Abstract
Rationale
The effectiveness of MDMA and its enantiomers to reinstate responding previously maintained by drug self-administration has not been thoroughly investigated.
Objectives
The present study was designed to compare the reinstatement effects of amphetamine, the piperazine-analog BZP, SR(+/−)-MDMA, S(+)-MDMA, R(−)-MDMA, and fenfluramine on behavior maintained under a second-order schedule of intravenous amphetamine self-administration in rhesus monkeys (n=4).
Methods
Following saline substitution and extinction, a range of doses of amphetamine, BZP, SR(+/−)-MDMA, S(+)-MDMA, R(−)-MDMA, and fenfluramine were administered i.v. as non-contingent priming injections in order to characterize their effectiveness to reinstate responding previously maintained by amphetamine self-administration.
Results
Priming injections of amphetamine, BZP, SR(+/−)-MDMA, and S(+)-MDMA induced significant reinstatement effects. In contrast, neither R(−)-MDMA nor fenfluramine effectively reinstated behavior. Pretreatment with the selective serotonin transporter inhibitor, fluoxetine, attenuated the reinstatement effects of SR(+/−)-MDMA, S(+)-MDMA, and BZP but had no significant effect on amphetamine-primed reinstatement.
Conclusions
Given the profile of neurochemical effects published previously, these findings suggest that the reinstatement effects of MDMA are mediated primarily by dopamine release; however, the attenuation of MDMA-induced reinstatement by fluoxetine supports previous research demonstrating the complex behavioral pharmacology of MDMA-like drugs and that the reinstatement effects of MDMA are at least partially mediated by serotonergic mechanisms.
Keywords: Amphetamine, MDMA, BZP, Reinstatement, Rhesus monkey
Introduction
3,4-methylenedioxymethamphetamine (MDMA) is a phe-nethylamine derivative that is structurally similar to both amphetamine and mescaline. Racemic MDMA binds to monoamine transporters, resulting in the release of dopamine (DA), serotonin (5-HT), and norepinephrine (NE), with its highest affinity at the serotonin transporter (SERT) (Steele et al. 1987; Rudnick and Wall 1992). Interestingly, the enantiomers of MDMA have distinct neurochemical and behavioral effects. S(+)-MDMA is more potent at releasing 5-HT and DA, compared to R(−)-MDMA (Steele et al. 1987). Conversely, R(−)-MDMA has a higher affinity for postsynaptic 5-HT receptors, compared with S(+)-MDMA (Battaglia and De Souza 1989). In rats, administration of racemic MDMA produces dose-dependent increases in locomotor activity (Callaway et al. 1990; McNamara et al. 1995; De Souza et al. 1997); however, the motor pattern induced by MDMA differs from that of traditional psychostimulants, such as amphetamine (Gold and Koob 1989; Callaway et al. 1990; McCreary et al. 2003), and is often accompanied by stereotypic behaviors similar to those seen in the serotonin syndrome (Slikker et al. 1989). In regards to the enantiomers of MDMA, the S(+) enantiomer is more potent in producing hyperactivity and stereotypies than R(−)-MDMA (Hiramatsu et al. 1989; Paulus and Geyer 1992). Moreover, pretreatments with the selective SERT inhibitor, fluoxetine (Callaway et al. 1990), or 5-HT2A antagonists (Fantegrossi et al. 2003) suppress MDMA-induced locomotion. These findings suggest a role for both DA and 5-HT in mediating the locomotor-stimulant effects of MDMA.
Drug discrimination studies have provided further insight into the behavioral pharmacology and mechanism of action of racemic MDMA. SR(+/−)-MDMA has been shown to substitute for the dopamine releaser, amphetamine, in rats, pigeons, and rhesus monkeys (Evans and Johanson 1986; Kamien et al. 1986; Glennon and Misenheimer 1989; Easton and Marsden 2006). Likewise, amphetamine generalized to racemic MDMA in rats trained to discriminate SR(+/−)-MDMA from saline (Glennon 1989). However, other studies have reported only partial or no substitution between MDMA and amphetamine in rats (Schechter 1987; Oberlender and Nichols 1988; Baker and Taylor 1997). In humans, the subjective effects of SR (+/−)-MDMA have consistently been reported as amphetamine- or stimulant-like (Cami et al. 2000; Tancer and Johanson 2001; Tancer and Johanson 2003). However, racemic MDMA has also been reported to produce similar subjective effects as the serotonin releaser, meta-chlorophenylpiperazine (mCPP) (Tancer and Johanson 2001; Tancer and Johanson 2003; Johanson et al. 2006). Similarly, drug discrimination studies in animals consistently show generalization between MDMA and the serotonin releaser, fenfluramine (Schechter 1986; Schechter 1988; Goodwin and Baker 2000). Three-way drug discrimination procedures are commonly used to evaluate drugs with complex neurochemical effects (Stolerman 1993) and have provided additional insight into the stimulus effects of MDMA. For example, in pigeons trained to discriminate amphetamine and fenfluramine from saline, racemic MDMA produced both amphetamine- and fenfluramine-appropriate responding (Evans and Johanson 1986). Likewise, in human participants trained to discriminate amphetamine from mCPP, half of the participants identified MDMA as amphetamine and the other half as mCPP (Johanson et al. 2006). Pretreatment with selective SERT inhibitors can attenuate the subjective (Liechti et al. 2000; Liechti and Vollenweider 2001) and euphoric (Stein and Rink 1999) effects of MDMA in human subjects. Overall, the stimulus effects of racemic MDMA appear to be mediated through both serotonergic and dopaminergic mechanisms.
The enantiomers of MDMA have been shown to substitute completely (Schechter 1987; Oberlender and Nichols 1988; Baker et al. 1995) or partially (Baker et al. 1997) for racemic MDMA, with the S(+) enantiomer being more potent, and with a faster onset of action than the R(−) enantiomer (Baker et al. 1997; Fantegrossi et al. 2009). Cross generalization between the enantiomers has been shown in animals trained to discriminate the individual enantiomers from saline (Baker et al. 1995; Baker and Taylor 1997; Fantegrossi et al. 2009). These results indicate similarities between the MDMA compounds; however, there is also considerable evidence that the enantiomers produce distinct stimulus effects. For example, R(−)-MDMA did not generalize to racemic MDMA; however, full generalization occurred between S(+)-MDMA and racemic MDMA (Fantegrossi et al. 2009). In addition, generalization to amphetamine occurs for S(+)-MDMA but not R(−)-MDMA (Glennon and Young 1984; Glennon et al. 1988; Murnane et al. 2009), which may reflect the higher affinity of the S(+) enantiomer for the DAT. However, others have reported that neither enantiomer fully substitutes for amphetamine (Oberlender and Nichols 1988). Conversely, the serotonin releasers, fenfluramine and p-chloroamphetamine, substitute for both enantiomers of MDMA (Baker et al. 1995; Baker and Taylor 1997). Overall, the drug discrimination literature suggests that although there is some overlap, the enantiomers of MDMA produce distinct stimulus effects and that the S (+) enantiomer may be more amphetamine-like than the R (−) enantiomer.
Despite numerous reports on the discriminative stimulus properties of MDMA, few studies have examined the effectiveness of racemic MDMA to reinstate responding previously maintained by drug self-administration, and to our knowledge, no study has characterized the reinstatement effects of the enantiomers of MDMA. In the reinstatement paradigm, non-contingent administration of a drug can reinstate responding previously maintained by drug self-administration. Importantly, drugs with pharmacological mechanisms similar to the self-administered drug can reinstate responding. For example, responding previously maintained by cocaine self-administration can be reinstated by non-contingent infusions of cocaine, amphetamine, or methamphetamine (Gerber and Stretch 1975; de Wit and Stewart 1981; Slikker et al. 1984). Moreover, drugs capable of inducing reinstatement typically share discrimitive stimulus effects with the self-administered drug. In the present study, the effectiveness of non-contingent priming injections of MDMA and its enantiomers to reinstate responding previously maintained by amphetamine self-administration was assessed in rhesus monkeys. Drug interactions with the selective SERT inhibitor, fluoxetine, evaluated the potential role of serotonin in reinstatement effects. It was hypothesized that priming injections of SR (+/−)-MDMA and S(+)-MDMA, but not R(−)-MDMA, would reinstate responding previously maintained by amphetamine self-administration.
Materials and methods
General methods
Subjects
Two female (RNa-4 and RMy-4) and two male (RVc-5 and ROk-5) adult rhesus monkeys (Macaca mulatta) weighing 8.0–15.0 kg were used as subjects. Each subject was housed individually and fed with Purina monkey chow (Ralston Purina, St. Louis, MO), fruit, and vegetables. Water was available continuously, and food restriction protocols were not used. Animal care procedures strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Emory University.
Surgery
Each subject was prepared surgically with a chronic indwelling venous catheter under sterile surgical conditions using a technique described previously (Howell and Wilcox 2001). Preoperative antibiotics [Rocephin (ceftriaxone, 25 mg/kg i.m.)] were given on the day of the surgery to help prevent infection. A silicone catheter (0.65 mm i.d., 1.75 mm o.d.; Access Technologies, Skokie, IL) was implanted into either a femoral or jugular vein under a combination of Telazol (tiletamine hydrochloride and zolazepam hydrochloride, 4.0 mg/kg i.m.) and isoflur-ane anesthesia using aseptic techniques. The proximal end of the catheter terminated in the vena cava above the right atrium, and the distal end was routed under the skin and attached to a subcutaneous vascular access port (Access Technologies) located in the center of the lower back. After surgery, the subject was returned to its home cage and received the analgesic Banamine (flunixin meglumine, 1.0 mg/kg i.m.) every 6 h for 24 h postoperatively. Catheters were flushed daily with 1.0 ml of heparinized (100 U/ml) saline to maintain patency.
Drugs
(+)-Amphetamine sulfate was purchased from Sigma-Aldrich (St. Louis, MO). SR(+/−)-MDMA HCl, S (+)-MDMA HCl, R(−)-MDMA HCl, and benzylpiperazine (BZP) were provided by the National Institute on Drug Abuse (Bethesda, MD). BZP was selected as a positive control based on its neurochemical and behavioral similarities to (+)amphetamine (Baumann et al. 2004; Fantegrossi et al. 2005; Brennan et al. 2007). Fluoxetine HCl was purchased from Spectrum Chemical MFG Corporation (Gardena, CA). The selective serotonin releaser, fenfluramine, was purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in 0.9% saline, and all drug doses were determined as salts.
Behavioral methods
Apparatus
During behavioral testing, each subject was seated in a commercially available primate chair (Primate Products, Miami, FL). A response panel with one lever was mounted on the front of the chair. Located above the lever in the center of the response panel were red, green, and white stimulus lights. Once the monkey was seated in the chair, a Huber needle (Access Technologies) was inserted into the venous access port. The polyvinyl chloride tubing attached to the Huber needle was connected to a motor-driven syringe (Coulbourn Instruments, Allentown, PA) located outside of the chamber containing the drug solution. Operation of the infusion pump delivered 2 ml of drug solution over 7 s. Testing during daily sessions occurred in a ventilated, sound-attenuating chamber. IBM-compatible computers controlled experimental events and recorded data.
Procedure
Responding was maintained under a second-order schedule of i.v. amphetamine injection. Each session began with a 5-min start delay during which a white noise generator was operational, but no stimulus lights were illuminated, and responding on the lever had no programmed consequences. After 5-min, the green light on the response panel was illuminated and indicated the availability of drug reinforcement. Each fixed ratio (FR) of 20 responses completed during a 10-min fixed interval (FI) changed the stimulus light from green to white for 2 s. The first FR20 completed after the 10-min FI had elapsed resulted in a drug infusion, and changed the stimulus light from green to white for 15 s. There was a 60 s limited hold for completion of the first FR20 after the 10-min FI had elapsed, and a drug infusion was not delivered if the limited hold expired. A 15-min time out separated subsequent drug components, and daily sessions were composed of four components. The maintenance doses of amphetamine were selected for the individual subjects as the doses that maintained peak rates of responding (0.03–0.1 mg/kg/infusion).
The conditions described above remained in effect until responding for amphetamine was stable (<20% variance in daily response rate over three consecutive sessions), after which saline was substituted for amphetamine and the paired-brief white light stimulus was removed until responding decreased to at least 20% of responding maintained during amphetamine self-administration. Typically, response rates declined gradually over two to five sessions. Tests for drug-induced reinstatement were begun once the criterion for extinction was satisfied. Reinstatement test sessions used procedures identical to the training conditions described above, except saline infusions were delivered upon completion of the first FR after the FI expired. Response-contingent presentations of the amphetamine-paired white stimulus light were restored during reinstatement test sessions. Extinction sessions were reimposed between reinstatement test sessions with different doses of a particular drug to ensure that monkeys maintained low rates of responding. Between experiments with different drugs, amphetamine self-administration was reestablished using the procedures described previously until responding stabilized (<20% variance in daily response rate over three consecutive sessions).
To evaluate the effectiveness of a drug to reinstate responding, tests were conducted with saline, and a range of doses of amphetamine (0.01–0.3 mg/kg), BZP (0.1–3.0 mg/kg), SR(+/−)-MDMA (0.01–1.7 mg/kg), S(+)-MDMA (0.03–1.0 mg/kg), R(−)-MDMA (0.1–1.0 mg/kg), and fenfluramine (1.0 and 3.0 mg/kg). Drugs were administered i.v. through vascular access ports immediately before the session and followed by a saline flush to clear the catheter of residual drug solution. Amphetamine reinstatement was characterized in all subjects first, and the reinstatement effects of BZP, SR(+/−)-, S(+)-, and R(−)-MDMA were randomized across subjects. Different doses of a particular drug were tested on different days, and each test session was separated by at least one extinction session. All doses of a given drug were characterized once before initiating testing with a different drug. To control for the effects of the amphetamine-paired stimulus, non-contingent priming injections of saline were administered several times in each subject, and individual data are the mean of three to four determinations. The order of doses tested within each drug was varied across subjects, with the exception that fenfluramine reinstatement was characterized last in all subjects.
For pretreatment experiments, fluoxetine HCl (1.0 or 3.0 mg/kg) was administered i.v. 15 min prior to a saline or drug prime. The priming dose of amphetamine, BZP, and SR(+/−)-MDMA was individually determined for each subject as the dose that resulted in the maximal reinstatement effect. Each test session was separated by at least one extinction session. Pretreatment with 3.0 mg/kg fluoxetine was characterized first, and stable self-administration was reestablished prior to pretreatment experiments with 1.0 mg/kg fluoxetine. (Note: Subject RVc-5 was not included in the fluoxetine pretreatment or fenfluramine reinstatement experiments due to problems with maintaining catheter patency.)
Data analysis
Response rates for amphetamine self-administration were analyzed for individual subjects. Responses and time elapsed during the time-outs and presentations of the white stimulus lights were not included in response rate determinations. For reinstatement studies, the response rate following non-contingent priming injections was derived as a percent of control rates maintained by amphetamine self-administration, and plotted as a function of drug dose in individual subjects. The shape of the dose–response functions for the individual drugs was comparable across subjects; however, the drug dose that resulted in the maximum reinstatement varied within the group. Therefore, the group mean dose–response functions for each drug were collapsed across subjects as to the dose that resulted in the maximum reinstatement effect, regardless of the drug dose. Statistical analysis was done with these group means normalized to dose. Repeated measures one-way analysis of variance (ANOVA) with the Dunnett’s post hoc test was used to determine statistical significance between the maximum reinstatement effects for each drug as compared to the saline control (Woolverton and Wang 2004; Wee et al. 2005). In fluoxetine pretreatment experiments, the mean maximum reinstatement effect for each drug was compared to the mean reinstatement effects following pretreatments with fluoxetine (1.0 mg/kg or 3.0 mg/kg). A repeated measures ANOVA with Dunnett’s post hoc test was used to determine statistical significance of the fluoxetine pretreatment conditions. For all tests, statistical significance was accepted at the p<0.05 level.
Results
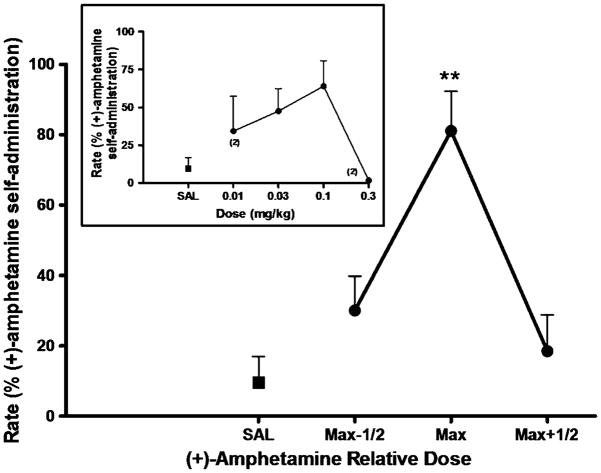
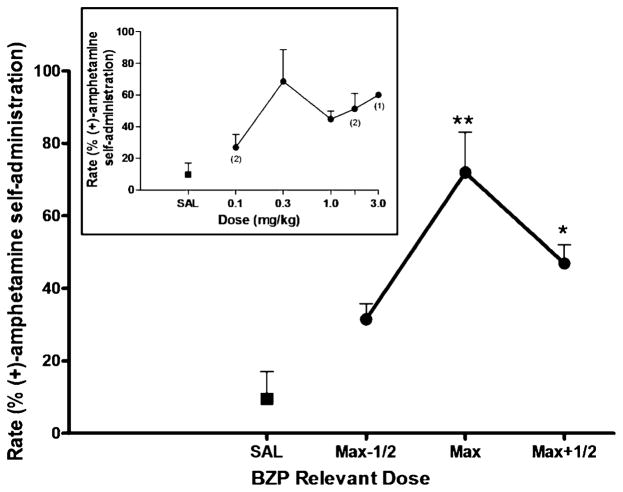
Amphetamine (0.03–0.1 mg/kg/infusion) reliably maintained responding in all four subjects under the second-order schedule of i.v. drug injection, with average response rates ranging from 0.49 to 0.88 responses/s in individual subjects, with a group (n=4) mean of 0.72±0.09. During extinction sessions, in which saline was substituted for amphetamine and the amphetamine-paired white light was omitted, response rates declined to less than 20% of the rate maintained by amphetamine self-administration in all subjects, meeting our criterion for extinction. Non-contingent priming injections of saline before extinction sessions, in which the amphetamine-paired stimulus was restored, induced minimal responding in all subjects. In contrast, priming injections of amphetamine reinstated responding in all subjects (Fig 1). Maximal reinstatement effects induced by 0.03 mg/kg (n=2) or 0.1 mg/kg (n=2) amphetamine resulted in 81±11% of the rates maintained by amphetamine self-administration [peak response rates induced by an amphetamine prime, 0.56±0.06 responses/s (n=4)]. A repeated measures ANOVA revealed a significant main effect for amphetamine [F(3, 9)=15.51, p=0.0007]. Post hoc analysis confirmed a significant reinstatement effect for the maximally effective priming dose of amphetamine (p=0.01). Similarly, the dopamine releaser BZP, significantly reinstated responding (Fig 2). A repeated measures ANOVA revealed a significant main effect for BZP [F(3, 9)=10.2, p=0.003]. Post hoc analysis revealed significant reinstatement for the maximally effective priming dose (p<0.01) and for the half-log higher dose of BZP (p <0.05). The peak response rates induced by priming injections of 0.3 mg/kg (n=2), 1.0 mg/kg (n=1), or 1.7 mg/kg (n=1) BZP were 69±12% of the rates maintained by amphetamine self-administration [peak response rates induced by BZP prime, 0.51±0.06 responses/s (n=4)].
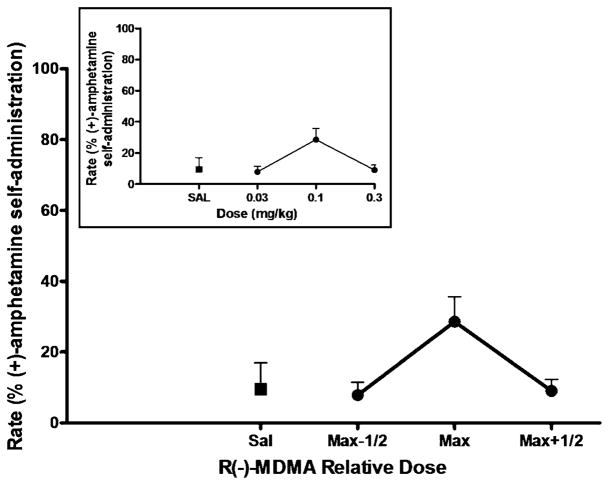
Fig. 1.
(+)-Amphetamine-primed reinstatement of responding previously maintained by (+)-amphetamine self-administration. Each data point represents the group (n=4) mean, and vertical error bars represent the S.E.M. values. Data were normalized as to dose to adjust for individual differences in potency. Max, dose that induced maximum reinstatement in each subject; Max-1/2, half-log dose lower than Max; Max+1/2, half-log higher dose than Max. **, p<0.01 compared with saline prime. Insert, dose–response function for (+)amphetamine-primed reinstatement. The doses of (+)-amphetamine tested in the individual subjected differed due to potency differences. When the data point was from less than three subjects, the number of subjects is indicated in parentheses
Fig. 2.
BZP-primed reinstatement of responding previously maintained by (+)-amphetamine self-administration. Each data point represents the group (n=4) mean, and vertical error bars represent the S.E.M. values. Data were normalized as to dose to adjust for individual differences in potency. Max, dose that induced maximum reinstatement in each subject; Max-1/2, half-log dose lower than Max; Max+1/2, half-log higher dose than Max. **, p<0.01; *, p<0.05 compared with saline prime. Insert, dose–response function for BZP-primed reinstatement. The doses of BZP tested in the individual subjected differed due to potency differences. When the data point was from less than three subjects, the number of subjects is indicated in parentheses
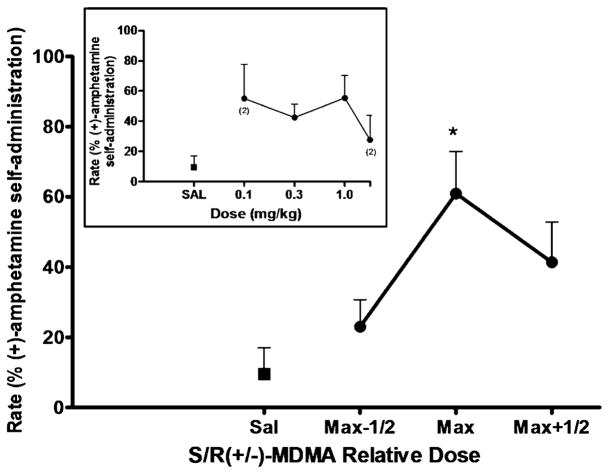
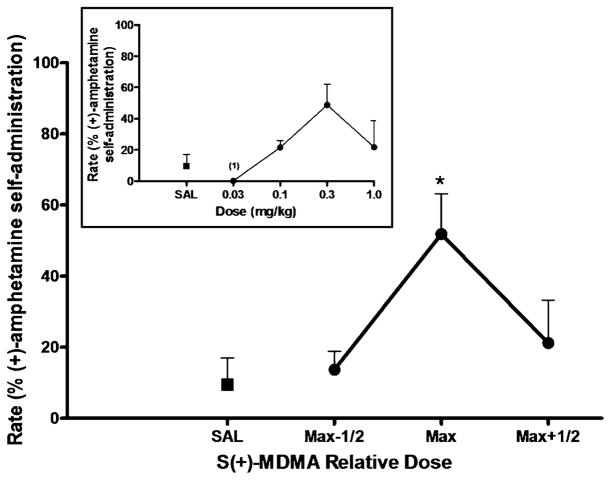
Priming injections with SR(+/−)-MDMA also resulted in significant reinstatement [F(3,9)=5.375, p=0.0214] (Fig 3). Post hoc analysis confirmed a statistically significant effect for the maximally effective priming dose of SR(+/−)-MDMA (p<0.05). The peak response rates induced by priming injections of 0.03 mg/kg (n=2) or 1.0 mg/kg (n=2) SR(+/−)-MDMA were 61±12% of the rates maintained by amphetamine self-administration [peak response rates induced by SR(+/−)-MDMA prime, 0.33±0.04 response/s (n=4)]. Likewise, priming injections with S(+)-MDMA induced significant reinstatement effects (Fig 4). A repeated measures ANOVA revealed a significant main effect of S (+)-MDMA [F(3,9)=4.328, p=0.0379], and post hoc analysis confirmed a statistically significant effect (p< 0.05) for the maximally effective priming dose of S(+)-MDMA. The peak response rates induced by priming injections 0.1 mg/kg (n=1) or 0.3 mg/kg (n=3) S(+)-MDMA were 51±12% of the rates maintained by amphetamine self-administration [peak response rates induced by S (+)-MDMA prime, 0.34±0.06 responses/s (n=4)].
Fig. 3.
SR(+/−)-MDMA-primed reinstatement of responding previously maintained by (+)-amphetamine self-administration. Each data point represents the group (n=4) mean, and vertical error bars represent the S. E.M. values. Data were normalized as to dose to adjust for individual differences in potency. Max, dose that induced maximum reinstatement in each subject; Max-1/2, half-log dose lower than Max; Max+1/2, half-log higher dose than Max. *, p<0.05 compared with saline prime. Insert, dose–response function for S/R(+/−)-MDMA-primed reinstatement. The doses of S/R(+/−)-MDMA tested in the individual subjected differed due to potency differences. When the data point was from less than three subjects, the number of subjects is indicated in parentheses
Fig. 4.
S(+)-MDMA-primed reinstatement of responding previously maintained by (+)-amphetamine self-administration. Each data point represents the group (n=4) mean, and vertical error bars represent the S.E.M. values. Data were normalized as to dose to adjust for individual differences in potency. Max, dose that induced maximum reinstatement in each subject; Max-1/2, half-log dose lower than Max; Max+1/2, half-log higher dose than Max. *, p<0.05 compared with saline prime. Insert, dose–response function for S(+)-MDMA-primed reinstatement. The doses of S(+)-MDMA tested in the individual subjected differed due to potency differences. When the data point was from less than three subjects, the number of subjects is indicated in parentheses
In contrast, R(−)-MDMA did not produce marked reinstatement in any subject (Fig 5) The peak response rates induced by priming injections of 0.3 mg/kg (n=4) R (−)-MDMA were 29±7% of the rates maintained by amphetamine self-administration [peak response rates induced by R(−)-MDMA prime, 0.17±0.04 responses/s (n=4)] Priming injections with the selective serotonin releaser, fenfluramine, were also ineffective at reinstating responding in the three animals tested (data not shown). The mean response rates induced by both priming doses (1.0 and 3.0 mg/kg) (n=3) of fenfluramine were less than 1% of the rates maintained by amphetamine self-administration [average response rates induced by 1.0 mg/kg fenfluramine, 0.02±0.02 responses/s (n=3), 3.0 mg/kg fenfluramine failed to elicit any responses (n=3)].
Fig. 5.
R(−)-MDMA-primed reinstatement of responding previously maintained by (+)-amphetamine self-administration. Each data point represents the group (n=4) mean, and vertical error bars represent the S.E.M. values. Data were normalized as to dose to adjust for individual differences in potency. Max, dose that induced maximum reinstatement in each subject; Max-1/2, half-log dose lower than Max; Max+1/2, half-log higher dose than Max. Insert, dose–response function for R(−)-MDMA-primed reinstatement. The doses of R(−)-MDMA tested in the individual subjected differed due to potency differences. When the data point was from less than three subjects, the number of subjects is indicated in parentheses
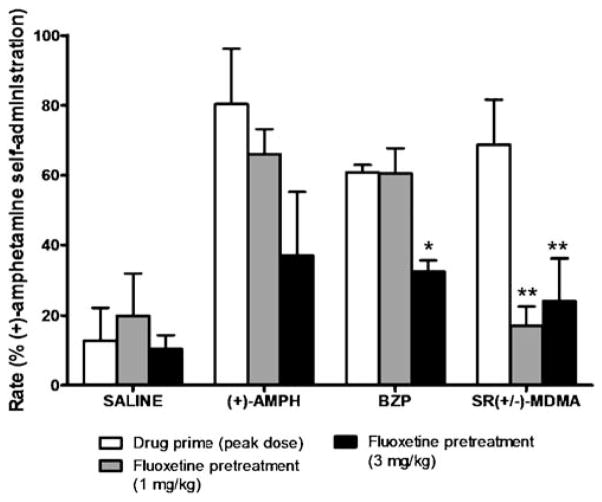
The effects of fluoxetine (1.0 and 3.0 mg/kg) pretreatment on reinstatement induced by the maximally effective doses of amphetamine, BZP and SR(+/−)-MDMA were assessed in three subjects (Fig 6). Fluoxetine significantly attenuated the reinstatement effects of BZP [F(2,4)=13.10, p=0.0175], and post hoc analysis revealed a significant (p< 0.05) effect for 3.0 mg/kg (but not 1.0 mg/kg) fluoxetine. Reinstatement by the maximally effective dose of BZP was reduced by 43±7% following pretreatment with 3.0 mg/kg fluoxetine. Fluoxetine pretreatments also significantly attenuated the reinstatement effects of SR(+/−)-MDMA [F(2,4)=41.17, p=0.0021]. Post hoc analysis revealed significant attenuation for 1.0 mg/kg (p<0.01) and 3.0 mg/kg fluoxetine (p<0.01). Reinstatement by the maximally effective dose of SR(+/−)-MDMA was reduced by 77±4% following pretreatment with the 1.0 mg/kg dose of fluoxetine, and by 70±4% following pretreatment with the 3.0 mg/kg dose of fluoxetine. In contrast, pretreatment with fluoxetine did not significantly alter amphetamine-primed reinstatement nor did it have an effect on responding following priming injections of saline.
Fig. 6.
Effects of fluoxetine pretreatments (1.0 or 3.0 mg/kg) on reinstatement induced by priming injections of saline, (+)-amphetamine, BZP, and SR(+/−)-MDMA. Each column represents the group mean (±SEM) of the maximal rate (% (+)-amphetamine self-administration) for each drug. **, p<0.01; *, p<0.05, compared with no pretreatment condition
Discussion
Reinstatement has been described as a preclinical animal model of relapse following a period of drug abstinence. It is well established that a non-contingent priming injection of a previously self-administered drug can reinstate responding. In the present study, non-contingent priming injections of amphetamine induced significant reinstatement of responding previously maintained by amphetamine self-administration. This finding is consistent with a number of other studies, which have demonstrated the effectiveness of a non-contingent injection of amphetamine to reinstate extinguished responding previously reinforced by amphetamine (Stretch and Gerber 1973; Gerber and Stretch 1975). Likewise, the dopamine releaser BZP induced significant reinstatement effects. Previous studies have shown that BZP generalizes to the discriminative cue induced by amphetamine in rhesus monkeys (Fantegrossi et al. 2005) and maintains self-administration in both rhesus monkeys (Fantegrossi et al. 2005) and rats (Brennan et al. 2007). Moreover, human subjects report the subjective effects of BZP as stimulant-like, similar to both amphetamine and MDMA (Lin et al. 2009). These findings further demonstrate concordance between the discrimitive stimulus and reinstatement effects of behavioral stimulants.
Importantly, non-contingent priming injections of SR(+/−)-MDMA significantly reinstated responding. In two subjects, the maximally effective dose of SR(+/−)-MDMA resulted in rates comparable to those maintained by amphetamine self-administration. In the other two subjects, the maximally effective dose of SR(+/−)-MDMA induced approximately 40% of the rate maintained by amphetamine self-administration. The effectiveness of a non-contingent priming injection of SR(+/−)-MDMA to reinstate responding previously maintained by amphetamine self-administration was predicted based on previous work indicating these compounds produce similar discriminative stimulus and subjective effects (Evans and Johanson 1986; Kamien et al. 1986; Cami et al. 2000; Tancer and Johanson 2001; Tancer and Johanson 2003). Although there are exceptions, the majority of drugs that reinstate responding previously maintained by self-administration share discrimitive stimulus effects with the self-administered drug. Accordingly, it was predicted that the more “stimulant-like” S(+) enantiomer would also reinstate responding. This prediction was confirmed in the present study as non-contingent priming injections of S(+)-MDMA induced significant reinstatement effects. In contrast, priming injections of R(−)-MDMA did not significantly increase response rates above extinction levels. It is likely that mechanistic differences between the enantiomers underlie the effectiveness of S(+)-MDMA, but not R(−)-MDMA, in reinstating responding. Specifically, the S(+) enantiomer has a higher affinity for the DAT and is more potent at releasing DA (Steele et al. 1987), and dopaminergic mechanisms likely play a significant role in mediating the effects of S(+)-MDMA.
As expected, priming injections of the selective serotonin releaser, fenfluramine (1.0 or 3.0 mg/kg), did not reinstate extinguished responding. Drug discrimination studies have consistently demonstrated that amphetamine and fenfluramine produce dissimilar stimulus effects (Schechter and Rosecrans 1973; White and Appel 1981; Chait et al. 1986), which likely reflects mechanistic differences between amphetamine (DA releaser) and fenfluramine (5-HT releaser). Although there are a number of conflicting reports regarding stimulus generalization between amphetamine and MDMA, generalization between fenfluramine and MDMA is a fairly consistent finding suggesting that 5-HT release is a salient feature mediating the stimulus effects of MDMA.
The attenuation of MDMA-induced reinstatement by the selective SERT inhibitor, fluoxetine, supports the importance of 5-HT release in mediating the effects of MDMA. However, the ineffectiveness of fenfluramine at reinstating extinguished responding indicates that mechanisms in addition to 5-HT release contribute to the reinstatement effects of MDMA. Interestingly, fluoxetine also attenuated the reinstatement effects of BZP, and to a lesser extent amphetamine, although the latter result did not reach statistical significance. It is possible that the limited sample size did not provide sufficient statistical power to detect a more subtle effect of amphetamine. Conversely, BZP may be more sensitive to the pretreatment effects of fluoxetine due to its greater potency at releasing 5-HT, as compared with amphetamine (Baumann et al. 2005). A recent study in rhesus monkeys suggested subtle differences in the behavioral effects of BZP compared to other stimulants (Negus et al. 2009). Taken together, the present findings support previous studies indicating that the behavioral profile of the MDMA compounds is complex and is mediated at least partially by 5-HT release. Although the present study was not specifically designed to evaluate the role of DA in MDMA-induced reinstatement, it is likely that dopaminergic mechanisms contributed to the reinstatement effects of MDMA. Both racemic and S(+)-MDMA release DA more potently than R(−)-MDMA, and this feature likely contributed to the effectiveness of SR(+/−)- and S(+)-MDMA, but not R(−)-MDMA, to reinstate extinguished responding.
In summary, non-contingent priming injections of amphetamine, BZP, racemic MDMA, and S(+)-MDMA significantly reinstated responding previously maintained by amphetamine self-administration. Conversely, R(−)-MDMA and fenfluramine failed to reinstate extinguished responding. Based on the profile of neurochemical effects and the relative potencies to release DA, it appears that the reinstatement effects were primarily mediated by DA release. However, the attenuation of MDMA-induced reinstatement by fluoxetine supports previous research indicating the complex behavioral pharmacology of MDMA-like drugs and that the reinstatement effects of MDMA are at least partially mediated by serotonergic-mechanisms. The finding that MDMA reliably reinstated responding previously maintained by amphetamine self-administration suggests that MDMA may precipitate relapse to amphetamine use in humans.
References
- Baker LE, Taylor MM. Assessment of the MDA and MDMA optical isomers in a stimulant-hallucinogen discrimination. Pharmacol Biochem Behav. 1997;57:737–748. doi: 10.1016/s0091-3057(96)00334-6. [DOI] [PubMed] [Google Scholar]
- Baker LE, Broadbent J, Michael EK, Matthews PK, Metosh CA, Saunders RB, West WB, Appel JB. Assessment of the discriminative stimulus effects of the optical isomers of ecstasy (3,4-methylenedioxymethamphetamine; MDMA) Behav Pharmacol. 1995;6:263–275. [PubMed] [Google Scholar]
- Baker LE, Virden TB, Miller ME, Sullivan CL. Time course analysis of the discriminative stimulus effects of the optical isomers of 3,4-methylenedioxymethamphetamine (MDMA) Pharmacol Biochem Behav. 1997;58:505–516. doi: 10.1016/s0091-3057(97)00287-6. [DOI] [PubMed] [Google Scholar]
- Battaglia G, De Souza EB. Pharmacologic profile of amphetamine derivatives at various brain recognition sites: selective effects on serotonergic systems. NIDA Res Monogr. 1989;94:240–258. [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB. Effects of “Legal X” piperazine analogs on dopamine and serotonin release in rat brain. Ann NY Acad Sci. 2004;1025:189–197. doi: 10.1196/annals.1316.024. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Budzynski AG, Partilla JS, Blough BE, Rothman RB. N-Substituted piperazines abused by humans mimic the molecular mechanism of 3,4-methylenedioxymethamphetamine (MDMA, or ‘Ecstasy’) Neuropsychopharmacology. 2005;30:550–560. doi: 10.1038/sj.npp.1300585. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Lake B, Hely LS, Jones K, Gittings D, Colussi-Mas J, Fitzmaurice PS, Lea RA, Schenk S. N-benzylpiperazine has characteristics of a drug of abuse. Behav Pharmacol. 2007;18:785–790. doi: 10.1097/FBP.0b013e3282f18d8f. [DOI] [PubMed] [Google Scholar]
- Callaway CW, Wing LL, Geyer MA. Serotonin release contributes to the locomotor stimulant effects of 3,4-methylenedioxymethamphetamine in rats. J Pharmacol Exp Ther. 1990;254:456–464. [PubMed] [Google Scholar]
- Cami J, Farre M, Mas M, Roset PN, Poudevida S, Mas A, San L, de la Torre R. Human pharmacology of 3,4-methylenedioxymethamphetamine (“ecstasy”): psychomotor performance and subjective effects. J Clin Psychopharmacol. 2000;20:455–466. doi: 10.1097/00004714-200008000-00010. [DOI] [PubMed] [Google Scholar]
- Chait LD, Uhlenhuth EH, Johanson CE. Human drug discrimination: D-amphetamine and other anorectics. NIDA Res Monogr. 1986;67:161–167. [PubMed] [Google Scholar]
- de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- Easton N, Marsden CA. Ecstasy: are animal data consistent between species and can they translate to humans? J Psychopharmacol. 2006;20:194–210. doi: 10.1177/0269881106061153. [DOI] [PubMed] [Google Scholar]
- Evans SM, Johanson CE. Discriminative stimulus properties of (+/−)-3,4-methylenedioxymethamphetamine and (+/−)-3,4-methylenedioxyamphetamine in pigeons. Drug Alcohol Depend. 1986;18:159–164. doi: 10.1016/0376-8716(86)90048-7. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Godlewski T, Karabenick RL, Stephens JM, Ullrich T, Rice KC, Woods JH. Pharmacological characterization of the effects of 3,4-methylenedioxymethamphetamine (“ecstasy”) and its enantiomers on lethality, core temperature, and locomotor activity in singly housed and crowded mice. Psychopharmacology (Berl) 2003;166:202–211. doi: 10.1007/s00213-002-1261-5. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Winger G, Woods JH, Woolverton WL, Coop A. Reinforcing and discriminative stimulus effects of 1-benzylpiperazine and trifluoromethylphenylpiperazine in rhesus monkeys. Drug Alcohol Depend. 2005;77:161–168. doi: 10.1016/j.drugalcdep.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murai N, Mathuna BO, Pizarro N, de la Torre R. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine and its enantiomers in mice: pharmacokinetic considerations. J Pharmacol Exp Ther. 2009;329:1006–1015. doi: 10.1124/jpet.109.150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA. Stimulus properties of hallucinogenic phenalkyl-amines and related designer drugs: formulation of structure-activity relationships. NIDA Res Monogr. 1989;94:43–67. [PubMed] [Google Scholar]
- Glennon RA, Misenheimer BR. Stimulus effects of N-monoethyl-1-(3,4-methylenedioxyphenyl)-2-aminopropane (MDE) and N-hydroxy-1-(3,4-methylenedioxyphenyl)-2-aminopropane (N-OH MDA) in rats trained to discriminate MDMA from saline. Pharmacol Biochem Behav. 1989;33:909–912. doi: 10.1016/0091-3057(89)90491-7. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R. MDA: a psychoactive agent with dual stimulus effects. Life Sci. 1984;34:379–383. doi: 10.1016/0024-3205(84)90627-1. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, Patrick G. Stimulus properties of 1-(3,4-methylenedioxyphenyl)-2-aminopropane (MDA) analogs. Pharmacol Biochem Behav. 1988;29:443–449. doi: 10.1016/0091-3057(88)90001-9. [DOI] [PubMed] [Google Scholar]
- Gold LH, Koob GF. MDMA produces stimulant-like conditioned locomotor activity. Psychopharmacology (Berl) 1989;99:352–356. doi: 10.1007/BF00445556. [DOI] [PubMed] [Google Scholar]
- Goodwin AK, Baker LE. A three-choice discrimination procedure dissociates the discriminative stimulus effects of d-amphetamine and (±)-MDMA in rats. Exp Clin Psychopharmacol. 2000;8:415–423. doi: 10.1037//1064-1297.8.3.415. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Nabeshima T, Kameyama T, Maeda Y, Cho AK. The effect of optical isomers of 3,4-methylendioxymethamphet-amine (MDMA) on stereotyped behavior in rats. Pharmacol Biochem Behav. 1989;33:343–347. doi: 10.1016/0091-3057(89)90511-x. [DOI] [PubMed] [Google Scholar]
- Howell LL, Wilcox KM. The dopamine transporter and cocaine medication development: drug self-administration in nonhuman primates. J Pharmacol Exp Ther. 2001;298:1–6. [PubMed] [Google Scholar]
- Johanson CE, Kilbey M, Gatchalian K, Tancer M. Discriminative stimulus effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans trained to discriminate among D-amphetamine, meta-chlorophenylpiperazine and placebo. Drug Alcohol Depend. 2006;81:27–36. doi: 10.1016/j.drugalcdep.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Johanson CE, Schuster CR, Woolverton WL. The effects of (+/−)-methylenedioxymethamphetamine and (+/−)-methylenedioxyamphetamine in monkeys trained to discriminate (+)-amphetamine from saline. Drug Alcohol Depend. 1986;18:139–147. doi: 10.1016/0376-8716(86)90046-3. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Vollenweider FX. Which neuroreceptors mediate the subjective effects of MDMA in humans? A summary of mechanistic studies. Hum Psychopharmacol Clin Exp. 2001;16:589–598. doi: 10.1002/hup.348. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology. 2000;22:513–521. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- Lin J, Bangs N, Lee H, Kydd R, Russell B. Determining the subjective and physiological effects of BZP on human females. Psychopharmacology. 2009;207:439–446. doi: 10.1007/s00213-009-1669-2. [DOI] [PubMed] [Google Scholar]
- McCreary AC, Filip M, Cunningham KA. Discriminative stimulus properties of (+/−)-fenfluramine: the role of 5-HT2 receptor subtypes. Behav Neurosci. 2003;117:212–221. doi: 10.1037/0735-7044.117.2.212. [DOI] [PubMed] [Google Scholar]
- McNamara MG, Kelly JP, Leonard BE. Some behavioural and neurochemical aspects of subacute (±)3,4-methylenedioxymethamphetamine administration in rats. Pharmacol Biochem Behav. 1995;52:479–484. doi: 10.1016/0091-3057(95)00206-c. [DOI] [PubMed] [Google Scholar]
- Murnane KS, Murai N, Howell LL, Fantegrossi WE. Discriminative stimulus effects of psychostimulants and hallucinogens in S(+)-3,4-methylenedioxymethamphetamine (MDMA) and R(−)-MDMA trained mice. J Pharmacol Exp Ther. 2009;331:717–723. doi: 10.1124/jpet.109.156174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Baumann MH, Rothman RB, Mello NK, Blough BE. Selective suppression of cocaine-versus food-maintained responding by monoamine releasers in rhesus monkeys: benzyl-piperazine, (+)phenmetrazine, and 4-benzylpiperidine. J Pharmacol Exp Ther. 2009;329:272–281. doi: 10.1124/jpet.108.143701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlender R, Nichols DE. Drug discrimination studies with MDMA and amphetamine. Psychopharmacology (Berl) 1988;95:71–76. doi: 10.1007/BF00212770. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Geyer MA. The effects of MDMA and other methylenedioxy-substituted phenylalkylamines on the structure of rat locomotor activity. Neuropsychopharmacology. 1992;7:15–31. [PubMed] [Google Scholar]
- Rudnick G, Wall SC. The molecular mechanism of “ecstasy” [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci USA. 1992;89:1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter MD. Discriminative profile of MDMA. Pharmacol Biochem Behav. 1986;24:1533–1537. doi: 10.1016/0091-3057(86)90480-6. [DOI] [PubMed] [Google Scholar]
- Schechter MD. MDMA as a discriminative stimulus: isomeric comparisons. Pharmacol Biochem Behav. 1987;27:41–44. doi: 10.1016/0091-3057(87)90474-6. [DOI] [PubMed] [Google Scholar]
- Schechter MD. Serotonergic-dopaminergic mediation of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) Pharmacol Biochem Behav. 1988;31:817–824. doi: 10.1016/0091-3057(88)90390-5. [DOI] [PubMed] [Google Scholar]
- Schechter MD, Rosecrans JA. D-amphetamine as a discriminative cue: drugs with similar stimulus properties. Eur J Pharmacol. 1973;21:212–216. doi: 10.1016/0014-2999(73)90228-8. [DOI] [PubMed] [Google Scholar]
- Slikker W, Jr, Brocco MJ, Killam KF., Jr Reinstatement of responding maintained by cocaine or thiamylal. J Pharmacol Exp Ther. 1984;228:43–52. [PubMed] [Google Scholar]
- Slikker W, Holson RR, Ali SF, Kolta MG, Paule MG, Scallet AC, McMillan DE, Bailey JR, Hong JS, Scalzo FM. Behavioral and neurochemical effects of orally administered MDMA in the rodent and nonhuman primate. Neurotoxicology. 1989;10:529–542. [PubMed] [Google Scholar]
- Souza D, Kelly JP, Harkin AJ, Leonard BE. An appraisal of the pharmacological and toxicological effects of a single oral administration of 3,4-methylenedioxymethamphetamine (MDMA) in the rat. Pharmacol Toxicol. 1997;80:207–210. doi: 10.1111/j.1600-0773.1997.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Steele TD, Nichols DE, Yim GK. Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharmacol. 1987;36:2297–2303. doi: 10.1016/0006-2952(87)90594-6. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Rink J. Effects of “Ecstasy” blocked by serotonin reuptake inhibitors. J Clin Psychiatry. 1999;60:485. doi: 10.4088/jcp.v60n0711a. [DOI] [PubMed] [Google Scholar]
- Stolerman IP. Components of drug dependence: reinforcement, discrimination and adaptation. Biochem Soc Symp. 1993;59:1–12. [PubMed] [Google Scholar]
- Stretch R, Gerber GJ. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can J Psychol. 1973;27:168–177. doi: 10.1037/h0082466. [DOI] [PubMed] [Google Scholar]
- Tancer ME, Johanson CE. The subjective effects of MDMA and mCPP in moderate MDMA users. Drug Alcohol Depend. 2001;65:97–101. doi: 10.1016/s0376-8716(01)00146-6. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. Reinforcing, subjective, and physiological effects of MDMA in humans: a comparison with D-amphetamine and mCPP. Drug Alcohol Depend. 2003;72:33–44. doi: 10.1016/s0376-8716(03)00172-8. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. J Pharmacol Exp Ther. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- White FJ, Appel JB. A neuropharmacological analysis of the discriminative stimulus properties of fenfluramine. Psychopharmacology. 1981;73:110–115. doi: 10.1007/BF00429199. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]