Abstract
The relationships between long-term intensive control of glycemia and indicators of skin collagen glycation (furosine), glycoxidation (pentosidine and N∊-[carboxymethyl]-lysine [CML]), and crosslinking (acid and pepsin solubility) were examined in 216 patients with type 1 diabetes from the primary prevention and secondary intervention cohorts of the Diabetes Control and Complications Trial. By comparison with conventional treatment, 5 years of intensive treatment was associated with 30–32% lower furosine, 9% lower pentosidine, 9–13% lower CML, 24% higher acid-soluble collagen, and 50% higher pepsin-soluble collagen. All of these differences were statistically significant in the subjects of the primary prevention cohort (P < 0 .006–0.001) and also of the secondary intervention cohort (P < 0.015–0.001) with the exception of CML and acid-soluble collagen. Age- and duration-adjusted collagen variables were significantly associated with the HbA1c value nearest the biopsy and with cumulative prior HbA1c values. Multiple logistic regression analyses with six nonredundant collagen parameters as independent variables and various expressions of retinopathy, nephropathy, and neuropathy outcomes as dependent variables showed that the complications were significantly associated with the full set of collagen variables. Surprisingly, the percentage of total variance (R2) in complications explained by the collagen variables ranged from 19 to 36% with the intensive treatment and from 14 to 51% with conventional treatment. These associations generally remained significant even after adjustment for HbA1c, and, most unexpectedly, in conventionally treated subjects, glycated collagen was the parameter most consistently associated with diabetic complications. Continued monitoring of these subjects may determine whether glycation products in the skin, and especially the early Amadori product (furosine), have the potential to be predictors of the future risk of developing complications, and perhaps be even better predictors than glycated hemoglobin (HbA1c).
The discovery that glycated hemoglobin provides a measure of cumulative glycemia over a period of 8–12 weeks has advanced our understanding of the association between glycemic control and long-term complications of diabetes. It has also made possible the design of long-term intervention trials such as the Diabetes Control and Complications Trial (DCCT) (1). The DCCT demonstrated that intensive glycemic control reduced the rate of development and progression of diabetic microvascular and neuropathic complications in type 1 diabetes (2). The observation that glycated proteins form stable advanced glycation/Maillard reaction end products (AGEs) that accumulate in tissues affected by diabetic complications (3) has led to the concept that glycation may be causal in the development of the complications. Moreover, quantitation of these end products could provide a tissue measure of integrated glycemia over several years and an estimate of the consequent risk of developing the above complications (4). In accordance with this concept, several studies have been carried out with small numbers of patients to investigate the relationship between skin levels of AGEs and diabetic complications. Skin collagen levels of nonspecific fluorescent and immunoreactive AGEs as well as the specific AGEs/glycoxidation products pentosidine and N∊-(carboxymethyl)-lysine (CML) were found to correlate with duration of diabetes and to various degrees with the severity of retinopathy and nephropathy in patients with type 1 diabetes (4-8). Although certain collagen variables, such as glycated collagen (Amadori product) and fluorescence, were also found to be positively associated with preceding levels of glycemic control in some studies (6,7), it is unclear whether long-term intensive glycemic control would prevent elevated levels of AGEs or even slow their increase. In one study, intensive treatment of glycemia in type 1 diabetic subjects over a period of 4 months resulted in decreased skin collagen glycation but had no effect on glycoxidation products (9). Similar results of intensive therapy on glycation of plasma protein and skin collagen were observed in type 2 diabetic subjects followed for 1–5 years but without improvement of tissue fluorescent AGEs, except in those subjects with the greatest decrease in fasting blood glucose (10).
Although in most studies the associations between skin levels of glycation products and complications remained significant after adjustment for age (8,9,11), and in some studies after adjustment for diabetes duration (7,11,12), no study has reported on whether the correlations were still significant after adjustment for HbA1c. This point is critical for two reasons: 1) because an independent effect of glycated proteins might point to specific pathways responsible for hyperglycemic tissue damage and 2) because it might suggest that the determination of skin AGEs would offer an advantage over HbA1c in predicting the risk of developing diabetic complications. Finally, none of the studies performed so far have addressed the question of whether glycemic control affects other variables of collagen crosslinking, such as collagen solubility and proteolytic digestibility. Because currently available probes of the Maillard reaction do not explain the precise mechanism of collagen crosslinking in diabetes, we have included chemical and physical measurements of collagen crosslinking in analyses of the correlation between complications and levels of specific AGEs.
The primary goal of this study was to determine whether long-term intensive control of glycemia, when compared with conventional therapy, would lead to improvements in skin collagen acid solubility, pepsin digestibility, glycation (fructosyl-lysine or furosine level), and the levels of the glycoxidation markers pentosidine, CML, and protein-bound fluorescence. The presumed biochemical relationship between these variables in skin collagen and glycemia is shown in Fig. 1. A second goal was to investigate whether these glycation variables correlated with cumulative glycemia, as measured by HbA1c levels at various time points before tissue sampling. Third, we sought to determine whether the prevalence, severity, and rate of development of retinopathy, nephropathy, or neuropathy are associated with the skin collagen variables and whether such associations would be independent of those previously described for HbA1c. A final goal was to determine the relationships among the skin collagen variables and whether clues to the mechanism of collagen crosslinking in diabetes would emerge based on the strength of such correlations.
FIG. 1.
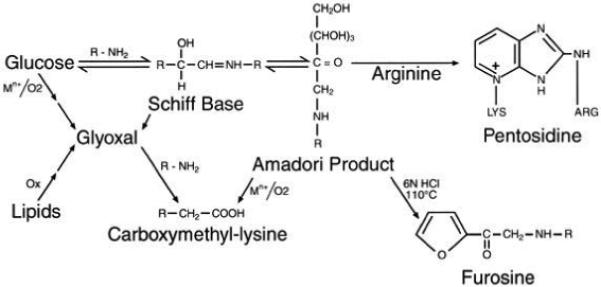
Postulated biochemical relationship between glucose, skin collagen glycation (furosine), and the AGEs CML and pentosidine. Fluorescence at 440 nm (excitation at 370 nm), collagen crosslinking, and CML may also originate from lipid peroxidation (29).
RESEARCH DESIGN AND METHODS
Study subjects
A total of 216 subjects with type 1 diabetes between the ages of 17 and 50 were recruited at or near closeout of the DCCT in eight DCCT Centers that elected to participate in this ancillary study (see APPENDIX). No selection criteria were imposed, and the participants represented 53% of the total number of DCCT subjects at these eight centers. Informed consent was obtained from each subject. A summary of selected DCCT baseline characteristics is presented in Table 1. Of the subjects, 122 had originally been assigned to the intensive therapy group and 94 to the conventional treatment group. Of the subjects in the intensive group, 65 were from the primary prevention with 1–5 years’ duration of type 1 diabetes and with no retinopathy or microalbuminuria at baseline, and 57 were from the secondary intervention cohort of the DCCT with 1–15 years’ duration of type 1 diabetes and with mild to moderate nonproliferative retinopathy and albumin excretion rates (AERs) <200 mg/24 h at baseline of the DCCT (2). Of those in the conventional group, 58 were from the primary prevention cohort and 36 were from the secondary prevention cohort. A total of 40 nondiabetic subjects between 20 and 51 years of age were also studied as age-matched control subjects.
TABLE 1.
Selected characteristics of study participants at baseline and closeout
| Primary cohort |
Secondary cohort |
|||||
|---|---|---|---|---|---|---|
| Intensive | Conventional | P value | Intensive | Conventional | P value | |
| n | 65 | 58 | 57 | 36 | ||
| Baseline | ||||||
| Age (years) | 28 ± 6 | 26 ± 7 | 0.019 | 30 ± 7 | 30 ± 6 | |
| Duration (months) | 31 ± 19 | 27 ± 14 | 108 ± 51 | 90 ± 43 | ||
| HbA1c (%) | 8.8 ± 1.9 | 9.1 ± 1.9 | 8.9 ± 1.5 | 8.3 ± 1.2 | 0.022 | |
| Mean blood glucose (mg/dl) | 221 ± 83 | 245 ± 85 | 237 ± 76 | 214 ± 72 | ||
| Triglycerides (mg/dl) | 79 ± 66 | 65 ± 23 | 97 ± 48 | 86 ± 56 | ||
| Cholesterol (mg/dl) | 182 ± 33 | 174 ± 32 | 181 ± 36 | 176 ± 37 | ||
| Retinopathy | ||||||
| 10/10: None | 100 | 100 | 0 | 0 | ||
| 20/≤20: Microaneurysms only | 0 | 0 | 58 | 61 | ||
| 30/≤30: Mild NPDR | 0 | 0 | 23 | 25 | ||
| 45/≤45: Moderate NPDR | 0 | 0 | 19 | 14 | ||
| Albuminuria (mg/24 h) | 13 ± 13 | 12 ± 8 | 19 ± 19 | 15 ± 13 | ||
| Confirmed clinical neuropathy | 6.2 | 1.7 | 17.9 | 11.1 | ||
| sBP (mmHg) | 116 ± 10 | 114 ± 11 | 115 ± 12 | 120 ± 12 | 0.042 | |
| dBP (mmHg) | 73 ± 9 | 72 ± 9 | 73 ± 8 | 76 ± 10 | ||
| Closeout | ||||||
| Age (years) | 34 ± 6 | 31 ± 7 | 0.013 | 36 ± 6 | 36 ± 7 | |
| Duration (months) | 101 ± 31 | 94 ± 23 | 189 ± 55 | 169 ± 46 | ||
| HbA1c (%) | 7.1 ± 0.8 | 9.5 ± 1.4 | <0.001 | 7.1 ± 0.8 | 8.8 ± 1.5 | <0.001 |
| Mean blood glucose (mg/dl) | 149 ± 48 | 239 ± 76 | < 0.001 | 152 ± 46 | 215 ± 83 | <0.001 |
| Triglycerides (mg/dl) | 72 ± 35 | 76 ± 34 | 91 ± 57 | 78 ± 36 | ||
| Cholesterol (mg/dl) | 175 ± 31 | 178 ± 33 | 181 ± 33 | 178 ± 33 | ||
| Retinopathy | ||||||
| 10/≤10: None | 58 | 36 | 4 | 3 | ||
| 20/≤20: Microaneurysms only | 37 | 41 | 0.004 | 30 | 36 | |
| 30/≤30: Mild NPDR | 2 | 19 | 44 | 36 | ||
| 45/≤45: Moderate NPDR | 3 | 3 | 23 | 25 | ||
| Albuminuria (mg/24 h) | 20 ± 72 | 16 ± 20 | 42 ± 176 | 22 ± 45 | ||
| Confirmed clinical neuropathy | 2.3 | 4.8 | 12.7 | 17.1 | ||
| sBP (mmHg) | 115 ± 9 | 115 ± 11 | 118 ± 9 | 121 ± 14 | ||
| dBP (mmHg) | 75 ± 9 | 74 ± 8 | 77 ± 8 | 77 ± 11 | ||
Data are means ± SD or %. P values are not significant (P < 0.05) unless indicated otherwise. The normal range for HbA1c is 4.0–6.1%. Mean blood glucose is based on the mean value of seven determinations in a 24-h period. Confirmed clinical neuropathy is defined as an abnormal neurological examination that was consistent with the presence of peripheral sensorimotor neuropathy plus either abnormal nerve conduction in at least two peripheral nerves or unequivocally abnormal autonomic nerve setting. dBP, diastolic blood pressure; NPDR, nonproliferative diabetic retinopathy; sBP, systolic blood pressure.
Baseline and closeout characteristics of the skin biopsy study participants
Baseline characteristics (at entry into the DCCT) of the 216 participating subjects from the eight DCCT centers are shown for the intensive and conventional treatment groups and within the primary prevention and secondary intervention cohorts (Table 1). Subjects in the two treatment groups of the primary cohort did not differ except that patients in the intensive treatment group were older (28 vs. 26 years). Within the secondary intervention cohort, entry HbA1c was higher (8.9 vs. 8.3%) and systolic blood pressure was lower (115 vs. 120 mmHg) in the intensive than in the conventional treatment group. Near the time of the biopsy, which was obtained within 6 months of the closeout of the DCCT, in both cohorts the intensive group subjects compared with the conventional group subjects had lower HbA1c and mean blood glucose levels (P < 0.001), as expected based on the overall DCCT results. In the primary prevention cohort, the intensive treatment group subjects also had significantly less retinopathy than the conventional treatment subjects; however, in the secondary cohort, there was no difference in the distribution of retinopathy scores between the two treatment groups.
The 216 skin collagen study participants were also compared with the 1,225 nonparticipating DCCT subjects. Within the intensive treatment group, skin collagen study participants were older (29 vs. 27 years), had slightly higher systolic blood pressure (116 vs. 113 mmHg), and had a higher incidence of neuropathy at baseline (12 vs. 6%). Within the conventional treatment group, skin collagen study participants had shorter duration of type 1 diabetes (51 vs. 68 months), modestly lower triglycerides (73 vs. 83 mg/dl), slightly but not significantly milder retinopathy, and slightly less nephropathy (AER of 13 vs. 16 mg/24 h). At DCCT closeout, participating subjects in the intensive treatment group had slightly lower HbA1c (7.1 vs. 7.3%) compared with nonparticipants. In the conventional treatment group, participants no longer differed from nonparticipants with respect to retinopathy and nephropathy levels, but they still had modestly lower levels of triglycerides (77 vs. 90 mg/dl).
With the exception of the few relatively small clinical differences noted between the skin collagen study participants and nonparticipants at baseline, the skin collagen study subjects were reasonably representative of the entire DCCT patient cohort. Moreover, any differences between the skin collagen study participants in the intensive and conventional treatment groups at baseline were quantitatively minor and would not likely bias the comparison of their skin collagen data or its relationship to diabetic complications. Also, the three major known factors that could be expected to affect glycation, i.e., age, diabetes duration, and duration of glycemic exposure during the DCCT, were not meaningfully greater in the conventional treatment group. In addition, all collagen data were adjusted for age and duration of diabetes. At closeout, the lack of difference between retinopathy levels in the intensive and conventional groups of the secondary cohort was discordant with the whole DCCT secondary cohort experience (2). It could reflect the lower entry HbA1c of the conventional group (13).
Skin biopsies
Skin biopsies (4-mm diameter) were obtained from the medial region of the right buttock ~8 cm below the iliac crest. Specimens were immediately rinsed twice with saline, frozen at −80°C, and shipped on dry ice to the Institute of Pathology at Case Western Reserve University, where they were stored under argon at −80°C until processing. Specimens were coded and processed without prior knowledge of the subjects’ DCCT treatment group assignments or their retinopathy, nephropathy, and neuropathy status.
The skin biopsies were processed for quantitation of acid-soluble, pepsin-soluble, and pepsin-insoluble collagen as described below. The levels of collagen glycation (furosine method), AGEs with fluorescence at 440 nm (excitation at 370 nm), and the glycoxidation products CML and pentosidine were determined in the acid hydrolysates of the combined pepsin-soluble and digested insoluble fractions, which represent more than 95% of total skin collagen.
The specimen was frozen in liquid nitrogen, and the stratum corneum and any noticeable fat were excised with a scalpel. The remaining portion of this biopsy was finely minced and suspended in 5 ml of CH2Cl:CH3OH (2:1 vol/vol) for delipidation on a rotary shaker at 4°C for 18 h. After centrifugation at 2,500 rpm, the supernatant was discarded. After storage in 50% methanol (15 h), each tissue sample was homogenized (Brinkman Polytron PT 10/35, Westbury, NY) in cold phosphate-buffered saline (pH 7.4) for 1 min and centrifuged at 11,000 rpm for 20 min. The supernatant was discarded. Samples were then sequentially extracted in salt, acetic acid, and pepsin, with each extraction being accomplished by rotary shaking for 18 h. After extraction, samples were centrifuged at 11,000 rpm for 1 h, and supernatants were frozen (−80°C). The order of the extractions was as follows: after homogenization, pellets were suspended in 1 ml NaCl (1 mol/l) with extraction at 4°C. Sequential extractions were done in 1 ml acetic acid (0.5 mol/l) at 4°C (acid-soluble collagen), 1 ml acetic acid (0.5 mol/l) with 30 μg pepsin at 4°C (pepsin-soluble collagen) (P-6887; Sigma, St. Louis, MO), and 1 ml acetic acid (0.5 mol/l) with 60 μg pepsin at 37°C (insoluble collagen) according to Dyer (14). Toluene:chloroform (1:1 vol/vol; 2 μl) was added to the second pepsin digestion as an antimicrobial agent. A small pellet remained after centrifugation of the second pepsin digestion. Control specimens without collagen were processed for correction of AGE levels normally present in the commercial enzyme preparations.
Acid hydrolysis and collagen assays
For quantitation of collagen content in each fraction, a portion of salt fraction (50%), acetic acid fraction (5%), pepsin-soluble fraction (5%), insoluble fraction (5%), and all of the remaining pellet were hydrolyzed in 1 ml HCl (6 N, deaerated) (Optima; Fisher, Silver Spring, MD). Tubes were flushed with N2 (g) before being sealed with Teflon-lined caps and heated at 110°C for 18 h. The HCl was evaporated with a Savant concentrator (model AS 160; Farmingdale, NY), and each pellet was resuspended in 1 ml of distilled water (Milli-Q Plus; Millipore, Bedford, MA). Aliquots (200–500 μl) were taken from each sample, and collagen content was determined by a hydroxyproline colorimetric assay as described earlier (5). Because the pepsin-soluble and the pepsin-digested insoluble fractions together contained more than 95% of the total collagen, they were combined for the quantification of pentosidine, CML, furosine, and relative fluorescence.
Equal volumetric portions of the remaining pepsin-soluble and pepsindigested insoluble fractions of each sample were combined (1:1) and hydrolyzed. After evaporation of HCl, each pellet was resuspended in 1 ml of distilled water and filtered with 4-μm microcentrifuge filters (Rainin Instrument, Woburn, MA). Aliquots (50 μl) were taken from each hydrolysate, and their collagen content was determined as described earlier (15).
Quantitation of furosine, pentosidine, and CML
Three high-performance liquid chromatography (HPLC) systems were used to measure furosine, pentosidine, and CML. Protein from the combined pepsin-soluble and pepsin-digested insoluble collagen hydrolysates were used for each measurement.
Furosine detection was carried out using a method modified from Resmini et al. (16,17). Equal amounts of protein (214 μg) from each sample were injected onto a reverse-phase column (Alltech specfuro; Sedriano, MI) that was attached to a Waters HPLC system (Milford, MA). Buffer A contained 0.4% acetic acid (vol/vol). Buffer B was the same as buffer A with 0.27% KCl (wt/vol). Initially, buffer A was run isocratically for 12.5 min, then by a gradient from 0 to 10% buffer B for 7 min, and finally isocratically at 10% buffer B for a further 15.5 min. The elution of furosine was monitored on a Waters absorbance detector model 486 (A280). The absorbance detector was interfaced to a computer to monitor furosine peak integration using Borwin chromatography software (Advanced Data Solutions, San Jose, CA). Furosine eluted at ~28 min.
For the detection of pentosidine by HPLC, equal amounts of protein (230 μg) from each sample were injected onto a reverse-phase column (Vydac 218TP104; Hesperia, CA). Buffer A contained heptafluorobutyric acid (0.01 mol/l; Sigma H-7133). Buffer B contained 60% acetonitrile (vol/vol) (Fisher A994SK-4) and heptafluorobutyric acid (0.01 mol/l). Initially, 2% buffer B was run isocratically for 10 min, followed by a gradient from 2 to 30% buffer B for 30 min, and then at 30% buffer B isocratically for 15 min. Elution of pentosidine was detected by fluorescence at 335/385 nm excitation/emission wavelength with a spectrofluorometer (Jasco 821-FP; Easton, MD) interfaced to a computer for peak integration. Pentosidine eluted at ~48 min.
For the detection of CML, two HPLC systems were used in sequence. CML was collected from the same HPLC system and hydrolysate injections that were used for the pentosidine determination. The retention time was ascertained by injection of a standard sample of CML detected by postcolumn derivatization with o-phthaldialdehyde (Aldrich, Milwaukee, WI) in the presence of 2-mercaptoethanol at 340/455 nm excitation/emission wavelength (18). Standard CML eluted at ~30.5 min. From the sample injections without postcolumn derivatization, CML was collected from 28 to 33 min and the solvent evaporated with a Savant concentrator. Each pellet was resuspended in distilled water (200 μl), and equal amounts were reinjected (50 μl) onto a reverse-phase column (Vydac 218TP54). Buffer A contained 5% 1-propanol (vol/vol) (Sigma 29,328-8), 3 g/l SDS (Fluka 71725, Ronkokoma, NY), and monobasic sodium phosphate (1 g/l) (Mallinckrodt 7892, Phillipsburg, NJ). Buffer B contained 60% 1-propanol (vol/vol) with the same amount of SDS and monobasic sodium phosphate as buffer A. Solvent flow consisted of a gradient from 15 to 22% buffer B for 30 min. CML eluted at ~28 min.
Relative fluorescence
Relative fluorescence was determined by diluting 60 μl of each sample with 2.0 ml of distilled water. Fluorescence was measured with a quartz cuvette (12.5 mm; Fisher 14-385-918) at 370/440 nm excitation/emission wavelength 370 nm with an Aminco-Bowman (Spectronic Instruments, Rochester, NY) spectrofluorometer. A control was used as a blank to subtract background levels of pepsin fluorescence.
Assessment of complications
Indices of retinopathy, nephropathy, and neuropathy used in prior DCCT analyses were quantitated as previously described (2,19-21). A sustained three-step change in Early Treatment of Diabetic Retinopathy Scale (ETDRS) score was the principal retinopathy outcome event in the DCCT; the sustained presence of three or more microaneurysms was an outcome event specific to the primary prevention cohort. The nephropathic outcome event was microalbuminuria, i.e., AER >40 mg/24 h. Confirmed clinical neuropathy, as previously defined (1), was an outcome event, while motor nerve conduction velocity was a continuous neuropathy variable. The rate of change of retinopathy was expressed as (ETDRS score at DCCT closeout – EDTRS score at DCCT baseline) ÷ years of follow-up. Rate of change in albumin excretion was similarly calculated as (AER at closeout – AER at baseline) ÷ years of follow-up. HbA1c was measured as previously reported (13,22).
Statistical methods
Characteristics of study participants and nonparticipants
The Wilcoxon rank-sum test (23) was used to compare the distributions of quantitative and ordinal characteristics between two independent groups.
Relationship of skin collagen variables with age and diabetes duration
Spearman rank-order correlations (23) were calculated to evaluate the relationship among pairs of quantitative or ordinal variables. Covariance adjustment for age and diabetes duration was performed by analysis of residuals from a simple linear model. To adjust for the differences between treatment groups, fractional ranks of the skin collagen levels were obtained separately within the intensive and conventional treatment groups.
Comparison of skin collagen variables between participants with and without complications
Differences in the skin collagen variables, covariance adjusted for age and duration of diabetes, between study participants with and without complications were assessed by a two-factor unbalanced analysis of variance (24) using type III sums-of-squares and partial F test with treatment group and complication status as the classification variables. Thus, the test of complication status provides a treatment group–adjusted test of the difference in collagen variables of those with and without complications.
Association of collagen variables with HbA1c at various time points relative to biopsy date
Univariate regression models (25) were used to determine the relationship between the age- and duration of diabetes– adjusted skin collagen variables and HbA1c at various time points relative to the date of biopsy. Each of the six collagen variables was analyzed against HbA1c levels at DCCT screening, HbA1c closest to the date of biopsy, the mean HbA1c over the period from the DCCT randomization to the date of the biopsy, and the mean HbA1c during the year before the biopsy. Linear and quadratic HbA1c effects were included, and the quadratic effect was dropped when not significant. The strength of the relationship of the collagen variables with the various HbA1c measures is assessed using the model R2.
Treatment group by collagen interaction terms (linear and possibly quadratic) were added to the model to evaluate whether the association of the collagen variables with complications was different in the intensive and conventional treatment groups. Difference between the two treatment groups in the association of collagens with complications was assessed using a likelihood ratio test (on df ≥ 6) of significance of the interaction terms.
Redundancy among collagen variables
For all seven collagen variables (acid-soluble, pepsin-soluble, pepsin-digested insoluble collagen, CML, pentosidine, fluorescence, and furosine), the variance-covariance matrices were significantly different (P < 0.01) between the intensive and conventional treatment groups, using a χ2 statistic (on 28 df) (26). Thus, principal components analyses (26) were conducted separately within each treatment group in order to eliminate variables that were linear functions of the others, i.e., redundant. The variance-covariance matrix is nearly singular in each treatment group, with the dominant linear redundancy occurring between pepsin-soluble and pepsin-insoluble collagen. This redundancy is an artifact of the assay, since the sum of acid-soluble, pepsin-soluble, and pepsin-insoluble collagen is ~100%. Pepsin-insoluble collagen was excluded from subsequent analyses because it has a greater coefficient of multiple determination (R2 > 0.98) with the other six collagen variables compared with pepsin-soluble collagen.
Association of collagen variables with complications
Associations of skin collagen variables with the prevalence of complication outcomes were assessed using logistic regression models (27), while associations with rates of worsening of complications were evaluated using linear regression models.
Significance levels
Unless otherwise noted, nominal Pvalues are presented without adjustment for multiple tests. To adjust for tests, the Bonferroni correction was used, for which P < α/κ is required for significance.
RESULTS
Do age and diabetes duration affect the collagen variables?
The relationship between each collagen variable and the age of the subjects is shown in Fig. 2 and Table 2. As demonstrated in previous studies, furosine, pentosidine, CML, and relative fluorescence were positively associated with age, and acid and pepsin solubility of skin collagen were inversely correlated with age in nondiabetic and diabetic subjects (5,14). The only exception was furosine, which had no significant correlation with age in the diabetic subjects (Table 2). Duration of diabetes was similarly related with the skin collagen variables, except for acid-soluble collagen and furosine, which were not significantly correlated with duration (Table 2).
FIG. 2.
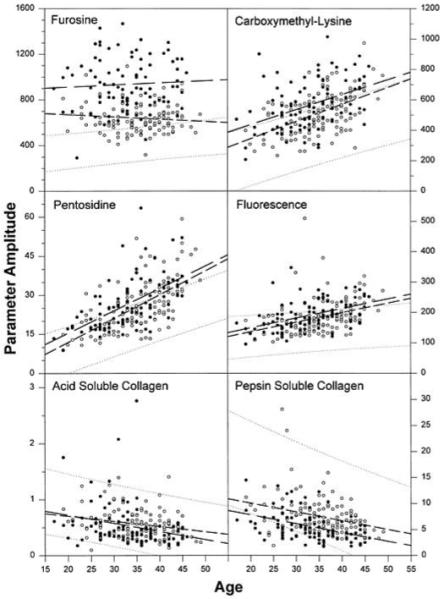
Correlation between collagen variables and age of study participants at the time of the skin biopsy. The 95% CI for nondiabetic control subjects is indicated with punctuated lines. ●, conventional treatment group; ○, intensive treatment group. Regression lines are indicated by dashed lines for both treatment groups, with longer dashes for the conventional group. Units are picomoles per milligram collagen for furosine, CML, and pentosidine; arbitrary units for fluorescence; and percent of total collagen for acid- and pepsin-soluble collagen.
TABLE 2.
Spearman correlations among age- and duration-adjusted skin collagen levels (combined groups)
| Attained | Age (years) | Duration (months) | Acid-soluble | Pepsin-soluble | Fluorescence | Furosine | Pentosidine |
|---|---|---|---|---|---|---|---|
| Acid-soluble (%) | −0.289 (<0.001) | −0.020 (NS) | — | ||||
| Pepsin-soluble (%) | −0.357 (<0.001) | −0.279 (<0.001) | 0.674 (<0.001) | — | |||
| Relative fluorescence | 0.489 (<0.001) | 0.411 (<0.001) | −0.183 (0.007) | −0.333 (<0.001) | — | ||
| Furosine (pmol/mg) | −0.012 (NS) | 0.106 (NS) | −0.183 (0.007) | −0.370 (<0.001) | 0.296 (<0.001) | — | |
| Pentosidine (pmol/mg) | 0.627 (<0.001) | 0.560 (<0.001) | −0.298 (<0.001) | −0.595 (<0.001) | 0.450 (<0.001) | 0.335 (<0.001) | — |
| CML (pmol/mg) | 0.483 (<0.001) | 0.406 (<0.001) | −0.290 (<0.001) | −0.521 (<0.001) | 0.404 (<0.001) | 0.320 (<0.001) | 0.588 (<0.001) |
Correlations are computed based on the within-treatment group fractional ranks of the collagens. Correlations (of fractional ranks) of age and duration are made versus unadjusted skin collagens. Numbers in parentheses are P values for testing the significance of the correlation coefficients.
In Fig. 2, the 95% CIs obtained from regressions of skin collagen variables of nondiabetic control subjects with their age are shown by dotted lines. The Spearman correlations and the tests of their significance are shown in Table 2. Similar regressions were performed in diabetic subjects, and the slopes of regression lines of CML, pentosidine, and fluorescence were steeper than the comparable slopes in age-matched control subjects (P < 0.01), indicating that these variables increase at a greater rate in type 1 diabetic patients than in nondiabetic individuals. The fairly horizontal regression lines of furosine suggest that collagen glycation (furosine) is not correlated with age and represents a steady state between synthesis and degradation as previously proposed (9). Pentosidine, CML, and relative fluorescence were also positively associated with diabetes duration, whereas pepsin-soluble and acid-soluble collagen are inversely related to duration (Table 2).
In the diabetic subjects, the collagen variables, adjusted for age and duration, were highly correlated with each other (Table 3). Pepsin-soluble and acid-soluble collagens showed the strongest association with each other, followed by pentosidine associations with CML, pepsin-soluble collagen (inverse), and fluorescence. CML and pepsin-soluble collagens were also strongly associated with each other.
TABLE 3.
Univariate regression models of age- and duration-adjusted skin collagen variables versus HbAlc
| Collagen versus HbA1c | R2 (%) | P |
|---|---|---|
| Acid-soluble (%) versus | ||
| Mean HbA1c up to biopsy† | 4.2 | <0.011 |
| Mean HbA1c over the past year† | 2.4 | <0.078 |
| HbA1c nearest to biopsy | 2.0 | <0.041 |
| Screening HbA1c | 0.4 | NS* |
| Pepsin-soluble (%) versus | ||
| Mean HbA1c up to biopsy† | 15.0 | <0.001 |
| Mean HbA1c over the past year | 16.4 | <0.001 |
| HbA1c nearest to biopsy | 17.0 | <0.001 |
| Screening HbA1c | 0.4 | NS* |
| Relative fluorescence versus | ||
| Mean HbA1c up to biopsy | 6.5 | <0.001 |
| Mean HbA1c over the past year | 6.6 | <0.001 |
| HbA1c nearest to biopsy | 5.5 | <0.001 |
| Screening HbA1c | 3.1 | <0.010 |
| Furosine (pmol/mg) versus | ||
| Mean HbA1c up to biopsy | 19.4 | <0.001 |
| Mean HbA1c over the past year | 72.3 | <0.001 |
| HbA1c nearest to biopsy | 69.4 | <0.001 |
| Screening HbA1c | 3.0 | <0.012 |
| Pentosidine (pmol/mg) versus | ||
| Mean HbA1c up to biopsy | 8.9 | <0.001 |
| Mean HbA1c over the past year | 9.6 | <0.001 |
| HbA1c nearest to biopsy | 9.6 | <0.001 |
| Screening HbA1c | 1.7 | <0.058 |
| CML (pmol/mg) versus | ||
| Mean HbA1c up to biopsy | 16.1 | <0.001 |
| Mean HbA1c over the past year | 14.4 | <0.001 |
| HbA1c nearest to biopsy | 13.8 | <0.001 |
| Screening HbA1c | 2.6 | <0.020 |
HbA1c effect not significant.
Based on a regression model with significant quadratic HbA1c effect.
Do long-term intensive treatment and conventional treatment result in differences in collagen variables?
The comparative effects of intensive versus conventional therapy on the age- and diabetes duration–adjusted collagen variables are shown in Fig. 3. In the primary prevention cohort, long-term intensive therapy was associated with significantly lower levels of furosine, pentosidine, CML, and fluorescence and higher levels of acid-soluble and pepsin-soluble collagens (P < 0.006, all significant at α = 0.05 for six tests) compared with conventional therapy. In the secondary intervention cohort, intensive therapy was associated with significantly lower levels of furosine, fluorescence, and pentosidine and with higher levels of pepsin-soluble collagens (P < 0.003, significant at α = 0.05 for six tests). However, the levels of skin collagen variables associated with intensive therapy remained abnormal by comparison with the mean levels of the age-matched control subjects.
FIG. 3.
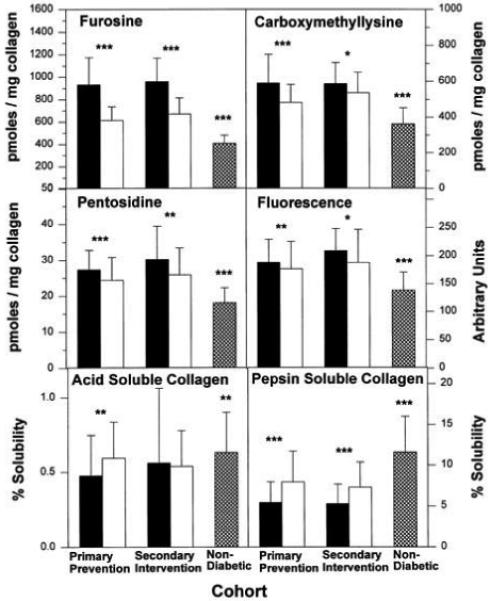
Effect of long-term glycemic control on diabetes- and duration-adjusted means ± SD values of skin collagen glycation (furosine in picomoles per milligram collagen), glycoxidation (CML and pentosidine, both in picomoles per milligram collagen), fluorescence (in arbitrary units per milligram collagen), and crosslinking (acid- and pepsin-soluble collagen in percent) adjusted for covariance with age and diabetes duration. ■, conventional treatment group; □, intensive treatment group;  , nondiabetic age-matched control subjects. For mean values *P < 0.05, **P < 0.01, ***P < 0.001 comparing intensive vs. conventional and nondiabetic vs. intensive treatment groups.
, nondiabetic age-matched control subjects. For mean values *P < 0.05, **P < 0.01, ***P < 0.001 comparing intensive vs. conventional and nondiabetic vs. intensive treatment groups.
Is there a temporal relationship between collagen variables and HbA1c?
The relationship between collagen variables and cumulative glycemia (HbA1c) was assessed by performing univariate regression analyses on each of the collagen variables, adjusting for age, duration, and treatment group, with HbA1c measurements taken at various time points relative to the skin biopsy date. AGEs might be expected to correlate best with HbA1c values that had been averaged over long periods of time as opposed to a single measurement at the time of tissue sampling. HbA1c values that were chosen included the single screening value at DCCT baseline, the value nearest the biopsy date, the mean cumulative HbA1c value from DCCT randomization to biopsy, and the mean HbA1c over the 12 months preceding the biopsy (Table 3). With few exceptions, all the age-adjusted collagen variables were significantly correlated with the various HbA1c measures. Not surprisingly, the weakest, and for some collagen variables insignificant, correlations were noted with the screening HbA1c obtained on average 6 years earlier. The strongest relationships (R2 ~70%) were observed between furosine and the HbA1c value over the preceding 1 year and that closest to biopsy, higher than the R2 values of any other collagen variable. This befits furosine’s position as reflecting directly glycosylation of the collagen molecule, i.e., glycemic exposure at the tissue level. The other variables correlated similarly, although less strongly, with the mean HbA1c over 1 year before biopsy, the mean HbA1c over the entire 6 years of DCCT treatment, or with HbA1c values closest to biopsy.
Is there a quantitative relationship between diabetic complications and collagen variables with and without adjustment for HbA1c?
Nine individual retinopathy, nephropathy, and neuropathy outcomes used in prior DCCT analyses were each modeled for their association with and potential dependence on the full panel of six collagen variables simultaneously. The purpose of this analysis was to determine whether there was any possibility that the levels of collagen variables could be used to predict the risk of diabetic complications.
Table 4 shows the complications outcomes used as dependent variables in the statistical modeling and their prevalences. In the primary prevention cohort, the lower prevalences at DCCT closeout in the intensively treated than conventionally treated subjects mirrored the results seen in the entire DCCT cohort analyses. In the secondary intervention cohort, there were no differences in the prevalences of the retinopathic and nephropathic outcomes between the two treatment groups. This observation was discordant with the overall DCCT results. It could be partly explained by the lower entry HbA1c level of this conventional group, since the risk of retinopathywas related to the initial HbA1c in the DCCT (13), or it could be due to an unknown self-selection factor.
TABLE 4.
Prevalence and severity of complications at DCCT closeout in the skin biopsy cohort
| Primary cohort |
Secondary cohort |
|||
|---|---|---|---|---|
| Intensive | Conventional | Intensive | Conventional | |
| n | 65 | 58 | 57 | 36 |
| Retinopathy | ||||
| Sustained ≥3-step progression | 4 (6) | 10 (17) | 7 (12) | 4 (11) |
| Sustained ≥3 microaneurysms | 12 (18) | 19 (33) | 50 (88) | 32 (89) |
| Rate of change of ETDRS | 0.13 ± 0.03 | 0.27 ± 0.04 | 0.11 ± 0.03 | 0.16 ± 0.06 |
| Nephropathy | ||||
| AER >40 (mg/24 h) | 2 (3) | 6 (10) | 8 (14) | 4 (11) |
| Rate of change of AER | 0.54 ± 0.90 | 0.89 ± 0.62 | 3.91 ± 4.10 | 1.38 ± 1.23 |
| Neuropathy | ||||
| n | 44 | 42 | 55 | 35 |
| Confirmed clinical neuropathy | 1 (2) | 2 (5) | 7 (13) | 6 (17) |
| Median NCV (m/s) | 55.9 ± 0.5 | 53.4 ± 0.6 | 53.4 ± 0.5 | 51.0 ± 0.9 |
Data are n (%) or means ± SD of (closeout – baseline)/length of follow-up. Values in the table represent the number of subjects with complications. Numbers in parentheses represent the proportion with complications. Sustained 3 microaneurysms includes the primary cohort only. Confirmed clinical neuropathy is defined as an abnormal neurological examination that was consistent with the presence of peripheral sensorimotor neuropathy plus either abnormal nerve conduction in at least two peripheral nerves or unequivocally abnormal autonomic nerve setting (at 5 years). NCV, nerve conduction velocity.
For each of the two treatment groups, the risk of each complication outcome was modeled by multiple logistic regression against the six collagen variables using a two-step process seeking to identify significant individual collagen variables. First, a 12-parameter model using linear and quadratic terms of the six collagen variables was fit separately for the intensive and conventional treatment groups. Quadratic terms that were nominally significant at the 0.10 level based on the Wald χ2 test were retained, together with the linear terms. Then, a model with the linear and nominally significant quadratic terms was fit; the models for the intensive and conventional treatment groups differed significantly in most cases and the data were therefore not combined. The significance of each set of collagen coefficients was assessed using a likelihood ratio test. Additional models were adjusted for HbA1c by including screening HbA1c, HbA1c nearest to biopsy, and the mean HbA1c from randomization to biopsy. Likelihood ratio tests (on df ≥ 6) of the significance of each set of collagen variables were again performed. Models for nephropathy were also adjusted for the log baseline AER and only for the mean HbA1c up to the time of biopsy, because the other two HbA1c variables do not add significantly to the models after adjusting for the baseline AER. For purposes of comparison, a logistics regression model was also constructed in which the association of each complication outcome in Table 4 with only the three HbA1c terms described above was assessed.
When the collagen variables were treated statistically as a group of independent variables, the prevalence of various complication outcomes was significantly associated with the full set of age- and duration-adjusted skin collagen variables (Table 5). The results are presented separately for each treatment group. The explained variances (R2) in the relationship between the prevalence of complications and the collagen variables were often greater in the conventionally treated subjects (up to 51% unadjusted) compared with the intensively treated subjects (up to 36% unadjusted). Models that adjusted for screening HbA1c, HbA1c nearest the biopsy date, and mean HbA1c from randomization to biopsy date were then tested. These analyses revealed that in spite of the adjustment for HbA1c, significant associations remained between sustained ≥3 microaneurysms, clinical neuropathy, and median nerve conduction velocity and the collagen variables in both treatment groups. Similar associations remained between AER and rate of change of AER and the collagen variables in the conventional treatment group. In several instances, particularly in the conventional treatment group, the adjustment for HbA1c led to little loss of explained variance (e.g., sustained ≥3 microaneurisms, R2 = 47% without and 43% after HbA1c adjustment). Moreover, the variance in complications explained by the collagen variables after adjustment for HbA1c was comparable to or greater than the variance explained by HbA1c itself (Table 5).
TABLE 5.
Likelihood ratio test of significance of HbA1c, collagen variables, and collagen variables after adjusting for HbA1c
| Intensive treatment |
Conventional treatment |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HbA1c |
Collagen variables |
Collagen variables after adjusting for HbA1c |
HbA1c |
Collagen variables |
Collagen variables after adjusting for HbA1c |
|||||||||||||
| df | R2 | P | df | R2 | P | df | R2 | P | df | R2 | P | df | R2 | P | df | R2 | P | |
| Retinopathy | ||||||||||||||||||
| Sustained ≤3-step progression | 3 | 17 | <0.011 | 6 | 28 | <0.005 | 6 | 15 | NS | 3 | 13 | <0.024 | 6 | 7 | NS | 6 | 5 | NS |
| Sustained ≤3 microaneurysms | 3 | 37 | <0.001 | 6 | 26 | <0.019 | 6 | 33 | <0.005 | 3 | 14 | <0.023 | 10 | 47 | <0.001 | 10 | 43 | <0.001 |
| Rate of change of ETDRS | 3 | 27 | <0.001 | 7 | 19 | <0.002 | 7 | 10 | <0.030 | 3 | 24 | <0.001 | 6 | 14 | <0.040 | 6 | 7 | NS |
| Nephropathy | ||||||||||||||||||
| AER >40 (mg/24 h) | 2 | 55 | <0.001 | 7 | 23 | NS | 7 | 6 | NS | 2 | 10 | <0.037 | 7 | 40 | <0.001 | 7 | 32 | <0.006 |
| Rate of change of AER | 2 | 5 | NS | 7 | 10 | NS | 7 | 7 | NS | 2 | 4 | NS | 6 | 20 | <0.005 | 6 | 16 | <0.019 |
| Neuropathy | ||||||||||||||||||
| Confirmed clinical neuropathy | 3 | 7 | NS | 6 | 36 | <0.004 | 6 | 37 | <0.003 | 3 | 5 | NS | 6 | 51 | <0.001 | 6 | 67 | <0.001 |
| Median NCV (m/s) | 3 | 4 | NS | 8 | 16 | <0.013 | 8 | 14 | <0.034 | 3 | 18 | <0.001 | 7 | 31 | <0.001 | 7 | 18 | <0.004 |
df = 6 corresponds to a model with six linear (collagen) effects. df > 6 corresponds to a model with six linear and (df – 6) quadratic effect(s). Sustained ≥3 microaneurysms includes the primary cohort only. Rate of change is (closeout – baseline)/length of follow-up. Confirmed clinical neuropathy is defined as an abnormal neurological examination that was consistent with the presence of peripheral sensorimotor neuropathy plus either abnormal nerve conduction in at least two peripheral nerves or unequivocally abnormal autonomic nerve setting (at 5 years). NCV, nerve conduction velocity.
Can any single collagen variable or subgroup of variables substitute for the full set of collagen variables?
Having found strong associations between the prevalence of complications and the collagen variables as a group, it was of interest to investigate which collagen variables contributed significantly to these associations. The purpose of this exploratory analysis was to determine if any one or a small subgroup of the collagen variables could serve as a surrogate(s) for the complete set of six. This was accomplished by performing a backwards elimination procedure on the six linear collagen variables. Any quadratic term that had been identified as nominally significant in the full model without adjusting for HbA1c was included. All the linear and quadratic terms that were nominally significant at the 0.05 level based on the Wald χ2 test were retained in this final model. The strength of association of complications with these individual collagen variables was evaluated using the R2 from these logistic regression models.
The results of this analysis are summarized as a matrix in Table 6. In the conventional treatment group, furosine was the collagen variable with which the presence or prior rate of progression of at least one expression of retinopathy, nephropathy, and neuropathy was most frequently associated. In addition, worsening of AER and confirmed clinical neuropathy were associated with pentosidine, while the appearance of retinopathy (≥3 microaneurysms) and confirmed clinical neuropathy were associated with CML. In the intensive treatment group, acid-soluble collagen was the variable with which some expression of each complication was most frequently associated. In both treatment groups, virtually all the collagen variables contributed significantly to the neuropathy outcomes.
TABLE 6.
Summary of age and duration-adjusted collagen variables that have nominally significant association (P < 0.05) with complications of diabetes in the reduced model (unadjusted for HbA1c)
| Model R2 (%) |
Significant collagen predictors |
|||||||
|---|---|---|---|---|---|---|---|---|
| Complication | Full | Reduced | Acid-soluble | Pepsin-soluble | Fluorescence | Furosine | Pentosidine | CML |
| Intensive treatment | ||||||||
| Retinopathy | ||||||||
| Sustained ≥3-step progression | 28 | 20 | ■ | |||||
| Sustained ≥3 microaneurysms | 26 | 18 | ■ | |||||
| Rate of change of ETDRS | 19 | 14 | ■ † | |||||
| Nephropathy | ||||||||
| AER >40 mg/24 h | 23 | 10 | ■ | |||||
| Rate of change of AER | 10 | 8 | ■ † | |||||
| Neuropathy | ||||||||
| Confirmed clinical neuropathy | 36 | 33 | ■ | ■ | ■ | ■ | ■ | |
| Median NCV (m/s) | 16 | 11 | ■ | ■ † | ■ | |||
| Conventional treatment | ||||||||
| Retinopathy | ||||||||
| Sustained ≥3-step progression | 7 | NS* | ||||||
| Sustained ≥3 microaneurysms | 47 | 30 | ■ † | ■ | ||||
| Rate of change of ETDRS | 14 | 7 | ||||||
| Nephropathy | ||||||||
| AER >40 mg/24 h | 40 | 27 | ■ | |||||
| Rate of change of AER | 20 | 18 | ■ | ■ | ||||
| Neuropathy | ||||||||
| Confirmed clinical neuropathy | 51 | 50 | ■ | ■ | ■ | ■ | ■ | |
| Median NCV (m/s) | 31 | 31 | ■ | ■ † | ■ | |||
Columns marked with blocks indicate collagen parameters that remain significant (P < 0.05) after a backwards elimination procedure. Sustained ≥3 microaneurysms includes the primary cohort only. Rate of change is (closeout – baseline)/length of follow-up. Confirmed clinical neuropathy is defined as an abnormal neurological examination that was consistent with the presence of peripheral sensorimotor neuropathy plus either abnormal nerve conduction in at least two peripheral nerves or unequivocally abnormal autonomic nerve setting (at 5 years).
No significant collagen parameter remained in the model after a backwards elimination procedure.
Quadratic effect significant at P < 0.05. NCV, nerve conduction velocity.
The strength of association of complications with the unadjusted collagen variables, either individually or as subgroups (reduced model) or as the whole group (full model), was indicated by the model R2 (Table 6). In the intensive group, the reduced model R2’s are similar to or only slightly lower than the full model R2’s, indicating that the strength of association of the various complications outcomes with individual or subgroups of collagens is comparable to that with the full set of collagen variables. On the other hand, in the conventional treatment group, the reduced R2 values are considerably lower than the full model R2 values for retinopathy outcomes, indicating that the strength of association of these outcomes with particular collagen variables is weaker compared with the association with the full set of collagen variables. In both treatment groups, for neuropathy outcomes, there is little difference in R2’s between full and reduced model collagen variables, but most of the latter remained significant in the reduced model.
DISCUSSION
The DCCT offered an opportunity to assess skin collagen abnormalities in a large group of patients with type 1 diabetes who had been exceptionally well characterized both with regard to their glycemic control and the status of retinopathy, nephropathy, and neuropathy over the preceding 5- to 6-year period.
The first and most readily apparent finding is that long-term intensive treatment of hyperglycemia, as compared with conventional treatment, is associated with lower levels of both early and advanced glycation products in skin collagen. This was also the first demonstration that the physicochemical characteristics of skin collagen, as assessed by its pepsin and acid solubilities, are also less abnormal in long-term intensively treated subjects. Thus, intensive treatment leads to lower levels of skin collagen AGEs in parallel with a reduction in HbA1c and in the risks of retinopathy, nephropathy, and neuropathy (2).
The magnitude of the effect of improved glycemic control on glycated collagen (furosine, i.e., Amadori product) was similar in the primary prevention and secondary intervention cohorts, despite the differences in diabetes duration and initial complications status of these subjects. In contrast, with the exception of pentosidine and relative fluorescence, the effects of intensive versus conventional therapy on the late products of the Maillard reaction and the collagen crosslinks (Fig. 1) were slightly greater in primary prevention subjects who had shorter diabetes durations and had essentially no detectable complications at study onset. Pretreatment DCCT baseline levels of the collagen variables were not measured. Therefore, we cannot know whether the lower levels of slowly turning over glycooxidation products and crosslinking resulted from an actual decrease from baseline levels or resulted from a slower rate of age-related accumulation in the intensively treated subjects. The average age-related increases of pentosidine and CML over these subjects’ age span during the DCCT (Fig. 2) suggest that at least some of the intensive treatment effect can be accounted for by slower accumulation. In contrast, the effect of intensive treatment on the levels of glycated collagen (the Amadori product furosine), which is reversible, is not related to age (Fig. 2) and likely reflects decreased formation of furosine because of less hyperglycemia. Because it is likely that those glycation sites on collagen that are reversible are unable to be converted to advanced reaction products (28), the pathological significance of reduced furosine levels is unclear.
The second finding of clinical importance is that quantitation of both early and advanced glycation products in skin provides a biological index of prior glycemic status over a considerable period of time. In particular, quantitation of collagen-bound glucose (furosine) provides very significant information on cumulative glycemia for at least 1 year before its measurement; CML, pentosidine, and pepsin-soluble collagen provide some information over several years but reflect glycemia less strongly (Table 3). Somewhat unexpected, however, is the fact that in univariate analyses, R2 values (strength of correlation with HbA1c) are the highest for glycated collagen when compared with the values for pentosidine and CML at every analyzed time point during the DCCT (Table 2). This suggests that in spite of their reversibility, glycated residues in collagen have the surprising ability to reflect rather long-term cumulative glycemia, although with diminishing strength as time goes on (Table 3). In view of the reversibility of the reaction that forms the Amadori product, a likely explanation for this observation is that steady-state levels of glycemia tend to change little within an individual over very long periods of time, at least in a research setting of glycemic therapy. Yet another explanation for the fact that the correlations with mean HbA1c levels of AGEs (CML, pentosidine) are weaker than those of collagen glycation (furosine) is that AGEs are not simply a reflection of mean glycemia, but that their formation rate is modulated by tissue levels of catalytic and inhibitory factors, such as transition metals and antioxidants (14). We also now know that CML can originate not only through glycoxidation but also through lipoxidation (29).
Previous studies have demonstrated higher skin levels of AGEs in patients with diabetic complications versus those without complications (4-8). However, the reverse question was not addressed, i.e., whether complications status as the dependent variable could be correlated with the levels of skin furosine and AGEs as the independent variables, and whether such correlations are independent of HbA1c levels. The latter point is crucial because HbA1c itself predicts the risk of developing complications (13,30). Therefore, we performed exploratory analyses of our data separately in each DCCT treatment group. These analyses demonstrated correlations with R2’s as high as 67% between prevalence of retinopathy, nephropathy, and neuropathy, expressed as various outcomes, and collagen glycation products; furthermore, these correlations were often independent of HbA1c and virtually as strong or stronger after this adjustment (Table 8). This finding could implicate one or more of these products in mechanisms of tissue damage that may operate beyond the initial step of protein glycosylation. These correlations also tended to be stronger in the conventional compared with the intensive treatment group, perhaps because prior conventional treatment during the DCCT had little impact on the levels of these accumulating collagen AGEs. These results at least raise the possibility that determination of skin collagen glycation and AGEs could be used to evaluate the future risk of developing complications or their severity (7). In this regard, serum AGE levels have also been proposed as a predictor of future pathological changes in the kidneys of subjects with type 1 diabetes (31).
The outcome “sustained ≥3 microaneurysms,” a reliable indicator of the first appearance of retinopathy—and therefore only analyzable in the primary prevention cohort—was especially consistent and relatively strong (R2 = 43% after adjustment for HbA1c in the conventional treatment group) in its relationship to collagen variables (Table 5). This observation suggests that any skin collagen index of risk would be especially useful early in type 1 diabetes. It is also noteworthy that in both treatment groups, each of the two neuropathy outcome measures used by the DCCT, one primarily clinical and one electro-physiological, correlated strongly in multivariate HbA1c-adjusted analyses, but the model required virtually the entire array of measured skin collagen variables (50% variance explained in conventional, and 33% in intensive treatment groups, respectively) (Table 6). This relationship between neuropathy and AGEs parallels some experimental data (32).
A practical clinical question is whether measurement of skin collagen variables would yield additional useful information to that provided by HbA1c, as to the individual’s risk of developing retinopathy, nephropathy, and neuropathy. In the conventional treatment group, which is more representative of the natural history of type 1 diabetes, the variable that stands out in the multivariate analyses is furosine (glycated collagen). The levels of furosine contributed significantly to the correlations between almost all the DCCT indices of retinopathy, nephropathy, and neuropathy and the full panel of collagen variables. However, our data are cross-sectional and only consistent with the possibility that furosine would add predictive value to that of HbA1c. This possibility can only be tested in a prospective study.
Fortunately, in the Epidemiology of Diabetes Interventions and Complications Study, virtually the entire DCCT cohort of patients is continuing to be monitored for these complications as well as for glycemic control with the same DCCT methods. At the time of their skin biopsy, a number of subjects had no retinopathy and/or no microalbuminuria. Thus, in these individuals it should be possible to correlate the future development of these complications with the present levels of skin collagen variables and with appropriate adjustment for covariates such as HbA1c. If a reliable skin collagen predictor emerges, it could identify subjects who are particularly in need of intensive glycemic control or other anticomplication therapy, and those at very low risk of complications could be spared the rigors and risks of intensive therapy of type 1 diabetes.
ACKNOWLEDGMENTS
This study was supported by a grant from the American Diabetes Association and grants from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK UO1 AM 30628), the National Institute of Aging (AG 05601 and AG 11080), and the participating Clinical Research Center at University Hospitals of Cleveland (MO1 RR00080), Massachusetts General Hospital (MO1 RR01066), Albert Einstein College of Medicine (RR12248), Mayo Clinic (RR01066), University of California San Diego (RR00827), University of Iowa (RR00059), and Washington University (RR00036). We thank Dr. David Nathan for his critical review of the manuscript.
Glossary
- AER
albumin excretion rate
- AGE
advanced glycation end product
- CML
N∊-(carboxymethyl)-lysine
- DCCT
Diabetes Control and Complications Trial
- ETDRS
Early Treatment of Diabetic Retinopathy Scale
- HPLC
high-performance liquid chromatography
APPENDIX: THE DCCT SKIN COLLAGEN ANCILLARY STUDY GROUP
Albert Einstein College of Medicine, Bronx, NY: H. Shamoon and H. Duffy; Case Western Reserve University, Cleveland, OH: S. Genuth and E. Brown; Massachusetts General Hospital, Boston, MA: D. Nathan and M. Larkin; Mayo Foundation, Rochester, MN: F.J. Service and A.L. Schmidt; University of California, San Diego, CA: O. Kolterman and G. Lorenzi; University of Iowa, Iowa City, IA: W. Sivitz, R. Zeitler, and M. Bayless; University of South Florida, Tampa, FL: J. Malone and N. Grove; Washington University, St. Louis, MO: J. Santiago†, J. McGill, and L. Levandoski.
Footnotes
Deceased.
REFERENCES
- 1.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310:341–346. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial (DCCT) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biological, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- 4.Monnier VM, Vishwanath V, Frank KE, Elmets CA, Dauchot P, Kohn RR. Relation between complications of type 1 diabetes mellitus and collagen-linked fluorescence. N Engl J Med. 1986;314:403–408. doi: 10.1056/NEJM198602133140702. [DOI] [PubMed] [Google Scholar]
- 5.Sell DR, Lapolla A, Odetti P, Fogarty J, Monnier VM. Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes. 1992;41:1286–1292. doi: 10.2337/diab.41.10.1286. [DOI] [PubMed] [Google Scholar]
- 6.Beisswenger PJ, Moore LL, Curphey TJ. Relationship between glycemic control and collagen-linked advanced glycosylation end products in type I diabetes. Diabetes Care. 1993;16:689–694. doi: 10.2337/diacare.16.5.689. [DOI] [PubMed] [Google Scholar]
- 7.Beisswenger P, Makita Z, Curphey TJ, Moore LL, Jean S, Brinck-Johnsen T, Bucala R, Vlassara H. Formation of immunochemical advanced glycosylation end products precedes and correlates with early manifestations of renal and retinal disease in diabetes. Diabetes. 1995;44:824–829. doi: 10.2337/diab.44.7.824. [DOI] [PubMed] [Google Scholar]
- 8.McCance DR, Dyer DG, Dunn JA, Bailie KE, Thorpe SR, Baynes JW, Lyons TJ. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J Clin Invest. 1993;91:2470–2478. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyons TJ, Bailie KE, Dyer DG, Dunn JA, Baynes JW. Decrease in skin collagen glycation with improved glycemic control in patients with insulin-dependent diabetes mellitus. J Clin Invest. 1991;87:1910–1915. doi: 10.1172/JCI115216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salmela PI, Oikarinen AI, Ukkola O, Karjalainen A, Linnaluoto M, Puukka R, Ryhanen L. Improved metabolic control in patients with non-insulin-dependent diabetes mellitus is associated with a slower accumulation of glycation products in collagen. Eur J Clin Invest. 1995;25:494–500. doi: 10.1111/j.1365-2362.1995.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 11.Vishwanath V, Frank KE, Elmets CA, Dauchot PJ, Monnier VM. Glycation of skin collagen in type I diabetes mellitus: correlation with long-term complications. Diabetes. 1986;35:916–921. doi: 10.2337/diab.35.8.916. [DOI] [PubMed] [Google Scholar]
- 12.Buckingham B, Reiser KM. Relationship between the content of lysyl oxidase-dependent cross-links in skin collagen, nonenzymatic glycosylation, and long-term complications in type I diabetes mellitus. J Clin Invest. 1990;86:1046–1054. doi: 10.1172/JCI114807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The DCCT Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression in the Diabetes Control and Complications Trial. Diabetes. 1995;44:978–983. [PubMed] [Google Scholar]
- 14.Dyer DG, Dunn JA, Thorpe SR, Bailie KE, Lyons TJ, McCance DR, Baynes JW. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984;81:583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resmini P, Pellegrino L, Battelli G. Accurate quantification of furosine in milk and dairy products by a direct HPLC method. Ital J Food Sci. 1990;3:173–183. [Google Scholar]
- 17.Wu YC, Monnier VM, Friedlander MA. Reliable determination of furosine in human serum and dialysate proteins by high-performance liquid chromatography. J Chrom. 1995;B667:328–332. doi: 10.1016/0378-4347(95)00038-k. [DOI] [PubMed] [Google Scholar]
- 18.Glomb MA, Monnier VM. Mechanism of protein modification by glyoxal and glycolaldehyde reactive intermediates of the Maillard reaction. J Biol Chem. 1995;270:10017–10026. doi: 10.1074/jbc.270.17.10017. [DOI] [PubMed] [Google Scholar]
- 19.The DCCT Research Group The Diabetes Control and Complications Trial (DCCT) Diabetes. 1986;35:530–545. [PubMed] [Google Scholar]
- 20.The Diabetes Control and Complications Trial Research Group The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019. [DOI] [PubMed] [Google Scholar]
- 21.The Diabetes Control and Complications Trial Research Group Progression of retinopathy with intensive versus conventional treatment in the diabetes control and complications trial. Ophthalmology. 1995;102:647–661. doi: 10.1016/s0161-6420(95)30973-6. [DOI] [PubMed] [Google Scholar]
- 22.The DCCT Research Group Feasibility of centralized measurements of glycated hemoglobin in the Diabetes Control and Complications Trial: a multicenter study. Clin Chem. 1987;33:2267–2271. [PubMed] [Google Scholar]
- 23.Snedecor GW, Cochran WC. Statistical Methods. Iowa State University Press; Ames, IA: 1967. [Google Scholar]
- 24.Searle SR. Linear Models for Unbalanced Data. Wiley; New York: 1987. [Google Scholar]
- 25.Neter J, Wasserman W. Applied Linear Statistical Models. Irwin; Homewood, IL: 1974. [Google Scholar]
- 26.Morrison DF. Multivariate Statistical Methods. McGraw-Hill; New York: 1976. [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Applied Logistic Regression. Wiley; New York: 1989. [Google Scholar]
- 28.Acharya AS, Roy RP, Dorai B. Aldimine to ketoamine isomerization (Amadori rearrangement) potential at the individual nonenzymic glycation sites of hemoglobin A: preferential inhibition of glycation by nucleophiles at sites of low isomerization potential. J Prot Chem. 1991;10:345–357. doi: 10.1007/BF01025633. [DOI] [PubMed] [Google Scholar]
- 29.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product N∊-(carboxymethyl)-lysine is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–9986. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 30.Klein WR, Klein BEK, Moss SE, Davis MD, DeMets DL. Glycosylated hemoglobin predicts the incidence and progression of diabetic retinopathy. JAMA. 1988;260:2864–2871. [PubMed] [Google Scholar]
- 31.Berg TJ, Bangstad H-J, Torjesen PA, Osterby R, Bucala R, Hanssen KF. Advanced glycation end products in serum predict changes in the kidney morphology of patients with insulin-dependent diabetes mellitus. Metabolism. 1997;46:661–665. doi: 10.1016/s0026-0495(97)90010-x. [DOI] [PubMed] [Google Scholar]
- 32.Brownlee M. Glycation products and the pathogenesis of diabetic complications. Diabetes Care. 1992;15:1835–1843. doi: 10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]


