Abstract
New mathematical model equations for O2 and CO2 saturations of hemoglobin (SHbO2 and SHbCO2) are developed here from the equilibrium binding of O2 and CO2 with hemoglobin inside RBCs. They are in the form of an invertible Hill-type equation with the apparent Hill coefficients KHbO2 and KHbCO2 in the expressions for SHbO2 and SHbCO2 dependent on the levels of O2 and CO2 partial pressures (PO2 and PCO2), pH, 2,3-DPG concentration, and temperature in blood. The invertibility of these new equations allows PO2 and PCO2 to be computed efficiently from SHbO2 and SHbCO2 and vice versa. The oxyhemoglobin (HbO2) and carbamino-hemoglobin (HbCO2) dissociation curves computed from these equations are in good agreement with the published experimental and theoretical curves in the literature. The model solutions describe that, at standard physiological conditions, the hemoglobin is about 97.2% saturated by O2 and the amino group of hemoglobin is about 13.1% saturated by CO2. The O2 and CO2 content in whole blood are also calculated here from the gas solubilities, hematocrits, and the new formulas for SHbO2 and SHbCO2. Because of the mathematical simplicity and invertibility, these new formulas can be conveniently used in the modeling of simultaneous transport and exchange of O2 and CO2 in the alveoli–blood and blood–tissue exchange systems.
Keywords: Mathematical modeling; Hill equation; Oxyhemoglobin and carbamino-hemoglobin dissociation curves; Effects of pH; 2,3-diphosphoglycerate, and temperature; Nonlinear O2–CO2 interactions; Bohr and Haldane effects
INTRODUCTION
As blood passes through capillaries, the affinity of hemoglobin (Hb) for O2 and CO2 changes along the length of the capillary. Each O2 and CO2 reduce the affinity of Hb for the other. Changes in pH and temperature have synergistic effects. In metabolizing tissue, the blood warms, becomes more acidic, and carries more CO2 as it progresses along the capillary; the rising temperature, the diminishing pH, and the rising PCO2 all reduce the affinity of Hb for O2 and foster O2 release from Hb into the tissue. The loss of O2 from Hb into the tissue fosters the uptake of CO2 by Hb, though this effect is small compared to the buffering by bicarbonate. In the lungs, the reduction in temperature, the loss of CO2, and the concordant rising of pH all foster increasing the affinity of Hb for O2. Thus the local influences in lung versus tissue capillaries are ideally suited to maximize the delivery of O2 from alveolar air to tissues and the removal of CO2 from tissue to alveolar air. The other solute having a significant influence on the binding of O2 to Hb is 2,3-diphosphoglycerate (2,3-DPG); raising [2,3-DPG] levels, as occurs with altitude and in diabetes, reduces the O2 binding to Hb, shifting the oxyhemoglobin (HbO2) dissociation curve to higher P50s, just like higher CO2, lower pH and higher temperature do.
Early Development of Oxyhemoglobin Dissociation Descriptions
Numerous mathematical models have been proposed in the literature to describe the standard and nonstandard HbO2 “equilibrium” dissociation curves since the pioneering work of Hill13 and Adair.1 These are reviewed extensively by Roughton,29 Antonini and Brunori,2 Baumann et al.,5 and Popel.27 We shall henceforth, for brevity, call the HbO2 “equilibrium” dissociation curves the HbO2 dissociation curves (ODC). Hill13 originally postulated an nth-order one-step kinetic hypothesis for O2 binding to Hb to derive the simplest model for standard ODC involving only two parameters. Hill’s equation for the ODC describes the oxygen saturation SO2 as a function of oxygen partial pressure PO2 relative to the half-saturation level P50:
| (1) |
where KO2 is the Hill coefficient and n is the Hill exponent. They are related by KO2 = (P50)−n where P50 is the level of PO2 at which Hb is 50% saturated by O2. The value n = 2.7 was found to fit well to the data for normal human blood in the saturation range of 20–98%29 for which the value of P50 is about 26.8 mmHg. This gives KO2 = 1.3933 × 10−4 mmHg−n. Hill’s equation is analytically invertible.
Subsequently, Adair1 postulated a more realistic four-step kinetic hypothesis (known as the intermediate compound hypothesis) and derived a more accurate formula for standard ODC involving four distinct parameters. Because of its better accuracy, Adair’s equation has been particularly useful in the analysis of experimental data at very low and very high PO2’s.28,30,40 Winslow et al.39 developed an algorithm for computing the nonstandard ODCs by analyzing fresh human whole blood data over a range of O2 and CO2 partial pressures, pH, and [DPG]/[Hb] concentration ratio using the Adair’s equation. O’Riordan et al.26 compared nine different models, including Hill’s equation and Adair’s equation, by fitting them to the data for normal human whole blood. Hill’s equation was found to give good characterization of the data over the saturation range of 20–98%, confirming the earlier finding of Roughton,29 which is the range of major physiological interest. However, Adair’s equation was found to be accurate at saturations approaching 100% and was good also down to a little less than 10% saturation (see also Baumann et al.,5 Roughton,28 Roughton et al.,29 Roughton and Severinghaus30).
The Need for a Model with Practical Accuracy and Convenience
The aim of this study is to provide an expression describing the relationship between hemoglobin saturation and PO2 over a wide range of not only PO2 but PCO2, pH, 2,3-DPG, and temperature, a total of five variables. It is furthermore important that this expression be invertible so that one can convert from observations on a blood sample to the relevant chemical driving forces and to the total contents of oxygen and carbon dioxide in the blood. Our efforts in searching for extensive data sets covering large ranges of these five variables met with failure: Winslow et al.’s40 data were by far the most extensive, but the original data tables have not been preserved, though of course they are summarized by the P50’s he reported. Consequently we have been reduced to fitting these “summaries” defined through other models rather than performing optimizations to parameterize our new, more broadly defined model against original experimental observations. This compromise is acceptable because of the many observations and analyses (referenced above and in the following paragraphs) on which it is based.
Development of Further Oxyhemoglobin Models
Margaria et al.25 and Margaria24 modified Adair’s equation by expressing the four Adair constants in terms of two distinct parameters, one representing the O2 affinity for first three heme sites and the other representing an increased affinity for fourth oxygenation. Subsequently, Kelman18 proposed an empirical formula (also see Kelman19) for converting O2 tension into its saturation; it is a little more complicated than Adair’s, using seven distinct parameters. For nonstandard physiological conditions, a virtual O2 tension was computed as a function of pH, PCO2, and temperature from the experimental data and curve-fitting results of Severinghaus.32 Kelman’s formula gives negative values of SO2 for PO2 < 10 mmHg, the physically unrealistic values indicating failure of the algorithm in the region 0 mmHg < PO2 < 10 mmHg. Later, Kelman20 proposed a quadratic formula for SO2 for this range. It is worth pointing out here that the models proposed by Adair,1 Margaria,24 and Kelman18 are not analytically invertible. Therefore, to obtain PO2 from SO2, an iterative numerical method had to be employed.
Severinghaus33 developed a simple and accurate empirical formula for standard ODC by modifying Hill’s equation. For nonstandard physiological conditions, appropriate PO2 factors for pH, base excess and temperature were used, assuming as usual that these variations do not alter the shape of the curve. The significance of this model is that it fits the normal human blood data to within ±0.0055 SO2 in the range 0 SO2 < 1. Later, Ellis9 and Severinghaus34 established that Severinghaus’s33 model is analytically invertible. The motivation behind Severinghaus’s33 new model is that Roughton was never satisfied with the Adair’s equation as he could not use the normal human blood data to generate the needed unique set of Adair’s constants and get a good O2 saturation curve (e.g., see Roughton et al.28,30 and Roughton and Severinghaus30). Adair’s equation does not accommodate the Hb affinity change for O2 which occurs when the second O2 is bound to Hb and the shape of the Hb molecule changes. Severinghaus’s33 new cubic formula accounts for this affinity change and fits the data far better. Later, Siggaard-Andersen et al.37 developed a different empirical formula for standard and nonstandard ODCs which fits very well to the model and data of Severinghaus. 33 However, Siggaard-Andersen et al.’s37 equation is not analytically invertible and requires an iterative numerical method for inversion (e.g., they used a Newton–Raphson method).
Easton8 proposed a new paradigm involving two parameters for characterizing the standard ODC. His mathematical description was based on the assumption that the formation of HbO2, and hence O2 saturation of Hb, is exponentially related to the O2 partial pressure. Buerk6 modified Easton’s formula and fitted it to normal human and dog blood data30,33,40 using a linear regression algorithm; the modified Easton’s model was found to fit well to the data in the saturation range of 0 to 95%, providing the same accuracy as that of Adair’s model. Buerk and Bridges7 further modified Easton’s formula and developed an algorithm for computing the nonstandard ODCs with varying pH, CO2 partial pressure, [DPG]/[Hb] concentration ratio, and temperature. The dissociation curves computed through this revised formula were found to agree well with those computed from the algorithms of Kelman18,19 and Winslow et al.39 The model proposed by Easton8 and subsequently modified by Buerk6 and Buerk and Bridges7 is analytically invertible, so one can efficiently calculate SO2 from PO2 and vice versa. However, since these models are good only up to 95% SO2, we have developed the present model which is good above this level.
Carboxyhemoglobin
There are few mathematical models available in the literature for computing the CO2 saturation of hemoglobin and CO2 content in whole blood. Kelman21 described an algorithm for computing the whole blood CO2 content from the levels of pH, CO2 tension, O2 saturation and temperature in blood. Forster et al.10 and Forster11 have studied the rate of reaction of CO2 with Hb to form HbCO2 (carbamino-hemoglobin) at various physiological conditions. Hill et al.14–16 and Salathe et al.31 have computed the concentration of HbCO2 during O2 and CO2 exchange through mathematical modeling by accounting for the physical and biochemical processes including the acid–base balance. Later, Singh et al.,38 extending their earlier work,36 developed mathematical formulas for nonstandard HbO2 and HbCO2 dissociation curves from the equilibrium binding of O2 and CO2 with Hb inside RBCs; these were similar to Hill’s equation but included the effects of PO2, PCO2 and pH. However, the effects of 2,3-DPG and temperature were not established. More recently, Huang and Hellums17 developed a computational model for convective-diffusive gas (O2 and CO2) transport in the microcirculation and in oxygenators by accounting for the acid–base regulation and the Bohr and Haldane effects (increasing PCO2 or pH reduces O2 affinity of Hb and increasing PO2 reduces CO2 affinity of Hb).
The Consequence of This Study
This study fulfills an important requirement in the physiological studies of simultaneous O2 and CO2 transport and exchange by including the influences of Hb-mediated nonlinear O2–CO2 interactions14–17,31 and the related changes in pH that occur with passage through capillaries in tissues and in the lung. Accounting for both Bohr and Haldane effects is crucial in the modeling of simultaneous transport and exchange of O2 and CO2 in the circulatory system. For example, an important clinical application using the results of the present work is in using 15O-oxygen positron emission tomography (PET) and in the analysis of signals from BOLD (blood oxygen level dependent) MRI (magnetic resonance imaging). Our governing equations account for O2 saturation of Hb as well as CO2 saturation of Hb, which are coupled or linked to each other through the kinetics of O2 and CO2 binding to Hb. With this motivation, by considering a detailed mathematical analysis of the equilibrium binding of O2 and CO2 with Hb inside RBCs, including the nonlinear O2–CO2 interactions and the effects of pH, 2,3-DPG and temperature, we end up with relatively simple model equations for nonstandard HbO2 and HbCO2 dissociation curves, as well as the O2 and CO2 contents in whole blood.
The equations for O2 and CO2 saturations of Hb (SHbO2 and SHbCO2) are of the form of a Hill-type equation which is invertible. The apparent Hill coefficients KHbO2 and KHbCO2 in the expression for SHbO2 and SHbCO2 are explicitly dependent on the levels of O2 and CO2 partial pressures, pH, 2,3-DPG concentration, and temperature in blood. The results show that, at normal physiological conditions, Hb is about 97.2% saturated by O2 and the amino group of Hb is about 13.1% saturated by CO2. The invertibility of our model equations for SHbO2 and SHbCO2 allows their convenient usage in computationally complex models of simultaneous transport and exchange of O2 and CO2 in the pulmonary and systemic circulations. The mathematical modeling language (MML) code for our model, which is implemented in our Java Simulation (JSim) interface, is available for download and public use at http://physiome.org/Models/GasTransport/.
MATHEMATICAL FORMULATION
Governing Biochemical Reactions
The dynamics of hemoglobin-facilitated transport of O2 and CO2 in blood and their nonlinear interactions are governed by the following biochemical reactions inside RBCs.2,14,29,31,38 Hemoglobin, Hb, consists of four heme-amino chains, two α and two β chains; each contains a heme group, Hm, which binds to an O2 molecule and has a terminal amino group, −NH2, which can bind to a CO2 molecule to form an ionizable carbamino terminus, −NHCOOH. We consider the α and β chains to be identical in their binding with CO2. The four heme sites for O2 binding show cooperativity so that an HbO2 saturation curve has a Hill exponent of about 2.7. Therefore, we consider Hb as of 4 Hm (i.e., Hb = Hm4). The governing reactions are:
- CO2 hydration reaction— , buffering of CO2:
(2a) - CO2 binding to HmNH2 chains—HmNHCOO− buffering of CO2:
(2b) - CO2 binding to O2HmNH2 chains—O2HmNHCOO− buffering of CO2:
(2c) - O2 binding to HmNH2 chains—one-step kinetics using the PO2-dependent values of the rates of association and dissociation to accounts for the cooperativity.
(2d) - Ionization of HmNH2 chains—pH buffering:
(2e) - Ionization of O2HmNH2 chains—pH buffering:
(2f)
HmNH2 and O2HmNH2 refer to the reduced and oxygenated heme sites attached to the amino chain, denote their ionized forms; HmNHCOOH and O2HmNHCOOH refer to the reduced and oxygenated carbamino chain, HmNHCOO− and O2HmNHCOO− denote their ionized forms; are the rate constants of forward and backward direction reactions; are the ionization constants of H2CO3, HmNHCOOH, O2HmNHCOOH, The units of are M−1 s−1; are in s−1; are in M.
In plasma, the CO2 hydration reaction (2a) is slow, but it is fast within RBCs because there is carbonic anhydrase in the cytosol and on the membrane. The uptake of O2 and CO2 by Hb inside RBCs is governed by reactions (2b), (2c) and (2d). Reactions (2e) and (2f) act as a buffer system and control the concentration of H+ in RBCs. The interaction between O2 and CO2 is mediated by the proton H+ within RBCs via reactions (2a) to (2f).
The binding of CO2 with the four amino groups of a hemoglobin molecule is noncooperative in nature. So the kinetics of CO2 uptake by Hb can be represented by reactions (2b) and (2c) with the rate and equilibrium constants fixed. However, the binding of O2 with Hb takes place in four intermediate steps and is cooperative in nature due to the interactions between the binding heme sites.2 To account for this through our one-step kinetic approach in reaction (2d), we use the equilibrium “constant” a function of O2 partial pressure PO2; also depends on the levels of pH, PCO2, 2,3-DPG concentration, and temperature inside the RBCs.4
Equilibrium Relations
The reactions (2a)–(2f) are not instantaneous and can be described through a set of ordinary differential equations. However, these reactions approach equilibrium within about 20 ms, so the equilibrium descriptions through algebraic equations are very close to the truth. Therefore, from here on, we use the ratios of the on-to-off rate constants, that is, the equilibrium constants, instead of the rate constants. At equilibrium, we obtain the following set of algebraic relations from the set of reactions (2a)–(2f), where we denote [·] as the concentration of a species in the water space of RBCs:
| (3a) |
| (3b) |
| (3c) |
| (3d) |
| (3e) |
| (3f) |
| (3g) |
| (3h) |
| (3i) |
where the equilibrium constants are defined by
| (4) |
Pure water (H2O) has a molecular weight of 18 g/mole so that its concentration in plasma, which is 94% water, is about 55.56 × 0.94 = 52.23 M which is very high as compared to the total solute concentration (280 mM) in plasma. This leads to being practically a constant. The units of is unitless; is dependent on [O2], [CO2], [H+], [2,3-DPG] and T.
The concentrations of total hemoglobin, total O2-bound hemoglobin, and total CO2-bound hemoglobin in RBCs are given by
| (5a) |
| (5b) |
| (5c) |
The values of −NHCOOH dissociation constants are usually higher than 10−6 M10,11 while the concentration of H+ in RBCs is about 5.75 × 10−8 M (i.e., pH in RBCs is about 7.24 when pH in plasma is about 7.4). So the concentrations of HmNHCOOH and O2HmNHCOOH are usually two orders of magnitude smaller than those of HmNHCOO− and O2HmNHCOO−.14 Nevertheless, extending the work of Singh et al.,38 we include the contributions of HmNHCOOH and O2HmNHCOOH for conceptual completeness and improved accuracy.
Using Eqs. (3c)–(3i) in Eqs. (5a), (5b) and (5c), we obtain the following explicit expressions for the concentrations of total hemoglobin, total O2-bound hemoglobin, and total CO2-bound hemoglobin in RBCs:
| (6a) |
| (6b) |
| (6c) |
Expressions for SHbO2 and SHbCO2
The fractional O2 and CO2 saturations of Hb (SHbO2 and SHbCO2) is obtained from Eqs. (6a), (6b) and (6c) as the ratios [HbO2]/[Hb] and [HbCO2]/[Hb]. Putting these in the form of the Hill13 equation gives the advantage of their being analytically invertible, allowing the O2 and CO2 concentrations ([O2] and [CO2]), or their partial pressures (PO2 and PCO2), to be calculated efficiently from their fractional saturations (SHbO2 and SHbCO2) and vice versa (see Appendix B). These expressions for SHbO2 and SHbCO2 are formulated as for single-site binding and a Hill exponent of unity and therefore using concentration-dependent Hill coefficients:
| (7a) |
| (7b) |
where the apparent Hill coefficients KHbO2 and KHbCO2 (with units M−1) account for the influences of PO2, PCO2, pH, [2,3-DPG] and T as well as the nonlinear O2–CO2 interactions on the binding of O2 and CO2 with Hb inside RBCs. These concentration-dependent Hill coefficients are quite different from the constant-valued Hill coefficient of Eq. (1). The expressions for KHbO2 and KHbCO2 are given by
| (8a) |
| (8b) |
KHbO2 and KHbCO2 depend on [2,3-DPG] and T through their dependency on (see below). The P50 for 50% HbO2 saturation is not a constant, but also a function of PCO2, pH, [2,3-DPG] and T. Note that the form of Eqs. (7a) and (7b) is first order and that the Hill exponent inferred by analogy to Eq. (1) is unity. This means that the fundamental S-shape of the HbO2 dissociation curve is built into the dependency of KHbO2 on [O2]. The linkage between KHbO2 and KHbCO2 can be deduced from the fact that the equilibrium constants for deoxygenated Hb are higher than for oxygenated Hb.
Expression for
The forward rate constant for the association of O2 with HmNH2 and the backward rate constant for the dissociation of O2 from O2HmNH2 depend on the levels of PO2, PCO2, pH, [2,3-DPG] and T in RBCs.4 We characterize the dependency of the equilibrium constant on [O2], [CO2], [H+], [2,3-DPG] and T through the following power-law proportionality equation:
| (9) |
where the proportionality equilibrium constant and the empirical exponents ni, i = 0, 1, …, 4, are to be determined. The subscript “S” refers to the values under standard physiological conditions in the arterial system: [O2]S = 146 µM (or PO2,S = 100 mmHg), [CO2]S = 1.31 mM (or PCO2,S = 40 mmHg), [H+]S = 57.5 nM (or pHS = 7.24), [2,3-DPG]S = 4.65 mM, and TS = 310 K = 37 °C in RBCs (see Table 1). At these conditions is also in the units of M−1. The standard hematocrit, Hct, is about 0.45 and the Hb concentration in blood, [Hb]bl, is about 0.15 g/mL or 2.33 mM taking the molecular weight of Hb to be 64458.19 Thus, [Hb]rbc is about 5.18 mM. Buerk and Bridges7 have used the molar concentration ratio [2,3-DPG]/[Hb] as 0.9 which corresponds to [2,3-DPG] = 4.65 mM.
TABLE 1.
The representative values of the parameters used in the model.
| Symbol | Definition | Value | Unit | Equation | Reference | |
|---|---|---|---|---|---|---|
|
|
Ionization constant of H2CO3 | 5.5 × 10−4 | M | 2a, 3b | 14–16 | |
|
|
Equilibrium constant for hydration of CO2 |
1.35 × 10−3 | Unitless | 3a, 4 | 14–16 | |
| K1 | Equilibrium constant for overall CO2 hydration reaction | 7.43 × 10−7 | M | * | ||
|
|
Ionization constant of HmNHCOOH | 1 × 10−6 | M | 2b, 3d | 10,11,14,15 | |
|
|
Equilibrium constant for uptake of CO2 by reduced hemoglobin |
29.5 | M−1 | 3c, 4 | * | |
| K2 | Equilibrium constant for overall uptake of CO2 by reduced hemoglobin |
2.95 × 10−5 | Unitless | 2 | ||
|
|
Ionization constant of O2HmNHCOOH | 1 × 10−6 | M | 2c, 3f | 10,11,14,15 | |
|
|
Equilibrium constant for uptake of CO2 by oxygenated hemoglobin |
25.1 | M−1 | 3e, 4 | * | |
| K3 | Equilibrium constant for overall uptake of CO2 by oxygenated hemoglobin |
2.51 × 10−5 | Unitless | 2 | ||
|
|
Equilibrium constant for uptake of O2 by hemoglobin under standard physiological conditions |
202123 | M−1 | 3g, 4, 9 | † | |
|
|
Proportionality equilibrium constant for uptake of O2 by hemoglobin | 202123 | M−1 | 9 | † | |
|
|
Ionization constant of HmNH3+ | 2.63 × 10−8 | M | 2e, 3h | 2 | |
|
|
Ionization constant of O2HmNH3+ | 1.91 × 10−8 | M | 2f, 3i | 2 | |
| SHbO2,S | O2 saturation of hemoglobin under standard physiological conditions | 97.2% | Unitless | 7a | † | |
| SHbCO2,S | CO2 saturation of hemoglobin under standard physiological conditions | 13.1% | Unitless | 7b | † | |
| Rrbc | Gibbs–Donnan ratio for electrochemical equilibrium across the RBC membrane |
0.69 | Unitless | 14,16,31,38 | ||
| n0,S | Exponent on [O2]/[O2]S in the expression for
under standard physiological conditions |
1.7 | Unitless | 9, 15 | † | |
| n1,S | Exponent on [H+]S/[H+] in the expression for
under standard physiological conditions |
1.06 | Unitless | 9, 16a | † | |
| n2,S | Exponent on [CO2]S/[CO2] in the expression for
under standard physiological conditions |
0.12 | Unitless | 9, 16b | † | |
| n3,S | Exponent on [DPG]S/[DPG] in the expression for
under standard physiological conditions |
0.37 | Unitless | 9, 16c | † | |
| n4,S | Exponent on TS/T in the expression for
under standard physiological conditions |
4.65 | Unitless | 9, 16d | † | |
| Hct | Hematocrit; volume fraction of blood occupied by RBCs | 0.45 | mL/mL | 14–16,31 | ||
| [Hb]bl | Hemoglobin concentration in whole blood | 2.33 or 0.15 | mM or g/mL | * | ||
| [Hb]rbc | Hemoglobin concentration in RBCs ([Hb]rbc = [Hb]bl / Hct) | 5.18 or 0.33333 | mM or g/mL | * | ||
| Wbl | Fractional water space of blood at Hct = 0.45 | 0.81 | mL/mL | * | ||
| Wpl | Fractional water space of plasma | 0.94 | mL/mL | 23 | ||
| Wrbc | Fractional water space of RBCs | 0.65 | mL/mL | 23 | ||
| αO2,S | Solubility coefficient of O2 in water at body temperature (T = 37 °C) |
1.46 × 10−6 | M × mmHg−1 | 10a | 12,19 | |
| αCO2,S | Solubility coefficient of CO2 in water at body temperature (T = 37 °C) |
3.27 × 10−5 | M × mmH−1 | 10a | 3,21 | |
| P50,S | The level of PO2, at which the hemoglobin is 50% saturated by O2 at STP |
26.8 | mmHg | 6,7,18,39,40 | ||
| P50,SCO2 | The level of PCO2 at which the hemoglobin is 50% saturated by CO2 at STP |
265 | mmHg | † |
Calculated using the formula in “definition” and data in “value” columns.
Estimated through the current model as described in the “Results ” section.
The standard physiological conditions are: [O2]S = 146 µM (or PO2,S = 100 mmHg), [CO2]S = 1.31 µM (or PCO2,S = 40 mmHg), [H+]S = 57.5 nM (or pHS = 7.24), [2,3-DPG]S = 4.65 µM, and TS = 37 °C inside the RBCs.
Now we see that Eqs. (8a) and (8b) along with Eq. (9) completely determine the apparent Hill coefficients KHbO2 and KHbCO2. Singh et al.38 did not account for the effects of [CO2], [2,3-DPG] and T on the equilibrium constant in their modeling. So their case corresponds to Eq. (9) with n2 = n3 = n4 = 0 and nonzero n0 and n1.
Expressions for αO2 and αCO2
The equations for fractional saturations, SHbO2 and SHbCO2, and apparent Hill coefficients, KHbO2 and KHbCO2, can be expressed in terms of O2 and CO2 partial pressures, PO2 and PCO2, by expressing the molar concentrations [O2] and [CO2] in water space of RBCs in terms of PO2 and PCO2 using Henry’s law: [O2] = αO2 PO2 and [CO2] = αCO2 PCO2, where αO2 and αCO2 are the solubilities of O2 and CO2 in water. At body temperature (T = 37 °C), αO2 = 1.46 × 10−6 M mmHg−1 and αCO2 = 3.27 × 10−5 M mmHg−1. The variation of αO2 and αCO2 with temperature (T) can be expressed through the following quadratic curve-fit equations19,21 based on the experimental data of Hedley-Whyte and Laver12 on αO2 and Austin et al.3 on αCO2, correcting for the plasma fractional water content, Wpl:
| (10a) |
| (10b) |
where Wpl = 0.94. The first term for αO2 is (1.37/0.94) × 10−6 or 1.46 × 10−6 M mmHg−1 and for αCO2 is (3.07/0.94) × 10−5 or 3.27 × 10−5 M mmHg−1, as mentioned above. The solubility of O2 and CO2 decreases as temperature increases.
The HbO2 and HbCO2 dissociation curves described by Eqs. (7a)–(7b) and (8a)–(8b) require knowing the equilibrium constants and the empirical exponents n0, n1, n2, n3 and n4. From the saturations, SHbO2 and SHbCO2, the total O2 and CO2 contents in whole blood can be calculated as described in Appendix A.
RESULTS: PARAMETER ESTIMATION
The O2 and CO2 saturations of Hb (SHbO2 and SHbCO2) and their whole blood contents ([O2]bl and [CO2]bl) depend on the physiological state variables PO2, PCO2, pH, Hct, [2,3-DPG]rbc and T, empirical exponents n0, n1, n2, n3 and n4, and equilibrium constants The literature does not provide consistent values for the equilibrium constants. Singh et al.38 estimated some of them in their theoretical studies of HbO2 and HbCO2 dissociation curves, but their estimates do not match with the experimental values obtained by Roughton,29 Forster et al.10 and Forster11 which are the values generally accepted.2,14–16,31 Singh et al.38 assumed that the Hb molecule has only one amino chain, −NH2, and is capable of binding to only one CO2 molecule, but Hb has four −NH2 chains and can bind to four CO2 molecules. Thus, Singh et al.’s estimates are not acceptable for the kinetic reactions (2a)–(2f). In the present study, we choose or calculate the equilibrium constant and Rrbc appropriately from the literature (see Table 1 for references) and then estimate the proportionality equilibrium constant and the empirical exponents n0, n1, n2, n3 and n4 so as to obtain the appropriate forms and shifts in the HbO2 dissociation curves with respect to the levels of pHrbc, PCO2, [DPG]rbc and T in accord with experimental observations and their summaries captured in the models of Kelman18 and Buerk and Bridges.7 The generality and comprehensiveness of the calculations might seem disadvantaged by their apparent complexity, but in fact the code for the equations is quite simple algebra and is viewable (and downloadable) within three clicks of http://physiome.org/Models/GasTransport.
Calculation of Equilibrium Constants
We choose the values (see Table 1 for references); K1 is the equilibrium constant of the overall CO2 hydration reaction (2a); K2 and K3 are the equilibrium constants of the overall reaction of CO2 with the reduced and oxygenated Hb, reactions (2b) and (2c). These give as shown in Table 1. Thus, the equilibrium constants for reduced Hb are higher than for oxygenated Hb. This indicates that the reduced Hb has a greater ability to bind to CO2 than does the oxygenated Hb (e.g., see Figs. 24 and 26 of Roughton,29 and Fig. 10.11 of Antonini and Brunori.2 The estimation of the proportionality equilibrium constant and the empirical exponents n0, n1, n2, n3 and n4 are described below.
Calculation of Oxygen P50
The P50 for O2 (the level of PO2 at which Hb is 50% saturated by O2) is conventionally used as a measure of O2 affinity of Hb and shift in the HbO2 dissociation curve (ODC). We first calculate the P50 values for nonstandard ODCs as a function of pHrbc, PCO2, [DPG]rbc and T from the model of Buerk and Bridges7 which agree well with those obtained experimentally by Winslow et al.39 We then employ the method of regression analysis to determine the curve-fit polynomials for these computed P50 values recursively with one of the variables varying and the other three fixed at their standard physiological values. We found that the P50(pHrbc), P50(PCO2) and P50([DPG]rbc) are best-fitted by quadratic polynomials, whereas P50(T) is best-fitted by a cubic polynomial. This is demonstrated through Fig. 1. The combined best-fit polynomial for P50(pHrbc,PCO2,[DPG]rbc,T) is given by
| (11) |
Figure 1.
The plot of oxygen P50 (pHrbc, PCO2, [DPG]rbc, T) with one of the variables varying and the other three fixed at their standard physiological values. The P50’s computed from Buerk and Bridges’7 model are shown by solid points, from our best-fit polynomial (11) are shown by solid lines, and from Kelman’s empirical equation (12) are shown by dashed lines.
The empirical equation for P50(pHrbc, PCO2, T) from Kelman’s18 model is given by
| (12) |
The P50(pHrbc) and P50(PCO2) computed from Eq. (11) are more accurate than those computed from Eq. (12). They agree closely near the standard physiological conditions, but deviate when the conditions are far different from the standard conditions, as seen in Figs. 1a and 1b. The P50(T) formulas agree well over the whole range of T, as seen in Fig. 1d. Kelman did not account for the effect of [DPG]rbc.
Figure 1 shows the variation of P50 with pHrbc for PCO2 = 40 mmHg, [DPG]rbc = 4.65 mM and T = 37 °C in panel A; P50 with PCO2 for pHrbc = 7.24, [DPG]rbc = 4.65 mM and T = 37 °C in panel B; P50 with [DPG]rbc for pHrbc = 7.24, PCO2 = 40 mmHg and T = 37 °C in panel C; and P50 with T for pHrbc = 7.24, PCO2 = 40 mmHg and [DPG]rbc = 4.65 mM in panel D. The P50 values from the model of Buerk and Bridges7 are shown by solid points and the corresponding best-fit polynomials are shown by solid lines. The P50 values from the model of Kelman18 are shown by dashed lines. This figure illustrates that the O2 affinity of Hb decreases (i.e., the P50 level increases) as pH decreases (or [H+] increases) and PCO2, [DPG] or T increases. Also, the O2 affinity is significantly affected by the pH and temperature T. The values of P50 computed from Eq. (11) are used here to estimate the values of , n0, n1, n2, n3 and n4.
Equations for Parameter Estimation
Now eliminating KHbO2 from Eqs. (7a) and (8a), then substituting the expression for from Eq. (9), and finally evaluating the resulting equation at 50% HbO2 saturation (SHbO2 = 0.5), we obtain the following equation relating , n0, n1, n2, n3 and n4 to P50:
| (13) |
where Kratio and Kfact below characterize the nonlinear O2–CO2 interactions:
| (14a) |
| (14b) |
P50 = P50 ([H+], [CO2], [DPG], T) on the right hand side of Eq. (13) is based on the model of Buerk and Bridges7 which is plotted in Fig. 1 and is given by the polynomial (11). We use Eqs. (13), (14a) and (14b) to estimate the values of , n0, n1, n2, n3 and n4.
Estimation of and n0
Now the proportionality equilibrium constant and empirical exponent n0 can be estimated simultaneously by fixing the physiological state variables at their standard values: [H+] = [H+]S (or pH = pHS), [CO2] = [CO2]S (or PCO2 = PO2 ), [DPG] = [DPG]S, and T = TS. Then Eq. (13) becomes independent of the exponents n1, n2, n3 and n4 (because all the ratios in Kfact are 1) and so depends only on the exponent n0. On comparing the resulting equation with the relation between the Hill exponent and Hill coefficient (given below Eq. 1), we obtain the following estimations for and n0:
| (15) |
Under standard physiological conditions in the arterial system, we have [O2] = [O2]S = 146 µM (or PO2 = PO2,S = 100 mmHg), [CO2] = [CO2]S = 1.31 mM (or PCO2 = PCO2,S = 40 mmHg), [H+] = [H+]S = 57.5 nM (or pH = pHS = 7.24), [DPG] = [DPG]S = 4.65 mM, and T = TS = 37 °C in RBCs; pH = 7.24 in RBCs corresponds to pH = 7.4 in plasma, because the Gibbs–Donnan ratio Rrbc for the electrochemical equilibrium condition across the RBC membrane is about 0.6914–16,31,38; the value [DPG] = 4.65 mM corresponds to the ratio [DPG]/[Hb] = 0.9 which is used as the standard ratio by Winslow et al.39 and Buerk and Bridges.7 Under these conditions, P50 = P50,S = 26.8 mmHg for human blood,6,7,39,40 which when substituted into Eq. (15) gives the estimation that is independent of the choices of n1, n2, n3 and n4. It can be noted here that one can use PO2,S = P50,S = 26.8 mmHg as the referencePO2 instead of PO2,S = 100 mmHg. However, this will give a different estimate of while the estimate n0 = 1.7 will remain unchanged.
The current model can be made to fit the Adair model (or Severinghaus’s33 model) by adjusting parameters so that Eqs. (7a), (8a) and (9) fit Adair’s equation (or Severinghaus’s33 equation). In this case, the exponent n0 will be obtained as a function of PO2. However, we avoid this because the SHbO2 to PO2 relationship will then no longer be analytically invertible. See Appendix A for the calculation of blood O2 content from saturation, SHbO2, and PO2, and see Appendix B for calculating PO2 from SHbO2 and the P50, taking into account PCO2, pH, [2,3-DPG], Hct, and T.
Estimation of n1, n2, n3 and n4
The empirical exponents n1, n2, n3 and n4 are estimated as independent of each other. These are estimated recursively by varying one of the physiological state variables and fixing the others at their standard values. For example, the exponent n1 is estimated by varying the level of pH and fixing the levels of PCO2, [DPG] and T at their standard values PCO2,S, [DPG]S and TS. In this case, Eq. (13) becomes independent of the exponents n2, n3 and n4 with the pH or [H+] as the only varying variable. From this resulting equation, the functional expression for n1 as a function of pH or [H+] can be obtained. In a similar fashion, the functional expressions for n2 as a function of PCO2 or [CO2], n3 as a function of [DPG], and n4 as a function of T can also be obtained. These are given by the following exact expressions:
| (16a) |
| (16b) |
| (16c) |
| (16d) |
where the P50’s are given by the polynomial expression (11) and the second subscript “1”, “2”, “3” or “4” indicates that a particular one of the variables pHrbc, PCO2, [DPG]rbc or T is varying.
Figure 2 shows the variation of n1 with pHrbc for PCO2 = 40 mmHg, [DPG]rbc = 4.65 mM and T = 37 °C in panel A; n2 with PCO2 for pHrbc = 7.24, [DPG]rbc = 4.65 mM and T = 37 °C in panel B; n3 with [DPG]rbc for pHrbc = 7.24, PCO2 = 40 mmHg and T = 37 °C in panel C; and n4 with T for pHrbc = 7.24, PCO2 = 40 mmHg and [DPG]rbc = 4.65 mM in panel D. It is depicted that the exponent n4 is considerably larger compared to the other exponents n1, n2 and n3, whereas the exponent n2 is very small. This is because the effect of temperature T on the O2 affinity is very significant and that of PCO2 is relatively small. These calculated functional forms of the exponents n1, n2, n3 and n4 are used here to compute SHbO2, SHbCO2, [O2]bl and [CO2]bl. However, for simplicity, the standard values n1,S = 1.06, n2,S = 0.12, n3,S = 0.37, and n4,S = 4.65 (see Table 1; calculated using the standard physiological conditions) can also be used. In this case, the shifts in the HbO2 saturation curves will not be very accurate. The results are summarized through Figs. 3–6, presented below.
Figure 2.
The exponents for Eq. (9); the plots of n1(pHrb c), n2(PCO2), n3([DPG]rbc) and n4(T) with the other three variables fixed at their standard physiological values. These are computed from Eqs. (16a)–(16d) using the estimated value of and n0 and the best-fit polynomial (11) for oxygen P50.
Figure 3.
The comparison of the HbO2 dissociation curves computed from our model, Kelman18 model, and Buerk and Bridges7 model for different values of pHrbc with PCO2, [DPG]rbc and T fixed at their standard physiological values.
Figure 6.
Total CO2 content of whole blood ([CO2]bl, mL CO2 per 100 mL blood) as a function of PCO2 at various physiological conditions (i.e., with varying levels of pHrbc, PO2, [DPG]rbc, T and Hct) as computed through Eqs. (A.2) and (A.3).
DISSOCIATION CURVES AND BLOOD GAS CONTENTS
Oxyhemoglobin (HbO2) Dissociation Curves
Figure 3 shows the comparison of the HbO2 dissociation curves computed from current model, Kelman18 model and Buerk and Bridges7 model for pHrbc = 6.92, 7.24 and 7.56 with PCO2 = 40 mmHg, [DPG]rbc = 4.65 mM and T = 37 °C. It is seen that these curves are in fairly good agreement with each other over the entire saturation range, although our curves are consistently slightly below the curves of Kelman and Buerk and Bridges when SHbO2 < 30%. In this range, our curves are not very accurate, since our model is based on the Hill’s equation, which is considered to be accurate only in the saturation range of 20 to 98%,29 and one might therefore prefer their models at very low PO2 even though inversion is more difficult. However, for SHbO2 > 30%, our curves agree closely with the curves of Buerk and Bridges which were fit to actual human and dog blood HbO2 saturation data of Roughton et al.,28 Roughton and Severinghaus,30 Winslow et al.,40 and Sveringhaus.33 Also Kelman’s model does not give accurate shifts in HbO2 dissociation curves when physiological variables deviate far from their standard values.
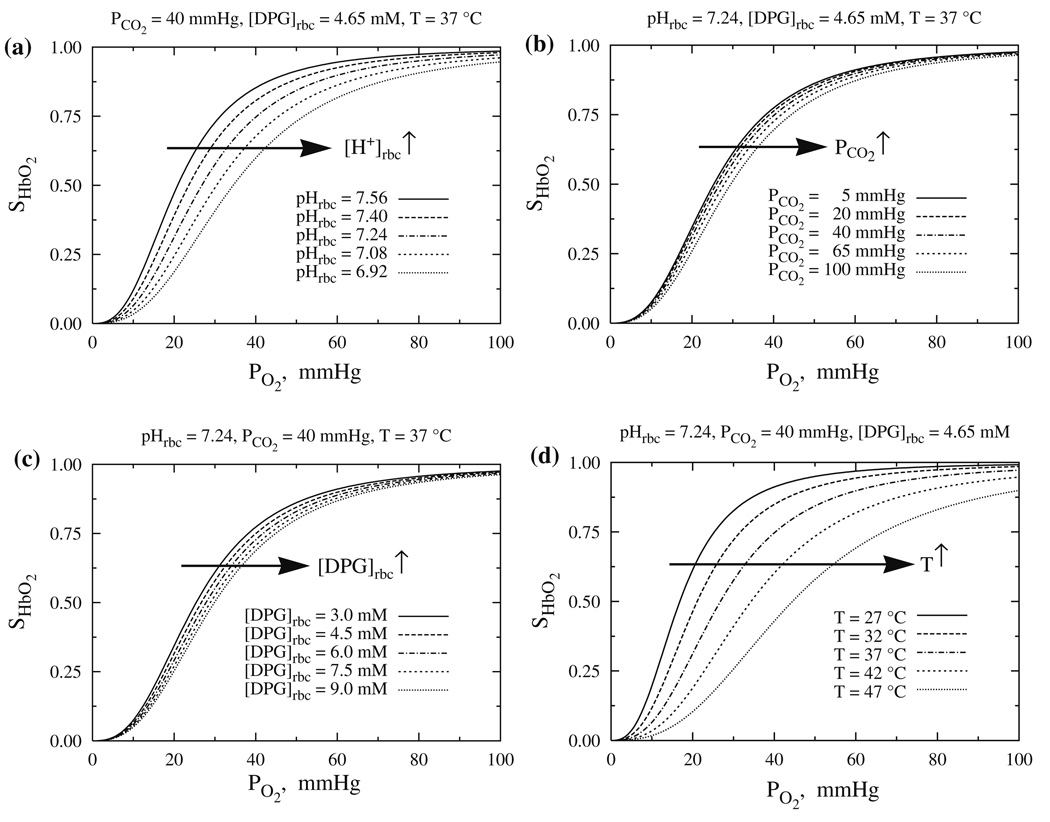
Figure 4 shows the variation of SHbO2 with PO2 for different values of pHrbc with PCO2 = 40 mmHg, [DPG]rbc = 4.65 mM and T = 37 °C in panel A; different values of PCO2 with pHrbc = 7.24, [DPG]rbc = 4.65 mM and T = 37 °C in panel B; different values of [DPG]rbc with pHrbc = 7.24, PCO2 = 40 mmHg and T = 37 °C in panel C; and different values of T with pHrbc = 7.24, PCO2 = 40 mmHg and [DPG]rbc = 4.65 mM in panel D. The HbO2 dissociation curve shifts to the right (i.e., the P50 for O2 increases, or O2 affinity of Hb decreases) as pH decreases (or [H+] increases) and PCO2, [DPG] or T increases. This diminution of Hb affinity for O2 as pH decreases or PCO2 increases is known as the Bohr effect, so our model provides a quantitative description of the Bohr effect. The shifts in the HbO2 dissociation curve with changes in PCO2 or [DPG] are small as compared to those occurring with changes in pH and T; the O2 affinity of Hb is most sensitive to temperature T. Undoubtedly the fit of these models to complete data sets (such as the unfortunately unavailable set of Winslow et al.40 would result in improved parameter estimates, but these curves should be accurate roughly to 2 or 3% for PCO2’s above 20 mmHg.
Figure 4.
The quantitative behavior of the HbO2 dissociation curves at various physiological conditions (i.e., with varying levels of pHrbc, PCO2, [DPG]rbc and T) as computed from Eqs. (7a), (8a), (9), (15), and (16a)–(16d).
Carbamino-Hemoglobin (HbCO2) Dissociation Curves
Figure 5 shows the variation of SHbCO2 with PCO2 for different values of pHrbc with PO2 = 100 mmHg, [DPG]rbc = 4.65 mM and T = 37 °C in panel A; different values of PO2 with pHrbc = 7.24, [DPG]rbc = 4.65 mM and T = 37 °C in panel B; different values of [DPG]rbc with pHrbc = 7.24, PO2 = 100 mmHg and T = 37 °C in panel C; and different values of T with pHrbc = 7.24, PO2 = 100 mmHg and [DPG]rbc = 4.65 mM in panel D. It is seen that the behavior of the HbCO2 dissociations curves (hyperbolic) is quite different than the HbO2 dissociation curves (sigmoid). Also, at fixed values of PO2, PCO2, pH, [DPG] and T, the Hb saturation of CO2 (SHbCO2) is considerably lower than the Hb saturation of O2 (SHbO2). This is because the CO2 binding to Hb is noncooperative in nature,2 whereas the O2 binding to Hb is cooperative.
Figure 5.
The quantitative behavior of the HbCO2 dissociation curves at various physiological conditions (i.e., with varying levels of pHrbc, PO2 [DPG]rbc and T) as computed from Eqs. (7b), (8b), (9), (15), and (16a)–(16d).
Figure 5a depicts that the CO2 saturation of Hb (SHbCO2) is greatly affected by pH, and is dependent only on the shift in affinity for single site binding. But raising [H+], reducing pH, greatly reduces the CO2 affinity for Hb, reducing the carbamino formation: so the P50 for CO2 shifts rightward. Likewise, Figs. 5b and 5d show that raising PO2 and T shift the P50 for CO2 rightward, indicating a reduction in carbamino formation and CO2 affinity for Hb. This shift in P50 for CO2 is higher at lower values of PO2 and T and negligible at higher values of PO2 and T. The alteration of the CO2 affinity of Hb with respect to PO2 is known as the Haldane effect, so our model provides a quantitative description of the Haldane effect.
The curve of SHbCO2 asymptotes at high PO2 to a limiting curve (Fig. 5b) because HbO2 saturation is almost complete by PO2 = 100 mmHg beyond which O2 has no direct effect on CO2 binding. For temperature (Fig. 5d), the apparent asymptotic behavior is due to a combination of factors: a reduction in the concentration of dissolved CO2 due a decrease in solubility of CO2 and an increase in the apparent Hill coefficient KHbCO2 due to a decrease in HbO2 saturation. These opposing trends in [CO2] and KHbCO2 with raising T diminish the shift in the HbCO2 dissociation curves. Figure 5c depicts that the effect of [DPG]rbc on the CO2 affinity of Hb can be neglected, as KHbCO2 and KHbCO2 are almost independent of [DPG]rbc.
CO2 Content in Whole Blood, [CO2]bl
Figure 6 shows the variation of [CO2]bl (ml of CO2 per 100 mL of blood) with PCO2, for different values of PO2 and [DPG]rbc with pHrbc = 7.24, T = 37 °C, and Hct = 0.45 in panel A; for different values of pHrbc with T = 37 °C, Hct = 0.45, and all PO2 and [DPG]rbc in panel B; for different values of T with pHrbc = 7.24, Hct = 0.45, and all PO2 and [DPG]rbc in panel C; and for different values of Hct with pHrbc = 7.24, T = 37 °C, and all PO2 and [DPG]rbc in panel D. Generally [CO2]bl varies linearly with PCO2, and varies negligibly with PO2 and [DPG]rbc. This is because most of the CO2 is carried as bicarbonate, and very little in the form of carbamino. Figure 6b shows that the [CO2]bl decreases as pH decreases and decreases, by Eq. (A.3). An increase in the temperature T (Fig. 6c) reduces the CO2 solubility and leads to a decrease in [CO2] and then [HCO3−]. Figure 6d shows that an increase in Hct leads to a decrease of plasma space, an increase in average pH in blood (since pHrbc = 7.24 and pHpl = 7.4), and hence a decrease in [HCO3−]. These lead to a decrease in total CO2 content in whole blood.
DISCUSSION AND CONCLUSIONS
The transport and exchange of O2 and CO2 in the circulatory system is highly influenced by the competitive binding of O2 and CO2 with hemoglobin (i.e., the Hb-mediated nonlinear O2–CO2 interactions) and by the levels of pH (acidity), 2,3-DPG concentration, and temperature in RBCs. Thus, in the modeling of simultaneous transport and exchange of O2 and CO2, one must consider suitable model equations for O2 and CO2 saturations of Hb (SHbO2 and SHbCO2) which are coupled or linked to each other through the kinetics of O2 and CO2 binding to Hb. Also, for computational efficiency, in simulating the blood-tissue gas exchange processes in changing physiological states, the SHbO2 to PO2 and SHbCO2 to PCO2 relationships should be analytically invertible. Again, since the SHbO2 is measurable spectrophotometrically, estimating PO2 from SHbO2 is of practical, even clinical, consequence. However, as SHbCO2 can not currently be estimated spectrophotometrically, there is no practical utility in calculating PCO2 from SHbCO2.
The models for standard HbO2 dissociation curve, valid for only fixed/standard values of pH, PCO2, [DPG]/[Hb] and T,1,6,8,13 are not very efficient to use in the simulation of dynamic blood–tissue gas exchange processes. To make them applicable, one must multiply all PO2 values of the standard curve by a factor which is composite for the variations in pH, PCO2, [DPG] and T (e.g., as in Kelman18 and Severinghaus33). The numerical algorithm of Winslow et al.39 for computing the nonstandard HbO2 dissociation curves is very complicated, because it needs numerical evaluation of all the four Adair constants as functions of pH, PCO2 and [DPG]/[Hb] by fitting the Adair’s equation with the experimental data on HbO2 saturation in human whole blood. Their quadratic fit regression analysis for the four Adair constants needs numerical evaluation of a total of 72 coefficients. The effect of temperature (T) were not established, but would need an estimation of 24 additional coefficients to extend their algorithm. Like Adair’s equation, the empirical equations of Kelman18 and Siggaard-Andersen et al.37 for nonstandard HbO2 dissociation curves require numerical inversion for computing PO2 from SHbO2, which is computationally expensive. The model of Buerk and Bridges,7 being analytically invertible, is more efficient. However, it does not describe the upper 5% of the dissociation curve accurately. Besides, all these models of HbO2 dissociation curves need a suitable coupled model of HbCO2 dissociation curves for integrating into a computational model of simultaneous or dynamic O2 and CO2 transport and exchange in the microcirculation.
The model of Singh et al.38 for nonstandard HbO2 and HbCO2 dissociation curves is based on the equilibrium binding of O2 and CO2 with Hb, but it does not account for the effects of 2,3-DPG concentration and temperature in blood. Also Singh et al.’s estimates of the equilibrium constants for the kinetic reactions do not agree well with the experimental values reported earlier.2,10,11,14–16,29,31 Their formulation for kinetic reactions of O2 and CO2 with Hb was based on the assumption that the Hb molecule has only one heme-amino chain, Hm-NH2, and is capable of binding to only one CO2 molecule, and therefore requires correction, since Hb actually has four Hm-NH2 chains and can bind to four CO2 molecules.
In an attempt to address these issues, a more appropriate and relatively simple mathematical model equations (Eqs. 7a and 7b) for O2 and CO2 saturations of Hb (SHbO2 and SHbCO2) are developed in this paper. These are derived by considering the equilibrium binding of O2 and CO2 with Hb inside RBCs,2,14,29,31,38 including the Hb-mediated nonlinear O2–CO2 interactions and the effects of pH, 2,3-DPG, and temperature. Unlike in the previous models mentioned above, Hb molecule is considered to have four heme-amino chains, Hm-NH2, each capable of binding to one O2 molecule and one CO2 molecule. The binding of O2 is considered to be cooperative and that of CO2 as non-cooperative. The new model equations for SHbO2 and SHbCO2 are of the form of Hill’s equation, which has the extra advantage of being analytically invertible, allows the O2 and CO2 partial pressures (PO2 and PCO2) to be computed directly from their saturations (SHbO2 and SHbCO2) and vice versa (see Appendix B). However, this new model is highly accurate only above 30% (SHbO2 or a PO2 of 20 mmHg, and leads to 1 to 2% underestimation of the saturation from the PO2 in computing intracapillary profiles of O2 and CO2 from models of simultaneous transport and exchange of O2 and CO2. Improvement is needed here, though the low range is not very commonly encountered in normal circumstances; the Severinghaus33 model would be an improvement within the range of conditions covered by that model.
The apparent Hill coefficients KHbO2 and KHbCO2 in the expressions for SHbO2 and SHbCO2 (Eqs. 8a and 8b) are explicitly dependent on the levels of PO2, PCO2, pH, [2,3-DPG] and T in RBCs and are dependent on the equilibrium constants of the chemical reactions involved in the binding O2 and CO2 with Hb. So these establish the linkage between SHbO2 and SHbCO2 and the nonlinear O2–CO2 interactions. The cooperativity of O2 binding and the effects of nonstandard physiological conditions are established in this model by considering the equilibrium constant for the reaction of O2 with an Hm-NH2 chain as a suitable function of PO2, PCO2, pH, [2,3-DPG] and T involving six adjustable parameters (see Eq. 9), including one proportionality equilibrium constant and five empirical exponents n0, n1, n2, n3 and n4. These were estimated using the P50 (pHrbc PCO2 [DPG]rbc T) values from the model of Buerk and Bridges7 for nonstandard HbO2 dissociation curves which agree closely with those obtained theoretically by Kelman18 and experimentally by Winslow et al.39 for normal human whole blood. These estimates are also influenced by the equilibrium constants for the other kinetic reactions in the uptake of CO2 and ionization of Hm-NH2 chains. These equilibrium constants are chosen or calculated appropriately to be consistent with those referred to largely in the literature.2,14–16,31
Our new model could be fitted to other sets of experimental data for characterizing the O2 and CO2 saturation of Hb. The report of Winslow et al.39 was based on a large dataset, and in their report, the data were summarized by the P50 values; the original data are no longer available, unfortunately, else they would have served as an excellent test of our descriptive equations. From an optimization using such large datasets, we would obtain more precise estimates of equilibrium constants and P50 (pHrbc, PCO2, [DPG]rbc, T) values, and presumably improved estimates of our model parameters, particularly the exponents n1, n2, n3 and n4.
The new model equations for SHbO2 and SHbCO2 are used here to calculate the O2 and CO2 contents in whole blood (see Appendix A). The HbO2 and HbCO2 dissociation curves and the O2 and CO2 contents in whole blood computed through these new equations are in good agreement with the published experimental and theoretical results in the literature. The result shows that at normal physiological conditions in arterial blood, the PO2 is about 100 mmHg and so Hb is about 97.2% saturated by O2 while the amino group of Hb is about 13.1% saturated by CO2. The invertibility (see Appendix B) of our new equations for SHbO2 and SHbCO2 allows their convenient use in computationally complex models of simultaneous or dynamic transport and exchange of O2 and CO2 in the alveoli–blood and blood–tissue exchange systems. This model has been implemented in our Java Simulation (JSim) interface, the mathematical modeling language (MML) code of which is available for download and public use at our physiome website, http://physiome.org/Models/GasTransport.
Our new model is unique in the sense that it is derived from the equilibrium binding of O2 and CO2 with Hb and it accounts for all the factors (e.g., PO2, PCO2, pH, [DPG] and T) that affect the O2 and CO2 binding to Hb. It also establishes the linkage between SHbO2 and SHbCO2 as well as the Hb-mediated nonlinear O2–CO2 interactions (or Bohr and Haldane effects), the effects of which are important in simulating the complex simultaneous or dynamic transport and exchange of O2 and CO2 in the microcirculation. The kinetic and equilibrium constants, compiled from the literature or estimated in this paper, can be used in the dynamically modeling of the complex gas exchange process in vivo. These can also used to compute the concentrations of all the reaction products at equilibrium from Eqs. (3a) to (3i).
In summary, our new multidimensional model equations for SHbO2 and SHbCO2 are relatively simple, lending themselves to simple equation-solving by spreadsheet or by a simple program in a handheld computer. Severinghaus32 provided a slide rule for this purpose, and later in his 1979 paper,33 he summarized all the earlier calculations through appropriate model equations, which gives the earlier references, and which stimulated Ellis9 to provide the inverted calculation (PO2 from SO2). The Appendix B provides a recipe for the inversion of our new SHbO2 to PO2 relationship that allows calculation of whole blood O2 content from the HbO2 saturation (e.g., provided spectrophotometrically) and other chemically or physically determined measures of pH, PO2 PCO2 or bicarbonate, [2,3-DPG], and temperature (T) in blood. Of particular value in analyzing the 15O-oxygen time course data from PET (positron emission tomography) using convection–diffusion distributed models (e.g., Li et al.23 is the analytical invertibility of the Hill-type expression for SHbO2, as given by Eqs. (B.1) to (B.3) in the Appendix B.
We measured the computation times for numerical inversion of Kelman’s18 equation. It took 20–25 times more computer time (because of the numbers of iterations required) than the analytical inversion of Hill’s equation for computing PO2 from SHbO2. This is more significant in the convection–diffusion distributed modeling of O2 and CO2 transport in blood–tissue exchange systems which involves large number of spatial and temporal grid points. Again, in the estimation of parameters, using such axially distributed models, accounting for the gradients in [O2], [CO2], pH and T along the capillary length, will require another level of iteration during the optimization process: this means that computational efficiency is doubly important.
ACKNOWLEDGMENTS
This research was supported by the National Simulation Resource for Circulatory Mass Transport and Exchange via grants NCRR/NIH P41-RR1243 and NIBIB/NIH R01-EB001973. The authors are grateful to the referees for their comments and suggestions, and to John Kenneth Baillie, University of Edinburgh for corrections to Eq. (11) of the 2004 publication.
APPENDIX A
Calculation of O2 Content in Whole Blood
To calculate the O2 content of whole blood, we need to sum the two forms of O2 in blood: O2 dissolved in plasma water and RBC water and O2 as oxyhemoglobin in RBCs. Thus, the molar O2 concentration of whole blood, [O2]bl, can be calculated as
| (A.1) |
where Wbl is the fractional water space of blood and is related to that of plasma and RBCs (Wpl and Wrbc) by Wbl = (1 − Hct) Wpl + Hct Wrbc; [Hb]bl is the molar concentration of hemoglobin in whole blood and is related to that in RBCs, [Hb]rbc, by [Hb]bl = Hct [Hb]rbc; Hct is the blood hematocrit. It is assumed that the O2 partial pressures in plasma and RBCs are equal. The factor 4 in Eq. (A.1) indicates that four molecules of O2 bind to one molecule of Hb. The whole blood O2 content in mL of O2 per mL of blood is 22.256 times [O2]bl.
At standard physiological conditions, Hct is about 0.45 and [Hb]bl is about 0.15 g/mL or 2.33 mM taking the molecular weight of Hb to be 64,458.19 Correspondingly, [Hb]rbc is about 5.18 mM that is assumed to be fixed and independent of Hct. With Wpl = 0.94, Wrbc = 0.65 and Hct = 0.45, we have Wbl = 0.81 mL water per mL blood. With these data and at a PO2 of 100 mmHg, the O2 saturation of Hb, SHbO2, is about 97.2%, the O2 content of whole blood is about 0.26 mL O2 per 100 mL blood in free form plus about 20.18 mL O2 per 100 mL blood bound to Hb, making a total O2 content in whole blood of 20.44 mL O2 per 100 mL blood.
Calculation of CO2 Content in Whole Blood
To calculate the CO2 content of whole blood, we need to sum the four forms of CO2 in blood: CO2 in dissolved form, as carbonic acid (H2CO3), as bicarbonate ions and as carbamino-hemoglobin (HbCO2). The dissociation constant for H2CO3 is about 5.5 × 10−4 M 14–16 while the concentration of H + in plasma is about 3.98 × 10−8 M and in RBCs is about 5.75 × 10−8 M. So the concentration of H2CO3 is usually four orders of magnitude smaller than that of . Therefore, its contribution to the CO2 content can be neglected.
Again four moles of CO2 bind with one mole of Hb in RBCs. So with the assumption that the CO2 partial pressures in plasma and RBCs are equal, the molar CO2 concentration of whole blood, [CO2]bl, can be calculated as
| (A.2) |
where the total bicarbonate in blood, [HCO3−]bl, is obtained by using the Henderson-Hasselbalch equation and Gibbs–Donnan electrochemical equilibrium condition as:
| (A.3) |
Here is the equilibrium constant for overall CO2 hydration reaction (2a) and is the Gibbs–Donnan ratio for electrochemical equilibrium condition across the RBC membrane. The value of Rrbc is about 0.69.14–16,31,38 Multiplying [CO2]bl by 22.256 converts the units of molar to mL of CO2 per mL of blood.
From the data used in Hill et al.,14–16 [H2O] Again, as shown in Table 1. Thus, pK1 = −log(K1) ≈ 6.13, which is close to the standard value pK1 = 6.1 reported in text books and literature. However, pK1 depends on the levels of pH and temperature T in plasma. This dependency was expressed by the following curve-fit equation by Kelman21 based on the experimental data of Austin et al.3:
| (A.4) |
At pHpl = 7.4 and T = 37, Eq. (A.4) gives pK1 = 6.09 which is close to the value pK1 = 6.13 calculated above and agrees well with the data of Severinghaus et al.35
At standard physiological conditions, the CO2 content of whole blood in free form is about 2.35 mL CO2 per 100 mL blood (i.e., 1.06 mM), in bicarbonate form is about 42.69 mL CO2 per 100 mL blood (i.e., 19.18 mM), and in carbamino form is about 2.72 mL CO2 per 100 mL blood (i.e., 1.22 mM), making a total CO2 content in whole blood of 47.76 mL CO2 per 100 mL blood (i.e., 21.45 mM). These estimates agree with the data reported in the literature.21,22,29 The CO2 saturation of Hb, SHbCO2, is computed to be about 13.1% at a PCO2 of 40 mmHg.
APPENDIX B
Algorithm for Inverting the Relationships Between SHbO2 and PO2
Equation (7a) for the fractional saturation SHbO2 is not convenient for analytical inversion because the apparent Hill coefficient KHbO2 depends on [O2] (see Eq. 8a). However, with the help of Eq. (9), the expression for SHbO2 can be rewritten in the following form, which is amenable for analytical inversion:
| (B.1) |
where (with units M−(1+n0)) is given by Eqs. (13) and (14):
| (B.2) |
The P50 is defined as before by Eq. (11) and the kinetic terms Kratio and Kfact are defined by Eqs. (14a) and (14b). The Hill exponent in the expression for SHbO2 is now 1 + n0 and apparent Hill coefficient is now independent of [O2]. This makes SHbO2 analytically invertible, when PCO2, pH, [DPG] and T are known. The inverted equation for [O2] is given by
| (B.3) |
It is clear from Eq. (B.2) that can be determined completely using only the P50 = P50 ([H+], [CO2], [DPG], T) data which is given by Eq. (11). This avoids the calculations of Kratio and Kfact which involves the complex computations of the empirical indices n1, n2, n3, and n4 using Eqs. (16a) to (16d). This also simplifies the computation of SHbO2 from [O2] and vice versa significantly. However, in the computation of SHbCO2 from [CO2] and vice versa (not shown in this appendix), the calculations of Kfact and Kratio are essential as the P50 data for 50% HbCO2 saturation is not available in the literature.
Here Eq. (B.1) further suggests that, in nonstandard physiological conditions, SHbO2 can be computed from the original Hill’s equation (Eq. 1) by scaling the [O2] axis by a factor (see Eq. B.2) which is composite for the variations in pH, PCO2, [2,3-DPG], and temperature (T), as in Kelman18 and Severinghaus.33 The same conclusion is valid for the computation of SHbCO2 from [CO2].
The inversion procedure for determining PO2 and then total O2 content in whole blood from the observations on SHbO2, PCO2, pHpl, [DPG]bl, Hct, and T in blood is as follows:
Calculate [H+]pl = 10−pHpl, [H+]rbc = [H+]pl/Rrbc, pHrbc = −log[H+]rbc, and [DPG]rbc = [DPG]bl/Hct, where Rrbc = 0.69.
Calculate P50 = P50 (PCO2, pHrbc, [DPG]rbc, T) from Eq. (11).
Calculate [O2] from Eq. (B.3), and then PO2 = [O2]/αO2, where αO2 is given by Eq. (10a), and n0 is 1.7. This is the inversion step.
Calculate [Hb]bl = Hct × [Hb]rbc = Hct × 5.18 mM, assuming that the RBC has standard hemoglobin concentration of 5.18 mM and that there is no methemoglobin or other abnormal type of hemoglobin.
Calculate Wbl = Wpl (1 − Hcts) + Wrbc Hct = 0.94 (1 − Hct) + 0.65 Hct, as the fractional water content of blood.
Calculate [O2]bl = Wbl [O2]bl + 4 [Hb]bl SHbO2, as the total O2 content in whole blood in M. To convert from [O2]bl M to mL gaseous O2 per mL blood, use mL O2 gas/100 mL blood = 22,256 × 100/1000 × [O2]bl = 2225 × [O2]bl.
This procedure can be set up in a spreadsheet, programmable hand calculator, or in any computer program such as the JSim MML code (available from the website http://www.physiome.org/Models/GasTransport/ for Linux, Unix, Macintosh and Windows). JSim, the general modeling system, and extensive manuals for it, can be downloaded from http://www.physiome.org/jsim/ for free.
Footnotes
History: This article corrects the errors made in the publication of the original article (Dash RK and Bassingthwaighte JB. Blood HbO2 and HbCO2 dissociation curves at varied O2, CO2, pH, 2,3-DPG and temperature levels. Ann Biomed Eng 32(12):1676–1693, 2004.) when the publisher failed to send galley proofs to the authors for review. Corrections were listed as an appendix to a later publication (Dash RK and Bassingthwaighte JB. Simultaneous blood–tissue exchange of oxygen, carbon dioxide, bicarbonate and hydrogen ion. Ann Biomed Eng 34(7): 1129–1148. 2006), except for one in Eq. 11 where it should have been specified that the DPG (bisphosphoglycerate) concentration was 4.65 mM, not molar. The equations here match the model code downloadable at www.physiome.org/model/.
The online version of the original article can be found under doi:10.1007/s10439-004-7821-6.
REFERENCES
- 1.Adair GS. The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J. Biol. Chem. 1925;63:529–545. [Google Scholar]
- 2.Antonini E, Brunori M. Hemoglobin and Myoglobin in Their Reactions with Ligands. Amsterdam: North Holland; 1971. p. 436. [Google Scholar]
- 3.Austin WH, Lacombe E, Rand PW, Chatterjee M. Solubility of carbon dioxide in serum from 15 to 38°C. J. Appl. Physiol. 1963;18:301–304. doi: 10.1152/jappl.1963.18.2.301. [DOI] [PubMed] [Google Scholar]
- 4.Bauer C, Klocke RA, Kamp D, Forster RE. Effect of 2,3-diphosphoglycerate and H+ on the reaction of O2 and hemoglobin. Am. J. Physiol. 1973;224:838–847. doi: 10.1152/ajplegacy.1973.224.4.838. [DOI] [PubMed] [Google Scholar]
- 5.Baumann R, Bartels H, Bauer C. Handbook of Physiology, Sect 3: The Respiratory System. Vol IV. Gas Exchange. Bethesda, Maryland: American Physiological Society; 1987. Blood oxygen transport; pp. 147–172. [Google Scholar]
- 6.Buerk DG. An evaluation of Easton’s paradigm for the oxyhemoglobin equilibrium curve. Adv. Exp. Med. Biol. 1984;180:333–344. doi: 10.1007/978-1-4684-4895-5_32. [DOI] [PubMed] [Google Scholar]
- 7.Buerk DG, Bridges EW. A simplified algorithm for computing the variation in oxyhemoglobin saturation with pH, PCO2, T and DPG. Chem. Eng. Commun. 1986;47:113–124. [Google Scholar]
- 8.Easton DM. Oxyhemoglobin dissociation curve as expoexponential paradigm of asymmetric sigmoid function. J. Theor. Biol. 1979;76:335–349. doi: 10.1016/0022-5193(79)90316-3. [DOI] [PubMed] [Google Scholar]
- 9.Ellis RK. Letter to the editor: determination of PO2 from saturation. J. Appl. Physiol. 1989;67:902. doi: 10.1152/jappl.1989.67.2.902. [DOI] [PubMed] [Google Scholar]
- 10.Forster RE, Constantine HP, Craw MR, Rotman HH, Klocke RA. Reaction of CO2 with human hemoglobin solution. J. Biol. Chem. 1968;243:3317–3326. [PubMed] [Google Scholar]
- 11.Forster RE. Rate of reaction of CO2 with human hemoglobin. In: Forster RE, Edsall JT, Otis AB, Roughton FJW, editors. CO2: Chemical, Biochemical, and Physiological Aspects. Washington, D.C.: Scientific and Technical Information Division, Office of Technology Utilization, NASA; 1969. pp. 55–59. [Google Scholar]
- 12.Hedley-Whyte J, Laver MB. O2 solubility in blood and temperature correction factors for PO2. J. Appl. Physiol. 1964;19:901–906. doi: 10.1152/jappl.1964.19.5.901. [DOI] [PubMed] [Google Scholar]
- 13.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910;40:iv–vii. [Google Scholar]
- 14.Hill EP, Power GG, Longo LD. Kinetics of O2 and CO2 exchange. In: West JB, editor. Bioengineering Aspects of the Lung. New York: Marcel Dekker; 1977. pp. 459–514. [Google Scholar]
- 15.Hill EP, Power GG, Longo LD. A mathematical model of carbon dioxide transfer in the placenta and its interaction with oxygen. Am. J. Physiol. Cell Physiol. 1973;224:283–299. doi: 10.1152/ajplegacy.1973.224.2.283. [DOI] [PubMed] [Google Scholar]
- 16.Hill EP, Power GP, Longo LD. Mathematical simulation of pulmonary O2 and CO2 exchange. Am. J. Physiol. Cell Physiol. 1973;224:904–917. doi: 10.1152/ajplegacy.1973.224.4.904. [DOI] [PubMed] [Google Scholar]
- 17.Huang NS, Hellums JD. A theoretical model for gas transport and acid/base regulation by blood flowing in microvessels. Microvasc. Res. 1994;48:364–388. doi: 10.1006/mvre.1994.1062. [DOI] [PubMed] [Google Scholar]
- 18.Kelman GR. Digital computer subroutine for the conversion of oxygen tension into saturation. J. Appl. Physiol. 1966;21:1375–1376. doi: 10.1152/jappl.1966.21.4.1375. [DOI] [PubMed] [Google Scholar]
- 19.Kelman GR. Calculation of certain indices of cardiopulmonary function using a digital computer. Respir. Physiol. 1966;1:335–343. doi: 10.1016/0034-5687(66)90050-8. [DOI] [PubMed] [Google Scholar]
- 20.Kelman GR. Computer program for the production of O2–CO2 diagrams. Respir. Physiol. 1968;4:260–269. doi: 10.1016/0034-5687(68)90057-1. [DOI] [PubMed] [Google Scholar]
- 21.Kelman GR. Digital computer procedure for the conversion of PCO2 into blood CO2 content. Respir. Physiol. 1967;3:111–115. doi: 10.1016/0034-5687(67)90028-x. [DOI] [PubMed] [Google Scholar]
- 22.Klocke RA. Handbook of Physiology, Sect. 3: The Respiratory System. Vol IV. Gas Exchange. Bethesda, Maryland: American Physiological Society; 1987. Carbon dioxide transport; pp. 173–197. [Google Scholar]
- 23.Li Z, Yipintsoi T, Bassingthwaighte JB. Nonlinear model for capillary-tissue oxygen transport and metabolism. Ann. Biomed. Eng. 1997;25:604–619. doi: 10.1007/bf02684839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margaria R. A mathematical treatment of the blood dissociation curve for oxygen. Clin. Chem. 1963;9:745–791. [PubMed] [Google Scholar]
- 25.Margaria R, Torelli G, Pini A. A possible mathematical definition of the O2 dissociation curve from blood or Hb solution. Exp. Med. Surg. 1963;21:127–142. [PubMed] [Google Scholar]
- 26.O’Riordan JF, Goldstick TK, Vida LN, Honig GR, Ernest JT. Modelling whole blood oxygen equilibirum: comparison of nine different models fitted to normal human data. Adv. Exp. Med. Biol. 1985;191:505–522. doi: 10.1007/978-1-4684-3291-6_51. [DOI] [PubMed] [Google Scholar]
- 27.Popel AS. Theory of oxygen transport to tissue. Crit. Rev. Biomed. Eng. 1989;17:257–321. [PMC free article] [PubMed] [Google Scholar]
- 28.Roughton FJW, Deland EC, Kernohan JC, Severinghaus JW. Some recent studies of the oxyhemoglobin dissociation curve of human blood under physiological conditions and the fitting of the Adair equation to the standard curve. In: Rørth M, Astrup P, editors. Oxygen Affinity of Hemoglobin and Red Cell Acid Base Status; Proceedings of the Alfred Benzon Symposium IV Held at the Premises of the Royal Danish Academy of Sciences and Letters; 17–22 May, 1971; Copenhagen. Copenhagen: Munksgaard; 1972. pp. 73–81. [Google Scholar]
- 29.Roughton FJW. Handbook of Physiology, Section 3: Respiration. Volume I. Washington, D.C.: American Physiological Society; 1964. Transport of oxygen and carbon dioxide; pp. 767–825. [Google Scholar]
- 30.Roughton FJW, Severinghaus JW. Accurate determination of O2 dissociation curve of human blood above 98.7% saturation with data on O2 solubility in unmodified human blood from 0° to 37° C. J. Appl. Physiol. 1973;35:861–869. doi: 10.1152/jappl.1973.35.6.861. [DOI] [PubMed] [Google Scholar]
- 31.Salathe EP, Fayad R, Schaffer SW. Mathematical analysis of carbon dioxide transfer by blood. Math. Biosci. 1981;57:109–153. [Google Scholar]
- 32.Severinghaus JW. Blood gas calculator. J. Appl. Physiol. 1966;21:1108–1116. doi: 10.1152/jappl.1966.21.3.1108. [DOI] [PubMed] [Google Scholar]
- 33.Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J. Appl. Physiol.: Respirat. Environ. Exercise Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- 34.Severinghaus JW. Letter to the editor: RE determination of PO2 from saturation. J. Appl. Physiol. 1989;67:902. doi: 10.1152/jappl.1989.67.2.902. [DOI] [PubMed] [Google Scholar]
- 35.Severinghaus JW, Stupfel M, Bradley AF. Variations of serum carbonic acid pK′ with pH and temperature. J. Appl. Physiol. 1956;9:197–200. doi: 10.1152/jappl.1956.9.2.197. [DOI] [PubMed] [Google Scholar]
- 36.Sharan M, Singh MP. Equivalence between one step kinetics and Hill’s equation. J. Biomed. Eng. 1984;6:297–301. doi: 10.1016/0141-5425(84)90078-5. [DOI] [PubMed] [Google Scholar]
- 37.Siggaard-Andersen O, Wimberley PD, Göthgen I, Siggard-Andersen M. A mathematical model of the hemoglobin-oxygen dissociation curve of human blood and of the oxygen partial pressure as a function of temperature. Clin. Chem. 1984;30:1646–1651. [PubMed] [Google Scholar]
- 38.Singh MP, Sharan M, Aminataei A. Development of mathematical formulae for O2 and CO2 dissociation curves in the blood. IMA J. Math. Appl. Med. Biol. 1989;6:25–46. [Google Scholar]
- 39.Winslow RM, Samaja M, Winslow NJ, Rossi-Bernardi L, Shrager RI. Simulation of continuous blood O2 equilibrium over physiological pH, DPG, and PCO2 range. J. Appl. Physiol.: Respirat. Environ. Exercise Physiol. 1983;54:524–529. doi: 10.1152/jappl.1983.54.2.524. [DOI] [PubMed] [Google Scholar]
- 40.Winslow RM, Swenberg M-L, Berger RL, Shrager RI, Luzzana M, Samaja M, Rossi-Bernardi L. Oxygen equilibrium curve of normal human blood and its evaluation by Adair’s equation. J. Biol. Chem. 1977;252:2331–2337. [PubMed] [Google Scholar]