Abstract
All cells possess long-term, steady-state voltage gradients across the plasma membrane. These transmembrane potentials arise from the combined activity of numerous ion channels, pumps and gap junction complexes. Increasing data from molecular physiology now reveal that the role of changes in membrane voltage controls, and is in turn controlled by, progression through the cell cycle. We review recent functional data on the regulation of mitosis by bioelectric signals, and the function of membrane voltage and specific potassium, sodium and chloride ion channels in the proliferation of embryonic, somatic and neoplastic cells. Its unique properties place this powerful, well-conserved, but still poorly-understood signaling system at the center of the coordinated cellular interactions required for complex pattern formation. Moreover, disregulation of ion channel expression and function is increasingly observed to be not only a useful marker but likely a functional element in oncogenesis. New advances in genomics and the development of in vivo biophysical techniques suggest exciting opportunities for molecular medicine, bioengineering and regenerative approaches to human health.
Keywords: ion channel, ion pump, membrane voltage, electric potential, current, mitosis, growth control
Introduction
Regulation of the cell cycle is of significant importance to many areas of biology. During development stem cells must maintain their proliferative potential, while some terminally differentiated cells such as neurons no longer divide once specified. In wound healing and regeneration, cells must initially proliferate in order to fill damaged areas or replace lost structures, and then downregulate growth once the proper pattern has been restored. Disruption of cell cycle checkpoints can lead to uncontrolled division of cells and is highly relevant to cancer biology and human health. While tremendous progress has been made on the molecular details of biochemical checkpoint machinery (e.g., kinase cascades),1 an important area of mitotic regulation still offers much opportunity for new discoveries: bioelectrical events controlling transmembrane voltage potential.2
The first correlations between membrane potential (the voltage gradient across the plasma membrane, Vmem) and proliferative ability came from observations that cell types with a very high resting potential such as muscle cells and neurons show little if any mitotic activity (reviewed in ref. 3). Though the early 1950’s it remained unclear whether there was a causal relationship between Vmem and proliferation, or if both were simply characteristics related to the specialized function of these cells. This question invoked more intense research in the late 1950’s and early 1960’s, following a number of studies in which multiple groups reported a decrease in the membrane potential of cells following malignant transformation.4–6 These results, in addition to observations that cultured cells under high growth conditions show a decrease in Vmem, were among the first to suggest a causal relationship between ion flow and the cell cycle.
These ideas were formally tested in a series of groundbreaking experiments by Clarence D. Cone, Jr. throughout the late 1960’s. He first observed that Vmem varied through the cell cycle and postulated that the variations were directly related to progression through G1/S and G2/M transitions in proliferating cells.7 In a follow up study to explicitly test causation, he altered the intracellular ionic concentration of cells and was able to induce a reversible mitotic block by mimicking Vmem to levels observed in neurons.8 Even more impressively, it was shown that sustained depolarization was able to induce DNA synthesis and mitosis in mature neurons.9,10
Cone would later synthesize these studies, as well as the pioneering prior work, into a theory on the basic mechanism of mitotic control and oncogenesis.11 This paper eloquently argued for a direct relationship between the cycle and electrical transmembrane potential and discussed possible mechanisms, both molecular and physiological. His commentary was notable given the limited information and methods available at the time, and Cone’s innovative work and ideas provided the foundation for formal inquiry into the relationship between membrane potential and the cell cycle.
Since then, molecular, physiological and pharmacological tools have become much more sophisticated, clarifying the molecular details of biochemical pathways involving cyclins, c-myc, c-fos, numerous tumor suppressors and oncogenes. While considerable modern work underscores the link between membrane potential and the cell cycle, this fascinating bioelectric control mechanism is still not well known in the field.
Here, we briefly summarize some of the recent studies examining the relationship between Vmem and cell proliferation in differentiated cells, and the modulation of expression and regulation of ion channels throughout development as cells progress from a stem cell state to terminal differentiation (Table 1). We also discuss data on the role of membrane potential in neoplastic cells, and suggest that this mechanism is an attractive target for modulation in regenerative medicine and developmental biology contexts.
Table 1.
A sample of some of the most well characterized modulations of membrane potential or ion channel activity, and the effect on cell proliferation
| Vmem or channel examined |
Effect | Cell type | Ref. |
|---|---|---|---|
| Vmem hyperpolarized through physiology |
arrest | vascular endothelial | 31 |
| Vmem depolarized through high K+ media |
proliferation | mouse macrophage cell line (PU5-1.8) |
32 |
| ClC-3 Cl− channel activity |
proliferation | glioma cell lines D54-MG and U251-MG |
86 |
| ENaC Na+ channel expression |
proliferation | mouse colonic epithelium |
89 |
| EAG K+ channel activity |
proliferation | multiple human melanoma lines |
83 |
| EAG K+ channel activity |
proliferation | multiple human carcinoma lines |
81 |
| EAG K+ channel expression |
proliferation | xenopus oocyte | 160 |
| ERG K+ channel activity |
proliferation | multiple human cancer lines |
73 |
| ERG K+ channel activity |
proliferation | quail neural crest | 33 |
| KCNK9 K+ channel activity |
proliferation | human colorectal cancer tissue |
79 |
| KV1.3, KV1.5 K+ channel activity |
proliferation | rat oligodendrocyte progenitors |
39 |
| C2+ dependent K+ channel activity |
proliferation | prostate cancer cell line LNCaP |
120 |
| Ca2+ sensitive K+ channels inhibition |
proliferation | colon carcinoma cell line T84 |
35 |
| K+ channel inhibition | arrest | human and mouse lymphocytes |
18, 22 |
| K+ channel inhibition | arrest | chick astrocytes | 20 |
| K+ channel inhibition | arrest | hamster fibroblasts | 21 |
| K+ channel inhibition | arrest | rat Schwann cells | 19 |
Membrane Potential as a Regulator of Cell Cycle in Healthy Cells
Vmem and cell proliferation
While rapid changes in membrane potential (Vmem) are best known in neurons and muscle, steady-state Vmem levels are associated with all cells, and exhibit cyclic fluctuations on much longer timescales than the familiar millisecond action potentials.12,13 Transmembrane potential arises from the combined actions of numerous channels and pumps, which segregate ions across the cell surface under constraints of concentration gradient and charge. Thus, modulation of specific ion channels and transporters is both an endogenous method for controlling cellular Vmem and a tractable technique for functional experiments. It should be noted that a number of channels and pumps are now known to have additional regulatory roles independent of their current-passing functions.14–17 In this review, we focus on functions specifically associated with charge transfer and voltage control.
Pharmacological blockade of ion channels has been a popular method of disrupting membrane potential; while not as specific as molecular approaches (knockout, RNAi or morpholinos), it has two advantages. First, it allows a more precise control of the timing of current inhibition. Second, it often produces more informative results than gene-specific loss of function because of the extensive compensation among multiple channel types: whereas phenotypes may be masked by redundancy in single-gene knockdowns, a pharmacological approach can reveal the function of membrane potential per se, which is not necessarily dependent on any one particular gene product.
Membrane potential has been examined as a key regulator of proliferation in a number of cell types, suggesting that modulation of Vmem is required for both G1/S phase and G2/M phase transitions. Depolarization of membrane through changes in extracellular ion concentration inhibits G1/S progression of lymphocytes, astrocytes, fibroblasts and Schwann cells suggesting hyper-polarization is a required step for S phase initation.18–21 In B cell lymphocytes, inhibition of channels induces a reversible cell cycle arrest;22 similar results have been noted in other cell types.22–27 Analysis of downstream targets has revealed that inhibition of potassium channels in these cells resulted in expression changes of a number of proteins, including IL-1/2 and transferrin, both of which are implicated in cell cycle control.18,22,23,26,28,29
Conversely, depolarization of the plasma membrane seems to be essential for the G2/M transition. Current models outline a rhythmic oscillation of membrane potential throughout the cell cycle, with a spike in hyperpolaration occurring before DNA synthesis followed by a prolonged period of depolarization necessary for mitosis, and appears to be conserved mechanism from early cell divisions in embryos through the normal division of differentiated tissues.30 The exact threshold of Vmem necessary to drive cells though proliferative stages is not known, and is likely to vary between cell type, and in development, the stage of differentiation.
In human endothelial cells, modulation of Vmem through applied electric fields revealed that hyper-polarizing currents arrest cell division.31 This arrest is characterized by downregulation of cyclin E and concurrent upregulation of the cyclin inhibitor p27, forging a direct link between transmembrane potential and known regulators of cell cycle. Conversely, depolarization of PUS-1.8 mouse macrophages results in DNA synthesis and progression through the cell cycle, and is marked by a subsequent upregulation of the transcription factors c-myc and c-fos.32 Both of these results fit the current Vmem oscillatory model of cell cycle, and suggest an intimate relationship between membrane potential and the well know cyclin-dependent pathways.
While a detailed consideration of the roles of bioelectric signals with differentiation is beyond the scope of this review, differentiation is often closely associated with changes in proliferative capacity. A number of cell types have shown a strong correlation between membrane potential and differentiation, with Vmem becoming more hyperpolarized as cells are specified. For example, the neural crest cells of quail embryos exhibit a −35 mV resting potential early in development, but as development progresses the resting potential shifts to −55 mV.33 These changes have been directly correlated to a turnover in potassium channels, with the early membrane voltage attributed to the expression of a K+ channel, ERG, while at later stages ERG expression is lost and replaced with inward-rectifying K+ channels associated with many differentiated tissues. Similarly, the normal course of human mesenchymal stem cell differentiation is accompanied by a progressive hyperpolarization; it has recently been shown that this is an instructive parameter, as artificial depolarization keeps the MSCs in the stem state, while hyperpolarization accelerates their differentiation.34
Vmem and cell cycle control in multi-cellular contexts
Cell cycle is a key parameter of cellular behavior that must be tightly regulated and coordinated during morphogenesis associated with development and regeneration. For example, in addition to normal turnover, changes in membrane potential have been linked to the proliferation of cells during wound healing. In cultured cells, modulation of Vmem using K+ channel-inhibiting drugs increases wound healing in cell monolayers;35 this is a related but distinct mechanism to the modulation of the voltage gradient across an epithelium,36 which is also a crucial component of wound healing and normal development.37 Regulation of gap junctional communication is known to control the proliferation of adult stem cells during regeneration in planaria,38 although the specific ions involved have not been identified. Whereas activation of currents is necessary for proliferation, inhibition of specific currents has been shown to disrupt normal development via changes of proliferative capacity. Knockdown or inhibition of two K+ channels (KV 1.3 and KV 1.5) resulted in cell cycle arrest at G1 in rat oligodendrocyte precursors;39,40 this effect was characterized by accumulation of p27 and p21. Blockade of other K+ channels results in similar signaling cascades, suggesting convergent mechanisms downstream of the activity of many diverse channels.40
Changes in Vmem and channel function during the cell cycle
Alongside the functional control of proliferation by ion channel function, observations from a wide array of cells have also shown the converse—control of physiology as a function of the cell cycle, suggesting bi-directional regulatory circuits. An increase in the expression or activity of potassium channels was observed after exposure to mitogens,19,41–48 demonstrating channel expression downstream of proliferation checkpoints. It remains unclear which mitogen activated pathways are responsible for the regulation of potassium channels, although the involvement of p21 and its downstream targets, the protein kinase Raf and GTPase Ras, has been shown.49 It is probable the multiple downstream signaling cascades exist, as the regulation of ion channels by mitogens is likely to be dependent on both cell type and the specific mitogenic signals used.
A number of ion channels show variation of expression or activity across stages of the cell cycle. During the G1/S transition, multiple families of K+ currents become active, including ATP-sensitive K+ channels, outward rectifying currents (KIR), and Ca2+ activated K+ channels.50–53 During the G2/M transition, increases in potassium channel EAG currents have been noted.54 In addition, progression to M phase is also characterized by an increase in chloride flux. Voltage activated chloride currents show a strong increase during G2; the NCC27 channel is activated, and ClC-2 shows M phase-specific expression and phosporylation.55–57
Alongside characterization of individual cells in vitro, ion channel and gap junction function changes across the cell cycle have also been observed in embryonic development.58–60 The complex bidirectional relationship between ion transporter function and cell cycle suggests this set of mechanisms as a powerful and versatile physiological network which can be used during pattern formation in a flexible and highly dynamic control mechanism to synchronize cell division.
Membrane Potential and Cancer
Neoplasia has long been associated with aberrant changes in cell cycle.61,62 Alterations in membrane potential and ion channel expression/function have been observed in a wide array of cancers.63 Likewise, alongside ion-independent roles of GJs in neoplasm,64 it is clear that gap junctions are an important component of Vmem, regulating its fluctuations,65 establishing iso-potential cell fields, and modulating cellular responses to external electric fields.66 For brevity, the well-known role of gap junctions in cancer is not discussed here (reviewed in refs. 67 and 68).
Potassium channels
The proliferation of some tumor cells is dependent on voltage-gated potassium channels.69,70 hERG channels are particularly implicated,71–76 as are 2-pore channels such as KCNK9.77 In the case of KCNK9, it is known that its oncogenic potential depends on K+ transport function, not some other role of the protein,78 and in human colorectal tissue KCNK9 K+ channel expression was shown to be significantly elevated.79 A screen of several cervical cancers found the K+ channel EAG expressed in 100% of the biopsies analyzed, and overexpression of EAG in human cells resulted in more quickly dividing progeny in culture.80,81 This result was replicated in vivo using mice implanted with human EAG (hEAG) expressing CHO cells. All of the mice receiving CHO hEAG injections formed tumors while none of CHO controls formed a growth greater than 1 mm in size.81 hEAG-1 is a true oncogene since its overexpression drives mammalian cells into uncontrolled proliferation and favors tumor progression in cells injected into immune-suppressed mice.81 Likewise the an EAG relative, hERG, is not normally present in most differentiated cells besides the heart but has been observed in a number of human cancers and neoplastic transformation in prostate epithelium.73,82 In these cells, hERG appears to recruit tumor necrosis factor receptor (TNFR) to the plasma membrane and cause a subsequent increase in NFκB, a known proliferation control gene. In addition to modulations of single channels, some cancers are characterized by the activation of multiple potassium currents, such as in the case human melanoma lines which express of both hEAG1 and Ca2+-activated K+ channels.83 Indeed, complex interactions by multiple channels likely exist, and currents driven by diverse families of potassium channels (including calcium activated, inward rectifying Kir, EAG and ERG) have all been correlated with cancerous tissue.
Proton, chloride and sodium flux
Manipulation of membrane H+ flux can confer a neoplastic phenotype upon cells,84 and voltage-gated sodium channels potentiate breast cancer metastasis.85 Studies in glioma lines have revealed a role of the ClC-3 chloride efflux channel in cellular division.86 Following a chloride buildup, Cl− efflux is a known driving force in cytoplasmic condensation and is required for mitosis to progress. Expression of ClC-3 is localized to both the plasma membrane and mitotic spindle of D54-MG and U251-MG glioma cells, and inhibition of the channel though hairpin RNA constructs resulted in the loss of premitotic condensation and arrest of the cell cyle. These results have been supported in studies of human prostate cancer lines, and support the role of chloride channels as key regulators of proliferation through cell size regulation.87,88
Sodium channels have been implicated in mouse cancers in vivo. The knockout APCmin/+ line share a mutation found in many human colorectal cancers, and subsequently develop multiple intestinal neoplasias.89 In vivo transepithelial voltage recordings in this line revealed an increase in Na+ compared to wild type mice that was the result of an increase in expression of the ENaC Na+ channel. The downstream targets of Na+ signaling are not well known, but it was noted that neither Cl− nor Ca2+ absorption were not altered in the APCmin/+ line.
Metastatic potential correlates with voltage-gated inward sodium current and it has been suggested that some sodium channels may be oncofetal genes.85,90–93
Molecular medicine
Unique bioelectrical properties of tumor tissue have been recognized for a long time.94–109 How relevant the changes of ion flux are to neoplasm in general will require much future work. The majority of studies has examined the presence of ion channels in cancerous tissues but have not explicitly examined the physiological role of Vmem changes in the cell. Nor do we know in many cases at which stage of neoplasm development the bioelectric signals are relevant. Cancer cells have been observed to be depolarized with respect to normal healthy epithelial tissue,110–113 and hyperpolarization therapy (molecular-genetic or pharmacological) remains to be tested as a therapeutic approach.
Characterization of ion channels involved in cancer will undoubtedly be of high interest in human health. First, expression of ion channels may prove to be highly relevant markers when screening tissue if unique among the transformed tissue, or if expression levels are significantly elevated.80,114–116 Voltage sensitive dyes have been used successfully in vivo to image the action potentials of the visual cortex, and modification of these techniques has been suggested for cancer screens. Double dye systems have more recently been developed to examine the membrane potential of non-nervous tissue in vivo.117 Application and analysis of these dyes could potentially reduce cost, invasiveness and time involved with diagnosis compared to more traditional biopsies, and in the case of epidermal malignancies such as melanoma may involve no invasiveness whatsoever if they could be applied and examined in intact skin.
Second, inhibition of ion channels through pharmacological treatment has been proposed as a potential cancer treatment.118 Drugs that target membrane voltage-generating transporters have shown clinical promise in cancer.119 Indeed, evidence in cancer lines supports this theory. In human prostate cells inhibition of Ca2+ dependent K+ channels lead to a decrease in cell proliferation,120 and knockdown of EAG with antisense oligonucloetides reduced division rates in somatic cancer lines.81 Growth suppression of pancreatic tumor cells occurs after selective blockade of IK-type channels.121 Control of tumor growth though pharmacology is especially exciting in cancers which display ion channels that are non-existent throughout the rest of the body as there would be little chance of the drug interacting with healthy tissue in the donor. For ion channels which are present in both tumors and surrounding tissues, targeted delivery remains an active area for investigation.
Challenges and Opportunities for Regenerative Medicine
Modulation of the membrane voltage as a novel parameter controlling cell proliferation offers unique opportunities for guiding morphogenesis in vitro (tissue engineering) and in vivo (regenerative medicine).2,122,123 For example, V-ATPase proton pump activity in the zebrafish eye is needed for retinoblast proliferation and survival,124 while induction of proton efflux from wound tissue has been shown to induce complete regeneration of the tail in Xenopus tadpoles.125 The molecular details of epigenetic bioelectrical pathways must be considered in developing strategies for rational modulation of cell behavior based on bioelectric controls.
Mechanisms
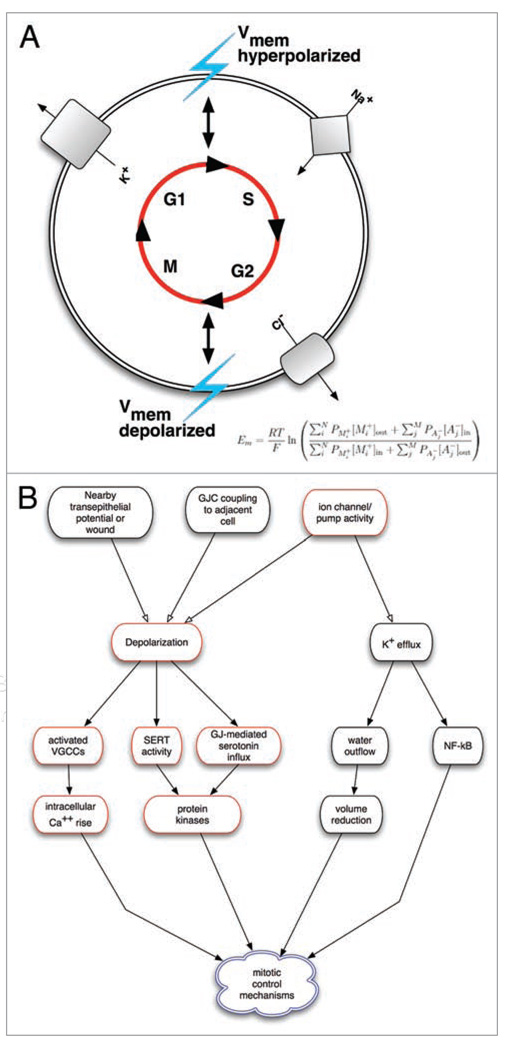
How are changes in Vmem transduced into alter-ations of mitotic behaviors (Fig. 1)? One likely mechanism is regulation of Ca2+ entry into the cell, and the positive feedback loop that would occur between Ca2+ entry and Ca2+ dependent potassium channels.126 For example, increasing intracellular calcium concentration with the ionomycin restored normal division in cells which were cultured in high potassium media, a condition normally inhibitory to division.28 In addition, Na+ influx is required for the uptake of metabolic substrates and subsequent progression through G1; hyper-polarization of the plasma membrane through potassium channels has been suggested to increase the rate influx and intracellular concentration of Na+.47 However, these links remain poorly understood and further research will be necessary to determine the direct and indirect downstream signal cascades resulting from Vmem flux.
Figure 1.
Linkage between bioelectric signals and cell cycle control via Vmem changes. (A) Schematic illustrating the linkage of membrane potential modulation to ion channel dynamics during the cell cycle. During G1/S transition, the membrane potential becomes hyperpolarized relative to the normal resting potential. Potassium channels from the ATP-sensitive, voltage gated and Ca2+-activated families become active allowing for potassium efflux from the cell; sodium channels also become activated. During the G2/S transition the membrane becomes depolarized, and there is a decrease in potassium channel activity. In addition, G2/M is characterized by the activation of chloride channels and a subsequent efflux of chloride. While the role of potassium channels are the most well-studied in relation to the cell cycle, a number other ion gradients are involved, each contributing to the net membrane potential as described by Goldman-Hodgkin-Katz equation. (B) Membrane voltage in a cell can be altered by a variety of factors, including channel/pump activity in it’s own membrane (cell-autonomous effects), gap junctional communication to neighboring cells of different potential, or nearby electric fields and ion flows from wounded and intact epithelia (the latter two being non-cell-autonomous control mechanisms). In turn, changes in ion flow can be transduced into alterations of the mitotic program by voltage-gated calcium channels and calcium-dependent second-messenger pathways, changes in cell volume, and alterations of transport of mitogens such as serotonin. These can arrive in cells by two voltage-dependent mechanisms: electrophoresis through gap junctions, or changes in the activity of transporters like SERT that are powered by transmembrane potential.
Additional mechanisms for sensing changes in plasma membrane polarization levels include: proteins that change conformation upon Vmem changes and activate integrin-dependent127 or PTEN phosphatase-dependent cascades,128,129 depolarization-dependent nuclear translocation of the NRF-2 transcription factor,130 induction of specific kinases such as KID-1,131,132 and the influx of mitogens such as serotonin,133–137 which is controlled by Vmem through the voltage-powered serotonin membrane transporter SERT and through gap-junctional paths via electrophoresis.138
Special features of Vmem signalling
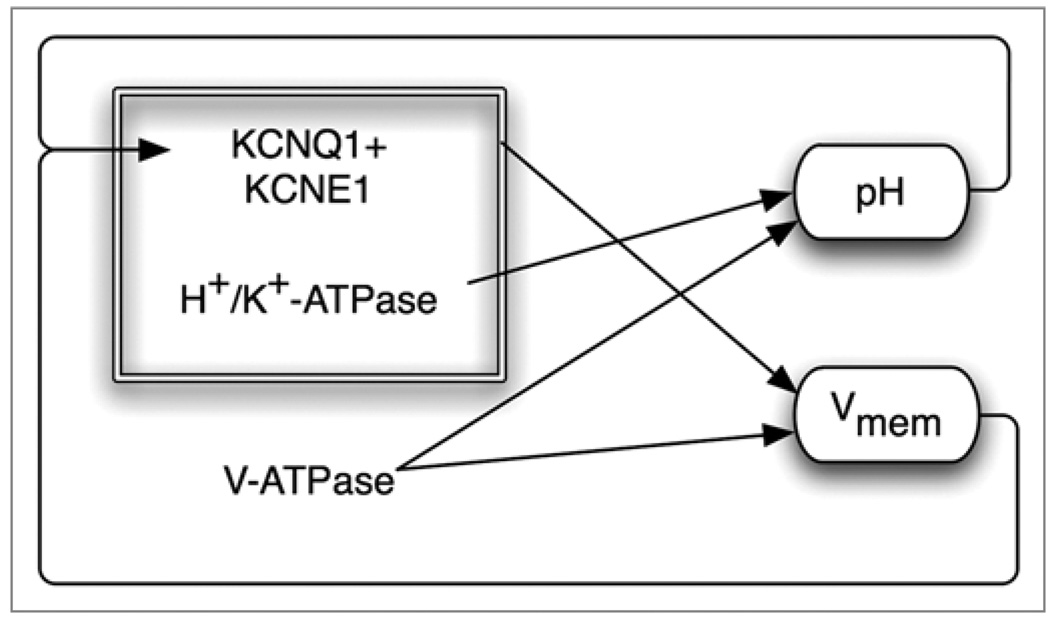
The control of cell functions by membrane voltage is highly non-linear because the existence of multiple feedback loops (Fig. 2). For example, many of the channels, pumps and gap junctions that determine transmembrane potential are themselves pH- and voltage-sensitive, leading to complex recursion of effects. This is a very powerful mechanism for buffering evolutionary control mechanisms, sometimes resulting in positive feedback loops (such as NFκB, which is turned on by K+ loss, downregulating transcription of the potassium importer HK-ATPase139) as well as negative feedback loops (e.g., depolarization can activate the V-ATPase hyperpolarizing pump). Thus, quantitative mathematical modelling will be necessary to integrate the temporal dynamics of multiple ion fluxes and the resulting Vmem changes and thus develop strategies for placing cells into specific Vmem states in biomedical applications.
Figure 2.
Sample of recursive feedback among physiological parameters and ion channel/pump activity. Bioelectric controls of cell functions are inherently non-linear because channels and pumps produce effects on voltage and pH that in turn regulate those same channels and pumps. Here is shown one example taken from a circuit used in vertebrate left-right patterning.166 The V-ATPase creates both a pH gradient and contributes to membrane hyperpolarization. At the same time, the H,K-ATPase functions together with a K+ channel to regulate Vmem; however, both of these components are themselves voltage- and pH-sensitive. Quantitative models of such networks, which take into account both the molecular biology of components expressed in relevant cells and the time-dependent physiology of the resulting circuit.
The non-linear and non-local aspects of bioelectric signals result in some very interesting features of Vmem control over proliferation during morphogenesis.140–143 For example, mitotic upregulation induced by the V-ATPase is limited to the regenerating region (not the rest of the tadpole) when Xenopus tadpoles regenerate their tails by a voltage-dependent mechanism.125 Alongside this spatial control, temporal control can also be used with high resolution: in depolarization-induced overproliferation of melanocytes, these cells undergo no more than 1 extra cell cycle, despite the continued presence of the depolarizing potassium channel mutant protein.117
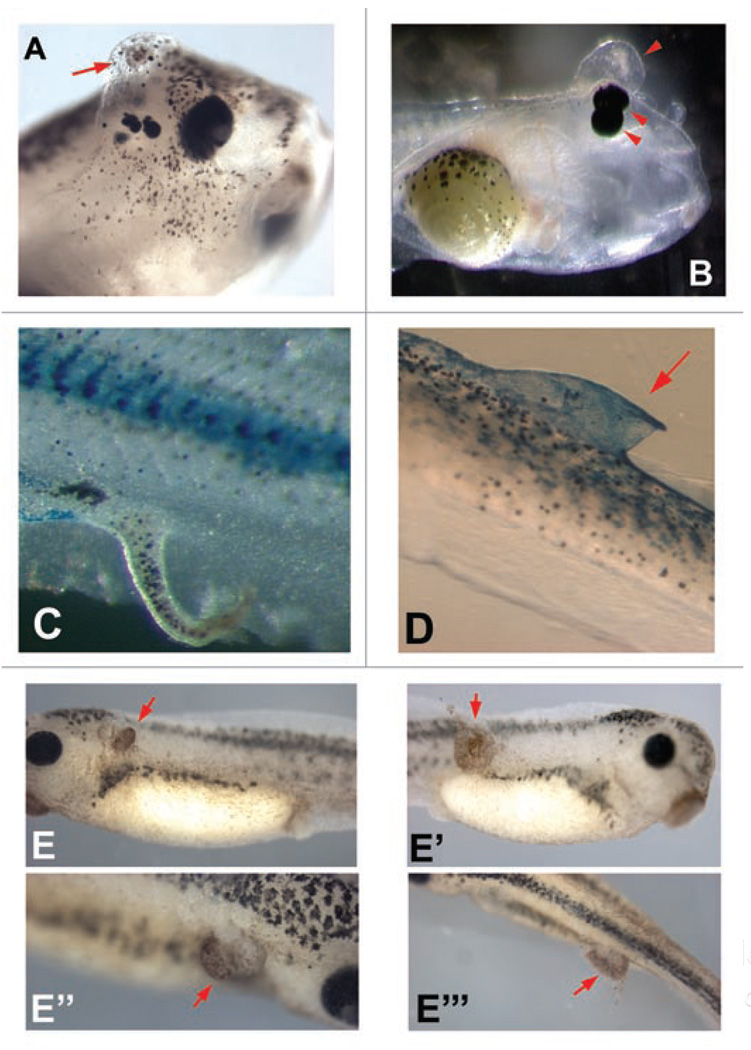
In our lab, gain-of-function studies altering bioelectric signals during embryogenesis by misexpression of specific ion transporters and their mutants have revealed a wide variety of subtle phenotypes related to proliferation and differentiation (Fig. 3). The ability to integrate growth control with morphogenesis via bioelectric parameters is only beginning to be understood, and further molecular investigation will surely reveal details of significant importance to biomedicine. Interestingly, we have observed that not only long-term, but also transient, modulation of Vmem has the ability to activate proliferation. Electroporation, a technique in which exogenous DNA is introduced into cells by square electric pulses on a millisecond timeframe, is widely used in cell biology, developmental biology and regenerative medicine.144–148 Surprisingly, the process of electroporation itself, using no DNA, is sufficient to activate not only de-differentiation149 but also hyperproliferation (Fig. 3E–E”’). This is surprising, given the rapid nature of electroporation compared to the time-scale of cell division, and indicates that significant care must be used using any methods that disrupt transmembrane potential.
Figure 3.
Perturbation of growth in Xenopus embryos caused by manipulation of ion channels and electroporation. A variety of mRNAs encoding wild-type and mutant channels were microinjected into frog embryo blastomeres to screen for bioelectrical signals with roles in growth and pattern control. The VSOP167 proton channel (kindly provided by Yasushi Okamura) (A), the Cx32 gap junction subunit (plasmid kindly provided by Dan Goodenough) (B and C), and the HERG K+ channel (plasmid kindly provided by Annarosa Arcangeli) (D) result in ectopic growth and abnormal duplication of body structures such as eyes, sometimes forming extensive fin-like protrusions that are clearly associated with increases in cell growth. (E–E”’) Electroporation of embryos at stage 33, with no DNA, (95 msec interval, 5 msec pulse, 10X repeated) results in significant areas of ectopic growth 24 hours later. Red arrows indicate hyperproliferation.
Future
Membrane voltage is a well-conserved and probably ancient control system, functioning in phyla ranging from plants150 to higher vertebrates. Several areas of this field suggest exciting future advances.
First, physiological parameters such as membrane voltage and specific ion content may be used as in vivo markers to identify special subpopulations of cell types. For example, human mammary tumor cells fall 4 Gaussian distributions of voltage with means of −9, −17, −24 and −2 mV.151 The functional significance and the value of this as a marker remain to be tested, but given the important regulatory roles of Vmem, it is likely that these data are informing us of important distinctions among subtypes of the population.
Second, it is abundantly clear that the original hypothesis of depolarization inducing growth3 is too simple. It is much more likely that types of cells (e.g., embryonic, committed, neoplastic, etc.,) can be sorted into distinct regions in a multi-dimensional state space with axes corresponding to physiological parameters (of which membrane potential is just one). Moreover, because of the Vmem oscillations occurring during the cell cycle, it is clear that temporal variation must be added to models of this signalling system.
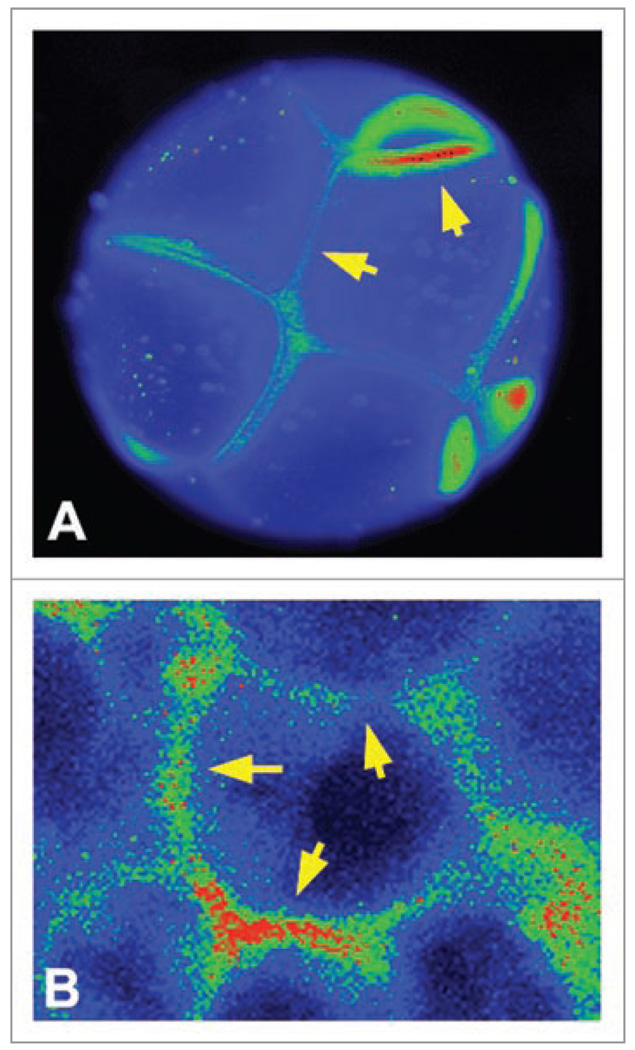
Third, it should be noted that assigning a single Vmem value to a cell is also a significant oversimplification. In fact, a number of embryonic blastomeres (Fig. 4A) and mammalian cells in culture (Fig. 4B) exhibit distinct domains of membrane voltage around the cell periphery. While the physiological literature often reports one particular mV reading for a cell this neglects the considerable complexity of microdomains of Vmem on cells, presumably established by distinct population of channel/pump proteins on lipid rafts152,153 and fence functions performed by plasma membrane and cortical/cytoskeletal components. Thus, since different Vmem values can be present in domains as small as 2 µ (reviewed in ref 154), each cell potentially contains a 2-dimensional surface which encodes a tremendous amount of information that can be transmitted to distinct intracellular second-messenger pathways as well as neighboring cells.155
Figure 4.
Membrane voltage levels are not homogenous around the cell surface. Using the voltage-sensitive fluorescent dye DiBAC,168 early frog embryo blastomeres (A) as well as COS cells in monolayer culture (B) exhibit significant variations of membrane voltage level around the cell surface, indicating that a single Vmem number for a given cell drastically under-estimates the amount of information that can be encoded in the plasma membrane’s physiological state and potentially communicated to neighboring cells. Images courtesy of Dany S. Adams.
Finally, it must be observed that transmembrane potential is only the best-known and most tractable of the cellular bioelectric parameters. Subcellular organelles such as mitochondria, endo-somes, phagosomes, ER and nucleus all possess a transmembrane potential due to specifically-localized ion channels.156–164 Future efforts must develop subcellularly-targeted voltage reporter proteins165 and mutant channels that can be used to study and functionally alter the bioelectric signalling in distinct intracellular locales.
The detailed understanding of the contribution of transmembrane potential to cell cycle control, and especially the integration of this mechanism into biochemical and genetic mechanisms occurring during complex morphogenesis, will reveal fascinating aspects of interdisciplinary biophysics and will offer significant opportunities for the biomedicine of birth defects, cancer and regenerative medicine.
Acknowledgements
This paper is dedicated to C.D. Cone, who was one of the first to study in detail the profound role of membrane voltage in proliferation control. We are grateful to the members of the Levin lab and the bioelectricity community for many useful discussions. We thank Dany Adams for the photos in Figure 4, and Ai-Sun Tseng for her comments on the manuscript. M.L. is supported by grants R01-GM077425, EY018168 and GM078484 from the NIH. D.B. is supported by Forsyth Institutes T32 grant 5T32DE007327-07. Kelly McLaughlin is supported by NSF grant IOS-0843355.
Glossary
- KID-1
a member of the pim family of proto-oncogenes.169
- Oncofetal
a protein form present in tumors and embryos, associated with the rapid growth and undifferentiated phenotype characteristic of neoplastic and embryonic states.
- EAG/ERG
Members of the ether-a-go-go gene family and major component of a delayed rectifiers current that allow potassium to exit the cell when active.170
- KIR
Inward rectifying potassium channel family involved in both depolarization and repolarization of the plasma membrane. The family is characterized by passing K+ ions more inward than outward.171
- ClC’s
Chloride channels involved in the maintenance of membrane potential that form a diverse family activated by various methods including Vmem, Ca2+ concentration, or ligand gating. Includes ClC-1 (NCC27), ClC-2, ClC-3.172
- KCNK9
a member of the two pore domain potassium channel family.173
- ENaC
a ion channel in epithelium permeable to Na+ and Li+ ions.174
- IK
Intermediate conductance, Ca2+-activated potassium channels.175
- V-ATPase
a protein pump that moves protons out of cells via ATP hydrolysis.176
- KV1.3/KV1.5
members of the voltage gated potassium channel family. Both are considered delayed rectifiers.177
References
- 1.Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003;36:131–149. doi: 10.1046/j.1365-2184.2003.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundelacruz S, Levin M, Kaplan DL. Role of membrane potential in the regulation of cell proliferation and differentiation. Stem Cell Rev Rep. 2009;5:231–246. doi: 10.1007/s12015-009-9080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Binggeli R, Weinstein R. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- 4.Balitsky KP, Shuba EP. Resting potential of malignant tumour cells. Acta Unio Int Contra Cancrum. 1964;20:1391–1393. [PubMed] [Google Scholar]
- 5.Johnstone BM. Micro-electrode penetration of ascites tumour cells. Nature. 1959;183:411. doi: 10.1038/183411a0. [DOI] [PubMed] [Google Scholar]
- 6.Tokuoka S, Morioka H. The membrane potential of the human cancer and related cells I. Gan. 1957;48:353–354. [PubMed] [Google Scholar]
- 7.Cone CD., Jr Electroosmotic interactions accompanying mitosis initation in sarcoma cells in vitro. Trans N Y Acad Sci. 1969;31:404–427. doi: 10.1111/j.2164-0947.1969.tb02926.x. [DOI] [PubMed] [Google Scholar]
- 8.Cone CD, Jr, Tongier M., Jr Control of somatic cell mitosis by simulated changes in the transmembrane potential level. Oncology. 1971;25:168–182. doi: 10.1159/000224567. [DOI] [PubMed] [Google Scholar]
- 9.Stillwell EF, Cone CM, Cone CD. Stimulation of DNA synthesis in CNS neurones by sustained depolarisation. Nat New Biol. 1973;246:110–111. doi: 10.1038/newbio246110a0. [DOI] [PubMed] [Google Scholar]
- 10.Cone CD, Cone CM. Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science. 1976;192:155–158. doi: 10.1126/science.56781. [DOI] [PubMed] [Google Scholar]
- 11.Cone CD., Jr Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30:151–181. doi: 10.1016/0022-5193(71)90042-7. [DOI] [PubMed] [Google Scholar]
- 12.Pandiella A, Magni M, Lovisolo D, Meldolesi J. The effect of epidermal growth factor on membrane potential. Rapid hyperpolarization followed by persistent fluctuations. J Biol Chem. 1989;264:12914–12921. [PubMed] [Google Scholar]
- 13.Lang F, Friedrich F, Kahn E, Woll E, Hammerer M, Waldegger S, et al. Bradykinin-induced oscillations of cell membrane potential in cells expressing the Ha-ras oncogene. J Biol Chem. 1991;266:4938–4942. [PubMed] [Google Scholar]
- 14.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arcangeli A, Becchetti A. Complex functional interaction between integrin receptors and ion channels. Trends Cell Biol. 2006;16:631–639. doi: 10.1016/j.tcb.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Xie Z, Askari A. Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem. 2002;269:2434–2439. doi: 10.1046/j.1432-1033.2002.02910.x. [DOI] [PubMed] [Google Scholar]
- 17.Baumgartner M, Patel H, Barber DL. Na(+)/H(+) exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol. 2004;287:844–850. doi: 10.1152/ajpcell.00094.2004. [DOI] [PubMed] [Google Scholar]
- 18.Freedman BD, Price MA, Deutsch CJ. Evidence for voltage modulation of IL-2 production in mitogen-stimulated human peripheral blood lymphocytes. J Immunol. 1992;149:3784–3794. [PubMed] [Google Scholar]
- 19.Wilson GF, Chiu SY. Mitogenic factors regulate ion channels in Schwann cells cultured from newborn rat sciatic nerve. J Physiol. 1993;470:501–520. doi: 10.1113/jphysiol.1993.sp019872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canady KS, Ali-Osman F, Rubel EW. Extracellular potassium influences DNA and protein syntheses and glial fibrillary acidic protein expression in cultured glial cells. Glia. 1990;3:368–374. doi: 10.1002/glia.440030508. [DOI] [PubMed] [Google Scholar]
- 21.Orr CW, Yoshikawa-Fukada M, Ebert JD. Potassium: effect on DNA synthesis and multiplication of baby-hamster kidney cells: (cell cycle-membrane potential-synchronization-transformation) Proc Natl Acad Sci USA. 1972;69:243–247. doi: 10.1073/pnas.69.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amigorena S, Choquet D, Teillaud JL, Korn H, Fridman WH. Ion channel blockers inhibit B cell activation at a precise stage of the G1 phase of the cell cycle. Possible involvement of K+ channels. J Immunol. 1990;144:2038–2045. [PubMed] [Google Scholar]
- 23.Chandy KG, DeCoursey TE, Cahalan MD, McLaughlin C, Gupta S. Voltage-gated potassium channels are required for human T lymphocyte activation. J Exp Med. 1984;160:369–385. doi: 10.1084/jem.160.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chiu SY, Wilson GF. The role of potassium channels in Schwann cell proliferation in Wallerian degeneration of explant rabbit sciatic nerves. J Physiol. 1989;408:199–222. doi: 10.1113/jphysiol.1989.sp017455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307:465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- 26.Price M, Lee SC, Deutsch C. Charybdotoxin inhibits proliferation and interleukin 2 production in human peripheral blood lymphocytes. Proc Natl Acad Sci USA. 1989;86:10171–10175. doi: 10.1073/pnas.86.24.10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YF, Jia H, Walker AM, Cukierman S. K-current mediation of prolactin-induced proliferation of malignant (Nb2) lymphocytes. J Cell Physiol. 1992;152:185–189. doi: 10.1002/jcp.1041520123. [DOI] [PubMed] [Google Scholar]
- 28.Gelfand EW, Cheung RK, Mills GB, Grinstein S. Role of membrane potential in the response of human T lymphocytes to phytohemagglutinin. J Immunol. 1987;138:527–531. [PubMed] [Google Scholar]
- 29.Lin CS, Boltz RC, Blake JT, Nguyen M, Talento A, Fischer PA, et al. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J Exp Med. 1993;177:637–645. doi: 10.1084/jem.177.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bregestovski P, Medina I, Goyda E. Regulation of potassium conductance in the cellular membrane at early embryo genesis. J Physiol Paris. 1992;86:109–115. doi: 10.1016/s0928-4257(05)80014-2. [DOI] [PubMed] [Google Scholar]
- 31.Wang E, Yin Y, Zhao M, Forrester JV, McCaig CD. Physiological electric fields control the G1/S phase cell cycle checkpoint to inhibit endothelial cell proliferation. FASEB J. 2003;17:458–460. doi: 10.1096/fj.02-0510fje. [DOI] [PubMed] [Google Scholar]
- 32.Kong SK, Suen YK, Choy YM, Fung KP, Lee CY. Membrane depolarization was required to induce DNA synthesis in murine macrophage cell line PU5-1.8. Immunopharmacol Immunotoxicol. 1991;13:329–339. doi: 10.3109/08923979109019708. [DOI] [PubMed] [Google Scholar]
- 33.Bauer CK, Schwarz JR. Physiology of EAG K+ channels. J Membr Biol. 2001;182:1–15. doi: 10.1007/s00232-001-0031-3. [DOI] [PubMed] [Google Scholar]
- 34.Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS ONE. 2008;3:3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotz MM, Wang H, Song JC, Pories SE, Matthews JB. K+ channel inhibition accelerates intestinal epithelial cell wound healing. Wound Repair Regen. 2004;12:565–574. doi: 10.1111/j.1067-1927.2004.012509.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 37.Robinson K, Messerli M. Electric embryos: the embryonic epithelium as a generator of developmental information. In: McCaig C, editor. Nerve Growth and Guidance. Portland: Portland Press; 1996. pp. 131–141. [Google Scholar]
- 38.Oviedo NJ, Levin M. Smedinx-11 is a planarian stem cell gap junction gene required for regeneration and homeostasis. Development. 2007;134:3121–3131. doi: 10.1242/dev.006635. [DOI] [PubMed] [Google Scholar]
- 39.Chittajallu R, Chen Y, Wang H, Yuan X, Ghiani CA, Heckman T, et al. Regulation of KVl subunit expression in oligodendrocyte progenitor cells and their role in G/S phase progression of the cell cycle. Proc Natl Acad Sci USA. 2002;99:2350–2355. doi: 10.1073/pnas.042698399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghiani CA, Yuan X, Eisen AM, Knutson PL, DePinho RA, McBain CJ, et al. Voltage-activated K+ channels and membrane depolarization regulate accumulation of the cyclin-dependent kinase inhibitors p27(Kip1) and p21(CIP1) in glial progenitor cells. J Neurosci. 1999;19:5380–5392. doi: 10.1523/JNEUROSCI.19-13-05380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decoursey TE, Chandy KG, Gupta S, Cahalan MD. Mitogen induction of ion channels in murine T lymphocytes. J Gen Physiol. 1987;89:405–420. doi: 10.1085/jgp.89.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deutsch C, Krause D, Lee SC. Voltage-gated potassium conductance in human T lymphocytes stimulated with phorbol ester. J Physiol. 1986;372:405–423. doi: 10.1113/jphysiol.1986.sp016016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enomoto K, Cossu MF, Maeno T, Edwards C, Oka T. Involvement of the Ca2+-dependent K+ channel activity in the hyperpolarizing response induced by epidermal growth factor in mammary epithelial cells. FEBS Lett. 1986;203:181–184. doi: 10.1016/0014-5793(86)80738-4. [DOI] [PubMed] [Google Scholar]
- 44.Grissmer S, Nguyen AN, Cahalan MD. Calcium-activated potassium channels in resting and activated human T lymphocytes. Expression levels, calcium dependence, ion selectivity and pharmacology. J Gen Physiol. 1993;102:601–630. doi: 10.1085/jgp.102.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lovisolo D, Bonelli G, Baccino FM, Peres A, Alonzo F, Munaron L. Two currents activated by epidermal growth factor in EGFR-T17 fibroblasts. Biochim Biophys Acta. 1992;1104:73–82. doi: 10.1016/0005-2736(92)90133-7. [DOI] [PubMed] [Google Scholar]
- 46.Magni M, Meldolesi J, Pandiella A. Ionic events induced by epidermal growth factor. Evidence that hyperpolarization and stimulated cation influx play a role in the stimulation of cell growth. J Biol Chem. 1991;266:6329–6335. [PubMed] [Google Scholar]
- 47.Mummery CL, Boonstra J, van der Saag PT, de Laat SW. Modulations of Na+ transport during the cell cycle of neuroblastoma cells. J Cell Physiol. 1982;112:27–34. doi: 10.1002/jcp.1041120106. [DOI] [PubMed] [Google Scholar]
- 48.Partiseti M, Korn H, Choquet D. Pattern of potassium channel expression in proliferating B lymphocytes depends upon the mode of activation. J Immunol. 1993;151:2462–2470. [PubMed] [Google Scholar]
- 49.Huang Y, Rane SG. Potassium channel induction by the Ras/Raf signal transduction cascade. J Biol Chem. 1994;269:31183–31189. [PubMed] [Google Scholar]
- 50.MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 51.Ouadid-Ahidouch H, Le Bourhis X, Roudbaraki M, Toillon RA, Delcourt P, Prevarskaya N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channel. Receptors Channels. 2001;7:345–356. [PubMed] [Google Scholar]
- 52.Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca(2+)-activated K(+) channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004;287:125–134. doi: 10.1152/ajpcell.00488.2003. [DOI] [PubMed] [Google Scholar]
- 53.Woodfork KA, Wonderlin WF, Peterson VA, Strobl JS. Inhibition of ATP-sensitive potassium channels causes reversible cell cycle arrest of human breast cancer cells in tissue culture. J Cell Physiol. 1995;162:163–171. doi: 10.1002/jcp.1041620202. [DOI] [PubMed] [Google Scholar]
- 54.Pardo LA, Bruggemann A, Camacho J, Stuhmer W. Cell cycle-related changes in the conducting properties of r-eag K+ channels. J Cell Biol. 1998;143:767–775. doi: 10.1083/jcb.143.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen L, Wang L, Zhu L, Nie S, Zhang J, Zhong P, et al. Cell cycle-dependent expression of volume-activated chloride currents in nasopharyngeal carcinoma cells. Am J Physiol Cell Physiol. 2002;283:1313–1323. doi: 10.1152/ajpcell.00182.2002. [DOI] [PubMed] [Google Scholar]
- 56.Valenzuela SM, Mazzanti M, Tonini R, Qiu MR, Warton K, Musgrove EA, et al. The nuclear chloride ion channel NCC27 is involved in regulation of the cell cycle. J Physiol. 2000;529:541–552. doi: 10.1111/j.1469-7793.2000.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zheng YJ, Furukawa T, Ogura T, Tajimi K, Inagaki N. M phase-specific expression and phosphorylation-dependent ubiquitination of the ClC-2 channel. J Biol Chem. 2002;277:32268–32273. doi: 10.1074/jbc.M202105200. [DOI] [PubMed] [Google Scholar]
- 58.Block ML, Moody WJ. A voltage-dependent chloride current linked to the cell cycle in ascidian embryos. Science. 1990;247:1090–1092. doi: 10.1126/science.2309122. [DOI] [PubMed] [Google Scholar]
- 59.Day ML, Pickering SJ, Johnson MH, Cook DI. Cell cycle control of a large-conductance K+ channel in mouse early embryos. Nature. 1993;365:560–562. doi: 10.1038/365560a0. [DOI] [PubMed] [Google Scholar]
- 60.Bittman K, Owens DF, Kriegstein AR, LoTurco JJ. Cell coupling and uncoupling in the ventricular zone of developing neocortex. J Neurosci. 1997;17:7037–7044. doi: 10.1523/JNEUROSCI.17-18-07037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kastan MB, Bartek J. Cell cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 62.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–348. doi: 10.1038/35077213. [DOI] [PubMed] [Google Scholar]
- 63.Kunzelmann K. Ion channels and cancer. J Membr Biol. 2005;205:159–173. doi: 10.1007/s00232-005-0781-4. [DOI] [PubMed] [Google Scholar]
- 64.McLachlan E, Shao Q, Wang HL, Langlois S, Laird DW. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res. 2006;66:9886–9894. doi: 10.1158/0008-5472.CAN-05-4302. [DOI] [PubMed] [Google Scholar]
- 65.Hulser DF, Lauterwasser U. Membrane potential oscillations in homokaryons. An endogenous signal for detecting intercellular communication. Exp Cell Res. 1982;139:63–70. doi: 10.1016/0014-4827(82)90318-4. [DOI] [PubMed] [Google Scholar]
- 66.Cooper MS. Gap junctions increase the sensitivity of tissue cells to exogenous electric fields. J Theor Biol. 1984;111:123–130. doi: 10.1016/s0022-5193(84)80200-3. [DOI] [PubMed] [Google Scholar]
- 67.Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 68.Levin M. Gap junctional communication in morphogenesis. Prog Biophys Mol Biol. 2007;94:186–206. doi: 10.1016/j.pbiomolbio.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conti M. Targeting K+ channels for cancer therapy. J Exp Ther Oncol. 2004;4:161–166. [PubMed] [Google Scholar]
- 70.Fraser SP, Grimes JA, Djamgoz MB. Effects of voltage-gated ion channel modulators on rat prostatic cancer cell proliferation: comparison of strongly and weakly metastatic cell lines. Prostate. 2000;44:61–76. doi: 10.1002/1097-0045(20000615)44:1<61::aid-pros9>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 71.Lin H, Xiao J, Luo X, Wang H, Gao H, Yang B, et al. Overexpression HERG K(+) channel gene mediates cell-growth signals on activation of oncoproteins SP1 and NFkappaB and inactivation of tumor suppressor Nkx3.1. J Cell Physiol. 2007 doi: 10.1002/jcp.21015. [DOI] [PubMed] [Google Scholar]
- 72.Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, et al. herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 1998;58:815–822. [PubMed] [Google Scholar]
- 73.Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, et al. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002;62:4843–4848. [PubMed] [Google Scholar]
- 74.Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H, et al. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res. 2004;64:606–611. doi: 10.1158/0008-5472.can-03-2360. [DOI] [PubMed] [Google Scholar]
- 75.Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- 76.Arcangeli A. Expression and role of hERG channels in cancer cells. Novartis Found Symp. 2005;266:225–232. [PubMed] [Google Scholar]
- 77.Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C, et al. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell. 2003;3:297–302. doi: 10.1016/s1535-6108(03)00054-0. [DOI] [PubMed] [Google Scholar]
- 78.Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, et al. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci USA. 2003 doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim CJ, Cho YG, Jeong SW, Kim YS, Kim SY, Nam SW, et al. Altered expression of KCNK9 in colorectal cancers. APMIS. 2004;112:588–594. doi: 10.1111/j.1600-0463.2004.apm1120905.x. [DOI] [PubMed] [Google Scholar]
- 80.Farias LM, Ocana DB, Diaz L, Larrea F, Avila-Chavez E, Cadena A, et al. Ether a go-go potassium channels as human cervical cancer markers. Cancer Res. 2004;64:6996–7001. doi: 10.1158/0008-5472.CAN-04-1204. [DOI] [PubMed] [Google Scholar]
- 81.Pardo LA, del Camino D, Sanchez A, Alves F, Bruggemann A, Beckh S, et al. Oncogenic potential of EAG K(+) channels. EMBO J. 1999;18:5540–5547. doi: 10.1093/emboj/18.20.5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Klezovitch O, Risk M, Coleman I, Lucas JM, Null M, True LD, et al. A causal role for ERG in neoplastic transformation of prostate epithelium. Proc Natl Acad Sci USA. 2008;105:2105–2110. doi: 10.1073/pnas.0711711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer R, Schonherr R, Gavrilova-Ruch O, Wohlrab W, Heinemann SH. Identification of ether a go-go and calcium-activated potassium channels in human melanoma cells. J Membr Biol. 1999;171:107–115. doi: 10.1007/s002329900563. [DOI] [PubMed] [Google Scholar]
- 84.Perona R, Serrano R. Increased pH and tumorigenicity of fibroblasts expressing a yeast proton pump. Nature. 1988;334:438–440. doi: 10.1038/334438a0. [DOI] [PubMed] [Google Scholar]
- 85.Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- 86.Habela CW, Olsen ML, Sontheimer H. ClC3 is a critical regulator of the cell cycle in normal and malignant glial cells. J Neurosci. 2008;28:9205–9217. doi: 10.1523/JNEUROSCI.1897-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jirsch J, Deeley RG, Cole SP, Stewart AJ, Fedida D. Inwardly rectifying K+ channels and volume-regulated anion channels in multidrug-resistant small cell lung cancer cells. Cancer Res. 1993;53:4156–4160. [PubMed] [Google Scholar]
- 88.Shuba YM, Prevarskaya N, Lemonnier L, Van Coppenolle F, Kostyuk PG, Mauroy B, et al. Volume-regulated chloride conductance in the LNCaP human prostate cancer cell line. Am J Physiol Cell Physiol. 2000;279:1144–1154. doi: 10.1152/ajpcell.2000.279.4.C1144. [DOI] [PubMed] [Google Scholar]
- 89.Ousingsawat J, Spitzner M, Schreiber R, Kunzelmann K. Upregulation of colonic ion channels in APC (Min/+) mice. Pflugers Arch. 2008;456:847–855. doi: 10.1007/s00424-008-0451-3. [DOI] [PubMed] [Google Scholar]
- 90.Brackenbury WJ, Djamgoz MB. Activity-dependent regulation of voltage-gated Na+ channel expression in Mat-LyLu rat prostate cancer cell line. J Physiol. 2006;573:343–356. doi: 10.1113/jphysiol.2006.106906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Onganer PU, Seckl MJ, Djamgoz MB. Neuronal characteristics of small-cell lung cancer. Br J Cancer. 2005;93:1197–1201. doi: 10.1038/sj.bjc.6602857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Onganer PU, Djamgoz MB. Small-cell lung cancer (human): potentiation of endocytic membrane activity by voltage-gated Na(+) channel expression in vitro. J Membr Biol. 2005;204:67–75. doi: 10.1007/s00232-005-0747-6. [DOI] [PubMed] [Google Scholar]
- 93.Diss JK, Stewart D, Pani F, Foster CS, Walker MM, Patel A, et al. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8:266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- 94.Stojadinovic A, Nissan A, Gallimidi Z, Lenington S, Logan W, Zuley M, et al. Electrical impedance scanning for the early detection of breast cancer in young women: preliminary results of a multicenter prospective clinical trial. J Clin Oncol. 2005;23:2703–2715. doi: 10.1200/JCO.2005.06.155. [DOI] [PubMed] [Google Scholar]
- 95.Gupta D, Lammersfeld CA, Burrows JL, Dahlk SL, Vashi PG, Grutsch JF, et al. Bioelectrical impedance phase angle in clinical practice: implications for prognosis in advanced colorectal cancer. Am J Clin Nutr. 2004;80:1634–1638. doi: 10.1093/ajcn/80.6.1634. [DOI] [PubMed] [Google Scholar]
- 96.Aberg P, Nicander I, Hansson J, Geladi P, Holmgren U, Ollmar S. Skin cancer identification using multi-frequency electrical impedance—a potential screening tool. IEEE Trans Biomed Eng. 2004;51:2097–2102. doi: 10.1109/TBME.2004.836523. [DOI] [PubMed] [Google Scholar]
- 97.Burr HS. Changes in the field properties of mice with transplanted tumors. Yale J Biol Med. 1941;13:783–788. [PMC free article] [PubMed] [Google Scholar]
- 98.Burr HS, Strong LC, Smith GM. Bioelectric correlates of methylcolantherene-induced tumors in mice. Yale J Biol Med. 1938;10:539–544. [PMC free article] [PubMed] [Google Scholar]
- 99.Rozengurt E, Mendoza S. Monovalent ion fluxes and the control of cell proliferation in cultured fibroblasts. Ann N Y Acad Sci. 1980;339:175–190. doi: 10.1111/j.1749-6632.1980.tb15977.x. [DOI] [PubMed] [Google Scholar]
- 100.Leffert HL, Koch KS. Ionic events at the membrane initiate rat liver regeneration. Ann N Y Acad Sci. 1980;339:201–215. doi: 10.1111/j.1749-6632.1980.tb15979.x. [DOI] [PubMed] [Google Scholar]
- 101.Koch KS, Leffert HL. Increased sodium ion influx is necessary to initiate rat hepatocyte proliferation. Cell. 1979;18:153–163. doi: 10.1016/0092-8674(79)90364-7. [DOI] [PubMed] [Google Scholar]
- 102.Cameron IL, Smith NK. Cellular concentration of magnesium and other ions in relation to protein synthesis, cell proliferation and cancer. Magnesium. 1989;8:31–44. [PubMed] [Google Scholar]
- 103.Jeter JR, Jr, Cameron IL, Smith NK, Steffens WL, Wille JJ. Cell cycle-fluctuations in concentration of various elements in cytoplasm and in nucleus/chromatin of Physarum polycephalum. Cytobios. 1982;35:47–62. [PubMed] [Google Scholar]
- 104.Cameron IL, Smith NK, Pool TB, Sparks RL. Intracellular concentration of sodium and other elements as related to mitogenesis and oncogenesis in vivo. Cancer Res. 1980;40:1493–1500. [PubMed] [Google Scholar]
- 105.Cameron IL, Pool TB, Smith NK. An X-ray microanalysis survey of the concentration of elements in the cytoplasm of different mammalian cell types. J Cell Physiol. 1979;101:493–501. doi: 10.1002/jcp.1041010315. [DOI] [PubMed] [Google Scholar]
- 106.Cameron IL, Smith NK. Energy dispersive x-ray microanalysis of the concentration of elements in relation to cell reproduction in normal and in cancer cells. Scan Electron Microsc. 1980:463–474. [PubMed] [Google Scholar]
- 107.Cameron IL, Smith NK, Pool TB. Element concentration changes in mitotically active and postmitotic enterocytes. An x-ray microanalysis study. J Cell Biol. 1979;80:444–450. doi: 10.1083/jcb.80.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith NR, Sparks RL, Pool TB, Cameron IL. Differences in the intracellular concentration of elements in normal and cancerous liver cells as determined by X-ray microanalysis. Cancer Res. 1978;38:1952–1959. [PubMed] [Google Scholar]
- 109.Sparks RL, Pool TB, Smith NK, Cameron IL. Effects of amiloride on tumor growth and intracellular element content of tumor cells in vivo. Cancer Res. 1983;43:73–77. [PubMed] [Google Scholar]
- 110.Binggeli R, Weinstein RC. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- 111.Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, Mini E, et al. A novel inward-rectifying K+ current with a cell cycle dependence governs the resting potential of mammalian neuroblastoma cells. J Physiol. 1995;489:455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O’Grady SM, Lee SY. Molecular diversity and function of voltage-gated (KV) potassium channels in epithelial cells. Int J Biochem Cell Biol. 2005;37:1578–1594. doi: 10.1016/j.biocel.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 113.Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–292. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- 114.Wissenbach U, Niemeyer BA, Fixemer T, Schneidewind A, Trost C, Cavalie A, et al. Expression of CaT-like, a novel calcium-selective channel, correlates with the malignancy of prostate cancer. J Biol Chem. 2001;276:19461–19468. doi: 10.1074/jbc.M009895200. [DOI] [PubMed] [Google Scholar]
- 115.Fixemer T, Wissenbach U, Flockerzi V, Bonkhoff H. Expression of the Ca2+-selective cation channel TRPV6 in human prostate cancer: a novel prognostic marker for tumor progression. Oncogene. 2003;22:7858–7861. doi: 10.1038/sj.onc.1206895. [DOI] [PubMed] [Google Scholar]
- 116.Stuhmer W, Alves F, Hartung F, Zientkowska M, Pardo LA. Potassium channels as tumour markers. FEBS Lett. 2006;580:2850–2852. doi: 10.1016/j.febslet.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 117.Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem. 2009;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- 119.Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol. 2005;67:929–936. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- 120.Skryma RN, Prevarskaya NB, Dufy-Barbe L, Odessa MF, Audin J, Dufy B. Potassium conductance in the androgen-sensitive prostate cancer cell line, LNCaP: involvement in cell proliferation. Prostate. 1997;33:112–122. doi: 10.1002/(sici)1097-0045(19971001)33:2<112::aid-pros5>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 121.Jager H, Dreker T, Buck A, Giehl K, Gress T, Grissmer S. Blockage of intermediate-conductance Ca2+-activated K+ channels inhibit human pancreatic cancer cell growth in vitro. Mol Pharmacol. 2004;65:630–638. doi: 10.1124/mol.65.3.630. [DOI] [PubMed] [Google Scholar]
- 122.Levin M. Errors of geometry: regeneration in a broader perspective. Semin Cell Dev Biol. 2009;20:643–645. doi: 10.1016/j.semcdb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Levin M. Bioelectric mechanisms in regeneration: Unique aspects and future perspectives. Semin Cell Dev Biol. 2009;20:543–556. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nuckels RJ, Ng A, Darland T, Gross JM. The vacuolar-ATPase complex regulates retinoblast proliferation and survival, photoreceptor morphogenesis and pigmentation in the zebrafish eye. Invest Ophthalmol Vis Sci. 2009;50:893–905. doi: 10.1167/iovs.08-2743. [DOI] [PubMed] [Google Scholar]
- 125.Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- 126.West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cherubini A, Hofmann G, Pillozzi S, Guasti L, Crociani O, Cilia E, et al. Human ether-a-go-go-related gene 1 channels are physically linked to beta1 integrins and modulate adhesion-dependent signaling. Mol Biol Cell. 2005;16:2972–2983. doi: 10.1091/mbc.E04-10-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Worby CA, Dixon JE. Phosphoinositide phosphatases: emerging roles as voltage sensors? Mol Interv. 2005;5:274–277. doi: 10.1124/mi.5.5.5. [DOI] [PubMed] [Google Scholar]
- 129.Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- 130.Yang SJ, Liang HL, Ning G, Wong-Riley MT. Ultrastructural study of depolarization-induced translocation of NRF-2 transcription factor in cultured rat visual cortical neurons. Eur J Neurosci. 2004;19:1153–1162. doi: 10.1111/j.1460-9568.2004.03250.x. [DOI] [PubMed] [Google Scholar]
- 131.Feldman JD, Vician L, Crispino M, Tocco G, Marcheselli VL, Bazan NG, et al. KID-1, a protein kinase induced by depolarization in brain. J Biol Chem. 1998;273:16535–16543. doi: 10.1074/jbc.273.26.16535. [DOI] [PubMed] [Google Scholar]
- 132.Liu W, Feldman JD, Machado HB, Vician LJ, Herschman HR. Expression of depolarization-induced immediate early gene proteins in PC12 cells. J Neurosci Res. 2003;72:670–678. doi: 10.1002/jnr.10626. [DOI] [PubMed] [Google Scholar]
- 133.Lieb K, Biersack L, Waschbisch A, Orlikowski S, Akundi RS, Candelario-Jalil E, et al. Serotonin via 5-HT7 receptors activates p38 mitogen-activated protein kinase and protein kinase C epsilon resulting in interleukin-6 synthesis in human U373 MG astrocytoma cells. J Neurochem. 2005;93:549–559. doi: 10.1111/j.1471-4159.2005.03079.x. [DOI] [PubMed] [Google Scholar]
- 134.Deraet M, Manivet P, Janoshazi A, Callebert J, Guenther S, Drouet L, et al. The natural mutation encoding a C terminus-truncated 5-hydroxytryptamine 2B receptor is a gain of proliferative functions. Mol Pharmacol. 2005;67:983–991. doi: 10.1124/mol.104.008268. [DOI] [PubMed] [Google Scholar]
- 135.Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N, et al. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate. 2004;59:328–336. doi: 10.1002/pros.10374. [DOI] [PubMed] [Google Scholar]
- 136.Nebigil CG, Launay JM, Hickel P, Tournois C, Maroteaux L. 5-hydroxytryptamine 2B receptor regulates cell cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci USA. 2000;97:2591–2596. doi: 10.1073/pnas.050282397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fanburg B, Lee S. A new role for an old molecule: serotonin as a mitogen. Am J Physiol. 1997;272:795–806. doi: 10.1152/ajplung.1997.272.5.L795. [DOI] [PubMed] [Google Scholar]
- 138.Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 139.Zhang W, Kone BC. NFkappaB inhibits transcription of the H(+)-K(+)-ATPase alpha(2)-subunit gene: role of histone deacetylases. Am J Physiol Renal Physiol. 2002;283:904–911. doi: 10.1152/ajprenal.00156.2002. [DOI] [PubMed] [Google Scholar]
- 140.Borgens RB, Shi R. Uncoupling histogenesis from morphogenesis in the vertebrate embryo by collapse of the transneural tube potential. Dev Dyn. 1995;203:456–467. doi: 10.1002/aja.1002030408. [DOI] [PubMed] [Google Scholar]
- 141.Hotary KB, Robinson KR. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol. 1994;166:789–800. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 142.Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- 143.Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- 144.Pazmany T, Murphy SP, Gollnick SO, Brooks SP, Tomasi TB. Activation of multiple transcription factors and fos and jun gene family expression in cells exposed to a single electric pulse. Exp Cell Res. 1995;221:103–110. doi: 10.1006/excr.1995.1357. [DOI] [PubMed] [Google Scholar]
- 145.Reed SD, Li S. Electroporation Advances in Large Animals. Curr Gene Ther. 2009 doi: 10.2174/156652309788921062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ferraro B, Cruz YL, Coppola D, Heller R. Intradermal delivery of plasmid VEGF(165) by electroporation promotes wound healing. Mol Ther. 2009;17:651–657. doi: 10.1038/mt.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gao Z, Wu X, Song N, Cao Y, Liu W. Electroporation-mediated plasmid gene transfer in rat incisional wound. J Dermatol Sci. 2007;47:161–164. doi: 10.1016/j.jdermsci.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 148.Echeverri K, Tanaka EM. Electroporation as a tool to study in vivo spinal cord regeneration. Dev Dyn. 2003;226:418–425. doi: 10.1002/dvdy.10238. [DOI] [PubMed] [Google Scholar]
- 149.Atkinson DL, Stevenson TJ, Park EJ, Riedy MD, Milash B, Odelberg SJ. Cellular electroporation induces dedifferentiation in intact newt limbs. Dev Biol. 2006;299:257–271. doi: 10.1016/j.ydbio.2006.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang Z, Ramirez J, Reboutier D, Brault M, Trouverie J, Pennarun AM, et al. Brassinosteroids regulate plasma membrane anion channels in addition to proton pumps during expansion of Arabidopsis thaliana cells. Plant Cell Physiol. 2005;46:1494–1504. doi: 10.1093/pcp/pci162. [DOI] [PubMed] [Google Scholar]
- 151.Wonderlin WF, Woodfork KA, Strobl JS. Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J Cell Physiol. 1995;165:177–185. doi: 10.1002/jcp.1041650121. [DOI] [PubMed] [Google Scholar]
- 152.Martens JR, O’Connell K, Tamkun M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol Sci. 2004;25:16–21. doi: 10.1016/j.tips.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 153.Davies A, Douglas L, Hendrich J, Wratten J, Tran Van Minh A, Foucault I, et al. The calcium channel alpha2delta-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: implications for localization and function. J Neurosci. 2006;26:8748–8757. doi: 10.1523/JNEUROSCI.2764-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gogan P, Schmiedel-Jakob I, Chitti Y, Tyc-Dumont S. Fluorescence imaging of local membrane electric fields during the excitation of single neurons in culture. Biophys J. 1995;69:299–310. doi: 10.1016/S0006-3495(95)79935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wallace R. Neural membrane microdomains as computational systems: Toward molecular modeling in the study of neural disease. Biosystems. 2007;87:20–30. doi: 10.1016/j.biosystems.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 156.Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev. 2001;81:1–19. doi: 10.1152/physrev.2001.81.1.1. [DOI] [PubMed] [Google Scholar]
- 157.Carrithers MD, Dib-Hajj S, Carrithers LM, Tokmoulina G, Pypaert M, Jonas EA, et al. Expression of the voltage-gated sodium channel NaV1.5 in the macrophage late endosome regulates endosomal acidification. J Immunol. 2007;178:7822–7832. doi: 10.4049/jimmunol.178.12.7822. [DOI] [PubMed] [Google Scholar]
- 158.Mohammad-Panah R, Harrison R, Dhani S, Ackerley C, Huan LJ, Wang Y, et al. The chloride channel ClC-4 contributes to endosomal acidification and trafficking. J Biol Chem. 2003;278:29267–29277. doi: 10.1074/jbc.M304357200. [DOI] [PubMed] [Google Scholar]
- 159.Schwake M, Friedrich T, Jentsch TJ. An internalization signal in ClC-5, an endosomal Cl-channel mutated in dent’s disease. J Biol Chem. 2001;276:12049–12054. doi: 10.1074/jbc.M010642200. [DOI] [PubMed] [Google Scholar]
- 160.Steinberg BE, Touret N, Vargas-Caballero M, Grinstein S. In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc Natl Acad Sci USA. 2007;104:9523–9528. doi: 10.1073/pnas.0700783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Thompson RJ, Akana HC, Finnigan C, Howell KE, Caldwell JH. Anion channels transport ATP into the Golgi lumen. Am J Physiol Cell Physiol. 2006;290:499–514. doi: 10.1152/ajpcell.00585.2004. [DOI] [PubMed] [Google Scholar]
- 163.Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan PG. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 164.Glab M, Lojek A, Wrzosek A, Dolowy K, Szewczyk A. Endothelial mitochondria as a possible target for potassium channel modulators. Pharmacol Rep. 2006;58:89–95. [PubMed] [Google Scholar]
- 165.Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat Methods. 2008;5:683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- 166.Levin M. Is the early left-right axis like a plant, a kidney or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today. 2006;78:191–223. doi: 10.1002/bdrc.20078. [DOI] [PubMed] [Google Scholar]
- 167.Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci USA. 2008;105:9111–9116. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Epps DE, Wolfe ML, Groppi V. Characterization of the steady-state and dynamic fluorescence properties of the potential-sensitive dye bis-(1,3-dibutylbarbi-turic acid)trimethine oxonol (Dibac4(3)) in model systems and cells. Chem Phys Lipids. 1994;69:137–150. doi: 10.1016/0009-3084(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 169.Saris CJ, Domen J, Berns A. The pim-1 oncogene encodes two related protein-serine/threonine kinases by alternative initiation at AUG and CUG. EMBO J. 1991;10:655–664. doi: 10.1002/j.1460-2075.1991.tb07994.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Perrin MJ, Subbiah RN, Vandenberg JI, Hill AP. Human ether-a-go-go related gene (hERG) K+ channels: function and dysfunction. Prog Biophys Mol Biol. 2008;98:137–148. doi: 10.1016/j.pbiomolbio.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 171.Isomoto S, Kondo C, Kurachi Y. Inwardly rectifying potassium channels: their molecular heterogeneity and function. Jpn J Physiol. 1997;47:11–39. doi: 10.2170/jjphysiol.47.11. [DOI] [PubMed] [Google Scholar]
- 172.Jentsch TJ, Poet M, Fuhrmann JC, Zdebik AA. Physiological functions of CLC Cl channels gleaned from human genetic disease and mouse models. Annu Rev Physiol. 2005;67:779–807. doi: 10.1146/annurev.physiol.67.032003.153245. [DOI] [PubMed] [Google Scholar]
- 173.Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:793–801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- 174.Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 175.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 176.Harvey WR, Wieczorek H. Animal plasma membrane energization by chemiosmotic H+ V-ATPases. J Exp Biol. 1997;200:203–216. doi: 10.1242/jeb.200.2.203. [DOI] [PubMed] [Google Scholar]
- 177.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]