Abstract
Sepsis is associated with an increase in circulating levels of bacterial endotoxin. Sepsis is a particularly serious problem in the geriatric population due to the high mortality associated with it. However, it remains unknown whether this phenomenon is related to an increase of apoptosis in splenic cells. To study this, male Fischer-344 rats (young: 3-months old; aged: 24-months old) were subjected to endotoxemia by injection of LPS. Splenic samples were collected 4 h thereafter. Apoptosis was determined by cleaved caspase-3 levels and TUNEL staining. The levels of 3 pro-inflammatory mediators, TNF-α, IL-6 and high mobility group box -1 (HMGB-1), were also measured. Our results showed that while splenic cell apoptosis increased in both young and aged rats with endotoxemia, aged animals had much higher levels of apoptotic cell death. The elevated expression of cell cycle inhibitory protein P21 was also observed in aged animals after treatment with LPS. Moreover, endotoxemia significantly increased TNF-α, IL-6 and HMGB-1. The accelerated apoptosis in aged animals was correlated with significantly higher levels of TNF-α, IL-6 and HMGB-1. It is possible that this accelerated rate of apoptosis contributes to age-related hyperinflammation in endotoxemia. To investigate the factors involving accelerated apoptosis in aged animals, we analyzed the Fas/Fas ligand (Fas-L) pathway. Our results showed that Fas and Fas-L gene expression were markedly higher in the spleen in aged animals after LPS. Similarly, cleaved caspase-8 expression, a downstream element of Fas and Fas-L, was also significantly higher in aged rats after LPS. Fas-L neutralizing antibodies markedly decreased apoptosis and proinflammatory cytokines in aged animals after endotoxemia. Thus, there is substantial evidence that the Fas/Fas-L pathway may play an important role in LPS-induced accelerated apoptosis in aged animals.
Keywords: Aging, apoptosis, spleen, lipopolysaccharide, cytokines, caspase-3, Fas/Fas-ligand
Introduction
Sepsis, often caused by systemic immune responses to severe bacterial infection, can result in multiple organ failure and even lead to death (1). A recent study has shown that the elderly (> or = 65 years of age) accounts for only 12% of the U.S. population but 65% of sepsis cases, yielding a much higher risk level compared to that of younger patients (2). Aging is characterized by changes in immune function and stress responses. It is associated with an increased susceptibility to infections, as well as increased incidence of autoimmune disorders and chronic inflammatory diseases (3,4). The elderly has an increased incidence of complications and mortality following bacterial infection (1). Our recent data showed that the levels of proinflammatory cytokines were much higher in aged animals after induction of endotoxemia, resulting in more severe organ damage and higher lethality (5). Thus, it appears that the alteration of immune responses in the aged population may contribute to a higher mortality rate after bacterial infection.
The pathogenesis of severe sepsis is characterized by tissue damage and accumulation of apoptotic lymphocytes in the spleen, thymus and other organs (6,7). A number of studies suggest that apoptosis plays an important role in the immune dysfunction and multiple organ failure observed in sepsis or endotoxemia (8-10). However, it remains unknown whether there is any connection between increased immune cell apoptosis in the spleen and hyperinflammation [e.g., increased proinflammatory cytokines, such as TNF-α, IL-6 and high mobility group box 1 (HMGB-1)] in aged animals during bacterial infection. Thus, the objective of this study was to determine the relationship between immune cell apoptosis and proinflammatory cytokines in aged animals after injection of LPS. We also investigated a pathway which may be responsible for the elevation of apoptosis in aged animals under such conditions.
Materials and Methods
Induction of endotoxemia in rats
Endotoxemia was induced by intravenous administration of lipopolysaccharide (LPS). Male Fischer-344 rats (young: 3-months-old; aged: 24-months-old) were obtained from the National Institute on Aging (NIA), housed in a temperature-controlled room on a 12-h light/dark cycle, and fed on a standard Purina rat chow diet. Prior to the induction of severe endotoxemia, rats were fasted overnight but allowed water ad libitum. Rats were then anesthetized with isoflurane inhalation, their inguinal regions were shaved and washed with 10% povidone-iodine and a short subinguinal incision was made. The femoral vein was carefully separated from the artery and cannulated with a catheter (PE-50 tubing). A bolus injection of LPS (15 mg/kg BW; E. coli 055:B5 in 200 μl normal saline; Sigma, St. Louis, MO) was given through the femoral vein catheter. The same operation was performed on the vehicle control animals but the control was injected with normal saline instead of LPS. Tissue samples were collected at 4 h after LPS injection. Please note that the LPS dosage used in our studies (15 mg/kg BW) produces septic shock since 90% of the aged rats died within 5 days after LPS injection (unpublished observation). The experiment described here was performed in adherence to the National Institutes of Health guidelines for the use of experimental animals. This project was approved by the Institutional Animal Care and Use Committee (IACUC) of The Feinstein Institute for Medical Research.
Administration of Fas ligand neutralizing antibodies
Endotoxemia was induced as described above. Fas ligand neutralizing antibodies or control IgG (R & D Systems, Minneapolis, MN) were administrated intravenousely immediately after the administration of LPS. The dosage of Fas ligand antibodies used was 200 μg/kg in 1-ml saline (11) and was infused for 30 min with the use of an infusion pump. Splenic tissues were collected at 4 h after LPS injection.
TUNEL assay
DNA breaks occur late in the apoptotic pathway and can be determined and analyzed by performing the terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) assay. The presence of apoptotic cells in splenic tissues was demonstrated using a TUNEL staining kit (Roche Diagnostics, Indianapolis, IN). Briefly, splenic tissues were fixed in 10% phosphate buffered formalin and were then embedded into paraffin and sectioned at 6 μm following the standard histology procedures. Spleen sections were dewaxed, rehydrated and equilibrated in Tris buffered saline (TBS). The sections were then digested with 20 μg/ml proteinase K for 20 min at room temperature. Following this, the sections were washed and incubated with a mixture containing terminal deoxynucleotidyl transferase and fluorescence labeled nucleotides and examined under a fluorescence microscope. The negative control was performed by incubating slides in a mixture containing only deoxynucleotidyl transferase as recommended by the provider.
Western blot analysis
Splenic tissues collected from animals at 4 h after LPS injection were homogenized in a lysis buffer, which contained protease inhibitor cocktail (1 tablelet/10 ml, Roche Diagnostics, Indianapolis, IN) in 10 mM Tris saline, PH 7.5 with 1% Triton-100X. In addition, 1 mM EDTA, 1 mM EGTA, 2 mM Na orthovanadate, 0.2 mM PMSF, 2 μg/ml leupeptin, 2 μg/ml aprotinin were added to the lysis buffer. After centrifugation at 16,000g for 10 min, the supernatant was collected, and the protein concentration was determined by using a Bio-Red DC Protein Assay kit (Bio-Rad, Hercules, CA). 36-75 μg protein from splenic tissue was separated by NuPage 4-12% Bis-Tris gel (Invitrogen, Carlsbad, CA) in MES-SDS running buffer (Invitrogen). The protein on the gel was then transferred onto a nitrocellulose membrane and blocked with 5% nonfat dry milk in 10 mM Tris saline with 0.1% Tween 20, pH 7.5 (TBST). Western blotting was performed using the following primary antibodies: rabbit anti-cleaved caspase-3 polyclonal antibody (1:2,000; Cell Signaling Technology, Danvers, MA), rabbit anti-caspase 8 polyclonal antibody (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA) and mouse anti-P21 monoclonal antibody (1:300, Santa Cruz Biotechnology). After incubation of primary antibodies overnight at 4°C, the membranes were washed with TBST. Immunoreactive bands were detected using HRP-linked anti-rabbit IgG or HRP-linked anti-mouse IgG (1:10,000, SouthernBiotech, Birmingham, AL) and the enhanced chemiluminescene (ECL) Western blot detection kit (Amersham, Piscataway, NJ). The immunoblots were exposed on X-ray film and analyzed with a digital image system (Bio-Rad, Hercules, CA). Mouse anti-β-actin monoclonal antibody (1:20,000; Sigma, Saint Louis, MO) was used as a loading control in all western blot experiments.
Measurement of proinflammatory cytokines TNF-α, IL-6, and HMGB-1
Splenic levels of TNF-α, IL-6 were quantified using an enzyme-linked immunosorbent assay (ELISA) kit specifically for rat TNF-α and IL-6 (BD Biosciences, San Diego, CA). Splenic tissues were homogenized in a lysis buffer, the supernatant was collected, and the protein concentrations were determined as described above. A 96-well plate was coated with a specific capture primary antibody for either rat TNF-α or IL-6. 100 μg protein/well (for TNF-α) or 20 μg protein/well (for IL-6) were loaded into the pre-coated plate, and the assay was carried out according to instructions provided by the manufacture. Circulating HMGB-1 levels were determined by Western blot analysis. 2 μl plasma in 10% SDS and loading buffer was boiled for 5 min. The sample was then separated by a NuPage 4-12% gel (Invitrogen) and transfer to a nitrocellulose membrane. Western blotting was performed using rabbit anti-HMGB-1 primary antibodies (1:500, a gift from Dr. Haichao Wang at the Feinstein Institute for Medical Research) following the Western blot procedures described above. We detected a HMGB-1 immunoreactive band at 30 kDa.
Real-time PCR analysis
Fas, Fas-ligand and P21 gene expression was determined by real-time PCR (Q-PCR). Total RNA was extracted from splenic tissue using TRIzol reagent (Invitrogen, Carlsbad, CA). Q-PCR was carried out on cDNA samples reverse transcribed from 2μg RNA using murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA). Using a SYBR Green PCR Master Mix (Applied Biosystems), reactions were carried out in 24 μl final volume containing 0.08 μmol concentration of each forward and reverse primer, 2 μl cDNA, 9.2 μl H2O and 12 μl SYBR Green PCR Master Mix. Amplification was performed with an Applied Biosystems 7300 real-time PCR machine under the thermal profile of 50 °C for 2 min, 95 °C for 10 min and followed by 40 cycles of 95 °C for 15 sec and 60 °C for 1 min. Rat GAPDH mRNA expression was used to normalize each sample, and analysis of each specific mRNA was conducted as duplicates. Relative expression of mRNA was calculated by the 2-ΔΔCt method, and results were expressed as fold change with respect to the corresponding experimental control. The following rat primers were used in the experiment (table below). In order to check the specificity of PCR products, a melting curve analysis was performed in each Q-PCR experiment. We did not detect any non-specific products from any of the primers used in our experiments. Primers for real-time PCR are listed as follows:
Statistical analysis
All data were expressed as means ± SE and compared by one-way or two-way ANOVA and Student-Newman-Keuls test. Differences in values were considered significant if P < 0.05.
Results
Alterations in splenic cell apoptosis
The extent of programmed cell death in the spleen was determined by cleaved caspase-3 Western blotting and TUNEL assay. As shown in Figure 1, splenic levels of cleaved caspase-3 increased significantly in both young and aged animals after LPS injection (P < 0.05 vs. the respective sham). In addition, both basal and LPS-induced cleaved caspase-3 expression is much higher in aged rats than young rats (P < 0.05). DNA breaks occur late in the apoptosis process and are expressed by almost all cell types. The occurrence of DNA breaks can be detected by the TUNEL assay. As shown in Figure 2, TUNEL positive cells in the spleen increased markedly after LPS injection in both young and aged rats. Similar to cleaved caspase-3 expression, both basal and LPS-induced TUNEL positive cells are more abundant in aged rats than young rats. Thus, cleaved caspase-3 measurement and the TUNEL assay indicated an aging-related accelerated apoptosis of splenic cells during endotoxemia.
Figure 1.
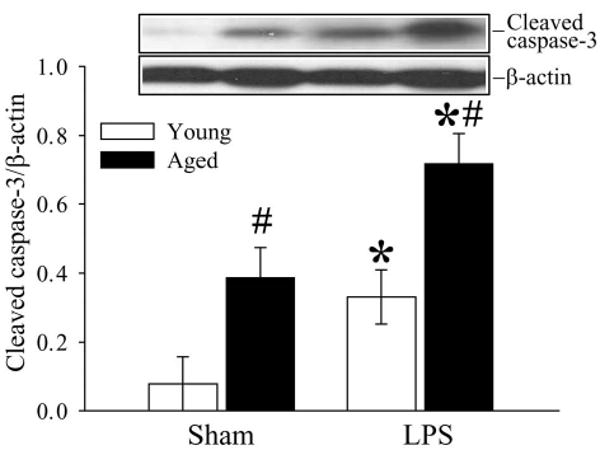
Alterations in splenic levels of cleaved caspase-3 in young (3-months-old) or aged (24-months-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection. Representative gels are presented. Data are presented as means ± SE (n=5-6/group) and compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus Young LPS group.
Figure 2.
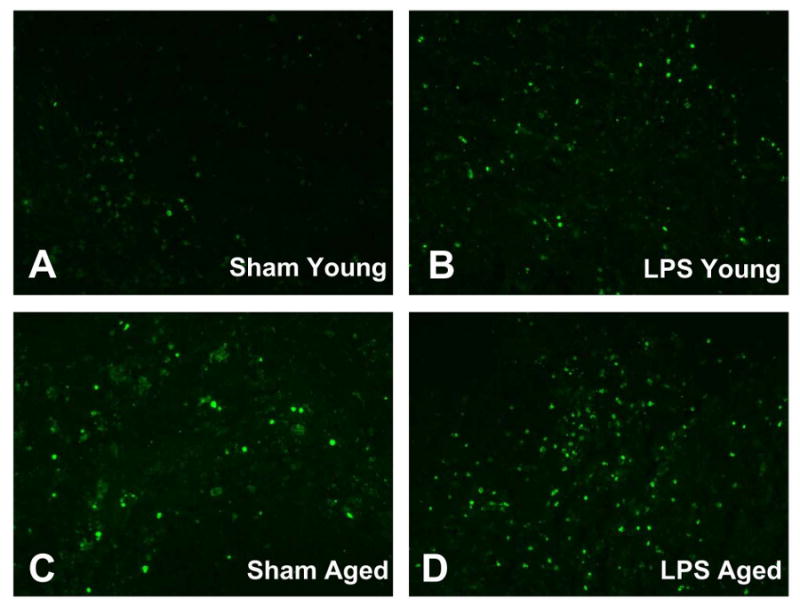
TUNEL staining in splenic tissues in young (3-months-old, A-B) or aged (24-months-old, C-D) rats at 4 h after normal saline (Sham) or LPS (LPS) injection. The TUNEL positive cells were mainly located in the red pulb of the spleen, and aging animals showed a marked increase in the number of TUNEL positive cells both in Sham (C) and LPS (D) groups. Original magnification: 200 X.
Alterations in P21 expression in the spleen
P21 is a cyclin-dependent kinase inhibitor, which controls the cell cycle (12,13). As shown in Figure 3, LPS significantly increased splenic levels of P21 gene (P < 0.05, Fig. 3A) and protein (P < 0.05, Fig. 3B) in both young and aged animals. However, the increase of P21 expression in aged animals was much greater than that in young rats (143% higher in gene expression and 206% higher in protein expression, P < 0.05).
Figure 3.
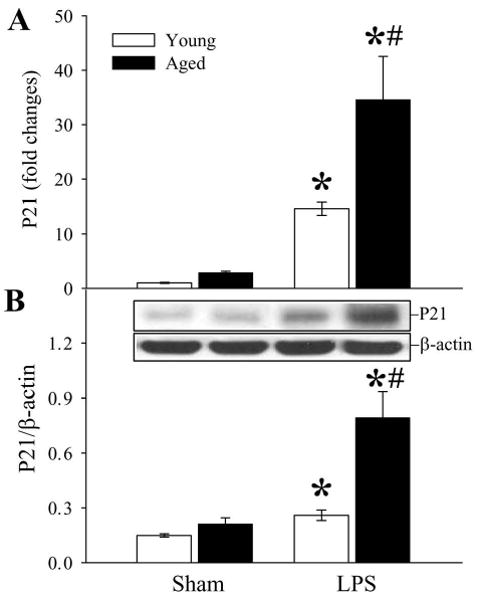
Alterations in splenic P21 gene (A) and protein (B) expression in young (3-months-old) or aged (24-months-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection. Representative gels are presented. Data are presented as means ± SE (n=5-6/group) and compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus Young LPS group.
Alterations in splenic levels of TNF-α and IL-6
Splenic levels of proinflammatory cytokines TNF-α and IL-6 were significantly elevated after administration of LPS (Figs. 4A-B). Compared with those in young rats, the levels of TNF-α (Fig. 4A) and IL-6 (Fig. 4B) were 4.1 and 1.9 fold higher, respectively, in aged animals (P < 0.05). Our results indicate that aged animals have a much greater inflammatory response to bacterial endotoxin than young animals.
Figure 4.
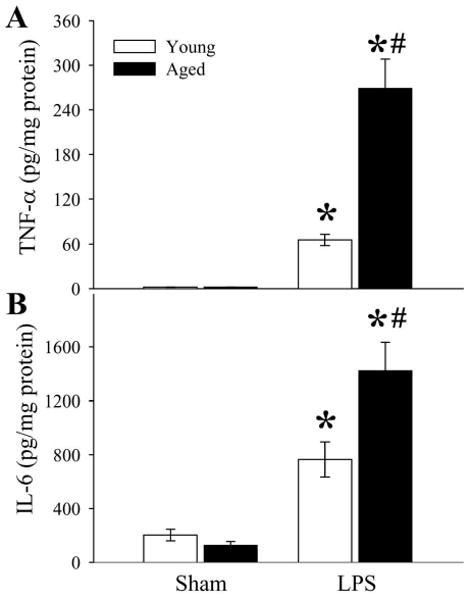
Alterations in splenic levels of TNF-α (A) and IL-6 (B) in young (3-months-old) or aged (24-months-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection. Data are presented as means ± SE (n=5-6/group) and compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus Young LPS group.
Alterations in circulating levels of HMGB-1
As indicated in Figure 5, circulating levels of HMGB-1 were significantly elevated in both young and aged animals after LPS injection. However, circulating levels of HMGB-1 in aged animals were markedly greater than those in young animals after LPS injection.
Figure 5.
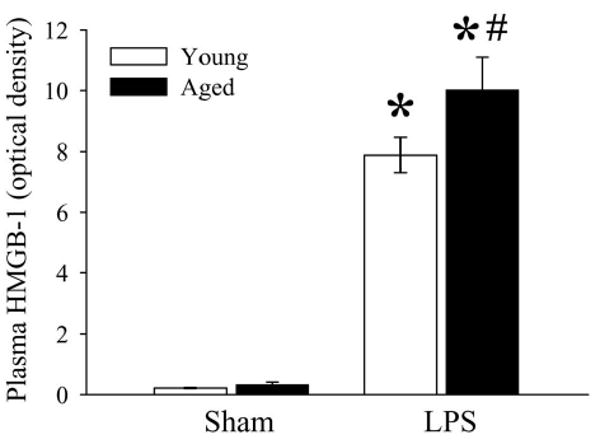
Alterations in plasma levels of HMGB-1 in young (3-months-old) or aged (24-months-old) rat at 4 h after normal saline (Sham) or LPS (LPS) injection. Data are presented as means ± SE (n=5-6/group) and compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus Young LPS group.
Alterations in Fas and Fas ligand expression in the spleen
To determine the mechanism involved in splenic cell apoptosis, we examined the Fas/Fas-L pathway. As shown in Figures 6A-B, Fas and Fas-L gene expression in the spleen was 1.6 and 10.1 fold higher respectively in aged rats compared to expression in young rats after LPS administration. The protein levels of Fas increased in both young and aged animals after LPS injection (P < 0.05, Fig. 6C). However, Fas-L protein levels only significantly increased in aged rats (Fig. 6D). The above data suggest that the Fas /Fas-L pathway may be involved in accelerated apoptosis in aged animals after LPS injection.
Figure 6.
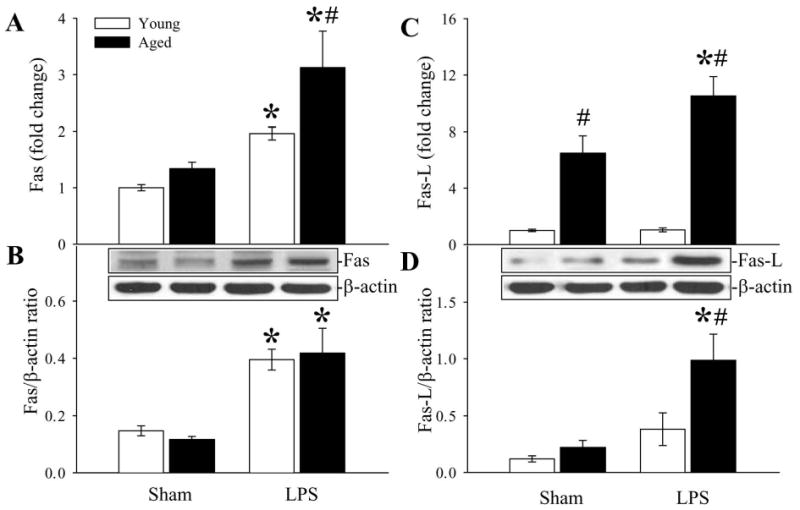
Alterations in Fas (A), Fas ligand (C) gene expressions and Fas (B), Fas ligand (D) protein levels in the spleen in young (3-months-old) or aged (24-months-old) rat at 4 h after normal saline (Sham) or LPS (LPS) injection. Representative blots are presented. Data are presented as means ± SE (n=5-6/group) and compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus Young LPS group.
Alterations in caspase-8 levels in the spleen
Caspase-8 is an important downstream caspase in the Fas/Fas-L pathway. Cleavage of caspase-8 occurs following the activation of the Fas/Fas-L pathway, which leads to the activation of caspase-3 and subsequent apoptosis. As shown in Figure 7, cleaved caspase-8 was significantly upregulated in aged animals after LPS injection (P < 0.05). In contrast, young animal only showed a slight increase in cleaved caspase-8 levels (no statistical significance, Fig. 7). We also observed higher cleaved caspase-8 levels in sham-operated aged rats as compared to sham-operated young rats, but the increase was not statistically significant (Fig. 7).
Figure 7.
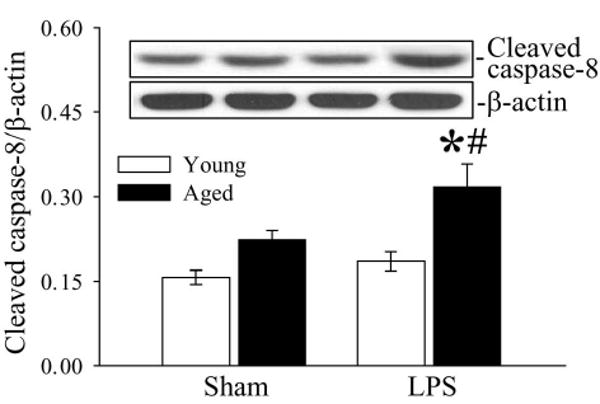
Alterations in splenic caspase-8 in young (3-months-old) or aged (24-months-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection. Representative blots are presented. Data are presented as means ± SE (n=5-6/group) and compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus Young LPS group.
Effects of Fas ligand neutralizing antibodies
To determine whether the Fas/Fas-L pathway plays a role in accelerated apoptosis in aged rats, Fas-L neutralizing antibodies were administrated immediately after LPS injection. As indicated in Figure 8, Fas-L neutralizing antibodies reduced cleaved caspase-3 to levels similar to that of sham-operated animals (P < 0.05). Thus, inhibition of the Fas/Fas-L pathway attenuated splenic cell apoptosis. As indicated in Figure 9, administration of Fas-L neutralizing antibodies at the time of LPS injection reduced splenic levels of TNF-α in aged animal by 50% (P < 0.05 vs. Vehicle). In contrast, levels of TNFα in young rat remained unchanged after treatment (Fig. 9). The treatment with Fas-L antibodies also decreased circulating HMGB-1 levels by 40.3% in young and 46.5% in aged animals (Fig. 10). Thus, inhibition of apoptosis by blocking the Fas/Fas-L pathway is associated with a significant decrease in aging-related LPS-induced TNF-α and HMGB-1 production.
Figure 8.
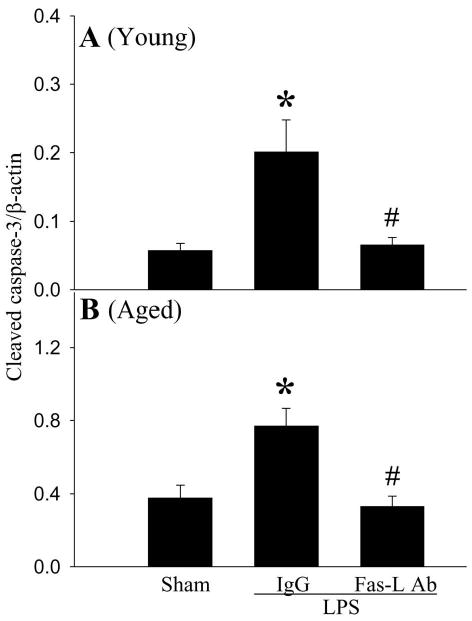
Alterations in splenic levels of cleaved caspase-3 in young (3-months-old, A) or aged (24-months-old, B) rats at 4 h after normal saline (Sham) or LPS (LPS) injection treated with normal IgG (IgG) or Fas-L neutralizing antibodies (Fas-L Ab). Data are presented as means ± SE (n=4-5/group) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus corresponding LPS+IgG group.
Figure 9.
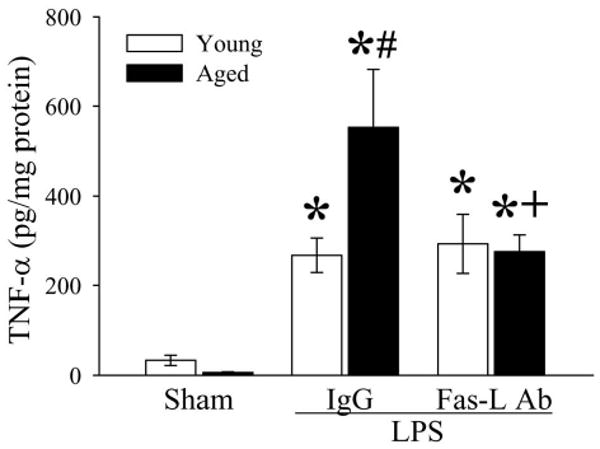
Alterations in splenic levels of TNF-α in young (3-months-old) or aged (24-months-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection treated with normal IgG (IgG) or Fas-L neutralizing antibodies (Fas-L Ab). Data are presented as means ± SE (n=4-5/group) and compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus Young LPS+IgG group; +P < 0.05 versus Aged LPS+IgG group.
Figure 10.
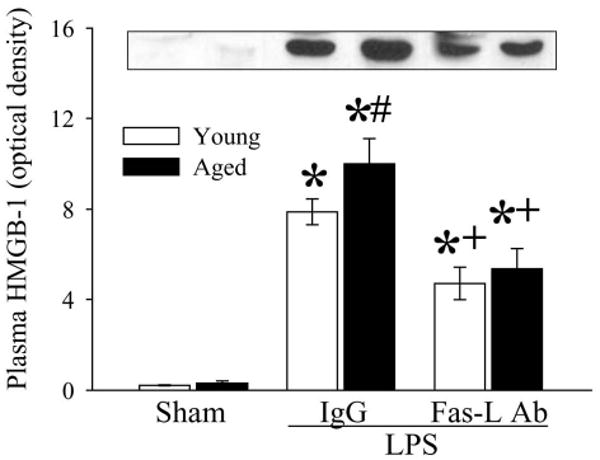
Alterations in circulating HMGB-1 levels in young (3-months-old) or aged (24-months-old) rats at 4 h after normal saline (Sham) or LPS (LPS) injection treated with normal IgG (IgG) or Fas-L neutralizing antibodies (Fas-L Ab). A representative blot is presented. Data are presented as means ± SE (n=4-5/group) and compared by two-way analysis of variance (ANOVA) and Student-Newman-Keuls test: *P < 0.05 versus corresponding Sham group; #P < 0.05 versus Young LPS+IgG group; +P < 0.05 versus Aged LPS+IgG group.
Discussion
Sepsis is a particularly serious problem in the geriatric population. Nearly 60% of all cases of sepsis occur in patients older than 65 years of age; also the mortality rate due to sepsis significantly increases with age, from 10-15% in patients 20-29 years-old to 38.4% in those over 85-years-old (1,2). Aging in human and animal models shows changes in many aspects of protective immunity during sepsis (4,14,15). It is assumed that the increased incidence and mortality rate of sepsis in the elderly is a direct result of an impaired immune response. We have recently reported that LPS injection causes an in vivo hyperinflammatory state in aged animals, characterized by a further increase in proinflammatory cytokines and more severe tissue injury (5). Current results also indicate that splenic levels of proinflammatory cytokines, TNF-α, IL-6 and circulating levels of HMGB-1, are markedly raised in aged animals and much higher than young animals after LPS administration. In this study, we focused on apoptotic immune cell death because of its crucial role in immune dysfunction and multiple organ failure during sepsis (8-10,16).
In our current study, we examined the effect of splenic cell apoptosis on the innate response, as well as the effects of Fas/Fas-L, caspase-8 as a signaling pathway by which apoptosis was activated. Aged septic animals had increased splenic cell apoptosis and higher levels of P21 as compared to either young septic animals or aged sham animals. Upregulation of P21, a cyclin-dependent kinase inhibitor, could result in cell cycle arrest and apoptosis (12,13). Therefore, significantly higher levels of P21 in aged animals may contribute to higher apoptosis levels in those animals after endotoxemia. Most importantly, we found that aging-related hyperinflammation is associated with splenic cell apoptosis. Splenic Fas-L expression was markedly higher in aged animals, indicating an impairment of the death receptor-related Fas/Fas-L apoptotic pathway. When we administered Fas-L neutralizing antibodies at the time of LPS injection, splenic apoptosis was significantly attenuated, and the aging-related elevation of TNF-α and HMGB-1 was also prevented. The aforementioned results clearly show that accelerated apoptosis contributes to age-related hyperinflammation during endotoxemia. Hotchikiss et al. reported that an increase in splenic lymphocyte apoptosis in septic mice is associated with an increase in mortality (17,18). This phenomenon may be related with the upregualtion of proinflammatory cytokines following splenic cell apoptosis. Since the Fas/Fas-L pathway plays an important role in age-related splenic apoptosis in endotoxemia, attenuating its activation may reduce age-related mortality in sepsis or bacterial infection.
Both aging and sepsis independently increase splenic cell apoptosis. Immune cells that undergo programmed cell death include various lymphocyte populations, macrophages, dendritic cells and neutrophils (4,19-21). Apoptotic loss of immune effector cells such as CD4 T and B cells is a key factor in the loss of immune competence in sepsis (22,23). An increase in splenic lymphocyte apoptosis in septic mice is thus associated with an increase in mortality (17,18). It has been reported that lymphocyte apoptosis is increased in CD4 and CD8 T cells, CD20 B cells, and NK cells (CD56) in septic patients as compared to their nonseptic counterparts (24). Samples taken from patients with sepsis also show that the level of CD3 T cell apoptosis correlates with the degree of severity of sepsis (24). Although we did not study the cell population that underwent programmed cell death in our current experiment, our preliminary data shows that a significantly larger number of splenic CD3+ T cells underwent apoptosis in aged animals after LPS injection. Thus, it appears that lymphocyte apoptosis does occur in aged animals after LPS injection. Thus, it is in this way that the combination of aging and bacterial infection leads to a disproportionate increase in immune cell death and alters the immune response, a factor which potentially plays a role in the marked increase in mortality seen in sepsis in the elderly.
Mammalian apoptotic cell death in sepsis proceeds through two distinct pathways, that is, the death receptor pathway and the mitochondrial pathway, that converge to activate caspases, resulting in intracellular demolition (25,26). The extrinsic, or death receptor, pathway is triggered by signaling through TNF, Trail, and Fas (19,27,28). The mitochondrial (intrinsic) death pathway responds to various stress stimuli, e.g. oxidative stress, radiation, and cytokine withdrawal (26). Both the death receptor and mitochondrial pathways are activated in sepsis (22,24). Although mediators such as steroids, tumor necrosis factor, nitric oxide, C5a, and Fas-L appear to contribute to the changes that occur during apoptosis, their effects are tissue and cell population-selective (19,29). Fas/Fas-L belongs to the death receptor mediated apoptosis pathway, which is heavily involved in lymphocyte apoptosis (9,29). Hsu et al. reported that the Fas/Fas-L pathway is impaired in aged animals, and Fas-L mediated apoptosis plays a major role in activation-induced cell death of T cells (30). Our current data shows that without LPS stimulation, splenic Fas-L expression is markedly higher in aged animals than in young animals without LPS stimulation. LPS administration further elevates the expression of Fas-L in aged animals. Our study also shows significantly upregulated levels of caspase 8 in these aged animals after LPS injection, indicating that the Fas/Fas-L pathway may play an important role in programmed cell death in aged animals. Inhibition of the Fas/Fas-L pathway by Fas-L neutralizing antibodies attenuated splenic cell apoptosis in aged rats, further supporting the idea that this pathway plays an important role in age-related accelerated apoptosis after LPS injection.
In summary, while splenic cell apoptosis increased in both young and aged rats during endotoxemia, aged animals had much higher levels of apoptotic cell death. This accelerated apoptosis in aged animals corresponded with significantly higher levels of TNF-α, IL-6 and HMGB-1. Moreover, the expression of Fas, Fas-L and caspase-8 was markedly higher in the spleen of aged animals than those in young animals after LPS injection. Fas-L neutralizing antibodies repressed apoptosis and proinflammatory cytokines in aged animals after endotoxemia. Therefore, it is possible that accelerated apoptosis contributes to age-related hyperinflammation in endotoxemia. The Fas/Fas-L pathway may play an important role in LPS-induced accelerated apoptosis in aged animals.
Acknowledgments
This study was supported by the National Institutes of Health grants R01 AG028352, R01 GM053008 and R01 GM057468 (P. Wang)
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis. Crit Care Med. 2006;34:15–21. doi: 10.1097/01.ccm.0000194535.82812.ba. [DOI] [PubMed] [Google Scholar]
- 3.Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437–3446. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 4.Weiskopf D, Weinberger B, Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009 doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 5.Wu R, Zhou M, Dong W, Ji Y, Miksa M, Marini CP, Ravikumar TS, Wang P. Ghrelin hyporesponsiveness contributes to age-related hyperinflammation in septic shock. Ann Surg. 2009;250:126–133. doi: 10.1097/SLA.0b013e3181ad85d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin S, Wang H, Yuan R, Li H, Ochani M, Ochani K, Rosas-Ballina M, Czura CJ, Huston JM, Miller E, Lin X, Sherry B, Kumar A, Larosa G, Newman W, Tracey KJ, Yang H. Role of HMGB1 in apoptosis-mediated sepsis lethality. J Exp Med. 2006;203:1637–1642. doi: 10.1084/jem.20052203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huston JM, Wang H, Ochani M, Ochani K, Rosas-Ballina M, Gallowitsch-Puerta M, Ashok M, Yang L, Tracey KJ, Yang H. Splenectomy protects against sepsis lethality and reduces serum HMGB1 levels. J Immunol. 2008;181:3535–3539. doi: 10.4049/jimmunol.181.5.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung CS, Yang S, Song GY, Lomas J, Wang P, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas signaling prevents hepatic injury and improves organ blood flow during sepsis. Surgery. 2001;130:339–345. doi: 10.1067/msy.2001.116540. [DOI] [PubMed] [Google Scholar]
- 9.Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74:344–351. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Zacks DN, Zheng QD, Han Y, Bakhru R, Miller JW. FAS-mediated apoptosis and its relation to intrinsic pathway activation in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2004;45:4563–4569. doi: 10.1167/iovs.04-0598. [DOI] [PubMed] [Google Scholar]
- 12.Min LJ, Mogi M, Iwai M, Horiuchi M. Signaling mechanisms of angiotensin II in regulating vascular senescence. Ageing Res Rev. 2009;8:113–121. doi: 10.1016/j.arr.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Prives C, Gottifredi V. The p21 and PCNA partnership: a new twist for an old plot. Cell Cycle. 2008;7:3840–3846. doi: 10.4161/cc.7.24.7243. [DOI] [PubMed] [Google Scholar]
- 14.Sawhney M, Mathew M, Valarmathi MT, Das SN. Age related changes in Fas (CD95) and Fas ligand gene expression and cytokine profiles in healthy Indians. Asian Pac J Allergy Immunol. 2006;24:47–56. [PubMed] [Google Scholar]
- 15.Grolleau-Julius A, Ray D, Yung RL. The Role of Epigenetics in Aging and Autoimmunity. Clin Rev Allergy Immunol. 2009 doi: 10.1007/s12016-009-8169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Tinsley KW, Karl IE. Role of apoptotic cell death in sepsis. Scand J Infect Dis. 2003;35:585–592. doi: 10.1080/00365540310015692. [DOI] [PubMed] [Google Scholar]
- 17.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-cell-deficient mice. Crit Care Med. 1997;25:1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Wesche DE, Lomas-Neira JL, Perl M, Chung CS, Ayala A. Leukocyte apoptosis and its significance in sepsis and shock. J Leukoc Biol. 2005;78:325–337. doi: 10.1189/jlb.0105017. [DOI] [PubMed] [Google Scholar]
- 20.Hsu HC, Scott DK, Zhang P, Zhou J, Yang P, Wu Q, Schroeder HW, Jr, Gerald LB, Ravussin E, Jazwinski SM, Mountz JD. CD8 T-cell immune phenotype of successful aging. Mech Ageing Dev. 2006;127:231–239. doi: 10.1016/j.mad.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Tinsley KW, Grayson MH, Swanson PE, Drewry AM, Chang KC, Karl IE, Hotchkiss RS. Sepsis induces apoptosis and profound depletion of splenic interdigitating and follicular dendritic cells. J Immunol. 2003;171:909–914. doi: 10.4049/jimmunol.171.2.909. [DOI] [PubMed] [Google Scholar]
- 22.Brahmamdam P, Watanabe E, Unsinger J, Chang KC, Schierding W, Hoekzema AS, Zhou TT, McDonough JS, Holemon H, Heidel JD, Coopersmith CM, McDunn JE, Hotchkiss RS. Targeted delivery of siRNA to cell death proteins in sepsis. Shock. 2009;32:131–139. doi: 10.1097/SHK.0b013e318194bcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parrino J, Hotchkiss RS, Bray M. Prevention of immune cell apoptosis as potential therapeutic strategy for severe infections. Emerg Infect Dis. 2007;13:191–198. doi: 10.3201/eid1302.060963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 25.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 26.Roy S, Nicholson DW. Cross-talk in cell death signaling. J Exp Med. 2000;192:F21–F25. [PMC free article] [PubMed] [Google Scholar]
- 27.Lavrik I, Golks A, Krammer PH. Death receptor signaling. J Cell Sci. 2005;118:265–267. doi: 10.1242/jcs.01610. [DOI] [PubMed] [Google Scholar]
- 28.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Maher S, Toomey D, Condron C, Bouchier-Hayes D. Activation-induced cell death: the controversial role of Fas and Fas ligand in immune privilege and tumour counterattack. Immunol Cell Biol. 2002;80:131–137. doi: 10.1046/j.1440-1711.2002.01068.x. [DOI] [PubMed] [Google Scholar]
- 30.Hsu HC, Scott DK, Mountz JD. Impaired apoptosis and immune senescence - cause or effect? Immunol Rev. 2005;205:130–146. doi: 10.1111/j.0105-2896.2005.00270.x. [DOI] [PubMed] [Google Scholar]


