Abstract
Delivering cells using semi-permeable hydrogels is becoming an increasingly important direction in cell based therapies and regenerative medicine applications. Synthetic hydrogels have been functionalized with bioactive motifs to render otherwise inert polymer networks responsive. However, little effort has been focused on creating immuno-isolating materials capable of retarding the transport of small antigenic molecules secreted from the cells delivered with the synthetic carriers. Toward the goal of developing a complete immuno-isolation polymeric barrier, affinity peptide-functionalized PEG hydrogels were developed with the ability to sequester monocyte chemotactic protein 1 (MCP-1), a chemokine known to induce the chemotaxis of monocytes, dendritic cells, and memory T-cells. Affinity peptides capable of sequestering MCP-1 were identified from CCR2 (a G protein-coupled receptor for MCP-1) and incorporated within PEG hydrogels via a thiol-acrylate photopolymerization. The release of encapsulated recombinant MCP-1 from PEG hydrogels is readily tuned by: (1) incorporating affinity peptides within the network; and/or (2) altering the spacer distance between the affinity peptide and the crosslinking site. Furthermore, when pancreatic β-cells were encapsulated within these novel peptide-functionalized hydrogels, the release of cell-secreted MCP-1 was significantly reduced, demonstrating the potential of this new gel formulation to reduce the host innate immune response to transplanted cells by decreasing the recruitment and activation of host monocytes and other immune cells.
Keywords: chemokine, affinity hydrogels, photopolymerization, tissue engineering, diabetes
1. Introduction
The long-term survival of transplanted islets is key to the successful reversal of type 1 diabetes [1]. To overcome graft rejection induced by host T-cells, the administration of immunosuppressants (e.g., sirolimus/rampamycin and tacrolimus) is inevitable, albeit these drugs are toxic to islet cells and may cause unfavorable systemic complications [2-6]. Recently, poly(ethylene glycol) (PEG) hydrogels are in development for many cell delivery and regenerative medicine applications [7, 8], including the encapsulation of pancreatic β-cells for cell transplantation to treat Type 1 diabetes [9, 10]. The needs for immunosuppressants following islet transplantation may be alleviated when the islet grafts are encapsulated and protected by a physical hydrogel barrier. One of the major characteristics of PEG hydrogels is their high water content that leads to preferential high diffusivity for small molecular weight nutrients and metabolic products, such as glucose and insulin [11, 12]. The crosslinked PEG polymer network provides a selective “immuno-isolation barrier” to protect the encapsulated cells from immune destruction initiated by host antibodies and immune cells [1]. The design of immuno-isolating hydrogels, however, requires consideration of not only antibodies and immune cells, but also small molecular weight antigenic, chemotactic, and cytotoxic immune-mediators (e.g., reactive oxygen species and pro-inflammatory cytokines) [13, 14]. While preventing direct cell-cell contact and antibody diffusion is relatively easily with size exclusive barriers, such as PEG hydrogels, it remains a challenging task to preclude the diffusion of small molecular weight soluble immune-mediators in permissive and bio-inert hydrogels [1, 11].
The concentration of a variety of cytokines and chemokines is known to be elevated at the site of injury, infection, or transplantation [15-17]. The increased cytokine/chemokine concentration plays an important role in the dynamic inflammatory response. For example, donor antigens (e.g., chemokines) secreted by transplanted cells are responsible for recruiting host inflammatory and immune cells [18-20]. In this regard, un-modified PEG hydrogels fail to prevent the leakage of small molecular weight chemokines secreted from cells encapsulated in the gel capsule. These chemokines, which readily diffuse out of the gel, can recruit additional immune cells that subsequently secrete excess cytotoxic cytokines that can damage the encapsulated cells [1, 11, 13]. Thus, implementing mechanisms to regulate the transport and escape of these small molecular weight antigenic/cytotoxic molecules produced by transplanted cells would be highly advantageous, and fabrication of new cell carriers that enable this suppression of transport of undesired molecules while maintaining cell viability would be highly desirable.
Chemokines are small proteins (∼ 8 to 13 kDa) known to attract inflammatory and immune cells. For example, the major function of monocyte chemotactic protein 1 (MCP-1 or CCL2), a member of the CC chemokine superfamily, is to recruit monocytes [21-23], dendritic cells [24, 25], and memory T-cells [26] to the site of injury, infection, and transplantation. These immune cells, when recruited and activated, secrete a variety of cytotoxic cytokines that subsequently cause damage to the allogenic or xenogenic cells, even when these cells are encapsulated in hydrogels. Chemokines are important immune-regulators in a variety of auto-immune and inflammatory diseases [23], including type 1 diabetes [27-29]. Constitutive expression of chemokines, such as MCP-1, from pancreatic islets has been demonstrated and is known to play a critical role in the induction of autoimmune response that leads to the onset of type 1 diabetes [30]. The secretion of MCP-1 from pancreatic islets, as well as insulin-producing INS-1E and RINm5F β-cells, is enhanced following the stimulation with angiotensin-II (Ang-II) [31, 32]. It has also been shown that the expression and secretion of MCP-1 from murine pancreatic β-cell line, MIN6, is up-regulated upon stimulation with pro-inflammatory cytokines [33]. Clinical studies have also revealed elevated serum MCP-1 concentration in type 1 diabetic patients [34], as well as reduced transplanted graft survival associated with increased circulating donor MCP-1 concentrations [35]. Given the importance of MCP-1 in type 1 diabetes, we hypothesize that it would be beneficial to design functional gel barriers that could eliminate or minimize the release of donor MCP-1 from transplanted allogenic/xenogenic β-cells.
Significant efforts have been dedicated to the design of bioactive materials for regulating local inflammation [13, 14, 36]. Previously, strategies were developed based on affinity protein-peptide binding to control growth factors release [37] from PEG-based hydrogels, as well as to antagonize a pro-inflammatory cytokine, tumor necrosis factor α (TNFα), within the local gel environment [13]. In the context of regulating the inflammatory response, affinity peptides were conjugated in photopolymerized PEG hydrogels, creating cytokine-antagonizing hydrogels that sequester and antagonize infiltrated TNFα, thereby decreasing apoptosis of the encapsulated cells, including pancreatic islets [13]. Here, we present the identification and characterization of affinity peptides capable of binding to murine MCP-1 and describe the fabrication of affinity PEG-peptide hydrogels to reduce the diffusion of chemokine MCP-1 from highly permissive PEG hydrogels. Specifically, several synthetic peptides derived from extracellular loops of murine CCR2, a 7 trans-membrane G-protein coupled receptor (GPCR) for murine MCP-1, were synthesized and co-polymerized with PEG-diacrylate (PEGDA), via a thiol-acrylate photopolymerization [10, 37, 38], to form peptide-functionalized PEG hydrogels. These affinity PEG-peptide hydrogels were found to sequester MCP-1 secreted by the encapsulated MIN6 β-cells and subsequently reduce the activation and chemotaxis of macrophages.
2. Materials and Methods
2.1 Materials
Standard amino acids (Fmoc-capped), Fmoc-Rink-amide MBHA resin, and reagents for solid phase peptide synthesis were obtained from Anaspec (Fremont, CA). Di-ethylene glycol spacer (Fmoc-PEG2-Suc-OH) was obtained from Bachem (Torrance, CA). Recombinant murine MCP-1 protein (M.W. 13.8 kDa) and ELISA development kits for murine MCP-1 and IL-1β were purchased from Peprotech (Rocky Hill, NJ). Rabbit anti-mouse MCP-1 antibody was acquired from Santa Cruz Biotechnology (Santa Cruz, CA). Goat anti-rabbit HRP conjugated antibody was purchased from Abcam (Cambridge, MA). AlamarBlue® reagent was obtained from Invitrogen (Carlsbad, CA). All other chemicals were received from Sigma-Aldrich (St. Louis, MO) unless noted otherwise.
2.2 In vitro cellular activities of monocytes in the presence of recombinant MCP-1
A monocyte/macrophage cell line, J774a.1 (ATCC: TIB-67TM), was used to test the dose-dependent response of monocytes to MCP-1. (1) Chemotaxis: J774a.1 cells were seeded in a 24-well trans-well device (pore size: 7μm) and placed in the wells containing different concentrations of recombinant murine MCP-1 in high glucose DMEM (with 10% FBS, 1% penicillin–streptomycin, and 0.5 μg/mL fungizone.) for 16hr. Migrating cells that adhered to the bottom of the trans-well devices were fixed with 4% formalin for 15min, washed with PBS, and stained with crystal violet (0.2wt% in 20% methanol/PBS) for 30 minutes. Excess crystal violet was removed by extensively washing with PBS. Stained cells were imaged with a phase contrast microscope and counted in 5 different fields per sample (n=4). The number of migrating cells without MCP-1 treatment was set as 100%. (2) Proliferation: J774a.1 cells were seeded in 24-well plates at 100,000 cells/well overnight. Cells were incubated in serum-free DMEM overnight prior to MCP-1 treatment. Various concentrations of murine MCP-1 were added to cells that were further incubated for 24hr. The relative cell proliferation was determined by Alamarblue® reagent according to the manufacturer's protocol. (3) IL-1β secretion: J774a.1 cells were seeded in a 24-well plate at 100,000 cells/well for overnight, followed by treatment of MCP-1 in serum-free DMEM. After 24hr of culture, media were collected and analyzed with murine IL-1β ELISA kit to determine the amount of IL-1β secretion.
2.3 Design, synthesis, and purification of peptide analogs
Peptide sequences were derived form murine CCR2, a GPCR for MCP-1. Prior studies have suggested that the first and second extracellular loops of CCR2 play an important role in MCP-1/CCR2 binding [39, 40]. Thus, two sequences derived from loop-1 and loop-2 of CCR2, respectively, were chosen as initial affinity peptides candidates for murine MCP-1 binding. While the complete sequence of loop-1 (112AHYAANEWVFGNIMCK127 or P1 in Figure 2A) was synthesized, only a portion of loop-2 (211WKNFQTI217 or P2 in Figure 2A) was selected and synthesized. This sequence (P2) is a comparison result of sequence alignment between murine and human CCR2, as a peptide sequence (WNNFHTIMR) derived from human CCR2 was previously found to exhibit high affinity for human MCP-1 [40]. Thus, it was hypothesized that the selected P2 sequence should contribute to affinity binding between murine MCP-1 and CCR2, due to the high sequence homology of chemokines and their receptors between species. A terminal cysteine residue and an addition glycine spacer were added for conjugating the peptide within crosslinked PEGDA hydrogels.
Figure 2.
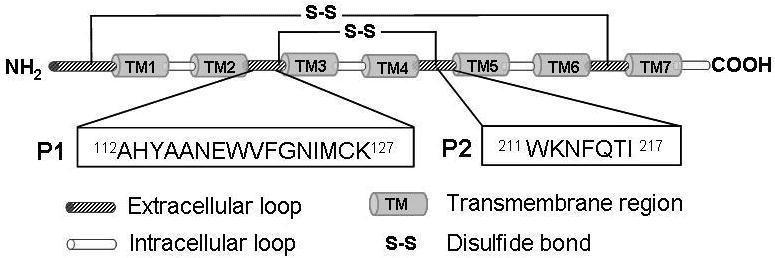

(A) Model of extracellular loops of murine CCR2, a murine MCP-1 receptor. Sequences P1 and P2 were derived from extracellular loop 1 and loop 2, respectively. (B) Affinity ELISA to determine binding affinity of P1 and P2 to recombinant MCP-1. BSA and a scrambled sequence were used as negative controls (Mean ± SEM, n=4).
All peptides were synthesized (Applied Biosystems 433A) using solid phase Fmoc chemistry with HBTU/HOBt amino acid activation. Peptides were deprotected and cleaved from the solid support using 5 wt% phenol in 95% TFA, 2.5% triisopropylsilane (TIPS), and 2.5% water. Peptides were purified by reverse-phase HPLC and characterized by MALDI-TOF mass spectroscopy. In all peptide analogs, an additional cysteine residue was added to the N-terminus for covalent conjugation within PEGDA hydrogel network via a thiol-acrylate photopolymerization [10, 37, 38].
2.4 Synthesis of PEGDA macromer, LAP photoinitiator, and PEG hydrogels
The synthesis of poly(ethylene glycol) diacrylate (PEGDA, 10kDa) has been described elsewhere [37, 41]. Briefly, hydroxyl-terminated PEG (PEG, 10kDa) was first dissolved in toluene at above 60°C and reacted with 4-molar excess (to hydroxyl group) of acryloyl chloride and triethylamine (TEA) at room temperature for overnight. The product was filtered through neutral alumina to remove TEA-HCl salt and precipitated in cold ether. The PEGDA macromer was then filtered and dried in vacuo. The degree of acrylation was over 95% as determined by 1H NMR. The photoinitiator lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) was synthesized by reacting equimolar amount of dimethyl phenylphosphonite with 2,4,6-trimethylbenzoyl chloride [42, 43]. Briefly, 2,4,6-trimethylbenzoyl chloride was added drop-wise into dimethyl phenylphosphonite and allowed to react for 18 hr, followed by the addition of 4 molar excess of lithium bromide in 2-butanone. The reaction mixture was heated to 50°C until a solid precipitate formed (∼10 min). The mixture was cooled to room temperature and allowed to rest for 4 hr. The resulting product was filtered and washed extensively with excess amount of 2-butanone and diethyl ether, then dried in vacuum. Previous studies have shown that the photoinitiator LAP is more efficient, compared to the widely used 2-Hydroxy-4′-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure-2959) in fabricating hydrogels from PEGDA with 365 nm light. The photo-curing time required to obtain a cured hydrogel is reduced to less than 2 min with ∼7 mW/cm2 of 365 nm light, while maintaining cytocompatibility [42].
PEG-peptide hydrogels were synthesized via a thiol-acrylate mixed-mode photopolymerization reaction [10, 38]. Briefly, 10 wt% of PEGDA (M.W. 10kDa) macromer was dissolved in pH 7.4 PBS containing 0.05wt% of photoinitiator (LAP) and selected amounts of peptides. The pre-polymer solutions were mixed and injected between two glass slides separated by 1mm thick Teflon spacers, followed by photopolymerization under a UV lamp (365nm, 7mW/cm2) at ambient temperature for 2 min. Equal sized hydrogels disks were obtained using a 7mm diameter biopsy punch.
2.5 Rheological analysis of hydrogel equilibrium properties
Hydrogels with or without peptides as described above were prepared for rheological property measurements. Prior to measurement, gels were incubated in PBS at room temperature for 48hr to reach equilibrium swelling. Rheological properties of the swollen hydrogels were measured using a modified ARES rotational rheometer (TA Instruments). Strain sweep experiments (ω=10 rad s-1) were conducted on equilibrated hydrogels between parallel plates. Gel moduli were determined within the materials linear viscoelastic regions.
2.6 Characterization of peptide affinity and incorporation into PEG hydrogels
Peptide affinity was qualitatively revealed by a modified ELISA similar to a prior study [44]. Briefly, purified peptides (100μM) were coated in a Maxisorp 96-well ELISA plate at 4°C overnight, washed 3 times (0.05% Tween-20 in PBS), and blocked with 1% BSA for at least 1 hr. The plate was then incubated with recombinant murine MCP-1 at various concentrations for 2 hr at room temperature. Rabbit anti-mouse MCP-1 antibody and goat anti-rabbit HRP-conjugated antibody were used as primary (2 hr) and secondary (1 hr) antibody, respectively. Finally, TMB substrate was added and the plate was incubated for 15-20 min before stop solution (0.2N HCl) was added. Absorbance of the plate at 450nm was measured with a microplate reader.
The degree of peptide incorporation in PEG hydrogels was determined by quantifying the concentrations of unconjugated peptides. Briefly, affinity peptides (1mM) were included in a 10 wt% PEGDA prepolymer solution and co-polymerized as described above. The resulting gels of identical size (7mm dia. × 1mm thickness) were incubated in pH 7.4 PBS at ambient temperature for 48 hr to allow for release of unconjugated peptides. The supernatants were collected and the concentrations of the unconjugated peptides were quantified by Fluoraldehyde reagent (PIERCE).
2.7 Characterization of recombinant MCP-1 release
Recombinant murine MCP-1 was photo-encapsulated in PEGDA hydrogels to characterize its in vitro release. Briefly, 100ng/mL of MCP-1 was mixed with 10wt% PEGDA (Mn∼10kDa), 0.05wt% LAP, and peptide analogs at desired concentrations. The mixed macromer solutions were injected between two glass slides separated by 0.5mm Teflon spacers and exposed to UV light (365nm, 7mW/cm2) for 2 minutes to allow photo-curing. The resulting gels were cut into identical circular disks with a 7mm diameter biopsy punch and placed in at least 2mL of release buffer (0.1% BSA, 1mM EDTA in PBS, pH7.4). To maintain the bioactivity of the released MCP-1, gel samples were placed at 4°C on an orbital shaker. After sampling at predetermined time intervals, gels were transferred into fresh release buffer. Samples were stored at -20°C until determination of released MCP-1 concentrations using a murine MCP1/JE ELISA kit. It should be noted that storage of the collected cytokine samples at low temperature (-20°C) for extended period of time often leads to the loss of immunoreactivity. However, for recombinant MCP-1 samples, we did not observe significant loss of immunoreactivity after 24hr of storage (93.8 ± 8.4 %). Around 50% decrease in immunoreactivity may occur after storage for 72hr at -20°C.
2.8 Photo-encapsulation of MIN6 cells and cytokine-stimulated MCP-1 secretion
MIN6 cells were trypsinized from flasks and dispersed into single cells, mixed with sterile-filtered macromer solutions containing 10wt% PEGDA, 0.05wt% LAP, and desired concentrations of peptide analogs. Mixed cell-macromer solutions (30μL per sample) were injected into 1mL sterile syringes with the tips cut-off and placed under UV light for 2 min to allow photo-gelation and encapsulation. Encapsulated cells were placed in RPMI-1640 medium for at least 1 hour to remove any un-encapsulated cells located on the gel surface. After which, the cell-laden hydrogels were transferred into fresh media or media containing a cytokine cocktail, including IFN-γ (750 units/mL), IL-1β (10 units/mL), and TNFα (500 units/mL) for 6hr or 24hr. Media were collected and the concentrations of the cell-secreted MCP-1 from gels were determined by ELISA. The amount of cell-secreted MCP-1 was normalized to cell viability determined by CellTiter-Glo reagent as described elsewhere.
2.9 Statistical analysis
Results were presented as Mean ± SEM. All experiments containing 3 to 4 replicates were repeated independently at least three times. Statistical analysis was performed using one-way ANOVA, followed by Turkey test with 95% confidence intervals.
3. Results and discussion
3.1 Effects of recombinant MCP-1 on monocyte activities in vitro
MCP-1 is known to exert a variety of cellular activities on monocytes, including chemotaxis, proliferation, and secretion of cytokines such as interleukin-1β (IL-1β). These cellular activities were verified in vitro using a model murine monocyte/macrophage cell line, J774a.1. As shown in Fig. 1A, J774a.1 cells migrate directionally in response to increasing MCP-1 concentration, demonstrating the chemotactic effect of MCP-1 to monocytes. In addition to chemotaxis, MCP-1 also induces enhanced cell proliferation (Fig. 1B) and IL-1β secretion (Fig. 1C). Collectively, these in vitro results confirm the importance of MCP-1 on recruiting and activating monocytes.
Figure 1.



Effects of recombinant MCP-1 on in vitro (A) chemotaxis, (B) proliferation, and (C) secretion of IL-1β of a monocyte/macrophage cell line, J774a.1 (Mean ± SEM, n=4). Asterisks represent statistical significance compared to non-treated cells (p<0.05).
3.2 Affinity peptide identification and peptide analog design rationale
Affinity peptides that bind to murine MCP-1 were identified from its receptor CCR2 (Fig. 2A) [39, 40]. The sequence P1 (112AHYAANEQVFQNIMCK127) was derived from the entire extracellular loop-1 of murine CCR2, while the sequence P2 (211WKNFQTI217) was only a partial sequence of the 2nd loop. The rationale of selecting P2 as a potential affinity peptide for murine MCP-1 was based on previous efforts in identifying human MCP-1 binding peptide [40]. It was shown that the sequence WNNFHTI (derived from loop-2 from human CCR2) binds to human MCP-1 with an equilibrium dissociation constant (Kd) of 22μM [40]. It was therefore hypothesized that a sequence derived from the same location on murine CCR2 may also contribute affinity binding to murine MCP-1, in part due to highly conserved sequences between murine and human MCP-1 and CCR2.
The two sequences (P1 and P2) were synthesized using a solid-phase peptide synthesizer and purified with reverse-phase HPLC. After purification, the identity of P1 and P2 were verified by MALDI-TOF (See supporting information). A modified ELISA was developed to determine whether P1 and P2 indeed exhibit affinity to murine MCP-1. As shown in Fig. 2B, P1 and P2 are both affinity peptides for murine MCP-1, as increasing the concentrations of soluble MCP-1 in the incubation buffer enhances its binding to the surface-adsorbed peptides. Wells coated with BSA, or a control peptide with identical amino acids but scrambled sequence of P2, were used as controls and only minimal non-specific interactions were observed at very high MCP-1 concentrations (Fig. 2B). Since both P1 and P2 exhibit similar affinity to MCP-1 and P2 (WKNFQTI) is a shorter peptide that allows facile synthesis and modification, only P2 were used in the subsequent studies.
3.3 Conjugation of peptide into hydrogels, peptide-functionalized hydrogel properties
Previously, several peptide-functionalized PEG hydrogels were engineered for a variety of controlled release and tissue engineering applications [10, 13, 37]. An efficient and convenient peptide conjugation scheme, namely thiol-acrylate photopolymerization (Fig. 3A), was utilized in this study to conjugate and crosslink affinity peptide analogs within PEG hydrogels. It has been revealed that the architecture of affinity peptides crosslinked in PEG hydrogels significantly affects their affinity binding to target proteins [37]. Since the main purpose of this study was to retain MCP-1 within hydrogels, peptide structures that offer high affinity binding are particularly favorable. Thus, three P2 analogs with an additional N-terminal cysteine and different spacers separating the cysteine residue (crosslinking site) and the affinity binding sequence (Fig. 3B) were synthesized and conjugated within PEG hydrogels to examine the relationship between peptide architecture and affinity binding, as well as to enhance the retention of MCP-1 within the permissive PEG hydrogels.
Figure 3.




(A) Reaction scheme of thiol-acrylate photopolymerization. (B) Chemical structure of a control peptide (CGKFIQWNT) with additional terminal cysteine and glycine residues. (C) Chemical structure of the affinity peptide (CGWKNFQTI) with an additional cysteine residue and a glycine spacer. (D) Chemical structure of the affinity peptide with an additional cysteine residue and three di-ethylene glycol spacers (COOOWKNFQTI, O: di-ethylene glycol spacer).
Prior to studying the affinity binding and retention of MCP-1 in PEG hydrogels, the efficiency of peptide incorporation within crosslinked PEG hydrogels was quantified to be at least 80% (data not shown). Further, it was determined that the incorporation of a small amount of P2 peptide analogs does not significantly alter the physical properties of the PEGDA-10kDa hydrogels (10wt% or 10mM). As shown in Fig. 4, rheological analysis revealed similar gel mechanical properties, implying a similar gel crosslinking density. Further, equilibrium gel swelling was not affected by the incorporation of the affinity peptides (data not shown), suggesting similar transport properties for small molecular weight molecules such as glucose, insulin, and most importantly in this study, cell-secreted MCP-1. It is important to note that maintaining the high permeability of hydrogels for small biomolecules is of paramount importance in the encapsulation of pancreatic β-cells due to the critical role of maintaining β-cells viability and facile insulin diffusion. Previously, it has been shown that the diffusion of insulin in the highly swollen PEG hydrogels was not affected by the incorporation of small quantities of crosslinkable peptides [10].
Figure 4.

Rheological analysis of PEG hydrogels incorporating with or without affinity peptides. (PEGDA: 10kDa, 10wt% ∼ 10mM; peptide concentration: 1mM) (Mean ± SEM, n=3)
3.4 Recombinant MCP-1 release from affinity hydrogels
Toward the goal of sequestering cell-secreted MCP-1 from PEG hydrogels, the efficiency of affinity peptide-functionalized PEG hydrogels in controlling the diffusion of in situ encapsulated recombinant MCP-1 was examined. Fig. 5 shows a rapid release of large quantities of MCP-1 from un-modified PEG hydrogels, revealing a major disadvantage of highly permeable PEG hydrogels in controlling the diffusion of small molecular weight biomolecules. Further, an intermediate release of MCP-1 was obtained when using affinity peptides with only one glycine spacer separating the crosslinking site (cysteine) and the affinity binding motif (WKNFQTI). Finally, very limited release of MCP-1 was observed from affinity peptide with three di-ethylene glycol spacers. Fig. 5 demonstrates that the release of MCP-1 from PEG hydrogels can be reduced significantly due to the binding of MCP-1 to network-immobilized affinity peptides. Furthermore, the binding can be further enhanced, at the same peptide incorporating concentration, by using affinity peptides with longer spacers. This demonstrates an important aspect of affinity binding in hydrogels using crosslinked peptide. Previously, work has shown that increasing spacer distance enhances the affinity binding of crosslinked peptides and soluble growth factor [37]. As shown in Fig. 5, enhanced binding leads to reduced MCP-1 release. The benefits of using peptides with specific affinity for cytokines are multifaceted. For example, similar tunable protein retention/release effects can be achieved by changing peptide affinity, concentration, architecture, and presentation in hydrogels. For example, increasing peptide concentrations in hydrogels or further increasing the length of the di(ethylene glycol) spacer may also result in similar enhanced protein retention, as suggested by prior research results [13, 37, 41, 45]. This former approach, however, will likely decrease the gel crosslinking density significantly due to the excessive chain transfer and termination during polymerization, while the latter may decrease the purity of the peptide due to the increased sequence length. Note that the absolute amounts of MCP-1 release shown in Fig. 5 may be skewed due to the practical limitation in the use of frozen MCP-1 aliquots and the short-term storage of the collected samples that result in decreased immunoreactivity in ELISA. However, the shape of the curves and the relative release amounts using different peptide architectures should remain the same, as all the samples were processed with the same conditions.
Figure 5.

Recombinant murine MCP-1 release from PEG gels (10%, 10kDa) with or without affinity peptide. Peptide incorporation concentration: 100μM. (Mean ± SEM, n=4).
3.5 MCP-1 secreted by MIN6 cells encapsulated in affinity hydrogels
MIN6 cells, a murine β-cell line, have been used in a variety of in vitro β-cell models focusing on understanding molecular and cell biology of type 1 diabetes. MIN6 cells have also been shown to secret MCP-1 under the stimulation of a cytokine cocktail containing tumor necrosis factor α (TNFα), interleukin-1β (IL-1β), and interferon γ (IFNγ) [33]. Here, MIN6 cells were encapsulated in either un-modified PEG hydrogels or gels functionalized with MCP-1 binding peptides. A high cell encapsulation density (200,000 cells/gel) was employed to increase cell-cell contact that maintains MIN6 cell viability during the course of study (data not shown). Following photoencapsulation, the cell-laden hydrogels were incubated in RPMI-1640 medium for one hour to allow for the removal of any sol fraction. Cell-laden hydrogels were transferred to media containing various concentrations of cytokine cocktails to stimulate the synthesis and secretion of MCP-1. Fig. 6A shows that the secretion of MCP-1 from MIN6s encapsulated in unmodified PEG hydrogels increases significantly and dose dependently under the stimulation of the cytokine cocktail, demonstrating that unmodified PEG hydrogels were unable to prevent the diffusion of small antigenic molecules, such as MCP-1, from permissive hydrogels network. The release of cell-secreted MCP-1 from affinity peptide-functionalized PEG hydrogels, however, was decreased significantly (Fig. 6B), with a trend similar to that shown in the release of recombinant MCP-1 (Fig. 5). Specifically, more than 60% of MCP-1 was sequestered and retained in PEG hydrogels after 24hr of cytokine stimulation.
Figure 6.

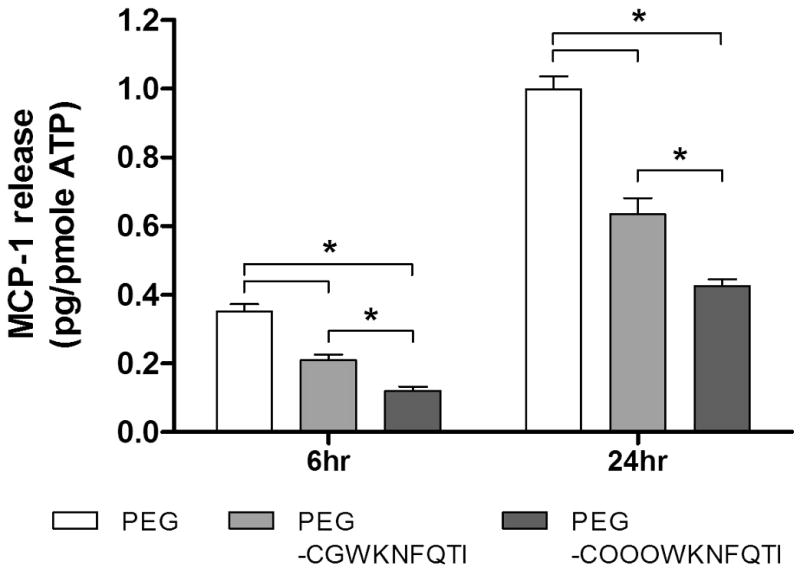
(A) MCP-1 release from MIN6 cells encapsulated in 10% PEG hydrogels incubated with or without a cytokine cocktail (1× cytokine: 750 units/mL IFN-γ, 10 units/mL IL-1β, and 500 units/mL TNFα). (B) MCP-1 release from MIN6 cells encapsulated in PEG hydrogels or PEG-peptide hydrogels incubated in 1× cytokine cocktail (peptide = 100μM, mean ± SEM, n=4). Asterisks represent statistical significance between indicated groups (p<0.05).
It should be noted that the purpose of using high concentrations of cytokine cocktail in Fig. 6 was to augment the effects of affinity binding on retaining MCP-1 within a short period of time (24hr). Clinically, the average amount of human MCP-1 (M.W. 8kDa) secreted by isolated islets is around 30 pg / islet / day (∼0.4 pmole) for high MCP-1 secreting islets. The affinity hydrogel formulation used in this study (100μM peptides in 30μL gels) theoretically is sufficient to sequester 3 nmole of MCP-1 in its maximal capacity. As noted before, increasing peptide affinity or concentration can further enhance the capacity of the affinity hydrogels in retaining cell-secreted MCP-1. Therefore, it is expected that the formulation of this affinity hydrogel platform can be readily adjusted to fulfill future clinically-relevant applications. Future studies will be focused on developing in vitro co-culture systems containing cell-laden hydrogels and monocytes, as well as in vivo studies to determine the therapeutic efficacy of this strategy in reducing local inflammation. Further, affinity peptides capable of binding to other chemokines (e.g., (IFNγ)-inducible protein-10 (IP-10), macrophage inflammatory protein (MIP), etc.) might be implemented together with MCP-1 binding peptides developed here to augment the effects of reducing chemokine diffusion.
4. Conclusions
An affinity hydrogel platform with the ability to sequester selectively a chemokine, MCP-1, was developed. In this study, two peptide sequences with binding affinity to murine MCP-1 were identified from extracellular loops of murine CCR2. A peptide analog, namely WKNFQTI, was incorporated within highly swollen PEG hydrogels to selectively and specifically bind to recombinant murine MCP-1 without affecting the bulk properties of hydrogels. Further, the release of in situ encapsulated MCP-1 was reduced by changing the peptide architecture (i.e., adding a tri-diethylene glycol spacer). Similarly, the release of MCP-1 secreted by the encapsulated MIN6 cells was reduced significantly. In addition to the encapsulation of pancreatic β-cells for type 1 diabetes, this strategy also has the potential to enhance the immuno-isolating function of PEG hydrogels, through selectively reducing diffusion of antigens, in the encapsulation and delivery of allogenic cell types.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (R01DK076084) and the Howard Hughes Medical Institute. The authors would like to thank the support to P.D.B. from the Research Experience for Undergraduates (REU) in Functional Materials Science and Engineering supported by the National Science Foundation. The authors also thank the support to A.A.A. from Graduate Assistance in Areas of National Need (GAANN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wilson JT, Chaikof EL. Challenges and emerging technologies in the immunoisolation of cells and tissues. Advanced Drug Delivery Reviews. 2008;60:124–145. doi: 10.1016/j.addr.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanemura M, Saga A, Kawamoto K, Machida T, Deguchi T, Nishida T, Sawa Y, Doki Y, Mori M, Ito T. Rapamycin Induces Autophagy in Islets: Relevance in Islet Transplantation. Transplantation Proceedings. 2009;41:334–338. doi: 10.1016/j.transproceed.2008.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Aronovitz A, Josefson J, Fisher A, Newman M, Hughes E, Chen F, Moons DS, Kiyokawa H, Lowe WL. Rapamycin Inhibits Growth Factor-Induced Cell Cycle Regulation in Pancreatic beta Cells. Journal of Investigative Medicine. 2008;56:985–996. doi: 10.2310/JIM.0b013e31818ce763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakata M, Yasuda H, Moriyama H, Yamada K, Kotani R, Kurohara M, Okumachi Y, Kishi M, Arai T, Hara K, Hamada H, Yokono K, Nagata M. Prevention of recurrent but not spontaneous autoimmune diabetes by transplanted NOD islets adenovirally Transduced with immunomodulating molecules. Diabetes Research and Clinical Practice. 2008;80:352–359. doi: 10.1016/j.diabres.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Larsen CP, Knechtle SJ, Adams A, Pearson T, Kirk AD. A new look at blockade of T-cell costimulation: A therapeutic strategy for long-term maintenance immunosuppression. American Journal of Transplantation. 2006;6:876–883. doi: 10.1111/j.1600-6143.2006.01259.x. [DOI] [PubMed] [Google Scholar]
- 6.Bell E, Cao XP, Moibi JA, Greene SR, Young R, Trucco M, Gao ZY, Matschinsky FM, Deng SP, Markman JF, Naji A, Wolf BA. Rapamycin has a deleterious effect on MIN-6 cells and rat and human islets. Diabetes. 2003;52:2731–2739. doi: 10.2337/diabetes.52.11.2731. [DOI] [PubMed] [Google Scholar]
- 7.Cushing MC, Anseth KS. Hydrogel cell cultures. Science. 2007;316:1133–1134. doi: 10.1126/science.1140171. [DOI] [PubMed] [Google Scholar]
- 8.Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Progress in Polymer Science. 2008;33:167–179. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weber LM, Hayda KN, Anseth KS. Cell-Matrix Interactions Improve beta-Cell Survival and Insulin Secretion in Three-Dimensional Culture. Tissue Engineering Part A. 2008;14:1959–1968. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CC, Anseth KS. Glucagon-Like Peptide-1 Functionalized PEG Hydrogels Promote Survival and Function of Encapsulated Pancreatic beta-Cells. Biomacromolecules. 2009 doi: 10.1021/bm900420f. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CC, Anseth KS. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharmaceutical Research. 2009;26:631–643. doi: 10.1007/s11095-008-9801-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin CC, Metters AT. Hydrogels in controlled release formulations: Network design and mathematical modeling. Advanced Drug Delivery Reviews. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Lin CC, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials. 2009;30:4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheung CY, McCartney SJ, Anseth KS. Synthesis of Polymerizable Superoxide Dismutase Mimetics to Reduce Reactive Oxygen Species Damage in Transplanted Biomedical Devices. Advanced Functional Materials. 2008;18:3119–3126. [Google Scholar]
- 15.Clark RAF. Basics of Cutaneous Wound Repair. Journal of Dermatologic Surgery and Oncology. 1993;19:693–706. doi: 10.1111/j.1524-4725.1993.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 16.Yang ZD, Chen M, Ellett JD, Carter JD, Brayman KL, Nadler JL. Inflammatory blockade improves human pancreatic islet function and viability. American Journal of Transplantation. 2005;5:475–483. doi: 10.1111/j.1600-6143.2005.00707.x. [DOI] [PubMed] [Google Scholar]
- 17.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: Molecular and cellular mechanisms. Journal of Investigative Dermatology. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 18.Miller MD, Krangel MS. Biology and Biochemistry of the Chemokines - a Family of Chemotactic and Inflammatory Cytokines. Critical Reviews in Immunology. 1992;12:17–46. [PubMed] [Google Scholar]
- 19.Murphy PM. The Molecular-Biology of Leukocyte Chemoattractant Receptors. Annual Review of Immunology. 1994;12:593–633. doi: 10.1146/annurev.iy.12.040194.003113. [DOI] [PubMed] [Google Scholar]
- 20.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: Basic chemokinese grammar for immune cells. Annual Review of Immunology. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 21.Jiang YL, Beller DI, Frendl G, Graves DT. Monocyte Chemoattractant Protein-1 Regulates Adhesion Molecule Expression and Cytokine Production in Human Monocytes. Journal of Immunology. 1992;148:2423–2428. [PubMed] [Google Scholar]
- 22.Rollins BJ, Walz A, Baggiolini M. Recombinant Human MCP-1/JE Induces Chemotaxis, Calcium Flux, and the Respiratory Burst in Human Monocytes. Blood. 1991;78:1112–1116. [PubMed] [Google Scholar]
- 23.Sozzani S, Locati M, Allavena P, VanDamme J, Mantovani A. Chemokines: A superfamily of chemotactic cytokines. International Journal of Clinical & Laboratory Research. 1996;26:69–82. doi: 10.1007/BF02592349. [DOI] [PubMed] [Google Scholar]
- 24.Xu LL, Warren MK, Rose WL, Gong WH, Wang JM. Human recombinant monocyte chemotactic protein and other c-c chemokines bind and induce directional migration of dendritic cells in vitro. Journal of Leukocyte Biology. 1996;60:365–371. doi: 10.1002/jlb.60.3.365. [DOI] [PubMed] [Google Scholar]
- 25.Vecchi A, Massimiliano L, Ramponi S, Luini W, Bernasconi S, Bonecchi R, Allavena P, Parmentier M, Mantovani A, Sozzani S. Differential responsiveness to constitutive vs. inducible chemokines of immature and mature mouse dendritic cells. Journal of Leukocyte Biology. 1999;66:489–494. doi: 10.1002/jlb.66.3.489. [DOI] [PubMed] [Google Scholar]
- 26.Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. Journal of Experimental Medicine. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arimilli S, Ferlin W, Solvason N, Deshpande S, Howard M, Mocci S. Chemokines in autoimmune diseases. Immunological Reviews. 2000;177:43–51. doi: 10.1034/j.1600-065x.2000.17716.x. [DOI] [PubMed] [Google Scholar]
- 28.Cameron MJ, Arreaza GA, Grattan M, Meagher C, Sharif S, Burdick MD, Strieter RM, Cook DN, Delovitch TL. Differential expression of CC chemokines and the CCR5 receptor in the pancreas is associated with progression to type I diabetes. Journal of Immunology. 2000;165:1102–1110. doi: 10.4049/jimmunol.165.2.1102. [DOI] [PubMed] [Google Scholar]
- 29.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocrine Reviews. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- 30.Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, Bianchi G, Sica A, Peri G, Melzi R, Aldrighetti L, Secchi A, Di Carlo V, Allavena P, Bertuzzi F. Human pancreatic islets produce and secrete MCP-1/CCL2: Relevance in human islet transplantation. Diabetes. 2002;51:55–65. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- 31.Chipitsyna G, Gong QK, Gray CF, Haroon Y, Kamer E, Arafat HA. Induction of monocyte chemoattractant protein-1 expression by angiotensin II in the pancreatic islets and beta-cells. Endocrinology. 2007;148:2198–2208. doi: 10.1210/en.2006-1358. [DOI] [PubMed] [Google Scholar]
- 32.Kutlu B, Darville MI, Cardozo AK, Eizirik DL. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic beta-cells. Diabetes. 2003;52:348–355. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- 33.Baker MS, Chen XJ, Rotramel A, Nelson J, Kaufman DB. Proinflammatory cytokines induce NF-kappa B-dependent/NO-independent chemokine gene expression in MIN6 beta cells. Journal of Surgical Research. 2003;110:295–303. doi: 10.1016/s0022-4804(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 34.Zineh I, Beitelshees AL, Silverstein JH, Haller MJ. Serum Monocyte Chemoattractant Protein-1 Concentrations Associate With Diabetes Status but Not Arterial Stiffness in Children With Type 1 Diabetes. Diabetes Care. 2009;32:465–467. doi: 10.2337/dc08-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogliari AC, Caldara R, Socci C, Sordi V, Cagni N, Moretti MP, Dell'Acqua A, Mercalli A, Scavini M, Secchi A, Bonifacio E, Bosi E, Piemonti L. High levels of donor CCL2/MCP-1 predict graft-related complications and poor graft survival after kidney-pancreas transplantation. American Journal of Transplantation. 2008;8:1303–1311. doi: 10.1111/j.1600-6143.2008.02240.x. [DOI] [PubMed] [Google Scholar]
- 36.Suggs LJ, Shive MS, Garcia CA, Anderson JM, Mikos AG. In vitro cytotoxicity and in vivo biocompatibility of poly(propylene fumarate-co-ethylene glycol) hydrogels. Journal of Biomedical Materials Research. 1999;46:22–32. doi: 10.1002/(sici)1097-4636(199907)46:1<22::aid-jbm3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 37.Lin CC, Anseth KS. Controlling Affinity Binding with Peptide-Functionalized Poly(ethylene glycol) Hydrogels. Advanced Functional Materials. 2009;19:2325–2331. doi: 10.1002/adfm.200900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salinas CN, Anseth KS. Mixed mode thiol-acrylate photopolymerizations for the synthesis of PEG-peptide hydrogels. Macromolecules. 2008;41:6019–6026. [Google Scholar]
- 39.Han KH, Green SR, Tangirala RK, Tanaka S, Quehenberger O. Role of the first extracellular loop in the functional activation of CCR2 - The first extracellular loop contains distinct domains necessary for both agonist binding and transmembrane signaling. Journal of Biological Chemistry. 1999;274:32055–32062. doi: 10.1074/jbc.274.45.32055. [DOI] [PubMed] [Google Scholar]
- 40.Kim MY, Byeon CW, Hong KH, Han KH, Jeong S. Inhibition of the angiogenesis by the MCP-1 (monocyte chemoattractant protein-1) binding peptide. FEBS Letters. 2005;579:1597–1601. doi: 10.1016/j.febslet.2005.01.070. [DOI] [PubMed] [Google Scholar]
- 41.Lin CC, Metters AT. Metal-chelating affinity hydrogels for sustained protein release. Journal of Biomedical Materials Research Part A. 2007;83A:954–964. doi: 10.1002/jbm.a.31282. [DOI] [PubMed] [Google Scholar]
- 42.Fairbanks BD, Schwartz MP, Bowman CN, Anseth KS. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.08.055. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Majima T, Schnabel W, Weber W. Phenyl-2,4,6-Trimethylbenzoylphosphinates as Water-Soluble Photoinitiators - Generation and Reactivity of O=P(C6h5)(O-) Radical-Anions. Makromolekulare Chemie-Macromolecular Chemistry and Physics. 1991;192:2307–2315. [Google Scholar]
- 44.Lee JY, Choo JE, Choi YS, Suh JS, Lee SJ, Chung CP, Park YJ. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials. 2009;30:3532–3541. doi: 10.1016/j.biomaterials.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Lin CC, Metters AT. Bifunctional monolithic affinity hydrogels for dual-protein delivery. Biomacromolecules. 2008;9:789–795. doi: 10.1021/bm700940w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


