Abstract
More than half a million Americans became newly infected with HIV in the first decade of the new millennium. The domestic epidemic has had the heaviest impact on men who have sex with men (MSM) and people from racial and ethnic minority populations, particularly African-Americans. For example, Black MSM represent <1% of the U.S. population but 25% of the new HIV cases, as per CDC estimates published in 2008. While Black and Hispanic women constitute 24% of all U.S. women, they accounted for 82% of HIV cases in women in 2005, based on data from 33 states with confidential name-based reporting. There is a nearly 23-fold higher rate of AIDS diagnoses for Black women (45.5/100,000 women) and nearly 6-fold higher rate for Hispanic women (11.2/100,000) compared to the rate for white women (2.0/100,000). Investigators from the HIV Prevention Trials Network (HPTN), an NIH-sponsored collaborative clinical trials group, have crafted a domestic research agenda with community input. Two new domestic studies are in progress (2009) and a community-based clinical trial feasibility effort is in development (2010 start date). These studies focus on outreach, testing, and treatment of infected persons as a backbone for HIV prevention. Reaching persons not receiving health message and service with novel approaches to both prevention and care/treatment is an essential priority for HIV control in the U.S.; our research is designed to guide the best approaches and assess the impact of bridging treatment and prevention.
Keywords: HIV, prevention, United States, homosexual, women, transmission, antiretroviral treatment, black, Hispanic
More than one million people in the U.S. are currently living with HIV, of whom an estimated one quarter are unaware of their HIV infection status. There have been an estimated 56,000 new HIV infections in the first years of the 21st century, with no evidence of a decrease in this number from year to year for the past decade. [1] As attempts to develop a number of biological and behavioral prevention measures against HIV have been largely disappointing to date, there is intense global interest in pursuing research in innovative HIV prevention strategies.
Current U.S. HIV Dynamics and Need for a Research Response
The HIV epidemic in the U.S. is geographically localized and is further focused in specific populations, creating “hot spots” of transmission nested within sexual and/or drug use risk networks. Two populations at highest risk are men who have sex with men (MSM), particularly Black MSM, and women of Black and Hispanic race/ethnicity.[2, 3] HIV transmission also persists in White MSM and substance users. The advent of antiretroviral therapy (ART) and the plummeting of HIV-related death rates in the United States have led at-risk persons to view HIV prevention to be less urgent. [4] Failure to lower HIV incidence in the U.S. during the potent antiretroviral therapy era likely reflects this “HIV complacency” alongside a systemic failure to effectively reach the persons at highest risk with risk reduction interventions and for those with recognized or unrecognized HIV infection with ART.
In 2008, the NIH-funded HIV Prevention Trials Network (HPTN) completed a systematic review of available literature and an analysis of available data on HIV prevalence and reported new HIV infections in the U.S .[5] Based on this review, an HPTN Domestic Prevention Research Agenda was developed (http://www.hptn.org/web%20documents/DPWG/HPTNDomesticResearchAgenda03-29-08.pdf, accessed September 28, 2009). In addition, based on results of the analysis of available data on reported new HIV infection in the U.S., it was apparent that approximately half of the newly reported cases were detected in MSM and that one quarter of cases were occurring in women of color. Thus, clearly these populations needed to be considered as special priorities for HIV prevention efforts because of their disproportionate disease burden. For example, Black MSM represent <1% of the U.S. population but 25% of the new HIV cases.[5] HIV in U.S. women is distributed in highly skewed geographical venues. While Black and Hispanic women constitute 24% of all U.S. women, they accounted for 82% of HIV cases in women in 2005, based on data from 33 states with confidential name-based reporting.[6–8] In addition, the heightened risk among women of color is dramatically demonstrated by the nearly 23-fold higher rate of AIDS diagnoses for Black women (45.5/100,000 women) and nearly 6-fold higher rate for Hispanic women (11.2/100,000) compared to the rate for white women (2.0/100,000).[6, 8]
HPTN are also exploring new research concepts for other key populations that have a heavy HIV burden in the U.S., such as non-minority MSM. For many at-risk persons, substance use and intercurrent mental health issues (e.g., depression) may play a major role in potentiating HIV spread. Future prevention clinical trials that address these primary drivers of risk-taking behavior and poor adherence could be helpful in enhancing HIV prevention.
As a most urgent priority, HPTN investigators have focused on Black MSM and women at risk as two relatively neglected populations at highest risk in the domestic U.S. HIV epidemic. Several questions were identified that required urgent attention prior to embarking on large HIV prevention effectiveness studies in these latter populations. These included: What are accurate estimates of HIV incidence in these populations? How can persons at risk who are least likely to be engaged in HIV prevention programs be reached? What are the types of prevention interventions that are likely to be feasible, acceptable, and effective in these individuals? How can individuals with unrecognized HIV be connected to HIV testing services? How can those with known HIV infection be linked and engaged in HIV care? How can those on ART achieve complete viral suppression? To begin to address these questions, several critical feasibility studies have been designed and launched to address these knowledge gaps.
Black Men Who Have Sex With Men: the HPTN 061 protocol (BROTHERS)
The HPTN 061 protocol (“BROTHERS” study) is designed to assess the feasibility of a community-level, multi-component intervention for reducing HIV incidence among Black MSM (protocol chairs are B.A.K., K.H.M., and D.P.W.). In recognition of the importance of this study, as well as the fact that Black MSM represent a neglected population for HIV risk reduction efforts, a diverse team was constituted in the design and implementation of this study inclusive of individuals from the target population as community representatives and investigators.
The protocol uses innovative community outreach methods to recruit Black MSM into a longitudinal study within a clinic-linked community setting, enabling prompt linkage to services of recruited men and their partners, as indicated. The recruitment approaches being utilized are both conventional (direct enrollment of sexually active Black MSM) and complex (enrolling persons who are part of the sexual/social network of directly recruited individuals). Men are, therefore, recruited in one of two ways – either directly from the community (“community recruited” participants) or as sexual network partners referred by participants (“referred” participants). A subset of community recruited and referred participants are considered “index” participants by virtue of having been newly identified with HIV infection through the study or having had previously diagnosed HIV infection but not receiving HIV care. These index participants are asked to refer network members into the study. A random sample of HIV-negative participants is recruited as a subset of those considered as index participants. Another component of the study includes conduct of focus groups (approximately 18–24 participants per site) and qualitative interviews (up to 30 participants at each site).
Field Research Plans for the HPTN 061 “BROTHERS” Study
The study began in mid-2009 and will continue for 12 months of participant accrual and up to 12 months of follow-up for each participant, with study visits at 6 and 12 months. Assessments include a behavioral and psychosocial assessments, as well as HIV and STI screening. At least 2,418 participants (403 per site) will be enrolled from 6 cities; San Francisco, Los Angeles, Atlanta, Boston, New York City, and Washington, D.C. (Table 1).
Table 1.
Sites for HPTN 061 (“BROTHERS”) and HPTN 064 (“ISIS”) studies to reduce HIV transmission in the US.
| Clinical Research Sites (CRS) | |
|---|---|
| HPTN 061 | HPTN 064 |
| The Fenway Institute CRS, Boston, MA | New Jersey Medical School- Adult CRS, Newark, NJ |
| Ponce de Leon Center CRS, Atlanta, GA | |
| Hope Clinic CRS, Emory Vaccine Center, Decatur, GA | |
| UCLA Vine Street CRS, Los Angeles, CA | Johns Hopkins University AIDS CRS, Baltimore, MD |
| Harlem Prevention CRS, New York, NY | |
| New York Blood Center/Union Square CRS, NY, NY |
Bronx-Lebanon Hospital Center CRS, Bronx, NY |
| San Francisco Vaccine and Prevention CRS, San Francisco, CA |
University of North Carolina AIDS CRS, Chapel Hill, NC |
| Wake County Health and Human Services CRS, Raleigh, NC |
|
| George Washington University CRS, Washington, DC | |
Another key feature of the study is the assessment of the feasibility and acceptability of use of peer health system navigators with this population. The success of such an intervention in engaging disenfranchised populations was first demonstrated in the U.S. by Freeman et al more than 20 years ago when they found increased mammography utilization by African-American women through use of trained peer assistance.[9] More recently, HIV-infected persons were found to be more likely to be retained in care and have suppressed plasma HIV RNA when health system navigators were available to assist them. [10] The HPTN study aims to determine whether peer health system navigation can assist with primary and secondary HIV prevention and whether this model is highly acceptable among Black MSM.
Study Objectives and Outcomes for the HPTN 061 “BROTHERS” Study
The key study objective is to assess the feasibility of recruiting and engaging Black MSM into prospective research studies designed to test novel approaches to reduce HIV risk and incidence.
Engagement of participants includes:
Referral of up to 5 sexual partners by index participants for study enrollment.
HIV risk reduction counseling, testing, and referral for HIV care, as needed.
Sexually transmitted infection (STI) testing and referral for care.
Screening for substance use, mental health issues, partner and/or homophobic violence, and counseling and referral for care as indicated.
Engagement with peer health care system navigators to facilitate uptake of health care and other services.
Key study outcomes include:
Effectiveness in recruitment and retention of black MSM.
Uptake of the intervention by black MSM, specifically the proportion of enrolled participants who agree to HIV testing, agree to STI testing, and use peer navigation.
Estimation of the following key measures: the proportion of participants newly diagnosed with HIV at enrollment; change in condom use from enrollment to week 52 of follow-up; change in HIV RNA levels at week 52 among HIV-infected participants who initiate potent ART; change in prevalence of STIs from enrollment to week 52; and satisfaction of Black MSM with intervention components.
Secondary objectives are also seen as important components of the HPTN 061 protocol and include:
Collecting samples, behavioral data, and HIV test results to improve laboratory measures of HIV incidence in cross-sectional surveys.
Estimating HIV incidence rates under intervention conditions.
Estimating the effects of the intervention on HIV incidence through mathematical modeling.
Describing social and sexual networks of Black MSM based on individually self-reported network data.
Describing risk behaviors of sexual network members of participants, especially of those who are newly diagnosed with HIV infection or were previously diagnosed but not in care.
Assessing attitudes of participants toward other HIV prevention interventions.
Examining individual, interpersonal, cultural, institutional, and geographic-specific processes that influence study participation and uptake of intervention components.
Understanding how and to what extent stigma and discrimination (and other emergent themes) influence HIV testing and access to care by geographic region.
As Black MSM represent a neglected population for HIV risk reduction efforts, we have engaged Black MSM themselves from communities and universities to help guide the efforts of the BROTHERS study investigators.
The Women’s HIV SeroIncidence Study (ISIS): the HPTN 064 protocol
The HPTN 064 protocol (“ISIS” study) is designed to estimate HIV incidence rates in women at risk for HIV acquisition in the U.S. and to determine the feasibility of enrolling and following at-risk women (protocol chairs are S.L.H. and J.E.J.). Measurement of HIV incidence in this study will be determined through the traditional multi-site, prospective observational cohort design of determining rate of HIV seroconversion over time, as well as use of novel laboratory assay algorithms to estimate recent HIV infection rates retrospectively at the time of enrollment. Quantitative as well as qualitative methods will be utilized for assessment of behavioral issues in the study. Semi-structured interviews of participants and focus group discussions with women enrolled in the cohort will be conducted as well as focus groups that include men recruited from the same communities.
A key innovative component of this study is the choice of a venue-based, time-space sampling (VBS) recruitment strategy. VBS is a cross-sectional survey of persons who attend venues within locally defined geographic areas and has been used to obtain large and diverse samples of MSM [11–13] and heterosexuals. [14] The use of VBS in ISIS permits the enrollment of women at high risk for HIV acquisition in a systematic and reproducible manner, and reflects the geographic patterns of the HIV epidemic in the U.S. Building on HIV surveillance data and community poverty rates, priority prevention areas with potentially high HIV incidence rates are identified in neighborhoods in several locations in the U.S. Within these defined neighborhoods, ethnographic work identifies venues, such as playgrounds, laundromats and grocery stores, where potential participants are approached. Women are enrolled based on geographic criteria (residence within specified census tracts) as well as their own or their partners’ behavioral characteristics. This recruitment strategy will result in the enrollment of more women with the highest geographic and behavioral risk profiles, rather than women who are primarily interested in enrolling in a clinical trial.
Field Research Plans for the HPTN 064 “ISIS” Study
The study aims to recruit 2,000 women from 10 geographically distinct communities in the Atlanta metropolitan area, Baltimore, Newark, New York City (Bronx and Manhattan), Chapel Hill/Raleigh metropolitan area, and Washington, D.C. (Table 1). Assuming approximately 40 HIV infections, the sample size provides a very precise (95% CI ±0.006/100 person-years) estimate of annual HIV incidence. Qualitative data will be collected from subsets of enrolled women from 4 (2 urban and 2 non-urban) of the 10 participating communities. In the first subset of women, focus groups will be conducted to explore ways to enhance recruitment of at-risk women for subsequent prevention trials. Incentives, barriers and facilitators to enrollment and retention (e.g., child care; transportation) will be discussed, as well as convenient times and locations for participating in HIV interventions. In a second subset of women, semi-structured, recorded interviews will be conducted with approximately 30 women in each of 4 communities to identify social, structural, and contextual factors likely to affect women’s HIV-related decision-making. In addition, male focus groups will explore barriers and facilitators to male-mediated factors of HIV prevention, such as male perceptions of community-level HIV testing efforts.
Study Objectives and Outcomes for the HPTN 064 “ISIS” Study
The study duration is 2 years with accrual for 1 year and follow-up for 6–12 months. Interventions to be provided include HIV testing, counseling for risk reduction, and referrals for substance use, domestic violence, mental health issues and HIV care, as needed. In addition to the primary objective of estimation of the overall HIV-1 incidence rate, important secondary objectives are as follows:
To evaluate new laboratory assays and algorithms for cross-sectional HIV-1 incidence determination, including use of combined laboratory methods and statistical adjustments based on population CD4+ cell count and ART coverage characteristics, anticipating future large-scale cross-sectional HIV intervention study in women and/or men [9].
To estimate recruitment and retention rates for use in the design of future interventional trials.
To describe sexual behaviors, alcohol and drug use, prevalence of domestic violence, and mental health indicators of women at risk of HIV acquisition.
To assess women’s preferred recruitment and retention strategies for future studies.
To describe social, structural and contextual factors in a subgroup of women to inform future intervention studies.
To estimate HIV prevalence rates among women who have not reported previously testing HIV-positive.
To explore facilitators and barriers to HIV testing among men residing in high risk areas to inform future intervention studies.
Past efforts to recruit high risk women into HIV prevention clinical trials in the U.S. have failed to identify women with >0.5% seroincidence per annum.[10, 15–18] This study aims to utilize an innovative multi-dimensional approach based on three elements, community, individual and partner characteristics. It is anticipated that the majority of women recruited in the study will include Black or Hispanic individuals, reflecting the characteristics of the U.S. HIV epidemic in women. (Table 1).
The Test and Treat “Plus” Study: the HPTN 065 protocol
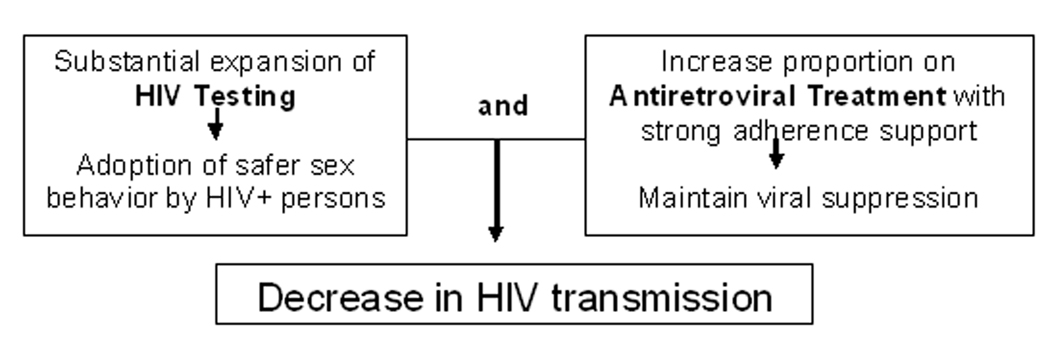
Persons who are unaware of their HIV infection are more likely to be diagnosed at advanced stages of HIV disease and thus are likely to have had uncontrolled HIV replication with consequent high plasma and genital tract HIV RNA levels for many years without access to ART.[19, 20] Suppression of plasma HIV RNA to undetectable levels through ART may lead to decreased risk of HIV transmission, offering a public health benefit alongside personal health benefits of ART.[21–31] In addition, several studies have demonstrated that individuals who know their HIV-positive status are likely to adopt safer behaviors [32, 33] and, thus, to reduce the risk of transmitting HIV to others. Therefore, potentially effective interventions to decrease HIV incidence in a community could involve a combination of interventions including intensive HIV testing efforts for early identification of HIV, linking HIV-infected individuals with access to ART, providing adherence support to maintain viral suppression combined with other prevention efforts. One model based on parameters relevant to the HIV epidemic in South Africa suggested a substantial impact on the trajectory of the global epidemic if testing and early treatment with antiretroviral medications were widely implemented.[34]
Expanded HIV testing and treatment coverage to prevent HIV transmission
Based on these promising elements for a cohesive HIV prevention strategy, the HPTN has embarked on the design of a Test aNd Treat (TNT) study that aims to determine the feasibility of such a strategy. The study involves expanded HIV screening and testing to identify individuals with HIV who are unaware of their status and then utilizing ART to decrease risk of HIV transmission. The study builds on ongoing efforts by community service providers and social mobilization to expand HIV testing, the community of HIV providers engaged in HIV care and treatment and the wealth of available HIV surveillance data to investigate various elements of this strategy. Key elements of enhanced TNT (“TNT-Plus”) include:
Substantial expansion of HIV testing with a goal of universal offering of testing to populations where testing is likely to have a high yield
Linkage of those identified with HIV to care sites using innovative approach
Prompt evaluation for ART eligibility and optimization of ART initiation
Provision of intervention to decrease risk of HIV transmission from those with HIV to others
Support for achievement of suppression of viral replication through innovative approach
The TNT Study team, co-chaired by W.M.E-S. and Bernard Branson of the CDC, has an expected launch in early 2010. The proposed study aims to use an innovative approach to demonstrate the feasibility of embarking on a large test and treat study through use of a community-focused approach, nested comparison of innovations and utilization of HIV surveillance data from the target communities. Implicit in the overarching test and treat approach are the following questions: can a substantial increase in HIV testing coverage be accomplished, will this effort identify those with undiagnosed infection in a community, can those with HIV infection be effectively linked and engaged in HIV care, can use of ART achieve decrease in community viral load levels, and consequently will these efforts result in decrease in HIV incidence? [34–36]
Need for New Prevention Research for MSM
While focusing on the studies described above, at the same time, the HPTN is exploring new research concepts for some of the other key populations that bear a heavy HIV burden in the U.S., such as white and Hispanic MSM who will not be targeted in HPTN 061 “BROTHERS” study, focused on Black MSM. For many of these men, substance use (e.g. methamphetamine, excessive alcohol) and intercurrent mental health issues (e.g. depression) may play a major role in potentiating HIV transmission, and thus need to inform design of future prevention trials. All of these efforts build on previous HPTN studies in the U.S. which have addressed behavioral and biological risk reduction in MSM, [37–39] injection drug users, [40] and at-risk women. [41–43] The studies described above demonstrate the importance of working through networks such as the HPTN in order to address questions in a variety of venues, and with a large enough sample size to derive meaningful results to guide the next stages of community- or individual-level clinical trials.
Stemming the HIV epidemic in the U.S. will require concerted research efforts, innovation and perseverance, assessment of complex multi-component strategies rather than traditional assessment of one intervention, embarking on critical feasibility studies, working at a community level rather than at a facility or research site level, working with diverse constituencies, use of new design methods, and establishment of meaningful partnerships with communities, often disenfranchised or stigmatized. We are optimistic that this research partnership has begun the journey toward our common goal and public health imperative.
Figure 1.
Test and Treat Hypothesis
Acknowledgements
The authors would like to acknowledge the contributions of members of the HPTN Domestic Prevention Working Group, the protocol teams involved in the studies described as well as community members who have contributed to this effort. Janeen Burlison and Meredith Bortz assisted in manuscript preparation. Support for this review was provided by the NIH through the HIV Prevention Trials Network (HPTN; grant # U01AI068619. The content of this publication or presentation does not necessarily reflect the views or policies of the NIH or HPTN Executive Committee.
Footnotes
Disclosures:
Sten Vermund: Consultant for Pfizer (Data Monitoring Committee), ended 2009
Sally Hodder: Research Support from BMS, Gilead, and Tibotec. Consultant/Advisory Boards for Boehringer Ingelheim, BMS, Gilead, and Tibotec.
Ken Mayer: Research support from Gilead and Merck; speaker’s fees from Inverness
All author authors have no potential conflicts.
REFERENCES
- 1.HIV prevalence estimates--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(39):1073–1076. [PubMed] [Google Scholar]
- 2.Qian HZ, Taylor RD, Fawal HJ, Vermund SH. Increasing AIDS case reports in the South: U.S. trends from 1981–2004. AIDS Care. 2006;18 Suppl 1:S6–S9. doi: 10.1080/09540120600839074. [DOI] [PubMed] [Google Scholar]
- 3.Holmes R, Fawal H, Moon TD, et al. Acquired immunodeficiency syndrome in Alabama: special concerns for black women. South Med J. 1997 Jul;90(7):697–701. doi: 10.1097/00007611-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Increases in unsafe sex and rectal gonorrhea among men who have sex with men--San Francisco, California, 1994–1997. MMWR Morb Mortal Wkly Rep. 1999;48(3):45–48. [PubMed] [Google Scholar]
- 5.Subpopulation estimates from the HIV incidence surveillance system--United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(36):985–989. [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services, CDC; HIV/AIDS Surveillance Report, 2005. (Revised edition) 2007 June;17:1–54.
- 7.Bridged-race vintage 2005 postcensal population estimates for July 1, 2000-July 2005, by year, county, single-year age, bridged-race, Hispanic origin, and sex. National Center for Health Statistics [Google Scholar]
- 8.HIV/AIDS among women: HIV/AIDS Fact Sheet. Department of Health and Human Services, CDC; 2008. Aug, pp. 1–7. [Google Scholar]
- 9.Loschen S, Batzing-Feigenbaum J, Poggensee G, et al. Comparison of the human immunodeficiency virus (HIV) type 1-specific immunoglobulin G capture enzyme-linked immunosorbent assay and the avidity index method for identification of recent HIV infections. J Clin Microbiol. 2008 Jan;46(1):341–345. doi: 10.1128/JCM.01055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown-Peterside P, Chiasson MA, Ren L, Koblin BA. Involving women in HIV vaccine efficacy trials: lessons learned from a vaccine preparedness study in New York City. J Urban Health. 2000 Sep;77(3):425–437. doi: 10.1007/BF02386751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacKellar D, Valleroy L, Karon J, Lemp G, Janssen R. The Young Men's Survey: methods for estimating HIV seroprevalence and risk factors among young men who have sex with men. Public Health Rep. 1996;111 Suppl 1:138–144. [PMC free article] [PubMed] [Google Scholar]
- 12.Muhib FB, Lin LS, Stueve A, et al. A venue-based method for sampling hard-to-reach populations. Public Health Rep. 2001;116 Suppl 1:216–222. doi: 10.1093/phr/116.S1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stueve A, O'Donnell LN, Duran R, San Doval A, Blome J. Time-space sampling in minority communities: results with young Latino men who have sex with men. Am J Public Health. 2001 Jun;91(6):922–926. doi: 10.2105/ajph.91.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lansky A, Sullivan PS, Gallagher KM, Fleming PL. HIV behavioral surveillance in the U.S.: a conceptual framework. Public Health Rep. 2007;122 Suppl 1:16–23. doi: 10.1177/00333549071220S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skurnick JH, Kennedy CA, Perez G, et al. Behavioral and demographic risk factors for transmission of human immunodeficiency virus type 1 in heterosexual couples: report from the Heterosexual HIV Transmission Study. Clin Infect Dis. 1998 Apr;26(4):855–864. doi: 10.1086/513929. [DOI] [PubMed] [Google Scholar]
- 16.Chirgwin KD, Feldman J, Dehovitz JA, Minkoff H, Landesman SH. Incidence and risk factors for heterosexually acquired HIV in an inner-city cohort of women: temporal association with pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol. 1999 Mar 1;20(3):295–299. doi: 10.1097/00042560-199903010-00013. [DOI] [PubMed] [Google Scholar]
- 17.Djomand G, Beyrer C, Buchbinder S. Low HIV seroincidence among female commercial sex workers: a barrier for measuring HIV vaccine efficacy. J Acquir Immune Defic Syndr. 2008 Dec 15;49(5):570. doi: 10.1097/QAI.0b013e31818d5f9e. [DOI] [PubMed] [Google Scholar]
- 18.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008 Nov 29;372(9653):1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magnus M, Kuo I, Shelley K, et al. Risk factors driving the emergence of a generalized heterosexual HIV epidemic in Washington, District of Columbia networks at risk. AIDS. 2009 Jun 19;23(10):1277–1284. doi: 10.1097/QAD.0b013e32832b51da. [DOI] [PubMed] [Google Scholar]
- 20.Battegay M, Fluckiger U, Hirschel B, Furrer H. Late presentation of HIV-infected individuals. Antivir Ther. 2007;12(6):841–851. [PubMed] [Google Scholar]
- 21.Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005 Sep 1;40(1):96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- 22.Fang CT, Hsu HM, Twu SJ, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004 Sep 1;190(5):879–885. doi: 10.1086/422601. [DOI] [PubMed] [Google Scholar]
- 23.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000 Mar 30;342(13):921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 24.Hosseinipour M, Cohen MS, Vernazza PL, Kashuba AD. Can antiretroviral therapy be used to prevent sexual transmission of human immunodeficiency virus type 1? Clin Infect Dis. 2002 May 15;34(10):1391–1395. doi: 10.1086/340403. [DOI] [PubMed] [Google Scholar]
- 25.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008 Oct 18;22(16):2179–2185. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.La Sala GB, Pilotti E, Nicoli A, et al. Dynamics of HIV viral load in blood and semen of patients under HAART: impact of therapy in assisted reproduction procedures. AIDS. 2007 Jan 30;21(3):377–379. doi: 10.1097/QAD.0b013e328012ccfb. [DOI] [PubMed] [Google Scholar]
- 27.Katz MH, Schwarcz SK, Kellogg TA, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002 Mar;92(3):388–394. doi: 10.2105/ajph.92.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson DP, Law MG, Grulich AE, Cooper DA, Kaldor JM. Relation between HIV viral load and infectiousness: a model-based analysis. Lancet. 2008 Jul 26;372(9635):314–320. doi: 10.1016/S0140-6736(08)61115-0. [DOI] [PubMed] [Google Scholar]
- 29.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006 Aug 5;368(9534):531–536. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 30.Fisher M, Sudarshi Darshan, Brown Alison, Pao David, Parry John, Johnson Anne, Cane Patricia, Gill Noel, Sabin Caroline, Pillay Deenan. HIV transmission amongst men who have sex with men: association with antiretroviral therapy, infection stage, viraemia and STDs in a longitudinal phylogenetic study; CROI; 2009. [Google Scholar]
- 31.Fisher M, Sudarshi D, Brown A, al E. HIV Transmission among men who have sex with men: Association with ART, infection stage, viremia, and sexually transmitted diseases, a longitudinal phylogenetic study; 16th Conference on Retroviruses and Opportunistic Infections; 2009. [Google Scholar]
- 32.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005 Aug 1;39(4):446–453. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 33.Weinhardt LS, Carey MP, Johnson BT, Bickham NL. Effects of HIV counseling and testing on sexual risk behavior: a meta-analytic review of published research, 1985–1997. Am J Public Health. 1999 Sep;89(9):1397–1405. doi: 10.2105/ajph.89.9.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan 3;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 35.Lima VD, Johnston K, Hogg RS, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis. 2008 Jul 1;198(1):59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 36.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009 Jun 10;301(22):2380–2382. doi: 10.1001/jama.2009.828. [DOI] [PubMed] [Google Scholar]
- 37.Koblin B, Chesney M, Coates T. Effects of a behavioural intervention to reduce acquisition of HIV infection among men who have sex with men: the EXPLORE randomised controlled study. Lancet. 2004 Jul 3–9;364(9428):41–50. doi: 10.1016/S0140-6736(04)16588-4. [DOI] [PubMed] [Google Scholar]
- 38.Trial finds microbicide promising as HIV prevention method for women. Microbicide Trials Network. 2009 [Google Scholar]
- 39.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008 Jun 21;371(9630):2109–2119. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latkin CA, Donnell D, Metzger D, et al. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Soc Sci Med. 2009 Feb;68(4):740–748. doi: 10.1016/j.socscimed.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer KH, Karim SA, Kelly C, et al. Safety and tolerability of vaginal PRO 2000 gel in sexually active HIV-uninfected and abstinent HIV-infected women. AIDS. 2003 Feb 14;17(3):321–329. doi: 10.1097/00002030-200302140-00005. [DOI] [PubMed] [Google Scholar]
- 42.Vermund SH, Allen KL, Karim QA. HIV-prevention science at a crossroads: advances in reducing sexual risk. Curr Opin HIV AIDS. 2009 Jul;4(4):266–273. doi: 10.1097/COH.0b013e32832c91dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abdool Karim S, Coletti A, Richardson B, al E. Safety and Effectiveness of Vaginal Microbicides BufferGel and 0.5% PRO 2000/5 Gel for the Prevention of HIV Infection in Women: Results of the HPTN 035 Trial; 16th Conference on Retroviruses and Opportunistic Infections; 2009. [Google Scholar]