Abstract
Background
Thymidine analog bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) has been widely used to label cells in culture and in tissue. The labeled cells can also be tracked when transplanted into a suitable host. In the present study we tested a new thymidine analog 5-ethynyl-2-deoxyuridine (EdU) for labeling and tracking of mesenchymal stem cells (MSC), specifically adipose tissue-derived stem cells (ADSC).
Methods
Labeling of ADSC was examined for dosage effect of EdU and stability of label by Alexa-594 staining followed by fluorescence microscopy. Labeling of various organs/tissues was done by intraperitoneal injection of EdU and examined by histology and fluorescence microscopy. Tracking of ADSC was done by intratissue or intravenous transplantation of EdU-labeled ADSC into various tissues and examined by histology and fluorescence microscopy.
Results
EdU was incorporated specifically into the nucleus in approximately 50% of ADSC and the percentage of cells that remained fully labeled declined with time. Peritoneal injection of EdU resulted in the appearance of EdU-positive cells in most organs and tissues. In the intestine, EdU-positive cells were found in both the epithelium and connective tissues 7h after injection. Long-term (2–6 w) follow-ups found EdU-positive cells only in the connective tissue. Tracking of ADSC was successful in tissues 10 weeks after intratissue or intravenous transplantation.
Discussion
Cell labeling with EdU in culture or living animal can be easily performed. Detection of EdU label requires no harsh treatment or immunological reaction as detection of BrdU label does. EdU can be used for long-term tracking of ADSC.
Keywords: EdU, mesenchymal stem cells, adipose tissue-derived stem cells, cell labeling, cell tracking
Introduction
Bromodeoxyuridine (5-bromo-2-deoxyuridine, BrdU) is a synthetic nucleoside that can be incorporated into the newly synthesized DNA of replicating cells. The BrdU-containing cells can be subsequently detected by immunochemistry using a BrdU-specific antibody [1, 2]. In earlier studies this BrdU labeling method was used mainly for analyzing cell cycle in cultured cells [3] and for visualizing proliferating cells in the central nervous system [4]. In more recent studies, it has been used to identify stem cells, which are believed to divide slowly or to segregate chromosomes asymmetrically, allowing the retention of BrdU in the slowly dividing stem cells or in the daughter (non-differentiating) stem cells but not in the differentiated daughter cells [5–8]. In addition, BrdU labeling has been used to track stem or non-stem cells that are labeled in vitro and subsequently transplanted in vivo [9, 10]. In the case of stem cells, such tracking allows the determination of whether the transplanted stem cells have differentiated into a particular cell type [10, 11].
However, despite its extensive usage in conventional cell biology and recent stem cell studies, the immunochemical detection of BrdU-labeled cells can be problematic because of the need to use strong DNA denaturing conditions, such as strong acids and heating, to expose the epitope so that it can bind to the antibody. This harsh treatment can distort the cell and tissue structure and can cause the loss of antigenicity of cellular proteins, preventing the immunochemical detection (co-localization) by their specific antibodies. Thus, in an effort to overcome these problems, Salic and Mitchison [12] recently introduced an alternative thymidine analogue, 5-ethynyl-2-deoxyuridine (EdU). The terminal alkyne group of EdU allows chemical detection using a fluorescent azide that covalently binds to the alkyne group. This detection method is fast and specific and does not require DNA denaturation. Its application for visualizing proliferating cells in the central nervous system has recently been demonstrated [13]. In the present study we show that it can also be used to label and tract stem cells - in this case, mesenchymal stem cells derived from the adipose tissue (adipose tissue-derived stem cells, ADSC) [14].
Material and Methods
Animals
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee at our institution. All animals were Sprague-Dawley rats purchased from Charles River Laboratories (Wilmington, MA). For in vivo EdU labeling, a total of 40 pups were used. These pups were given birth by rats that were made urinary incontinent (UI) as described previously [15, 16]. Briefly, immediately after delivery, the rats were subjected to vaginal balloon inflation to simulate birth trauma. One week later, the rats underwent ovariectomy to simulate menopause. Fat pads of the excised ovaries were used for ADSC isolation. For tracking transplanted cells in a high-fat diet-induced UI model, a total of twenty 3-month-old male rats were used. The experimental procedure for the establishment of the hyperlipidemic model has been described previously [17]. Briefly, rats were fed with a diet consisting of 2% cholesterol and 10% lard. Five months later, after body weight and blood lipid measurement, these rats were used for ADSC isolation and transplantation.
ADSC isolation and culture
ADSC were isolated from the above-harvested adipose tissue using a modified version of our previously published protocol [18]. Briefly, within 4 hours of harvest, the tissue was rinsed with phosphate-buffered saline (PBS) containing 1% penicillin and streptomycin, minced into small pieces, and then incubated in a solution containing 0.075% collagenase type IA (Sigma-Aldrich, St. Louis, MO, USA) for 1 hour at 37°C with vigorous shake. The top lipid layer was removed and the remaining liquid portion was centrifuged at 220×g for 10 min at room temperature. The pellet was treated with 160 mM NH4Cl for 10 minutes to lyse red blood cells. The remaining cells were suspended in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), filtered through a 40-μm cell strainer (BD Biosciences, Bedford, MA, USA), and plated at a density of 1 × 106 cells in a 10-cm dish. After reaching 80% confluence, the cells were trypsinized and reseeded at 25% confluence in successive passages. Cells between the 2nd and 5th passages were used for EdU labeling and tracking.
EdU labeling of ADSC
For EdU dosage study, 50,000 cells were seeded onto a coverslip in each well of a 6-well plate in DMEM supplemented with 10% FBS, 1% nonessential amino acid, 10,000 units/mL penicillin, 10,000 mcg/mL streptomycin SO4, 0.025 mg/mL fungizone, and 110 mg/mL sodium pyruvate. Twenty-four h later, EdU (Invitrogen, Carlsbad, CA) was added to the medium in concentrations of 0, 10, 20 and 50 μM. Twenty-four h later, cells were fixed for EdU staining (see below). For time course study (retention of label), 300,000 cells were seeded into a 10-cm dish in the same medium as above. Twenty-four later, EdU was added to the medium at 10 μM. Twenty-four h later, cells were washed three times with PBS followed by the addition of culture medium and resumption of incubation. At day 1, 4, 7, 14, and 21, the cells were trypsinized and reseeded onto coverslips as above. Six h after seeding, the cells were fixed with methanol, washed twice with PBS, incubated in 3% bovine serum albumin (BSA) in PBS, and then incubated in 0.5% Triton® X-100 in PBS for 20 minutes at room temperature. The cells were then incubated with freshly made Click-iT reaction cocktail, which contained Alexa-fluor 594 azide (Alexa-594, Cat #C10084, Invitrogen), for 30 minutes at room temperature without light. Cells were counterstained with Hoechst, mounted in standard mounting media and imaged by fluorescence microscopy.
EdU labeling of tissues
A total of 40 newborn (0-day-old) rat pups were randomly and equally divided into a test and a control group. Each pup in the test group received intraperitoneal injection of EdU (in 0.2 ml of PBS) at a dosage of 50 μg per g body weight. Each pup in the control group received injection of PBS without EdU. Five rats in each group were sacrificed at each of four time points (7h, 2w, 4w, and 6w post-injection). Major organs/tissues were harvested for histological examination.
Tracking of transplanted ADSC
ADSC were isolated from fat pads of ovaries excised from rats that underwent simulated birth trauma and ovariectomy. One week later, the cells were labeled with 10 μM EdU for 12 h and approximately 1×106 labeled cells injected autologously into the subcutaneous tissue on the right flank, which was then harvested at 1d, 3d, 4w, and 6 w for histological examination. The same number of EdU-labeled ADSC was also injected autologously into the bladder neck of rats one week after ovariectomy. Four weeks later, the bladder and urethra were harvested for histological examination. For tracking transplanted cells in the high-fat diet-induced UI model, rats were fed with a high-fat diet for 5 months. ADSC were then isolated from the paragonadal fat, cultured for one week, and labeled with EdU as above. Approximately 3×106 labeled cells were transplanted into each rat autologously by injection into the tail vein or into the urinary bladder (at 5 sites: anterior, posterior, bilateral and dome). The urinary bladders were harvested for histological examination at 4w and 10w post-transplantation.
Histology
Tissues were harvested at the indicated time points and fixed in cold 2% formaldehyde and 0.002% saturated picric acid in 0.1 M phosphate buffer, pH 8.0, for 4 h followed by overnight immersion in buffer containing 30% sucrose. The specimens were then embedded in OCT Compound (Sakura Finetic USA, Torrance, CA) and stored at −70 °C until use. Fixed frozen tissue specimens were cut at 10 microns, mounted onto SuperFrost-Plus charged slides (Fisher Scientific, Pittsburgh, PA) and air dried for 5 min. The tissue was then subjected to EdU staining with or without immunostaining for α-smooth muscle actin (α-SMA). For immunostaining, the slides were placed in 0.3% H2O2/methanol for 10 min, washed twice in PBS for 5 min and incubated with 3% horse serum in PBS/0.3% Triton X-100 for 30 min at room temperature. After draining this solution from the tissue section, the slides were incubated at room temperature with anti-α-SMA antibody (Abcam Inc., Cambridge, MA, 1:500) for 1.5 h. Control tissue sections were similarly prepared except no primary antibody was added. After rinses with PBS, the sections were incubated with FITC-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). After rinses with PBS, the slides were incubated with freshly made Click-iT reaction cocktail for 30 min at room temperature without light followed by staining with 4′,6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 μg/ml, Sigma-Aldrich, St. Louis, MO).
Image and statistical analysis
For image analysis, five randomly selected fields per slides for each treatment group were photographed and recorded using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY). The images were then quantified using Image-Pro Plus image soft ware (Media Cybernetics, Silver Spring, MD). Statistical analysis was performed according to the Primer of Biostatistics, 3rd edition (Glantz SA, McGraw-Hill, Inc, New York, NY, USA). Data were expressed as means ± standard deviation. One-way ANOVA was used to determine significance (p < 0.05).
Results
EdU labeling of ADSC
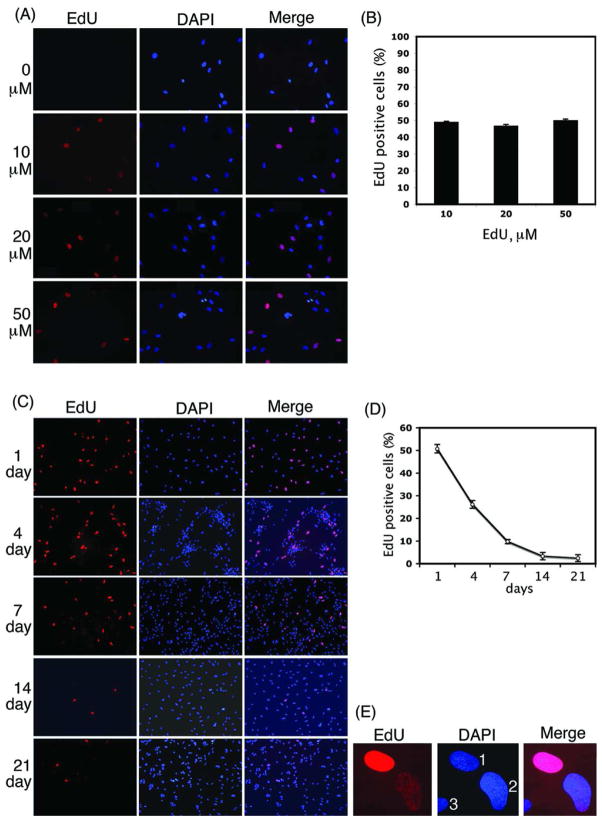
Incubation of ADSC in EdU-containing media resulted in intense red fluorescence in the nuclei when stained with Alexa-594 (Fig. 1). The detection of EdU was compatible with the blue fluorescent nuclear label, DAPI. The fluorescent probe for EdU was nuclear-specific, showing clear co-localization with DAPI. However, at the 3 tested EdU dosages (10, 20, and 50 μM), only ~50% of DAPI-positive nuclei were EdU-positive (Fig. 1B), suggesting that EdU incorporation took place only in replicating cells and was not enhanced by increased EdU concentrations. When cells labeled with 10 μM EdU were followed up for 21 days, the percentage of cells that remained intensely stained with Alexa-594 decreased from ~50 at day 1 to ~2 at day 21 (Fig. 1C & D). An example nucleus of these “label-retaining” cells is shown at a higher magnification in Fig. 1E “1”. The majority of the originally intensely stained cells, however, appeared to have “faded”, as seen in another example nucleus in Fig. 1E “2”.
Figure 1.
EdU labeling of ADSC. (A) ADSC labeled with EdU at 0, 10, 20, and 50 μM and stained with Alexa-594 (red fluorescence) and DAPI (blue fluorescence). 200× magnification. (B) Percentage of EdU-positive cells (number of red nuclei vs. number of blue nuclei). There was no significance difference among groups (n=3, P>0.05). (C) ADSC labeled with EdU at 10 μM, grown for 1, 4, 7, 14, and 21 days, and stained with Alexa-594 and DAPI. 100× magnification. (D) Percentage of EdU-positive cells (number of red nuclei vs. number of blue nuclei). There was significance difference among groups (n=3, P<0.01). (E) Nuclei of cells labeled with EdU at 10 μM and grown for 21 days. Nuclei 1, 2, and 3 show retention, fading and no EdU label, respectively. 1000× magnification.
EdU labeling of tissues
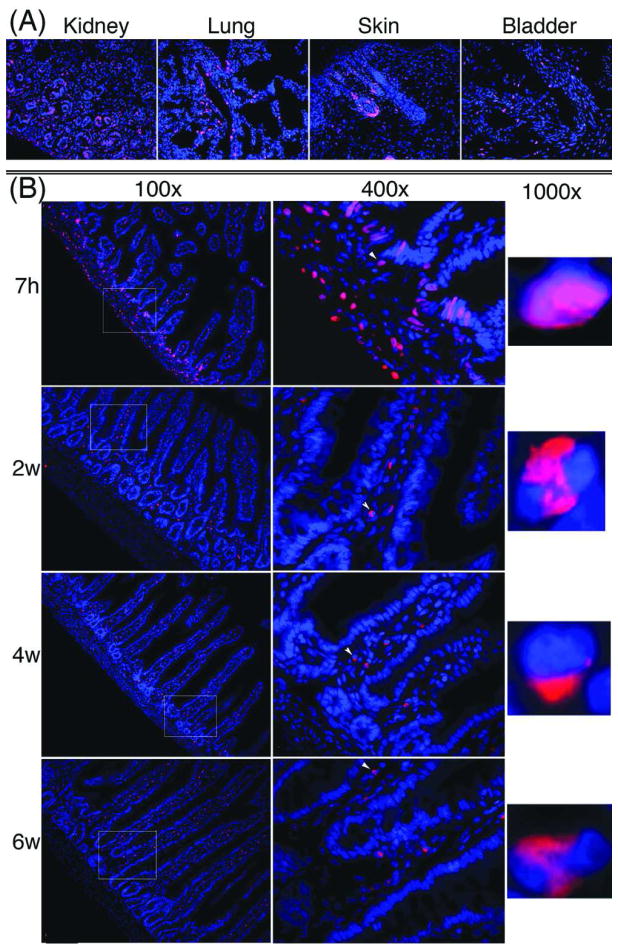
Alexa-594 staining of tissues from rats that received intraperitoneal injection of EdU and sacrificed 7h later resulted in intense red fluorescence in cellular nuclei in various tissues including kidney, lung, skin, and bladder (Fig. 2A). No such fluoresce was observed in rats that received injection of PBS (data not shown). Tissues harvested at 2, 4, and 6w all had much fewer EdU-positive nuclei. This loss of EdU-positive staining is best exemplified in the intestine. Similar to tissues shown in Fig. 2A, the 7h intestine had numerous EdU-positive nuclei (Fig. 2B). However, the 2, 4, and 6w intestines had few EdU-positive nuclei. Noteworthy is that EdU-positive nuclei in the 7h intestine were found mostly in the epithelium at the base of the villi and in the submucosa connective tissue. In contrast, EdU-positive nuclei in the 2, 4, and 6w intestines were found exclusively in the connective tissue. Also of interest is that while EdU was broadly and diffusely distributed in the nuclei of the 7h tissue, it was narrowly and densely distributed in the nuclei of the 2w, 4w, and 6w tissues.
Figure 2.
EdU labeling of tissues. Newborn rats received intraperitoneal injection of 50 μg of EdU per g body weight. Major organs and tissues were harvested at 7h, 2w, 4w, and 6w and stained with Alexa-594 (red fluorescence) and DAPI (blue fluorescence). The Alexa-594 and DAPI stained images were digitally merged. (A) Four representative 7h tissues at 200× magnification. (B) Intestine at all 4 time points. Boxed areas in the 100× graphs are shown in the corresponding 400× graphs. Arrowheads in the 400× graphs point to nuclei that are shown in the corresponding 1000× graphs.
Tracking of intratissue transplanted ADSC
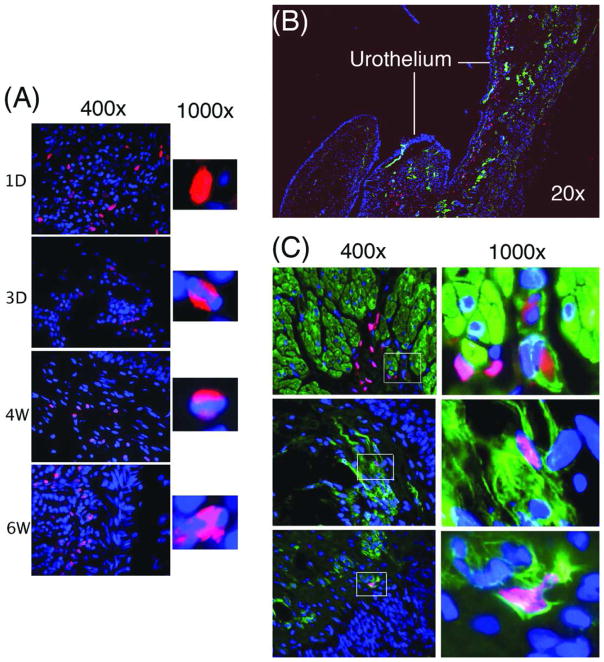
EdU-labeled ADSC were transplanted into the subcutaneous space and identified by Alexa-594 staining at 1d, 3d, 4w, and 6w post-transplantation. The results showed that transplanted ADSC were able to survive for at least 6 weeks (Fig. 3A). Interestingly, the nuclear distribution of EdU was also broad in the short-term tissue and narrow in the long-term tissues. EdU-labeled ADSC were also transplanted autologously into the bladder neck of SUI rats. Four weeks later, the rats were tested for urinary functions and their bladder and urethra examined histologically. Functional data indicated therapeutic efficacy and will be presented elsewhere. In the present study, the histological data indicated the presence of EdU-labeled cells in the submucosa connective tissue, particularly along and underneath the urothelium (Fig. 3B). A few EdU-positive nuclei appeared to co-localize with α-SMA (Fig. 3C), suggesting rare but possible smooth muscle differentiation.
Figure 3.
Tracking intratissue transplanted ADSC. (A) ADSC were labeled with EdU and autologously injected into the subcutaneous tissue, which was then harvested at 1d, 3d, 4w, and 6w and stained with Alexa-594 (red fluorescence) and DAPI (blue fluorescence). The Alexa-594 and DAPI stained images were digitally merged. (B) ADSC were labeled with EdU and autologously injected into the bladder neck, which was then harvested at 4w and stained with Alexa-594 (red fluorescence), DAPI (blue fluorescence), and anti-α-SMA antibody (green fluorescence). The stained images were digitally merged. (C) ADSC were labeled and transplanted as in panel B. Boxed areas in the 400× graphs are shown in the corresponding 1000× graphs.
Tracking of intravenously transplanted ADSC
EdU-labeled ADSC were autologously transplanted through tail vein injection into rats that suffered hyperlipidemia-associated overactive bladder. Four and ten weeks later, the rats were tested for urinary functions and their bladder examined histologically. Functional data indicated therapeutic efficacy and will be presented elsewhere. In the present study, the histological data indicated the presence of EdU-labeled cells in the submucosa connective tissue in both the 4w and 10w specimens (Fig. 4). For comparison, another group of hyperlipidemic rats received intratissue (intrabladder) injection of EdU-labeled ADSC and their bladder examined also at 4w and 10w. The results indicated the presence of EdU-positive cells in the connective tissue in both the 4w and 10w specimens (Fig. 4). Banded (narrow and dense) distribution of EdU was evident in all specimens (Fig. 4).
Figure 4.

Tracking intravenously transplanted ADSC. ADSC were labeled with EdU and autologously injected into the tail vein of hyperlipidemic rats. For comparison, EdU-labeled ADSC were also transplanted autologously into the bladder of hyperlipidemic rats. Four and ten weeks later, bladders were harvested, sectioned and stained with Alexa-594 (red fluorescence), DAPI (blue fluorescence), and anti-α-SMA antibody (green fluorescence). The stained images were digitally merged. IT and IV denote intratissue and intravenous, respectively. Boxed areas in the 100× graphs are shown in the corresponding 400× graphs. Arrowheads in the 400× graphs point to nuclei that are shown in the corresponding 1000× graphs.
Discussion
The first report on the use of EdU for cell labeling was published in February 2008, and we quickly adapted it for tracking ADSC in our animal models. While the therapeutic outcomes of these preclinical studies will be reported elsewhere, we thought that a timely report dedicated to the technical aspect of using EdU to track MSC could possibly lead to wider and faster adaptation of this novel technique. Thus, the present study started with EdU labeling of ADSC in vitro, progressed to EdU labeling in live animals, and ended with tracking of EdU-labeled ADSC in animal models. In the case of EdU labeling of ADSC, we found that the addition of 10 μM of EdU to the culture medium resulted in the labeling of ~50% of cells and higher EdU concentrations did not lead to increased labeling efficiency. Although similar observations have been made previously [12, 13], this apparent limit of labeling efficiency at ~50% has not been discussed. We therefore propose that the ~50% limit is probably a function of the cells’ replicative activity and the detection method’s limitation. We also for the first time examined the effect of prolonged cell culture on EdU labeling. Twenty-one days after the initial labeling, most cells expectedly became faintly stained; however, approximately 2% of cells remained intensely stained. Thus, it appears that regular ADSC cultures contain a small fraction of cells that are actively replicating at some point of time and then become dormant at other times. Whether these are “label-retaining cells” or possibly “true stem cells” may warrant further investigation.
For in vivo study, we conducted two lines of investigation, first, labeling of tissue cells by injection of EdU into the peritoneal cavity, and secondly, tracking of EdU-labeled cells after their autologous transplantation. Labeling of tissue cells by intraperitoneal injection of BrdU has been widely used to visualize replicating cells in the nervous system and to identify label-retaining stem cells in various tissues. A recent report has shown that EdU was an excellent alternative to BrdU for labeling brain cells [13]. In our hands, EdU labeling was not only easier to perform but also produced more reliable results than BrdU labeling (data not shown). As an extension of the present study, EdU labeling of various tissues of newborn rats was conducted. These tissues were harvested at 4 different time points and processed for tissue arrays, the results of which will be presented elsewhere. In the present study we showed that EdU-labeled cells were easily identifiable in tissues such as kidney, lung, skin, and intestine of rats that were sacrificed 7h after receiving EdU injection. We also used the intestine as an example to show that EdU-labeled cells were much less frequently observed in tissues of rats that were sacrificed at 2, 4, and 6 w after receiving EdU injection. In this example we also found that EdU-positive cells were localized in the connective tissue but not in the epithelium. Whether this is in any way contradictory to notions of the existence of label-retaining cells in the intestinal epithelium [19] will require a dedicated systematic analysis.
Our main interest in EdU is to use it to track transplanted cells in a preclinical setting. Because the subcutaneous tissue is easily accessible, it was chosen for our initial test, in which EdU-labeled ADSC were transplanted into the subcutaneous space. The results showed that EdU-labeled cells were identifiable for at least 6w post-transplantation. We then conducted our preclinical study, in which EdU-labeled ADSC were transplanted into the bladder neck of urinary incontinent rats. Four weeks later, EdU-positive cells were identifiable along and underneath the urothelium. While the great majority of these cells were localized in the connective tissue, a few appeared to co-localize with smooth muscle cells. Whether this latter observation indicates smooth muscle differentiation of the transplanted ADSC requires further investigation.
Because intravenous transplantation of therapeutic cells is potentially more convenient and less invasive (i.e., requiring no surgeries) than intratissue transplantation, we tested both methods side by side in a rat model of hyperlipidemia-associated overactive bladder. Functional analyses indicated that the two transplantation methods were similarly efficacious. Histological examination indicated the presence of EdU-positive cells in the bladder of both groups of rats at both 4w and 10w. Thus, EdU appears to afford the possibility of tracking transplanted cells for at least 10 weeks, whether the cells were administered intratissue or intravenously.
Throughout this study we have noticed that EdU-positive cells in the long-term tissues (≥2 weeks) exhibited “banded” EdU staining pattern in their nuclei. This occurred both with in vivo labeling through intraperitoneal EdU injection and with tracking of transplanted EdU-labeled ADSC. Similar observations have been made with BrdU labeling and have been discussed in details in a recent study [20]. However, while these authors recommended against the use of BrdU-labeled cells for transplantation studies when the survival time is longer than 2 weeks, our data indicated that EdU-labeled cells could be tracked for at least 10 weeks. As such, EdU labeling is superior to BrdU labeling in all aspects of consideration.
Acknowledgments
This work was supported by grants from the Arthur Rock Foundation and the National Institutes of Health (DK64538, DK045370, and DK069655).
Footnotes
Disclosure of interest: None.
References
- 1.Gratzner HG, Leif RC, Ingram DJ, Castro A. The use of antibody specific for bromodeoxyuridine for the immunofluorescent determination of DNA replication in single cells and chromosomes. Exp Cell Res. 1975;95:88–94. doi: 10.1016/0014-4827(75)90612-6. [DOI] [PubMed] [Google Scholar]
- 2.Gratzner HG. Monoclonal antibody to 5-bromo- and 5-iododeoxyuridine: A new reagent for detection of DNA replication. Science. 1982;218:474–5. doi: 10.1126/science.7123245. [DOI] [PubMed] [Google Scholar]
- 3.Dean PN, Dolbeare F, Gratzner H, Rice GC, Gray JW. Cell-cycle analysis using a monoclonal antibody to BrdUrd. Cell Tissue Kinet. 1984;17:427–36. doi: 10.1111/j.1365-2184.1984.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- 5.Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–37. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 7.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–63. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–87. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 9.Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci U S A. 1995;92:11879–83. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711–6. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ning H, Liu G, Lin G, Yang R, Lue TF, Lin CS. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–79. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salic A, Mitchison TJ. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci U S A. 2008;105:2415–20. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chehrehasa F, Meedeniya AC, Dwyer P, Abrahamsen G, Mackay-Sim A. EdU, a new thymidine analogue for labelling proliferating cells in the nervous system. J Neurosci Methods. 2009;177:122–30. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–63. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sievert KD, Emre Bakircioglu M, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol. 2001;166:311–7. [PubMed] [Google Scholar]
- 16.Banie L, Lin G, Ning H, Wang G, Lue TF, Lin CS. Effects of estrogen, raloxifene and levormeloxifene on alpha1A-adrenergic receptor expression. J Urol. 2008;180:2241–6. doi: 10.1016/j.juro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Rahman NU, Phonsombat S, Bochinski D, Carrion RE, Nunes L, Lue TF. An animal model to study lower urinary tract symptoms and erectile dysfunction: the hyperlipidaemic rat. BJU Int. 2007;100:658–63. doi: 10.1111/j.1464-410X.2007.07069.x. [DOI] [PubMed] [Google Scholar]
- 18.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–8. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 19.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–8. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 20.Sauerzweig S, Baldauf K, Braun H, Reymann KG. Time-dependent segmentation of BrdU-signal leads to late detection problems in studies using BrdU as cell label or proliferation marker. J Neurosci Methods. 2009;177:149–59. doi: 10.1016/j.jneumeth.2008.10.009. [DOI] [PubMed] [Google Scholar]