 | 1,354 | 4 | 1, 9 |
| 50–59 | 41 | 4,481 | 9 | 7, 12 |
| 60–69 | 75 | 2,552 | 29 | 23, 37 |
| 70+ | 110 | 1,065 | 103 | 85, 124 |
| 40+ | 231 | 9,452 | 24 | 21, 28 |
| Age | Perceived sexual problems | |||
| Incident cases | Person-years | Incidence/1,000 | 95% CI | |
| 40–49 | 9 | 1,362 | 7 | 3, 13 |
| 50–59 | 37 | 4,495 | 8 | 6, 11 |
| 60–69 | 53 | 2,598 | 20 | 15, 27 |
| 70+ | 56 | 1,360 | 41 | 31, 53 |
| 40+ | 155 | 9,815 | 16 | 13, 18 |
| Age | Low sexual satisfaction | |||
| Incident cases | Person-years | Incidence/1,000 | 95% CI | |
| 40–49 | 28 | 1,242 | 23 | 15, 33 |
| 50–59 | 119 | 3,796 | 31 | 26, 38 |
| 60–69 | 88 | 2,145 | 41 | 33, 51 |
| 70+ | 75 | 1,174 | 64 | 50, 80 |
| 40+ | 310 | 8,357 | 37 | 33, 41 |
Figure 2.
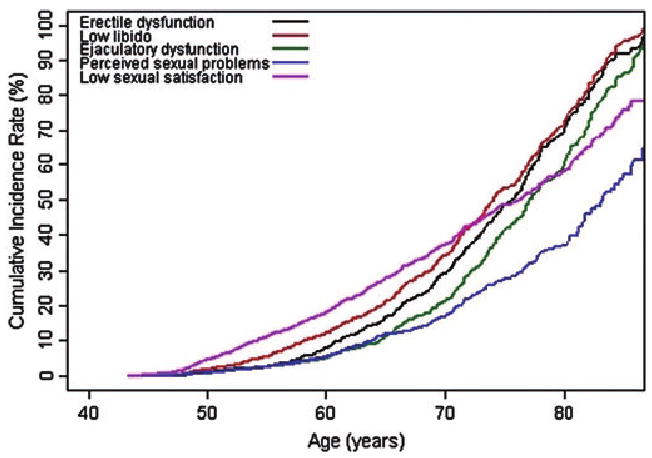
Cumulative incidence of sexual dysfunction with increasing age.
There was a significant rate of decline (points/year) in all of the sexual function domains, ranging from an annual decrease of 0.03 point per year for sexual satisfaction to an annual decrease of 0.23 point per year in erectile function (Table 3). Older men experienced larger annual declines in erectile function, sexual drive, and ejaculatory function compared to younger men. However, younger men experienced larger annual increases in perceived sexual problems compared to older men, and changes in sexual satisfaction did not differ by age group. Men who did not have a regular sexual partner at baseline experienced smaller annual declines in all sexual function domains compared to those who did have a sexual partner (Table 3). Similar results were seen when the analysis was limited to men who had no change in their partner status during follow-up (data not shown). Men who had ED at baseline experienced smaller declines in all sexual function domains compared to men who did not have ED at baseline (Table 3). Other baseline characteristics, including education level; smoking status; and presence of diabetes, hypertension, or coronary heart disease, were not significantly associated with the rate of decline in sexual function (data not shown).
Table 3.
Comparison of longitudinal sexual function slopes across baseline characteristics
| Baseline characteristic | Erectile function Mean, SD points/year | Sexual drive Mean, SD points/year | Ejaculatory function Mean, SD points/year | Sexual problem assessment Mean, SD points/year | Sexual satisfaction Mean, SD points/year |
|---|---|---|---|---|---|
| Overall* | −0.23, 0.15 | −0.07, 0.06 | −0.14, 0.13 | −0.09, 0.13 | −0.03, 0.03 |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Age* | |||||
| 40–49 | −0.16, 0.14 | −0.06, 0.05 | −0.05, 0.07 | −0.10, 0.11 | −0.02, 0.03 |
| 50–59 | −0.21, 0.15 | −0.06, 0.06 | −0.10, 0.10 | −0.13, 0.11 | −0.03, 0.03 |
| 60–69 | −0.32, 0.14 | −0.10, 0.05 | −0.19, 0.12 | −0.09, 0.14 | −0.02, 0.03 |
| 70+ | −0.26, 0.11 | −0.10, 0.05 | −0.28, 0.10 | 0.03, 0.12 | −0.03, 0.03 |
| P value | <0.0001 | 0.004 | <0.0001 | 0.0014 | 0.72 |
| Regular sexual partner* | |||||
| No | −0.12, 0.14 | −0.03, 0.06 | −0.10, 0.14 | 0.02, 0.15 | 0.02, 0.03 |
| Yes | −0.25, 0.15 | −0.08, 0.06 | −0.14, 0.13 | −0.11, 0.12 | −0.04, 0.03 |
| P value | <0.0001 | 0.0003 | 0.0002 | 0.004 | <0.0001 |
| Erectile dysfunction* | |||||
| No | −0.27, 0.18 | −0.08, 0.06 | −0.14, 0.14 | −0.13, 0.11 | −0.03, 0.03 |
| Yes | −0.01, 0.11 | −0.04, 0.06 | −0.13, 0.15 | 0.14, 0.13 | 0.01, 0.03 |
| P value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Age adjusted.
Moderate age-adjusted correlations were observed among all longitudinal changes in sexual function domains (Table 4). Change in erectile function was significantly correlated with change in all other sexual function domains (range in age-adjusted rs = 0.32–0.43). The weakest correlations were observed between change in sexual drive and change in both sexual problem assessment and sexual satisfaction (age-adjusted rs = 0.14 and rs = 0.16, respectively).
Table 4.
Age-adjusted Spearman correlations among longitudinal changes in sexual function slopes*
| Change in sexual drive | Change in ejaculatory function | Change in sexual problem assessment | Change in sexual satisfaction | |
|---|---|---|---|---|
| rs | rs | rs | rs | |
| Change in erectile function | 0.43 | 0.42 | 0.41 | 0.32 |
| Change in sexual drive | 1.0 | 0.27 | 0.14 | 0.16 |
| Change in ejaculatory function | 1.0 | 0.28 | 0.28 | |
| Change in sexual problem assessment | 1.0 | 0.38 |
All P values < 0.0001.
Correlations among changes in erectile function, sexual drive, and ejaculatory function were consistent across age groups (40–59, ≥60) (Table 5). Among men in their 40s and 50s, declines in erectile function, ejaculatory function, and sexual drive were moderately correlated with worsening problem assessment and increasing sexual dissatisfaction (range in age-adjusted rs = 0.24–0.51). However, significantly smaller correlations between these measures, and sexual satisfaction and problem assessment were observed among older men (range in age-adjusted rs = 0.03–0.29), suggesting that while sexual function may worsen among these men, older men may be less likely to perceive this as a problem and be dissatisfied compared to younger men (Table 5).
Table 5.
Spearman correlations among longitudinal changes in sexual function slopes among men 40–59 years and 60+ years of age
| 40–59 | 60+ | ||
|---|---|---|---|
| rs* | rs* | P† | |
| Change in erectile function and sexual drive | 0.39 | 0.42 | 0.46 |
| Change in erectile function and ejaculatory function | 0.44 | 0.39 | 0.22 |
| Change in erectile function and sexual problem assessment | 0.51 | 0.26 | <0.0001 |
| Change in erectile function and sexual satisfaction | 0.39 | 0.29 | 0.02 |
| Change in sexual drive and ejaculatory function | 0.23 | 0.24 | 0.83 |
| Change in sexual drive and sexual problem assessment | 0.27 | 0.05 | <0.0001 |
| Change in sexual drive and sexual satisfaction | 0.24 | 0.13 | 0.02 |
| Change in ejaculatory function and sexual problem assessment | 0.50 | 0.03 | <0.0001 |
| Change in ejaculatory function and sexual satisfaction | 0.32 | 0.22 | 0.03 |
| Change in sexual problem assessment and sexual satisfaction | 0.44 | 0.35 | 0.03 |
Age-adjusted Spearman correlations.
P value for test for a difference in the correlation coefficients by age group.
Discussion
In this study, we observed longitudinal declines in five measures of sexual function with greater declines for men who were older, who had a regular sexual partner at baseline, and who did not have ED at baseline. Our data also indicate that the annual declines in erectile function, ejaculatory function, and sexual drive track together. However, changes in the sexual problem assessment and changes in sexual satisfaction were less correlated with changes in the functional domains among older men, suggesting a possible acceptance of declines in sexual function with aging for some men.
Interestingly, the longitudinal rates of decline in sexual function were similar to those observed in this cohort cross-sectionally at baseline [10] with observed cross-sectional declines (points per year of age) of −0.21, −0.12, −0.10, −0.09, and −0.02 for erectile function, sexual drive, ejaculatory function, sexual problem assessment, and sexual satisfaction, respectively.
We observed age-related increases in the incidence of all five measures of sexual dysfunction. The overall crude incidence rate for ED in the MMAS [2] was very similar to our incidence rate for ED, with 26 cases per 1,000 years compared with 28 cases per 1,000 years, respectively. However, caution must be made in making this comparison as both studies reported strong age-related declines in sexual function, and the men in our study were older at baseline (43–87 years vs. 40–69 years at baseline in the MMAS). The MMAS reported an increased incidence of ED in aging men with a rate of 12.4/1,000 for men ages 40–49, 29.8/1,000 for men ages 50–59, and 46.4/1,000 for men ages 60–69 [2]. These rates are much greater than the age-specific rates of ED that we observed. These rate differences could be caused by differences in risk factors among the two populations; for example, the percentage of current smokers was 24% in the MMAS sample compared with 11% in ours.
We observed that men who did not have a regular sexual partner at baseline experienced smaller annual declines in all sexual function domains compared to those who did have a sexual partner. We had also noted significantly higher baseline levels of erectile function, sexual drive, ejaculatory function, and sexual satisfaction for men with a regular sexual partner [10], and therefore larger declines would be possible. Additionally, there may be more opportunity to observe a natural decline in those men who have a regular sexual partner and have been sexually active.
Men who had ED at baseline experienced less additional decline in all sexual function domains compared to men with normal erectile function at baseline. By definition, men who had ED at baseline had low initial erectile function domain scores, and the maximum decline in erectile function would be limited. Travison et al. [31] has also shown that some men with sexual dysfunction will experience improvement in their symptoms (35% of men with ED experienced a natural remission over time). Additionally, as there were strong correlations among the sexual function domains at baseline [10], men with ED at baseline may have also had lower initial scores in the other sexual function domains, and therefore limited maximum declines.
Unlike the MMAS studies, we did not observe differences in age-adjusted rates of decline in sexual function for levels of a number of baseline characteristics [31]. For example, the MMAS showed a higher incidence of ED for treated or untreated diabetics, men with treated heart disease, and men with treated hypertension [2]. In addition, Travison et al. [31] showed that the progression of ED was associated with aging, smoking, higher BMI, and worse general health. In our previous studies, we have observed cross-sectional associations between diabetes [23], smoking [27], and androgen levels [21] and sexual dysfunction, which support the idea that there are modifiable risk factors that lead to the development of sexual dysfunction. However, longitudinally, men with these characteristics at baseline did not have greater declines compared to those without these characteristics. It is possible that while these factors are associated with an increased risk of developing sexual dysfunction, once the dysfunction is present, the continued rate of decline does not differ from those without these factors.
The MMAS [30] also evaluated changes in sexual satisfaction. They reported a baseline score of 2.6 and a follow-up score of 2.3 over an average of 8.8 years of follow-up, for an approximate average decline of −0.03 point per year. Our study also observed an average decline of −0.03 point per year, which was consistent across age groups. Therefore, our studies have very similar results with regard to the change in sexual satisfaction scores.
Our results indicate that annual changes in erectile function, ejaculatory function, sexual drive, sexual problem assessment, and sexual satisfaction track together longitudinally. Overall, we observed moderate age-adjusted correlations between changes in erectile function and changes in all other sexual function domains. While the correlations between some of the sexual function domains were modest, we did observe stronger correlations for 40- to 59-year-old men than older men. Specifically, our data indicate that there may be an acceptance of sexual problems for some older men. For example, while the incidence of ED, low libido, and ejaculatory dysfunction showed strong increases with age, the incidence of perceived sexual problems was one-third, and the incidence of sexual dissatisfaction was one-half that of other domains for men ≥70. Second, older men experienced larger annual declines in erectile function, sexual drive, and ejaculatory function compared to younger men; however, younger men experienced larger increases in perceived sexual problems, and the rate of decline in sexual satisfaction was constant across age. Finally, the correlation between changes in sexual problem assessment and changes in sexual satisfaction with changes in the functional sexual domains was significantly decreased for older men. Other studies have suggested that older people place less importance on sex, possibly because of factors associated with aging, such as increased health problems [40,41] or loss of a regular sexual partner [42].
Conversely, in younger men, even though the incidence of functional sexual problems was low (among the 40–49 year olds, 6/1,000 reported ED and 4/1,000 reported ejaculatory dysfunction), the rate of low sexual satisfaction was high (23/1,000). Factors other than actual sexual function, such as depression, unrealized expectations, and heavy snoring could be contributing to increased dissatisfaction for these younger men [43–45].
There are several strengths of this study including the ability to examine longitudinal changes in multiple sexual function measures over 8 years of follow-up with up to five measures for each man. By looking at multiple measures of sexual function, we were able to assess not only the declines in the functional sexual measures, but also how the perceptions about these changes varied over time. Additionally, these subjects were randomly selected from the community and observations after treatment which could affect sexual function were truncated. Therefore, these data represent realistic estimates of how these sexual function measures change in the general population over time.
There are also potential limitations to this study. First, the potential for nonparticipation bias exists because of the low participation rate at baseline (55% response rate) for the entire cohort. However, previously we had documented only modest differences in baseline participants with regard to age, location, and prior history of urological conditions compared with nonparticipants [46]. Second, we utilized the BMSFI to assess sexual function. With the use of a questionnaire, subjects may overstate or understate their sexual function, and this could vary by age. Also, while the International Index of Erectile Function is commonly used to assess sexual function, we had longitudinal data available with multiple domains using the BMSFI. Third, we did observe greater dropout in men who reported having ED and men who reported existing comorbidities at baseline [47]; therefore, our results could be biased. If men with comorbidities who dropped out of the study subsequently developed sexual dysfunction, the result would be an underreporting of the sexual dysfunction and subsequent underestimation of incidence. Men who had ever suffered a stroke were most strongly associated with dropout, but this represented a small percentage of the cohort; only 22 men who suffered a stroke dropped out of the study. Therefore, it is unlikely that we are underestimating sexual dysfunction. Fourth, comorbidities, such as diabetes and hypertension, were based on self-report of medical diagnosis at baseline. Although there may be some lack of concordance between self-report and medical records [48], most studies show a strong concordance between self-report of chronic medical conditions such as hypertension and diabetes [49,50]. Finally, the generalizability may be limited, as all participants in this cohort study were Caucasian and were 40–79 years of age in 1990. Therefore, these findings may not be applicable to other ethnic populations and age groups.
Conclusion
Overall, these results demonstrate that longitudinal changes in five sexual function domains change together over time in our community-based cohort. Erectile function, ejaculatory function, and sexual drive decrease over time with greater rates of decline for older men. However, older men may be less likely to perceive these declines as a problem and be dissatisfied. These data may prove helpful to patients and clinicians in understanding and discussing changes in multiple aspects of sexual function.
Acknowledgments
The authors thank the men who participated in the Olmsted County study, the study personnel, and Ms. Sondra Buehler for her assistance in the preparation of this manuscript.
This project was supported by research grants from the Public Health Service, National Institutes of Health (grants DK58859, AR30582, and 1UL1 RR024150-01), and Merck Research Laboratories.
Footnotes
Conflict of Interest: Dr. Cynthia Girman is an employee of Merck Research Laboratories; Dr. Steven Jacobsen is an employee of Kaiser Permanente; and Dr. Ajay Nehra is a consultant for Pfizer, Glaxo Smith Klein, and Sanofi.
Statement of Authorship
- Conception and Design: Naomi M. Gades; Debra J. Jacobson; Jennifer L. St. Sauver; Steven J. Jacobsen
- Acquisition of Data: Debra J. Jacobson; Michaela E. McGree; Michael M. Lieber; Cynthia J. Girman; Steven J. Jacobsen
- Analysis and Interpretation of Data: Naomi M. Gades; Debra J. Jacobson; Michaela E. McGree; Jennifer L. St. Sauver; Michael M. Lieber; Ajay Nehra; Cynthia J. Girman; Steven J. Jacobsen
- Drafting the Article: Naomi M. Gades; Debra J. Jacobson; Jennifer L. St. Sauver; Michaela E. McGree; Steven J. Jacobsen
- Revising It for Intellectual Content: Naomi M. Gades; Debra J. Jacobson; Michaela E. McGree; Jennifer L. St. Sauver; Michael M. Lieber; Ajay Nehra; Cynthia J. Girman; Steven J. Jacobsen
- Final Approval of the Completed Article: Naomi M. Gades; Debra J. Jacobson; Michaela E. McGree; Jennifer L. St. Sauver; Michael M. Lieber; Ajay Nehra; Cynthia J. Girman; Steven J. Jacobsen
References
- 1.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: Results of the Massachusetts male aging study. J Urol. 1994;151:54–61. doi: 10.1016/s0022-5347(17)34871-1. [DOI] [PubMed] [Google Scholar]
- 2.Johannes CB, Araujo AB, Feldman HA, Derby CA, Kleinman KP, McKinlay JB. Incidence of erectile dysfunction in men 40 to 69 years old: Longitudinal results from the Massachusetts male aging study. J Urol. 2000;163:460–3. [PubMed] [Google Scholar]
- 3.Rosen RC, Fisher WA, Eardley I, Niederberger C, Nadel A, Sand M. The multinational men's attitudes to life events and sexuality (MALES) study: I. Prevalence of erectile dysfunction and related health concerns in the general population. Curr Med Res Opin. 2004;20:607–17. doi: 10.1185/030079904125003467. [DOI] [PubMed] [Google Scholar]
- 4.Aytac IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84:50–6. doi: 10.1046/j.1464-410x.1999.00142.x. [DOI] [PubMed] [Google Scholar]
- 5.Boswell-Smith V, Spina D, Page CP. Phosphodiesterase inhibitors. Br J Pharmacol. 2006;147(suppl 1):S252–7. doi: 10.1038/sj.bjp.0706495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrotra N, Gupta M, Kovar A, Meibohm B. The role of pharmacokinetics and pharmacodynamics in phosphodiesterase-5 inhibitor therapy. Int J Impot Res. 2007;19:253–64. doi: 10.1038/sj.ijir.3901522. [DOI] [PubMed] [Google Scholar]
- 7.Aversa A, Bruzziches R, Pili M, Spera G. Phosphodiesterase 5 inhibitors in the treatment of erectile dysfunction. Curr Pharm Des. 2006;12:3467–84. doi: 10.2174/138161206778343046. [DOI] [PubMed] [Google Scholar]
- 8.Shabsigh R, Seftel AD, Rosen RC, Porst H, Ahuja S, Deeley MC, Garcia CS, Giuliano F. Review of time of onset and duration of clinical efficacy of phosphodiesterase type 5 inhibitors in treatment of erectile dysfunction. Urology. 2006;68:689–96. doi: 10.1016/j.urology.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 9.O'Leary MP, Fowler FJ, Lenderking WR, Barber B, Sagnier PP, Guess HA, Barry MJ. A brief male sexual function inventory for urology. Urology. 1995;46:697–706. doi: 10.1016/S0090-4295(99)80304-5. [DOI] [PubMed] [Google Scholar]
- 10.O'Leary MP, Rhodes T, Girman CJ, Jacobson DJ, Roberts RO, Lieber MM, Jacobsen SJ. Distribution of the brief male sexual inventory in community men. Int J Impot Res. 2003;15:185–91. doi: 10.1038/sj.ijir.3900996. [DOI] [PubMed] [Google Scholar]
- 11.Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281:537–44. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 12.Enzlin P, Mak R, Kittel F, Demyttenaere K. Sexual functioning in a population-based study of men aged 40–69 years: The good news. Int J Impot Res. 2004;16:512–20. doi: 10.1038/sj.ijir.3901221. [DOI] [PubMed] [Google Scholar]
- 13.Smith LJ, Mulhall JP, Deveci S, Monaghan N, Reid MC. Sex after seventy: A pilot study of sexual function in older persons. J Sex Med. 2007;4:1247–53. doi: 10.1111/j.1743-6109.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 14.Mykletun A, Dahl AA, O'Leary MP, Fossa SD. Assessment of male sexual function by the brief sexual function inventory. BJU Int. 2006;97:316–23. doi: 10.1111/j.1464-410X.2005.05904.x. [DOI] [PubMed] [Google Scholar]
- 15.Chung WS, Nehra A, Jacobson DJ, Roberts RO, Rhodes T, Girman CJ, Lieber MM, Jacobsen SJ. Lower urinary tract symptoms and sexual dysfunction in community-dwelling men. Mayo Clin Proc. 2004;79:745–9. doi: 10.4065/79.6.745. [DOI] [PubMed] [Google Scholar]
- 16.Boyle P, Robertson C, Mazzetta C, Keech M, Hobbs R, Fourcade R, Kiemeney L, Lee C. The association between lower urinary tract symptoms and erectile dysfunction in four centres: The UrEpik study. BJU Int. 2003;92:719–25. doi: 10.1046/j.1464-410x.2003.04459.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosen R, Altwein J, Boyle P, Kirby RS, Lukacs B, Meuleman E, O'Leary MP, Puppo P, Robertson C, Giuliano F. Lower urinary tract symptoms and male sexual dysfunction: The multinational survey of the aging male (MSAM-7) Eur Urol. 2003;44:637–49. doi: 10.1016/j.eururo.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Stroberg P, Boman H, Gellerstedt M, Hedelin H. Relationships between lower urinary tract symptoms, the bother they induce and erectile dysfunction. Scand J Urol Nephrol. 2006;40:307–12. doi: 10.1080/00365590600642010. [DOI] [PubMed] [Google Scholar]
- 19.Gades NM, Jacobson DJ, Girman CJ, Roberts RO, Lieber MM, Jacobsen SJ. Prevalence of conditions potentially associated with lower urinary tract symptoms in men. BJU Int. 2005;95:549–53. doi: 10.1111/j.1464-410X.2005.05337.x. [DOI] [PubMed] [Google Scholar]
- 20.Lutz MC, Roberts RO, Jacobson DJ, McGree ME, Lieber MM, Jacobsen SJ. Cross-sectional associations of urogenital pain and sexual function in a community based cohort of older men: Olmsted County, Minnesota. J Urol. 2005;174:624–8. doi: 10.1097/01.ju.0000165386.26542.23. discussion 628. [DOI] [PubMed] [Google Scholar]
- 21.Gades NM, Jacobson DJ, McGree ME, St Sauver JL, Lieber MM, Nehra A, Girman CJ, Klee GG, Jacobsen SJ. The associations between serum sex hormones, erectile function, and sex drive: The Olmsted County study of urinary symptoms and health status among men. J Sex Med. 2008;5:2209–20. doi: 10.1111/j.1743-6109.2008.00924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrini RL, Barrett-Connor E. Sex hormones and age: A cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–4. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- 23.Burke JP, Jacobson DJ, McGree ME, Nehra A, Roberts RO, Girman CJ, Lieber MM, Jacobsen SJ. Diabetes and sexual dysfunction: Results from the Olmsted County study of urinary symptoms and health status among men. J Urol. 2007;177:1438–42. doi: 10.1016/j.juro.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 24.Shabsigh R, Shah M, Sand M. Erectile dysfunction and men's health: Developing a comorbidity risk calculator. J Sex Med. 2008;5:1237–43. doi: 10.1111/j.1743-6109.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- 25.Chew KK, Bremner A, Jamrozik K, Earle C, Stuckey B. Male erectile dysfunction and cardiovascular disease: Is there an intimate nexus? J Sex Med. 2008;5:928–34. doi: 10.1111/j.1743-6109.2007.00714.x. [DOI] [PubMed] [Google Scholar]
- 26.Esposito K, Giugliano F, Ciotola M, De Sio M, D'Armiento M, Giugliano D. Obesity and sexual dysfunction, male and female. Int J Impot Res. 2008;20:358–65. doi: 10.1038/ijir.2008.9. [DOI] [PubMed] [Google Scholar]
- 27.Gades NM, Nehra A, Jacobson DJ, McGree ME, Girman CJ, Rhodes T, Roberts RO, Lieber MM, Jacobsen SJ. Association between smoking and erectile dysfunction: A population-based study. Am J Epidemiol. 2005;161:346–51. doi: 10.1093/aje/kwi052. [DOI] [PubMed] [Google Scholar]
- 28.Verwoerdt A, Pfeiffer E, Wang HS. Sexual behavior in senescence. II. Patterns of sexual activity and interest. Geriatrics. 1969;24:137–54. [PubMed] [Google Scholar]
- 29.George LK, Weiler SJ. Sexuality in middle and late life. The effects of age, cohort, and gender. Arch Gen Psychiatry. 1981;38:919–23. doi: 10.1001/archpsyc.1981.01780330077008. [DOI] [PubMed] [Google Scholar]
- 30.Araujo AB, Mohr BA, McKinlay JB. Changes in sexual function in middle-aged and older men: Longitudinal data from the Massachusetts male aging study. J Am Geriatr Soc. 2004;52:1502–9. doi: 10.1111/j.0002-8614.2004.52413.x. [DOI] [PubMed] [Google Scholar]
- 31.Travison TG, Shabsigh R, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The natural progression and remission of erectile dysfunction: Results from the Massachusetts male aging study. J Urol. 2007;177:241–6. doi: 10.1016/j.juro.2006.08.108. discussion 246. [DOI] [PubMed] [Google Scholar]
- 32.Gan M, Smit M, Dohle GR, Bosch JL, Bohnen A. Determinants of ejaculatory dysfunction in a community-based longitudinal study. BJU Int. 2007;99:1443–8. doi: 10.1111/j.1464-410X.2007.06803.x. [DOI] [PubMed] [Google Scholar]
- 33.Chute CG, Panser LA, Girman CJ, Oesterling JE, Guess HA, Jacobsen SJ, Lieber MM. The prevalence of prostatism: A population-based survey of urinary symptoms. J Urol. 1993;150:85–9. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 34.Jacobsen SJ, Guess HA, Panser L, Girman CJ, Chute CG, Oesterling JE, Lieber MM. A population-based study of health care-seeking behavior for treatment of urinary symptoms. The Olmsted County study of urinary symptoms and health status among men. Arch Fam Med. 1993;2:729–35. doi: 10.1001/archfami.2.7.729. [DOI] [PubMed] [Google Scholar]
- 35.Melton LJ., 3rd History of the Rochester epidemiology project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 36.Arciero TJ, Jacobsen SJ, Reeder GS, Frye RL, Weston SA, Killian JM, Roger Vr VL. Temporal trends in the incidence of coronary disease. Am J Med. 2004;117:228–33. doi: 10.1016/j.amjmed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Bergstralh EJ, Offord KP, Kosanke JL, Augustine GA. Personyrs: A SAS procedure for person year analyses, Technical Report Series. No 31. Rochester, MN: Mayo Clinic; 1986. [Google Scholar]
- 38.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–74. [PubMed] [Google Scholar]
- 39.Feldman HA. Families of lines: Random effects in linear regression analysis. J Appl Physiol. 1988;64:1721–32. doi: 10.1152/jappl.1988.64.4.1721. [DOI] [PubMed] [Google Scholar]
- 40.Lindau ST, Schumm LP, Laumann EO, Levinson W, O'Muircheartaigh CA, Waite LJ. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357:762–74. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Camacho ME, Reyes-Ortiz CA. Sexual dysfunction in the elderly: Age or disease? Int J Impot Res. 2005;17(suppl 1):S52–6. doi: 10.1038/sj.ijir.3901429. [DOI] [PubMed] [Google Scholar]
- 42.Gott M, Hinchliff S. How important is sex in later life? The views of older people. Soc Sci Med. 2003;56:1617–28. doi: 10.1016/s0277-9536(02)00180-6. [DOI] [PubMed] [Google Scholar]
- 43.Hanak V, Jacobson DJ, McGree ME, Sauver JS, Lieber MM, Olson EJ, Somers VK, Gades NM, Jacobsen SJ. Snoring as a risk factor for sexual dysfunction in community men. J Sex Med. 2008;5:898–908. doi: 10.1111/j.1743-6109.2007.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santtila P, Wager I, Witting K, Harlaar N, Jern P, Johansson A, Varjonen M, Sandnabba NK. Discrepancies between sexual desire and sexual activity: Gender differences and associations with relationship satisfaction. J Sex Marital Ther. 2008;34:29–42. doi: 10.1080/00926230701620548. [DOI] [PubMed] [Google Scholar]
- 45.Haavio-Mannila E, Kontula O. Correlates of increased sexual satisfaction. Arch Sex Behav. 1997;26:399–419. doi: 10.1023/a:1024591318836. [DOI] [PubMed] [Google Scholar]
- 46.Panser LA, Chute CG, Guess HA, Larsonkeller JJ, Girman CJ, Oesterling JE, Lieber MM, Jacobsen SJ. The natural history of prostatism: The effects of non-response bias. Int J Epidemiol. 1994;23:1198–205. doi: 10.1093/ije/23.6.1198. [DOI] [PubMed] [Google Scholar]
- 47.Gades NM, Jacobson DJ, McGree ME, Lieber MM, Roberts RO, Girman CJ, Jacobsen SJ. Dropout in a longitudinal, cohort study of urologic disease in community men. BMC Med Res Methodol. 2006;6:58. doi: 10.1186/1471-2288-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckles GL, Williamson DF, Brown AF, Gregg EW, Karter AJ, Kim C, Dudley RA, Safford MM, Stevens MR, Thompson TJ. Agreement between self-reports and medical records was only fair in a cross-sectional study of performance of annual eye examinations among adults with diabetes in managed care. Med Care. 2007;45:876–83. doi: 10.1097/MLR.0b013e3180ca95fa. [DOI] [PubMed] [Google Scholar]
- 49.El Fakiri F, Bruijnzeels MA, Hoes AW. No evidence for marked ethnic differences in accuracy of self-reported diabetes, hypertension, and hypercholesterolemia. J Clin Epidemiol. 2007;60:1271–9. doi: 10.1016/j.jclinepi.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Skinner KM, Miller DR, Lincoln E, Lee A, Kazis LE. Concordance between respondent self-reports and medical records for chronic conditions: Experience from the veterans health study. J Ambul Care Manage. 2005;28:102–10. doi: 10.1097/00004479-200504000-00002. [DOI] [PubMed] [Google Scholar]




