Abstract
The concomitant use of alcohol (EtOH) and the psychotherapeutic agent dl-methylphenidate (MPH) has risen as a consequence of an increase in ADHD diagnoses within the drinking age population. It was recently found that the combination of MPH and EtOH increases the self-report of pleasurable feelings relative to MPH alone. This finding raises concerns regarding the combined abuse liability for these two widely used drugs. The present behavioral study reports on the development of an adult male C57BL/6J (B6) mouse model to further characterize this MPH-EtOH interaction. We examined the effects of MPH on EtOH consumption in a limited access paradigm and EtOH stimulation of locomotor activity. B6 mice consumed about 2 g/kg EtOH daily and MPH dose-dependently reduced drinking. The most effective dose of MPH was 1.25 mg/kg, which produced a 41% decrease in drinking and had no effect on locomotor activity. However, when the 1.25 mg/kg dose of MPH was combined with a stimulatory dose of ethanol (1.75 g/kg) by intraperitoneal injection, there was a significantly enhanced stimulation of locomotor activity. The drug combination increased activity compared to the vehicle or MPH injections by 45% and increased the activity relative to EtOH alone by an additional 25%. The results of the EtOH and MPH interactions observed with the mouse model appear to be behaviorally relevant and suggest several converging mechanisms that may underlie MPH-EtOH interactions.
Introduction
Alcohol drinking frequently occurs in combination with the administration of other drugs (McCabe et al., 2006), including the psychostimulant methylphenidate (MPH). Although use of this specific combination of psychoactive drugs has been reported in the context of abuse or misuse (Barrett & Pihl, 2002), concomitant use of MPH and alcohol (ethanol, EtOH) also occurs when MPH is used therapeutically in adults who consume EtOH. In fact, the use of MPH in patients of legal drinking age has substantially risen as a consequence of the increasing recognition that Attention-Deficient/Hyperactivity Disorder (ADHD) commonly persists into adulthood (Biederman & Faraone, 2005; Biederman & Spencer, 2002). Additionally, particular health concerns arise with the frequent experimentation or co-abuse of illicit MPH with EtOH in high school students and college undergraduates (Godfrey, 2009; Johnston et al., 2008; Mallonee & Calvin, 2005; McCabe et al., 2004; Teter et al., 2006), as well as in individuals across the lifespan (Hasin et al., 2007). Approximately one third of prescribed ADHD medications are to individuals at least 20 years of age, with the most common medication being racemic (i.e., dl-) MPH (Okie, 2006). In addition, MPH abuse appears to be in part driven by the “widespread” belief that ADHD stimulant medications permit the consumption of more EtOH, thus facilitating late-night partying [see (Godfrey, 2009)].
Although the interactive effects of MPH and EtOH on behavior are largely unexplored, emerging findings indicate that they deserve further attention. Previous work established that a MPH and EtOH combination interacted to increase self-report of pleasurable feelings in normal subject volunteers when compared to MPH administered alone (Patrick et al., 2007). Although the study by Patrick and colleagues (2007) did not examine the effects of EtOH alone, numerous human studies support the position that EtOH by itself produces pleasurable effects [e.g. (Gilman et al., 2008; King et al., 2002; Thomas et al., 2004)]. Therefore, the findings regarding the enhanced pleasurable feelings of the MPH-EtOH combination carry implications for abuse liability.
The mechanism for these interactive effects is likely multi-faceted in nature. On the one hand, the altered subjective effects of this drug combination may be mediated through converging mechanisms of action of EtOH and MPH on multiple neurotransmitters in the central nervous system, including the dopaminergic (Pierce & Kumaresan, 2006) and noradrenergic (Markowitz & Patrick, 2008) systems. Additionally, it is known that the combination of MPH and EtOH leads to the production of a transesterification metabolite, ethylphenidate, in humans (Markowitz et al., 2000; Patrick et al., 2007), with the preponderance being the pharmacologically inactive l-isomer (Patrick et al., 2007; Patrick et al., 2005; Williard et al., 2007). In contrast, the pharmacologically active d-isomer of ethylphenidate, although only detected in the picogram/ml range in humans given a standard EtOH drink and a therapeutically relevant MPH dose (Patrick et al., 2007), may be more important in overdose situations when higher levels of both drugs are present (Markowitz et al., 1999). Importantly, it was determined that levels of the active d-isomer of MPH were significantly elevated in human subjects when dl-MPH was combined with EtOH (Patrick et al., 2007). Thus, the mechanisms underlying the increase in pleasurable feelings reported following the drug combination are likely to be complex, involving neurobiological and pharmacokinetic interactions.
Given such complexity, preclinical models become complementary to human studies allowing more systematic and experimentally controlled examination of these interactions. In this regard, C57BL/6J (B6) mice exhibit dose-dependent increases in motor activity when challenged with MPH (Williard et al., 2007) as previously shown for rats (Patrick et al., 1987). B6 mice also biotransform MPH into ethylphenidate upon concomitant administration of MPH and EtOH (Williard et al., 2007). Further, B6 mice avidly self-administer EtOH under free-access (Belknap et al., 1993; Middaugh et al., 1999) and limited access (Griffin et al., 2009a; Griffin et al., 2009b; Griffin et al., 2007) conditions. In addition to the increase in activity noted for MPH (Williard et al., 2007), B6 mice also demonstrate an increase in activity when challenged with low doses of EtOH (Middaugh et al., 1992; Phillips & Shen, 1996), which may in part model the stimulant effects of EtOH in humans (Davidson et al., 2002; Martin et al., 1993; Thomas et al., 2004). Accordingly, this particular mouse strain appears to provide an appropriate model system for investigating MPH and EtOH interactions.
The present study examined the effect of MPH on motor stimulation produced by low EtOH doses and on voluntary EtOH consumption. Taken together, these two well-characterized behaviors in B6 mice provide a behavioral probe for evaluating potential interactive effects of MPH and EtOH. We hypothesized MPH would enhance the effects of EtOH as reflected in enhanced stimulation of motor activity produced by low doses of ethanol and enhanced reward value of ethanol as reflected in reduced EtOH consumption in a free access paradigm.
Methods
Subjects
Male C57BL/6J mice (8 weeks of age) were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were individually housed in polycarbonate cages (in cm: 17.1 w × 28.3 l × 12.4 d) with wood shavings and stainless steel wire lids, and maintained in a temperature- and humidity-controlled AAALAC accredited animal facility under a 12 hr light cycle (lights off 0700 hr). Mice had free access to food and water at all times during experimental procedures. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina and were consistent with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996). Mice were acclimated to the vivarium for a minimum of one week prior to the start of the experiments. Separate groups of mice were used to examine the effects of MPH on EtOH consumption (Experiment 1, n=15) and to examine the effects of MPH on EtOH stimulation (Experiment 2, n=24).
Limited Access to EtOH
Mice were given limited access to ethanol in the home cage five days per week, Monday through Friday, similar to methods previously described (Griffin et al., 2009a; Griffin et al., 2009b; Griffin et al., 2007) although access to EtOH was given later in the dark cycle than the previous studies. It should also be noted that sucrose or saccharin fading procedures were not used. At 1300hr, water bottles were removed from the home cage and replaced with 15 ml graduated bottles, one containing EtOH (15% v/v) and the other tap water. Following the 2 hr access period, the graduated bottles were removed from the home cage and replaced with water bottles. The volume consumed over the 2 hour period was recorded and converted to g/kg amounts based on a weekly body weight measurement of the mouse. Spillage and evaporation were accounted for in the calculations by subtracting fluid loss measured from bottles placed on empty cages. The position of the water and EtOH bottles was alternated daily to avoid potential side preferences exhibited by the mice.
Locomotor Activity Monitoring
Locomotor activity was assessed with a Digiscan Animal Activity Monitor system, model RXYZCM(8) TAO with a two-animal option (Accuscan Instruments, Columbus, OH). Each activity chamber contained 2 arrays of 16 photobeams spaced 5 cm apart, one array was located 1.5 cm above floor level to capture horizontal activity and the other was located 6.5 cm above the floor to capture vertical activity (i.e. rearing behavior) of the mice. The Versamax Analyser (Version 4.00-1375E) recorded the interruption of each beam during testing. Each activity chamber was partitioned into 20 × 20 cm quadrants with acrylic dividers to allow two mice to be tested together; however, in these studies only a single mouse was tested within an activity chamber at one time. Each of the activity chambers were enclosed in 90 × 54 × 35 cm sound-attenuated boxes.
Drug Administration
d,l-MPH•HCl was purchased from Sigma, Inc, d,l-ethylphenidate•HCl was synthesized in-house (Patrick et al., 2005) and EtOH was purchased from Pharmaco-Aaper, Inc. MPH and ethylphenidate doses are expressed as their hydrochloride salts and administered by intraperitoneal (ip) injection using 0.9% saline as the vehicle. For the EtOH consumption experiment, the injection volume was 0.01ml/kg. For the EtOH stimulation experiment, EtOH and MPH were injected simultaneously (ip.) using a volume of 0.02ml/kg. EtOH (95%) was diluted with 0.9% saline prior to injection to a concentration of 12.17% (v/v).
Experiment 1: Effects of MPH on EtOH Consumption
This experiment evaluated the effects of MPH on EtOH consumption while mice were maintained on the limited access paradigm. In order to habituate the mice to the injection procedure, saline injections [0.01 ml/kg] began several days prior to the introduction of limited access drinking and continued prior to each drinking session throughout the remainder of the study. The injections were given 15 minutes prior to EtOH access which was based on ongoing MPH discrimination studies indicating this pretreatment time supports the interoceptive cue of MPH (Griffin & Patrick, unpublished). The experiment began after 8 weeks of limited access sessions and MPH was administered each Wednesday, according to a Latin-square design such that each mouse experienced all MPH doses and vehicle (Bradley, 1958).
After the MPH portion of the experiment, mice continued drinking on the same schedule but received only vehicle injections Monday through Friday for 1 week. Following this washout period, mice were randomly assigned to be challenged with a single dose of 2.5 mg/kg ethylphenidate or vehicle each Wednesday for 2 weeks, using a procedure similar to that described above for MPH. This dose of ethylphenidate was chosen based on previous work indicating that it did not increase locomotor activity in C57BL/6J mice (Williard et al., 2007).
Experiment 2: Effects of MPH on EtOH Stimulation
This experiment evaluated the effects of MPH, EtOH and their combination on locomotor activity. In order to approximate the conditions of the first experiment in which mice were drinking ethanol in the 2 hr limited access paradigm, mice in experiment 2 were also given access to ethanol in the limited access paradigm for 3 weeks and experienced daily saline injections for habituation to the injection procedure. It should be noted that the stimulatory effects of EtOH in B6 mice have been observed whether mice are maintained with access to ethanol for consumption or not (Middaugh et al., 1992; Middaugh et al., 1987; Middaugh et al., 1989). After this period of EtOH drinking, on Monday and Thursday of each week, mice were exposed to the locomotor activity chambers for 20 minute sessions, but were not given access to ethanol for drinking. The mice resumed drinking on Tuesday, Wednesday and Friday in the limited access paradigm as usual. The testing design was modeled after a previous study (Middaugh et al., 1992), although food restriction was not used in the present study.
Locomotor activity was assessed in the mice under 4 conditions according to a Latin-Square design. The 4 conditions were: 1) Vehicle, 2) 1.25 mg/kg MPH, 3)1.75 g/kg EtOH and 4) 1.25 mg/kg MPH + 1.75 g/kg EtOH. Each condition was represented during each activity session except the first session in which all mice received vehicle injections in order to habituate them to the testing equipment. Injections were given five minutes prior to being placed into the chamber because this pretreatment interval consistently produces an increase in locomotor activity in C57BL/6J mice (Middaugh et al., 1992; Middaugh et al., 1987; Middaugh et al., 1989). The activity assessments occurred at the time when mice would have been given EtOH to drink in the limited access paradigm. The dose of MPH (1.25 mg/kg) for this study was chosen based on results from the first study showing it substantially reduced EtOH consumption and was not expected to increase locomotor activity based on our previous work (Williard et al., 2007). The EtOH dose (1.75 g/kg) was chosen based on previous studies indicating it increases locomotion (Jerlhag, 2008; Middaugh et al., 1992; Middaugh et al., 1987) and supports expression of place preference (Middaugh & Bandy, 2000; Nocjar et al., 1999) in B6 mice, consistent with the stimulant and pleasurable effects of low ethanol doses in humans (Gilman et al., 2008; King et al., 2002; Martin et al., 1993; Thomas et al., 2004).
Data Analysis
The primary data for the EtOH consumption study was g/kg and data from the active drug day (Wednesday) were analyzed by one-way Repeated measures Analysis of Variance (ANOVA) using Dose as a grouping factor. For the EtOH stimulation study, horizontal and vertical activity measures were analyzed using Two-way ANOVA with Group as a between-subjects factor and Time Bin as a repeated measure. Bodyweights from both studies were analyzed using One-way ANOVA using experimental day as a grouping factor. In both experiments, post hoc comparisons of significant main effects or factor interactions were made using pairwise comparisons with Bonferroni’s correction, when appropriate. For all analyses, significance levels were set at p< 0.05.
Results
Experiment 1: Effects of MPH on EtOH Consumption
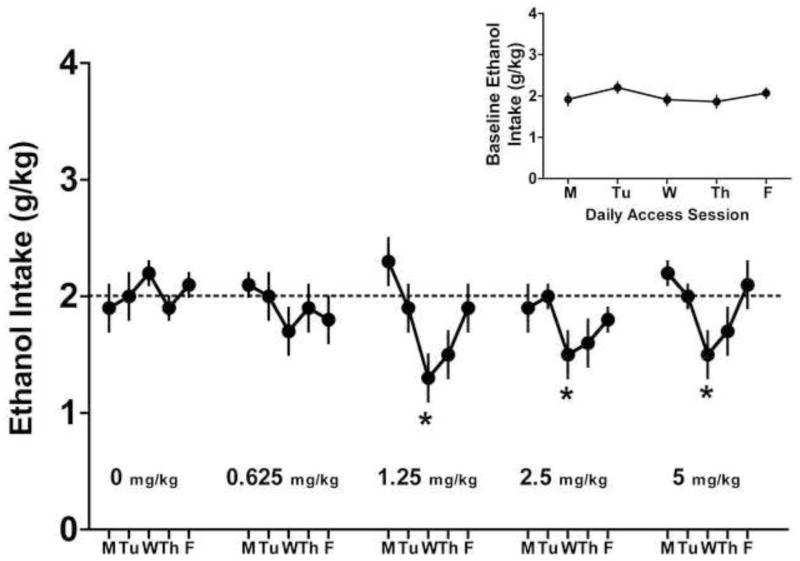
Before beginning the experiment, mice were trained to drinking ethanol in the limited access paradigm and habituated to the injection procedure. As can be seen in the inset of Figure 1, ethanol intake during the last week of baseline drinking was quite consistent across days and averaged 2.0 ± 0.14 g/kg for the 5 days of access. Two mice showed a steady decline in drinking over 2–3 weeks after completing baseline to daily ethanol intake consistently below 1.5 g/kg and were excluded, leaving n = 13 for all analyses.
Figure 1.
Methylphenidate (MPH) dose-dependently reduced ethanol (EtOH) consumption in EtOH-preferring C57BL/6J mice (n=13) in a 2 hour, 2 bottle-choice limited access procedure. The inset of the figure shows the last week of baseline drinking before the experiment began. Average drinking during this week was 2.0 ± 0.14 g/kg and is indicated by the dashed line in the main figure. MPH significantly reduced ethanol drinking at the 3 highest doses (*p<0.05) and there was a trend for a reduction in drinking the day after MPH challenge. Values are means ± S.E.M.
The results of Study 1 are summarized in Figure 1. The data show that MPH, given each Wednesday, dose-dependently reduced EtOH intake (g/kg) during the limited access procedure. This observation was supported by a One Way (5 Doses) Repeated Measures ANOVA which indicated a significant effect of MPH dose on EtOH consumption on Wednesdays (F (4,48) = 3.6, p = 0.012). Post hoc analysis indicated that consumption, relative to the vehicle injection, was significantly reduced at the four highest doses (all ps<0.05) but not at the lowest dose tested (0.625 mg/kg; p=0.06). Additionally, visual inspection of the data in Figure 1 suggests that EtOH consumption appeared to be suppressed on Thursdays, particularly after the 1.25 mg/kg dose was administered. However, one way repeated measures analysis on these data did not substantiate this effect (F (5, 60) = 1.24, p = 0.302) of MPH on EtOH drinking on the day after active drug administration. The lowest effective dose of MPH (1.25 mg/kg) which tended to have the greatest effect on drinking (41% reduction) was not expected to be behaviorally active based on previous locomotor activity studies (Williard et al., 2007).
Following the washout period (see methods), the effect of a single ethylphenidate dose (2.5 mg/kg) was evaluated similarly to MPH using the Latin square design. After ethylphenidate was administered, mice consumed 1.9 ± 0.2 g/kg EtOH compared with 2.2 ± 0.2 g/kg EtOH after vehicle injection. Although there appeared to be a trend for ethylphenidate to reduce drinking, this was not statistically significant (t-test: t = 0.995, df = 26, p = 0.329). Thus, we found that 2.5 mg/kg ethylphenidate did not affect drinking under these conditions. Further doses were not tested in this experiment due to limited availability of ethylphenidate.
Consistent with a previous report (Griffin et al., 2009b), mice directed nearly all of their consummatory behavior to the ethanol bottle because water intake during the 2 hour period was quite low (generally <0.2ml). Thus, the effects of MPH or ethylphenidate on water intake were not evaluated.
Experiment 2: Effects of MPH on EtOH Stimulation
Prior to locomotor activity testing, mice consumed an average of 1.9 ± 0.1 g/kg EtOH during the limited access procedure for the last 5 days of the baseline period. During the active period of locomotor testing, mice drank on average 2.0 ± 0.1 g/kg, essentially the same amount of EtOH, when given access to EtOH on the limited access schedule (see methods). As noted for the first study, mice drank little water during the limited access sessions (<0.3 ml).
Initial analysis of total horizontal activity measures from this study indicate that the combination of MPH and EtOH increased locomotion compared to either drug administered alone. Vehicle and MPH administration produced similar total activity measures of 5927 ± 223 and 5771 ± 446, respectively, indicating that MPH at this dose did not stimulate locomotor activity. On the other hand, EtOH increased total activity to 6903 ± 452 (16% greater than vehicle) and the drug combination further increased total activity to 8632 ± 422 (45% greater than vehicle and 25% greater than EtOH).
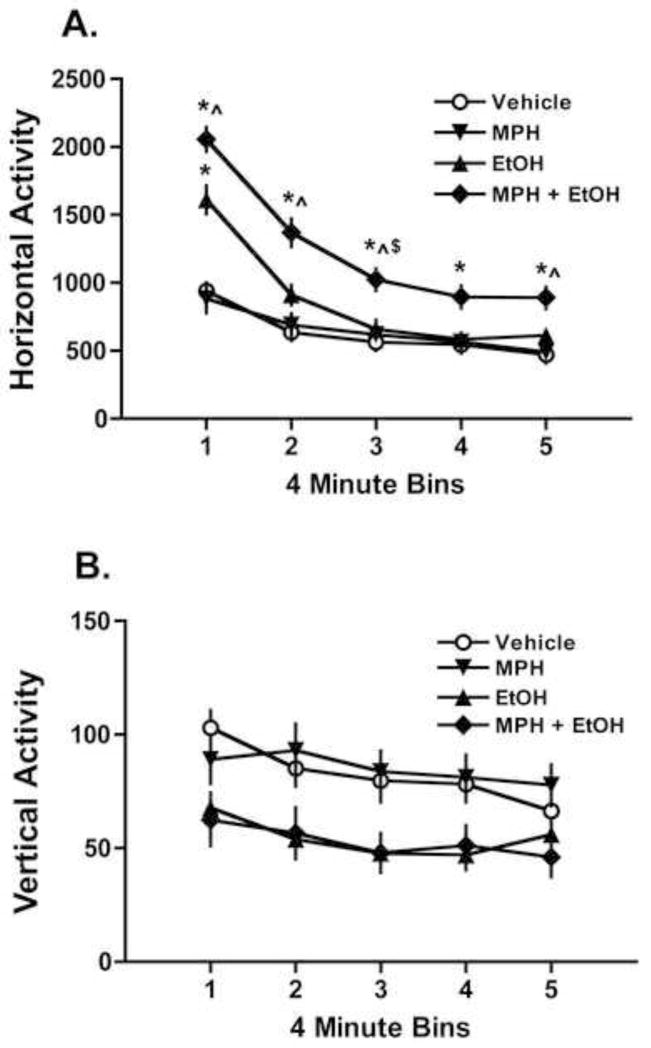
The horizontal activity data were further analyzed by examining changes in activity across time for the different treatment groups and are summarized in Figure 2. Figure 2A shows horizontal activity in 4 minute bins for the mice after vehicle, EtOH 1.75 g/kg, MPH 1.25 mg/kg and the drug combination with the same doses. When administered alone, 1.25 mg/kg MPH did not alter horizontal activity relative to treatment with vehicle. On the other hand, 1.75 g/kg EtOH increased horizontal activity compared to the vehicle injection, consistent with previous reports (Jerlhag, 2008; Middaugh et al., 1992; Middaugh et al., 1987). The combination of 1.25 mg/kg MPH and 1.75 mg/kg EtOH significantly increased activity compared to EtOH alone. These observations were supported by a significant factor interaction in the 4 (Group) × 5 (Time Bin) repeated measures ANOVA (F(12,368) = 6.895, p<0.001). As shown in Figure 2A, post-hoc analysis indicated a significant EtOH stimulation effect which resolved by approximately 10 minutes into the test session. Post-hoc analysis also indicated a significant increase in locomotor activity when the MPH and EtOH combination was administered, which declined over time, but activity was still elevated relative to MPH alone and saline by the end of the test session.
Figure 2.
Methylphenidate (MPH) enhanced the locomotor stimulating effects of EtOH in C57BL/6J mice (n=24). Mice were administered vehicle (0.9% saline) EtOH (1.75 g/kg), MPH (1.25 mg/kg) or the combination in a Latin-square design. A) EtOH increased horizontal activity but MPH did not. The drug combination significantly enhanced horizontal activity relative to the increase caused by EtOH alone, indicating a significant interaction between EtOH and MPH. B) EtOH treatment reduced vertical activity, consistent with known ataxic effects of EtOH at this dose. However, the drug combination did not further enhance this effect. (*p<0.05 versus vehicle; ^p<0.05 versus MPH; $p<0.05 versus EtOH). Values are means ± S.E.M.
Also shown in Figure 2 is vertical activity (2B), which reflects rearing behavior by the mice during the activity session. Not unexpectedly, vertical activity was reduced by 1.75 g/kg EtOH compared with vehicle exposure, consistent with the known ataxic effects of EtOH at this dose (Linsenbardt et al., 2009; Middaugh et al., 1992). Acute exposure to MPH did not alter vertical activity and the effects of EtOH on vertical activity were not additive when combined with MPH under these conditions. The 4 (Group) × 5 (Time Bin) repeated measures ANOVA did not indicate a significant factor interaction (F(12,368) = 1.241, p=0.253) but did indicate significant effects of Group (F(3,92) = 5.035, p = 0.003) and Time Bin (F(4,368) = 9.514, p <0.001). Taken together, the data from the EtOH stimulation study (Figure 2) indicate that the drug combination of EtOH and MPH increases locomotion relative to either drug given alone.
Subject bodyweights
In clinical studies, treatment with MPH has been associated with reduced weight and height gain in children under chronic therapy regimens (Mattes & Gittelman, 1983; Poulton & Cowell, 2003), suggesting reduced caloric intake during a rapid growth phase. Because the B6 mice in our experiments were challenged multiple times with MPH, albeit with different doses on widely spaced intervals, we examined bodyweights on testing days for both experiments. For Experiment 1, the mean bodyweights (in grams, ± S.E.M.) across consecutive weeks of testing were as follows: 28.8 ± 0.6, 30.1 ± 0.7, 29.9 ± 0.7, 30.0 ± 0.7 and 29.9 ± 0.7. Likewise, bodyweights (in grams, ± S.E.M.) across the consecutive weeks of testing for Experiment 2 were: 28.1 ± 0.5, 27.8 ± 0.5, 28.3 ± 0.5, 28.4 ± 0.5 and 28.8 ± 0.5. Inspection of the data for both experiments shows there was not a systematic change in bodyweight in either case, which was supported by separate One Way ANOVA’s on these two data sets [Experiment 1: F(4,60) = 0.576, p = 0.681; Experiment 2: F(4,115) = 0.565, p = 0.689]. Thus, bodyweights were not affected by MPH administered once per week in these experiments.
Discussion
In the present study, MPH altered both EtOH consumption and EtOH stimulation of locomotor activity in B6 mice suggesting an interaction of the two drugs. MPH dose-dependently reduced voluntary EtOH consumption by mice in a 2 bottle choice, limited access procedure. The lowest effective dose (1.25 mg/kg) of MPH did not increase locomotor activity, yet this dose was slightly more effective in reducing EtOH intake than the highest MPH dose tested (5 mg/kg). Importantly, the effect of MPH on EtOH motor stimulation was examined using the same B6 mouse strain, and maintained on the same limited access drinking paradigm used for the EtOH drinking experiment. In these EtOH-experienced mice, MPH significantly enhanced the motor stimulation produced by EtOH alone. Taken together, these experiments indicate that MPH and EtOH interact in a behaviorally relevant and potentiated manner.
In the first study, acute administration of MPH dose-dependently reduced voluntary EtOH consumption in B6 mice. This reduced EtOH consumption after MPH treatment is consistent with reports indicating that MPH reduces consumption of sucrose (Bello & Hajnal, 2006; Wayner et al., 1979; Wooters et al., 2006) and condensed milk (Eckerman et al., 1991) in rats and palatable foods in humans (Goldfield et al., 2007). The combined results of these studies suggest that acute MPH may generally reduce consumption of reinforcers (i.e. rewarding substances). The mechanism of action for the reduced EtOH consumption is unclear; however, our experiment indicating that the reduction in EtOH consumption produced by MPH was most robust at 1.25 mg/kg, a dose that does not alter locomotor activity (Figure 2), suggests that the effect of MPH on consumption of EtOH is not likely related to changes in locomotor activity. However, the possibility of increased activity cannot be completely ruled out since some EtOH consumption did occur and both drugs were probably present together, at least in small concentrations. Nevertheless, reduced EtOH consumption appeared to persist to the day after MPH challenge, although this was statistically non-significant, again suggesting that acute locomotor activating effects of MPH does not play a major role in its effects on EtOH consumption. Rather, substantial evidence suggests that B6 mice and other EtOH-preferring rodents consume EtOH for its post-ingestive, rewarding effects (Middaugh et al., 1999; Samson et al., 2004); consistent with EtOH interacting with neurobiological systems related to reward functions. Because MPH has rewarding properties in its own right, as indicated by the fact it supports reinforced behaviors (Botly et al., 2008; Nielsen et al., 1984), the results of our study suggest that pretreatment with low doses of MPH may promote satiation from less EtOH (or palatable foods). This interpretation is also consistent with our previous human study indicating that adding a single EtOH drink (0.6 g/kg) to a typical therapeutic dose of MPH (0.3 mg/kg) significantly increased the subjective feelings of pleasure compared with the dose of MPH alone (Patrick et al., 2007). This interactive effects of the two drugs could imply that the two drugs interact with similar neurobiological systems relevant to reinforcement and suggests that in the presence of MPH, less EtOH is required to produce similar effects. The extent of the interactive effects of the two drugs remains an area of active investigation.
It is important to note that at the doses used in the present study, MPH did not eliminate, but did lower EtOH consumption, leaving open the possibility of pharmacodynamic or pharmacokinetic interactions between MPH and EtOH accounting for the altered behavioral effect of drug combination. This possibility was further investigated in the second experiment which determined the effects of the 1.25 mg/kg MPH dose on a stimulating dose of EtOH in B6 mice maintained in the limited access drinking paradigm. At the doses tested, injected EtOH increased motor activity, while MPH did not. Both findings are consistent with the known stimulatory effects of EtOH in mice (Jerlhag, 2008; Middaugh et al., 1992; Middaugh et al., 1987), and our previous study with MPH in which the lowest dose tested (2.5 mg/kg) only modestly increased locomotion (Williard et al., 2007). Most importantly, however, the MPH dose that was without effect on locomotor activity markedly potentiated the stimulating effects of EtOH. This finding is consistent with MPH and EtOH interacting in a functionally important manner to alter behavior. It should be noted that these observations were obtained from EtOH-experienced mice, leaving open the possibility of qualitative or quantitative differences in the interactive effects of the two drugs on EtOH-naïve mice. Although the influence of EtOH drinking history remains to be tested, our results provide clear evidence of an interactive effect of these two psychoactive drugs.
The underlying mechanism for this interactive effect of MPH and EtOH was not ed in the current studies but is the subject of ongoing investigations. The present findings indicate that MPH doses having no effect on motor activity of mice, can engage central neurotransmitter systems thereby enhancing the stimulant effects of EtOH. This interpretation is consistent with a recent report that low doses of MPH (0.5 to 1 mg/kg) injected into rats improved cognitive task performance without increasing locomotor activity, yet, increased extracellular dopamine and norepinephrine in the nucleus accumbens, prefrontal cortex and medial septal brain regions (Berridge et al., 2006). Indeed, it is established that MPH injected into B6 mice blocks dopamine and norepinephrine transporters (Williard et al., 2007) leading to elevated levels of these neurotransmitters. Although the specific mechanism of action differs, EtOH likewise increases extracellular dopamine in the nucleus accumbens (Yim et al., 2000), and there is some evidence that it increases norepinephrine in the prefrontal cortex (Rossetti et al., 1992), but not the nucleus accumbens (Marinelli et al., 2003). Particularly relevant to the current study is that dopamine plays an important role in reward processes including those associated with MPH and EtOH intake (Pierce & Kumaresan, 2006; Vengeliene et al., 2008) as well as EtOH stimulation (Phillips & Shen, 1996). Accordingly, dopamine may assume a pivotal and common neuropharmacological substrate that underlies the interactive effects of these two drugs on behavior.
In addition to neurobiological systems accounting for the interaction effects of EtOH and MPH, pharmacokinetic interactions may also be accountable. We have identified a transesterification metabolite of MPH, ethylphenidate, which forms in the presence of EtOH in both humans (Patrick et al., 2007) and B6 mice (Williard et al., 2007). Furthermore, like in humans, mice enantioselectively form l-ethylphenidate, although only the d-isomer increases locomotor activity (Williard et al., 2007). The significance of ethylphenidate formation is not yet fully understood since the majority produced is the inactive l-isomer and only a small portion is the active d-isomer (Patrick et al., 2007). In the present study, a single dose of ethylphenidate chosen on the basis of the absence of its effect on locomotor activity of B6 mice (Williard et al., 2007) also had no effect on EtOH consumption. Because only a single dose was tested, we cannot rule out the possibility that higher ethylphenidate doses may affect drinking. In fact, the formation of ethylphenidate could be an important factor in the toxicological sequelae following concomitant high doses of EtOH and MPH (Markowitz et al., 1999).
Furthermore, the combination of EtOH and MPH was reported to increase the maximum plasma concentrations of d-MPH in human volunteers by 40% (Patrick et al., 2007). The increase in d-MPH may occur by competitive inhibition by EtOH of the MPH hydrolysis pathway which produces the inactive metabolite ritalinic acid, essentially “sparing” d-MPH from its primary metabolic fate and leading to increased plasma levels of d-MPH (Patrick et al., 2009; Zhu et al., 2008). Importantly, if this elevation of d-MPH also occurs in B6 mice, this might contribute to the enhanced stimulatory effects observed for B6 mice exposed to the drug combination because there is more of the active isomer available to interact with the target neurotransmitter systems. Studies are underway to further examine the pharmacokinetic interactions of MPH and EtOH that lead to the formation of ethylphenidate as well as increased plasma levels of d-MPH.
In summary, we have demonstrated that low doses of MPH can significantly reduce EtOH consumption and enhance EtOH stimulation of locomotor activity. These results in combination with our previous reports of studies in B6 mice (Williard et al., 2007) and humans (Markowitz et al., 2000; Patrick et al., 2007), indicate significant interactions between MPH and EtOH. The underlying mechanisms for these interactive effects likely involve a complex interplay of neurobiological and pharmacokinetic mechanisms. The relative degree to which each of these components contributes to the interactive effects observed for the two drugs in the current experiments is currently unknown. Further, as has recently been reviewed (Kuczenski & Segal, 2005), attention to tissue levels of MPH (and EtOH), as well as the route of administration, will be critical extensions of our present studies. To this end, studies are aimed at tissue measurement of MPH and ethylphenidate levels, after oral and topical administration of MPH [MPH Transdermal System (Daytrana®) see Patrick et al., 2009] in combination with EtOH as well as measuring behavioral and neurobiological changes in mice and humans. Ultimately, growing evidence indicates that the interactive effects of MPH and EtOH deserve increased attention by clinicians who prescribe MPH or who may be involved in the treatment of substance abuse.
Acknowledgments
This work was supported by NIH grant RO1AA016707.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Barrett SP, Pihl RO. Oral methylphenidate-alcohol co-abuse. J Clin Psychopharmacol. 2002;22:633–634. doi: 10.1097/00004714-200212000-00020. [DOI] [PubMed] [Google Scholar]
- Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacol. 1993;112:503–510. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- Bello NT, Hajnal A. Acute methylphenidate treatments reduce sucrose intake in restricted-fed bingeing rats. Brain Res Bull. 2006;70:422–429. doi: 10.1016/j.brainresbull.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Devilbiss DM, Andrzejewski ME, Arnsten AF, Kelley AE, Schmeichel B, Hamilton C, Spencer RC. Methylphenidate preferentially increases catecholamine neurotransmission within the prefrontal cortex at low doses that enhance cognitive function. Biol Psychiatry. 2006;60:1111–1120. doi: 10.1016/j.biopsych.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Biederman J, Spencer T. Methylphenidate in treatment of adults with Attention-Deficit/Hyperactivity Disorder. J Atten Dis. 2002;6 (Suppl 1):S101–107. doi: 10.1177/070674370200601s12. [DOI] [PubMed] [Google Scholar]
- Botly LC, Burton CL, Rizos Z, Fletcher PJ. Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacol. 2008;199:55–66. doi: 10.1007/s00213-008-1093-z. [DOI] [PubMed] [Google Scholar]
- Bradley JV. Complete counterbalancing of immediate sequential effects in a Latin square design. J Amer Statist Assoc. 1958;53:525–528. [Google Scholar]
- Davidson D, Hutchison K, Dagon C, Swift R. Assessing the stimulant effects of alcohol in humans. Pharmacol Biochem Behav. 2002;72:151–156. doi: 10.1016/s0091-3057(01)00758-4. [DOI] [PubMed] [Google Scholar]
- Eckerman DA, Moy SS, Perkins AN, Patrick KS, Breese GR. Enantioselective behavioral effects of threo-methylphenidate in rats. Pharmacol Biochem Behav. 1991;40:875–880. doi: 10.1016/0091-3057(91)90100-g. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey J. Safety of therapeutic methylphenidate in adults: a systematic review of the evidence. J Psychopharmacol. 2009;23:194–205. doi: 10.1177/0269881108089809. [DOI] [PubMed] [Google Scholar]
- Goldfield GS, Lorello C, Doucet E. Methylphenidate reduces energy intake and dietary fat intake in adults: a mechanism of reduced reinforcing value of food? Am J Clin Nutr. 2007;86:308–315. doi: 10.1093/ajcn/86.2.308. [DOI] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009a;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacol. 2009b;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, 3rd, Middaugh LD, Becker HC. Voluntary ethanol drinking in mice and ethanol concentrations in the nucleus accumbens. Brain Res. 2007;1138:208–213. doi: 10.1016/j.brainres.2006.12.071. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Jerlhag E. The antipsychotic aripiprazole antagonizes the ethanol- and amphetamine-induced locomotor stimulation in mice. Alcohol. 2008;42:123–127. doi: 10.1016/j.alcohol.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the Future national results on adolescent drug use: Overview of key findings 2007. Bethesda, MD: National Institute on Drug Abuse; 2008. [Google Scholar]
- King AC, Houle T, de Wit H, Holdstock L, Schuster A. Biphasic alcohol response differs in heavy versus light drinkers. Alcohol Clin Exp Res. 2002;26:827–835. [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention-deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Linsenbardt DN, Moore EM, Gross CD, Goldfarb KJ, Blackman LC, Boehm SL., 2nd Sensitivity and tolerance to the hypnotic and ataxic effects of ethanol in adolescent and adult C57BL/6J and DBA/2J mice. Alcohol Clin Exp Res. 2009;33:464–476. doi: 10.1111/j.1530-0277.2008.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallonee E, Calvin S. Emergency Department Visits Involving Underage Drinking 2004. The New DAWN Report. 2005:1–4. [Google Scholar]
- Marinelli PW, Quirion R, Gianoulakis C. A microdialysis profile of beta-endorphin and catecholamines in the rat nucleus accumbens following alcohol administration. Psychopharmacol. 2003;169:60–67. doi: 10.1007/s00213-003-1490-2. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, DeVane CL, Boulton DW, Nahas Z, Risch SC, Diamond F, Patrick KS. Ethylphenidate formation in human subjects after the administration of a single dose of methylphenidate and ethanol. Drug Metab Dispos. 2000;28:620–624. [PubMed] [Google Scholar]
- Markowitz JS, Logan BK, Diamond F, Patrick KS. Detection of the novel metabolite ethylphenidate after methylphenidate overdose with alcohol coingestion. J Clin Psychopharmacol. 1999;19:362–366. doi: 10.1097/00004714-199908000-00013. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Patrick KS. Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers: does chirality matter? J Clin Psychopharmacol. 2008;28:S54–61. doi: 10.1097/JCP.0b013e3181733560. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Mattes JA, Gittelman R. Growth of hyperactive children on maintenance regimen of methylphenidate. Arch Gen Psychiatry. 1983;40:317–321. doi: 10.1001/archpsyc.1983.01790030087011. [DOI] [PubMed] [Google Scholar]
- McCabe SE, Cranford JA, Morales M, Young A. Simultaneous and concurrent polydrug use of alcohol and prescription drugs: prevalence, correlates, and consequences. J Stud Alcohol. 2006;67:529–537. doi: 10.15288/jsa.2006.67.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe SE, Teter CJ, Boyd CJ, Guthrie SK. Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. J Adol Health. 2004;35:501–504. doi: 10.1016/j.jadohealth.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Bandy AL. Naltrexone effects on ethanol consumption and response to ethanol conditioned cues in C57BL/6 mice. Psychopharmacol. 2000;151(4):321–327. doi: 10.1007/s002130000479. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Bao K, Shepherd CL. Comparative effects of ethanol on motor activity and operant behavior. Pharmacol Biochem Behav. 1992;43:625–629. doi: 10.1016/0091-3057(92)90202-q. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Boggan WO, Randall CL. Stimulatory effects of ethanol in C57BL/6 mice. Pharmacol Biochem Behav. 1987;27:421–424. doi: 10.1016/0091-3057(87)90343-1. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Favara JP, Boggan WO. Ethanol stimulation after chronic exposure in C57 mice. Pharmacol Biochem Behav. 1989;34:331–335. doi: 10.1016/0091-3057(89)90321-3. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Kelley BM, Bandy AL, McGroarty KK. Ethanol consumption by C57BL/6 mice: influence of gender and procedural variables. Alcohol. 1999;17:175–183. doi: 10.1016/s0741-8329(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Duda NJ, Mokler DJ, Moore KE. Self-administration of central stimulants by rats: a comparison of the effects of d-amphetamine, methylphenidate and McNeil 4612. Pharmacol Biochem Behav. 1984;20:227–232. doi: 10.1016/0091-3057(84)90247-8. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Middaugh LD, Tavernetti M. Ethanol consumption and place-preference conditioning in the alcohol- preferring C57BL/6 mouse: relationship with motor activity patterns. Alcohol Clin Exp Res. 1999;23:683–692. [PubMed] [Google Scholar]
- Okie S. ADHD in adults. N Engl J Med. 2006;354:2637–2641. doi: 10.1056/NEJMp068113. [DOI] [PubMed] [Google Scholar]
- Patrick KS, Caldwell RW, Ferris RM, Breese GR. Pharmacology of the enantiomers of threo-methylphenidate. J Pharmacol Exp Ther. 1987;241:152–158. [PubMed] [Google Scholar]
- Patrick KS, Straughn AB, Minhinnett RR, Yeatts SD, Herrin AE, DeVane CL, Malcolm R, Janis GC, Markowitz JS. Influence of ethanol and gender on methylphenidate pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2007;81:346–353. doi: 10.1038/sj.clpt.6100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick KS, Straughn AB, Perkins JS, Gonzalez MA. Evolution of stimulants to treat ADHD: transdermal methylphenidate. Hum Psychopharmacol. 2009;24:1–17. doi: 10.1002/hup.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick KS, Williard RL, VanWert AL, Dowd JJ, Oatis JE, Jr, Middaugh LD. Synthesis and pharmacology of ethylphenidate enantiomers: the human transesterification metabolite of methylphenidate and ethanol. J Med Chem. 2005;48:2876–2881. doi: 10.1021/jm0490989. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Shen EH. Neurochemical bases of locomotion and ethanol stimulant effects. Int Rev Neurobiol. 1996;39:243–282. doi: 10.1016/s0074-7742(08)60669-8. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Poulton A, Cowell CT. Slowing of growth in height and weight on stimulants: a characteristic pattern. J Paediatr Child Health. 2003;39:180–185. doi: 10.1046/j.1440-1754.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Longu G, Mercuro G, Hmaidan Y, Gessa GL. Biphasic effect of ethanol on noradrenaline release in the frontal cortex of awake rats. Alcohol Alcohol. 1992;27:477–480. [PubMed] [Google Scholar]
- Samson HH, Cunningham CL, Czachowski CL, Chappell A, Legg B, Shannon E. Devaluation of ethanol reinforcement. Alcohol. 2004;32:203–212. doi: 10.1016/j.alcohol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Voronin K, Anton RF. Following alcohol consumption, nontreatment-seeking alcoholics report greater stimulation but similar sedation compared with social drinkers. J Stud Alcohol. 2004;65:330–335. doi: 10.15288/jsa.2004.65.330. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner MJ, Mintz RB, Jolicoeur FB, Rondeau DB. Effects of methylphenidate on schedule dependent and schedule induced behavior. Pharmacol Biochem Behav. 1979;10:299–302. doi: 10.1016/0091-3057(79)90104-7. [DOI] [PubMed] [Google Scholar]
- Williard RL, Middaugh LD, Zhu HJ, Patrick KS. Methylphenidate and its ethanol transesterification metabolite ethylphenidate: brain disposition, monoamine transporters and motor activity. Behav Pharmacol. 2007;18:39–51. doi: 10.1097/FBP.0b013e3280143226. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacol. 2006;188:18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]
- Yim HJ, Gonzales RA Department of Pharmacology CoPUoTaAUSA. Ethanol-induced increases in dopamine extracellular concentration in rat nucleus accumbens are accounted for by increased release and not uptake inhibition. Alcohol. 2000;22(2):107–115. doi: 10.1016/s0741-8329(00)00121-x. [DOI] [PubMed] [Google Scholar]
- Zhu HJ, Patrick KS, Yuan HJ, Wang JS, Donovan JL, DeVane CL, Malcolm R, Johnson JA, Youngblood GL, Sweet DH, Langaee TY, Markowitz JS. Two CES1 gene mutations lead to dysfunctional carboxylesterase 1 activity in man: clinical significance and molecular basis. Am J Hum Genet. 2008;82:1241–1248. doi: 10.1016/j.ajhg.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]