Abstract
Noroviruses (NoV) cause the great majority of epidemic nonbacterial gastroenteritis in humans. Expression of the capsid protein in recombinant systems, including insect and plant cells, yields assembly of virus-like particles (VLPs) that mimic the antigenic structure of authentic virions, and are relatively acid- and heat-stable. Norwalk virus (NV), the prototype NoV, has been studied extensively, and Norwalk virus-like particles (NVLPs) produced in insect cells and plants are immunogenic in mice and humans when delivered orally, stimulating the production of systemic and mucosal anti-NV antibodies. NVLPs are also highly immunogenic when delivered intranasally, provoking antibodies at levels similar to orally delivered VLP at much lower doses. Oral and nasal delivery of NVLPs efficiently produces antibodies at distal mucosal sites, which suggests that NVLPs could be used to deliver heterologous peptide antigens by production of genetic fusion chimeric capsid proteins. Examination of norovirus VLP surface structures and receptor binding motifs facilitates identification of potential sites for insertion of foreign peptides that will minimally affect the efficiency of VLP assembly and receptor binding. Thus, there is strong potential to use norovirus VLPs as vaccine-delivery vehicles.
Keywords: chimeric vaccines, intranasal delivery, mucosal antibody, mucosal immunity, multivalent vaccines, norovirus, oral delivery, vaccine platforms, virus-like particle, VLP
Noroviruses (NoV) are the most common agent of nonbacterial gastroenteritis in humans, being responsible for more than 95% of epidemic viral gastroenteritis in adults [1–5]. The virus is highly infectious, and outbreaks are associated with various social settings, including catered events, restaurants, schools and cruise ships, as well as nursing homes and daycare centers. In addition, there are several high-risk groups that include young children, the elderly, military personnel and immunocompromised individuals.
Norovirus variants are numerous: a recent paper proposed a classification system based on amino acid sequences of the major capsid protein from 164 different strains, with 29 genetic clusters distributed among five genogroups [6]. The most prevalent are genogroups I and II (GI and GII), and GII.4 viruses are currently circulating widely [7–9].
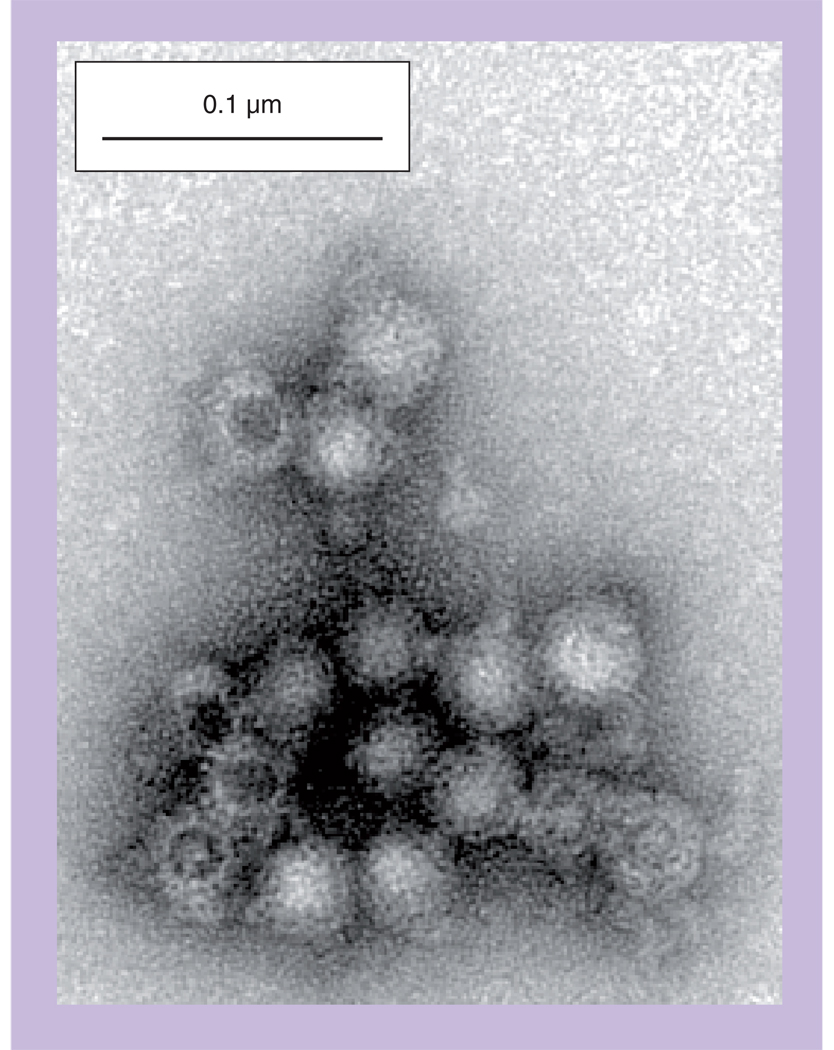
The NoV genomes are single-stranded, positive-sense RNA containing three open-reading frames and a poly(A) tail, as first characterized in the prototype Norwalk virus (NV) [10]. The NV capsid is a 38-nm icosahedral arrangement of 90 dimers of the 58-kDa capsid protein VP1 in a T = 3 symmetry [11,12]. Expression of recombinant NoV capsid proteins in insect cells using baculovirus vectors [13,14] and in plants using tobamovirus [15] and geminivirus [16] vectors showed that VP1 alone can self-assemble virus-like particles (VLPs) that antigenically and morphologically resemble authentic virus particles (Figure 1). The plant-derived VLPs appear to be very similar to insect cell-derived particles. A recent paper used VLPs generated from 26 different GI and GII NoV strains to evaluate the cross-reactivity of antisera produced in rabbits [13].
Figure 1. Virus-like particles assembled from Norwalk virus capsid protein expressed in plants.
Norwalk virus capsid protein was transiently expressed in leaves of Nicotiana benthamiana using a geminiviral vector [16]. The leaf extract was sedimented on a sucrose gradient, and the peak virus-like particle fraction collected and examined by transmission electron microscopy following negative staining with uranyl acetate. The particles observed are very similar to those expressed in insect cells [21].
Furthermore, VLPs were used to show that NoV bind in a strain-dependent manner to carbohydrate histo-blood group antigen (HBGA) displayed on mucosal cell surfaces [17–20]. Recent studies have suggested a correlation between susceptibility, secretor status (secretor enzyme α [1,2] fucosyltransferase) and HBGA profile, suggesting a role for HBGA as putative receptors for NoV as with many other pathogens that utilize similar carbohydrates as receptors [21]. In addition, the strong correlation between genetic polymorphisms in potential receptor genes on host susceptibility or resistance to microbes is high-lighted by the well-established link between resistance to HIV and polymorphisms in the C-C chemokine receptor type 5, later found to be a coreceptor for HIV [21].
There is substantial interest in developing NoV vaccines based on VLPs, especially in view of the success of VLP vaccines against human papillomaviruses [22,23]. VLPs are highly immunogenic because they efficiently trigger B- and T-cell responses [24]. The ordered and repetitive VLP surface promotes activation of B cells and binding of antibodies, thus enhancing uptake by antigen-presenting cells (APCs). Moreover, APC uptake is enhanced by the macromolecular structure of VLP. A variety of studies (reviewed later) demonstrate the immunogenicity of Norwalk VLP (NVLP) delivered by oral or nasal routes, and thus the potential for vaccine development [25–28]. Although rapid evolution of new NoV strains presents a ‘moving target’ for vaccine development, the problem is similar to that encountered with influenza viruses. Analogous to influenza viruses, NoV accumulate genetic point mutations in the capsid protein that may result in unique antibody-binding sites [29]. This antigenic drift results in diverse strains that would potentially escape immunity against a previously vaccinated strain, especially between genogroups where there is little cross-reactivity [30]. Thus, vaccines may require reformulation each year as updated NoV epidemiological data allow identification of the most prevalent strains that could be used as a reference vaccine strain(s) to provide optimal protection [5,7,21,30].
Immunogenicity of NVLP in mice
Insect cell-derived NVLPs
Many studies have demonstrated serum and mucosal antibody responses in animals against NVLPs that were delivered orally or intranasally [25–27]. The convenience of oral delivery is an attractive strategy because NVLPs, which are the result of evolutionary selection for enteric infection, are stable at low pH and thus can survive the harsh gastric environment [14,31]. Studies on insect cell-derived NVLPs showed they are stable over a pH range of 3–7 and at temperatures up to 55°C [31]. Ball and colleagues examined oral doses of insect cell-derived NVLPs between 5 and 500 µg, either with or without the mucosal adjuvant cholera toxin (CT; 10 µg) delivered by gastric intubation in CD1 outbred mice [25]. Even without CT, 5 µg doses on days 1, 2, 11 and 28 provoked serum anti-NV IgG in eight out of 11 mice, indicating the strong mucosal immunogenicity of NVLP. Maximal serum anti-NV geometric mean titers (GMT; 1168) were observed at 200 µg NVLP doses without CT, while inclusion of CT adjuvant increased GMT to 6400 [18]. Moreover, substantial amounts of NV-specific intestinal IgA (up to 0.1% of total IgA) were measured in the fecal extracts of mice immunized orally with 200 µg doses. These data indicate that NVLPs are potent oral immunogens in mice, and provoke local antibody responses that can potentially neutralize NV and inhibit infectivity.
Another study compared intranasal and oral delivery of insect cell NVLPs in mice [26]. Intranasal delivery of two 10-µg doses of NVLP (on days 0 and 21) without adjuvant elicited 100% IgG seroconversion, and anti-NV titers at this dose were greatly enhanced by codelivery of the adjuvant LTR192G (mutant enterotoxin similar to CT). Similar serum IgG responses were observed with 200 µg NVLP delivered orally, indicating that intranasal delivery induces more potent responses at a low dose. Furthermore, these immunization regimens produced strong mucosal anti-NV IgA responses in feces and in vaginal washes, which were still detectable after 1 year in the adjuvanted groups [26]. This finding supports the use of NVLP as carriers of heterologous epitopes, for example from sexually transmitted pathogens, which can be delivered intranasally in order to generate neutralizing antibodies in the reproductive mucosa.
Stable transgenic plant-derived NVLP
The use of plants for expression of NVLP allows the possibility of oral delivery of edible tissues without the expense of purification. NVLP expressed in transgenic potato tubers and fed directly to mice (~40–80 µg doses in 4 g raw tuber at days 1, 2, 11 and 28) provoked serum anti-NV at titers up to 200 [32]. In the same study, purified NVLP expressed in transgenic tobacco leaves delivered orally at 50 µg doses yielded serum anti-NV titers up to 800, or up to 3200 when CT adjuvant was used. Since only approximately 50% of the Norwalk virus capsid protein (NVCP) in the potato tubers was assembled into VLP [32], the benefit of purification of VLP is obvious. It is also possible that NVLP contained in the potato tissue was not as effectively delivered to the gut-associated lymphoid tissue as purified VLP delivered by gavage.
Later, a plant-optimized NVCP gene was used to produce NVLP in transgenic tomato fruit in higher yields of 100 µg per gram of freeze-dried fruit [28]. Four doses (days 1, 4, 17 and 20) of 0.8 g freeze-dried tomato fruit reconstituted in water and fed to mice resulted in 100% seroconversion, with anti-NV IgG GMT of 350, and also stimulated anti-NV intestinal IgA in all mice. Interestingly, delivery of 0.8 g of air-dried NV tomato fruit by ingestion provoked substantially higher serum anti-NV IgG with a GMT of 4400, and higher fecal IgA titers than the freeze-dried fruit-fed mice [28]. It is unclear what physical attribute of the air-dried fruit caused the enhanced immunogenicity, but it is possible that freeze-drying altered the stability of NVLP in tomato fruit. Alternatively, air-drying fruit instead of freeze-drying may preserve the tissue structure in a way that provides greater protection from proteolytic enzymes in the gut until the NVLP can be taken up into the gutassociated lymphoid tissue [28]. Regardless of how the tomato fruit is dried, this plant provides a good system for expression of orally immunogenic NVLP.
Transient expression with plant viral vectors
It should be noted that the use of food plants for the production of pharmaceutical proteins like vaccines has generated substantial concern among regulatory agencies [33]. It is generally agreed that the necessary regulatory and good manufacturing practice requirements are already in place for plant-derived vaccines. However, fears persist that noncompliance events could compromise food security. Thus, many plant vaccine workers have shifted more effort to the use of non-food plants (tobacco and relatives) for production of vaccine antigens. The change is facilitated by the development of robust transient expression using plant virus replicons [16,34,35], which provide expression levels that support purification of antigens. The tobacco relative Nicotiana benthamiana is a favorite host for the viral vectors. NVCP was obtained at levels of 800 mg per kg of leaf tissue using a tobacco mosaic virus RNA replicon system, and the purified NVLP were orally immunogenic in outbred CD1 mice at 100 µg per dose without adjuvant [15]. A DNA replicon from the geminivirus bean yellow dwarf virus generated NVCP at approximately 400 mg per kg of leaves in N. benthamiana [16]. These studies and purification of NVLP from plant leaves are discussed further below.
Mammalian cell-derived NVLP using Venezuelan encephalitis virus replicons
Venezuelan encephalitis virus replicon particles (VRPs) have been utilized as vectors for expression of NVLP in mammalian cells and as potential vaccines [36–38]. The system is analogous in many respects to the viral replicon vectors for insect and plant cells that we described earlier, and generates a high level of expression of inserted genes. The VRP system can be used both as a means to generate NVLP that can be purified and delivered as immunogens, or as a vaccine itself that replicates and produces recombinant NVLP in target cells, which can then process and present antigenic peptides to T and B cells [37]. Subcutaneous (footpad) inoculation of NVCP VRP in mice (two doses, 107 infectious units each) provoked strong serum anti-NV IgG and intestinal IgA responses that were substantially higher than those induced by oral delivery of 75 µg VRP-generated NVLP [37]. Thus, the VRP system is a functional alternative to insect- or plant-based production of NVLP, although direct comparisons of efficiency and costs of production have not been made.
Clinical trials with NVLP
Clinical studies using NVLP show that the material is safe and immunogenic in humans (Table 1). An initial clinical study by Ball et al. was the first report that demonstrated that NVLPs were safe and immunogenic when delivered by the oral route [39]. In a subsequent study, transgenic potatoes described previously [32] were fed to human volunteers in doses of 150 g of sliced raw tubers on days 0 and 7, or on days 0, 7 and 21 [40]. The NVCP content of tubers was variable (215–750 µg per dose) and VLP content was up to 325 µg per dose. Nonetheless, 19 out of 20 subjects who ingested transgenic potato developed measurable, albeit relatively weak, increases in anti-NV antibody-secreting cells and the majority of those subjects mounted a response following the first dose. Overall, 20% of subjects produced serum anti-NV IgG (12-fold mean increase), and 30% produced fecal anti-NV IgA (17-fold mean increase) (Table 1). A later trial using purified insect cell-derived NVLP examined antibody responses to oral doses of 250, 500 or 2000 µg [41]. Interestingly, there was no apparent dose-related effect, as all doses induced similar anti-NV antibody levels (serum IgG and mucosal IgA). The responses at 250 µg per dose were stronger than those obtained from fed potato [40], which suggests presentation of NVLP in the potato tissue was detrimental. Another interesting finding of the later study was that cell-mediated responses, as measured by IFN-γ production by peripheral blood mononuclear cells stimulated with NVLP, increased inversely with the dose, with the 2000 µg dose showing no increase in response. The reason for this finding is unclear, but the authors emphasized that it is consistent with the lack of a dose–response effect on antibody production (Table 1). It is possible that the high dose induced regulatory T cells that mediate tolerance [42], but this is pure speculation in the absence of any data from NVLP-immunized individuals.
Table 1.
Immunogenicity of Norwalk virus-like particle produced in plants and insect cells following mucosal delivery in Phase I trials.
| Study (year) |
Production system for NVLP |
Number of subjects/ group (n) |
Dosage of NVCP (µg) |
Formulation (adjuvant) |
Number of doses and route of administration |
Serum IgG GMT peak titer (response rate %) |
Serum IgA GMT peak titer (response rate %) |
Salivary IgA GMT peak titer (response rate %) |
Stool IgA GMT peak titer (response rate %) |
Vaginal IgA GMT peak titer (response rate %) |
Semenal IgA GMT peak titer (response rate %) |
Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ball et al. (1999) |
Baculovirus | 5 | 100 | Liquid in Milli-Q water (none) |
Two oral doses (days 1 and 21) |
320 (60%) | 90 (80%) | nd | (0%)† | nd | nd | [39] |
| Ball et al. (1999) |
Baculovirus | 15 | 250 | Liquid in Milli-Q water (none) |
Two oral doses (days 1 and 21) |
453 (100%) | 249 (80%) | nd | nr (10%) | nd | nd | [39] |
| Tacket et al. (2000) |
Transgenic potato |
10 | ~215– 542‡ |
Diced, raw potato (none) |
Two oral doses (days 1 and 21) |
3200 (10%) | nd | nd | 38 (20%) | nd | nd | [40] |
| Tacket et al. (2000) |
Transgenic potato |
10 | ~215– 542‡ |
Diced, raw potato (none) |
Three oral doses (days 0, 7, 21) |
468 (30%) | nd | nd | 48 (40%) | nd | nd | [40] |
| Tacket et al. (2003) |
Baculovirus | 10 | 250 | Liquid in Milli-Q water (none)§ |
Two oral doses (days 0 and 21) |
3140 (90%) | nr (90%) | 541 (40%) | 977 (28.5%) |
2256 (80%) | 1913 (25%) |
[41] |
| Tacket et al. (2003) |
Baculovirus | 10 | 500 | Liquid in Milli-Q water (none)§ |
Two oral doses (days 0 and 21) |
2670 (70%) | nr (60%) | 319 (30%) | 1245 (42.9%) |
265 (66.7%) |
(0%)† | [41] |
| Tacket et al. (2003) |
Baculovirus | 10 | 2000 | Liquid in Milli-Q water (none)§ |
Two oral doses (days 0 and 21) |
2731 (80%) | nr (100%) | 308 (50%) | 454 (30%) | (0%)† | 460 (25%) | [41] |
| El-Kamary et al. (2007) Tacket et al. (2009) |
Baculovirus | 5 | 5 | Dry powder containing chitosan (MPL at 25 µg) |
Two doses intranasally (days 0 and 21) |
nr (0%) | nr (0%) | nr | nr | nd | nd | [60,61] |
| El-Kamary et al. (2007) Tacket et al. (2009) |
Baculovirus | 5 | 15 | Dry powder containing chitosan (MPL at 25 µg) |
Two doses intranasally (days 0 and 21) |
nr (20%) | nr (40%) | nr | nr | nd | nd | [60,61] |
| El-Kamary et al. (2007) Tacket et al. (2009) |
Baculovirus | 5 | 50 | Dry powder containing chitosan (MPL at 25 µg) |
Two doses intranasally (days 0 and 21) |
nr (75%) | nr (25%) | nr | nr | nd | nd | [60,61] |
| Tacket et al. (2009) |
Baculovirus | 20 | 50 | Dry powder containing chitosan (MPL at 25 µg) |
Two doses intranasally (days 0 and 21) |
nr (56%) | nr (72%) | nd | nd | nd | nd | [61] |
| Tacket et al. (2009) |
Baculovirus | 20 | 100 | Dry powder containing chitosan (MPL at 25 µg) |
Two doses intranasally (days 0 and 21) |
nr (63%) | nr (79%) | nd | nd | nd | nd | [61] |
No converters.
150 g of potato corresponded to ~215–542 µg NVCP.
Preceded by a sodium bicarbonate buffer (500 mg in 60 ml of milli-Q water).
GMT: Geometric mean titer; MPL: Monophosphoryl lipid A; nd: Not determined; nr: Not reported; NVCP: Norwalk virus capsid protein; NVLP: Norwalk virus-like particle.
Two Phase I clinical trials have been conducted utilizing intranasal delivery of a dry powder formulation of baculovirus-derived NVLP containing chitosan (ChiSys®; Archimedes Development Limited, Nottingham, UK), a muco-adhesive, and a TLR4 agonist adjuvant, monophosphoryl lipid A (MPL™ [GSK, Middlesex, UK]). The data have not been published, but have been presented at international conferences (the 3rd International Calicivirus in Cancun, Mexico in 2007, and ‘Vaccines for Enteric Diseases’ in Malaga, Spain, 2009) and intranasal delivery of NVLP has been reported to be safe and immunogenic at higher than expected doses (see Table 1). A Phase III trial has been initiated to determine the safety and immunogenicity of intranasally delivered NVLP, followed by a live virus challenge to determine if this is a viable vaccine approach to preventing or limiting NV infection in humans [Robert Atmar, Pers. Comm.] [101].
Plant-based production & purification of NVLP
In the last decade, significant progress has been made in the enhancement of plant-based recombinant protein expression, especially with the development of virus-based transient expression systems [33,35,43,44], and specifically for the production of NVLP [15,16,34]. As expression levels increase, purification costs become an increasingly significant proportion (>80%) of the total cost of production of NVCP and other vaccines [45–47]. Thus, much attention has shifted to the development of technologies for efficient NVLP purification and delivery. While processed (e.g., dried) plant-derived antigens still represent a viable opportunity to deliver vaccines by ingestion, regulatory concerns motivate the development of processing technologies to produce NVLP with a more rigorously defined unit dosage [45]. A well-developed downstream process will increase manufacturing productivity, reduce cost of operations, enhance scalability for processing large volumes of NVLP preparations with high yield, preserve the stability of the VLP, and ensure the compliance of the manufacturing procedures with the US FDA’s current Good Manufacturing Practices (cGMP) regulations. Similar to other pharmaceutical proteins, the goal of downstream processing is to recover the maximum amount of highly purified NVLP with a minimal number of steps, including tissue harvesting, protein extraction, purification and product formulation.
Despite the employment of the different production systems and the availability of diverse methods for VLP extraction and purification, NVLPs have been produced based only on a few variations of centrifugation and precipitation methods [12,14,15,41]. The centrifugation-based purification methods were originally used to isolate viruses, but later expanded to VLP purification. VLPs, like their native viruses, are purified on the basis of their size and density using ultracentrifugation techniques, with sucrose and cesium chloride (CsCl) being the most commonly used reagents for gradient generation [48]. Ultracentrifugation and density gradient methods are useful analytical tools for characterizing VLP size, assembly status, and effective isolation of bench-scale quantities of NVLP for research [15,31,41]. However, they are not practical for large-scale commercial vaccine manufacture because they are time-consuming, difficult to scale up and produce poor yields [49]. Furthermore, density gradient-based methods have the added complication of requiring specific technical expertise for their preparation.
To circumvent the limitations of traditional VLP purification methods, we and others have been working to develop alternative downstream processing strategies, aiming to optimize the scalability and robustness of VLP purification. We have explored chromatography for its ability to achieve high purity and recovery rates, and its facile adaptability for scaling up and cGMP manufacturing [45,50,51]. Ongoing research by our group has established a scalable purification process for NVLP based on low pH precipitation and conventional chromatographic steps [45]. This purification procedure can produce fully assembled VLP with high product purity (>98%), yet eliminates the laborious and time-consuming steps of sucrose and CsCl gradients, and can be carried out in 12 h or less rather than several days [Chen Q, Unpublished Data]. NVLP purified by this process has been shown to maintain VLP structure and immunogenicity in mice following mucosal administration [Herbst-Kralovetz et al., Manuscript in Preparation]. Standard operating procedures based on this purification scheme have been established and used in cGMP production of NVLP for anticipated clinical trials.
To further optimize the recovery and binding capacities of VLP, including NVLP, novel chromatographic matrices and strategies are being developed to accommodate their unusually large size. For example, membrane chromatography is increasingly being explored as the leading alternative to conventional chromatographic resins for VLP purification [52,53]. Membrane chromatography is particularly useful for purification of VLPs or other large molecules with low diffusivities because the interaction of the binding sites on the membrane and the target molecules occurs in convective flow-through pores, instead of inside diffusional pores of classical beads [53].
One of the factors for the ultimate clinical success of norovirus VLP (NoVLP) vaccines will depend on the availability of efficient large-scale downstream manufacturing processes, in which significant advances for VLP have been made in recent years. While general purification methods can be adapted from one decorated NoVLP to another, there will be no single universal purification scheme that can be exploited for a broad range of decorated NoVLP products that have diverse physicochemical structures and properties [54]. Specific adjustments must be made to the purification process to accommodate the final VLP size and charge, while excluding host-contaminating proteins. Nevertheless, novel chromatography materials and technologies are being developed, with promising results for robust, cost-effective and cGMP-compliant VLP extraction and purification.
Potential for heterologous epitope display on NVLP
The potent immunogenic nature of NVLP delivered at mucosal surfaces (oral and intranasal) and the induction of anti-VLP secretory IgA at distal mucosal sites suggest that VLPs can be used as a platform to display heterologous peptide epitopes [25–28]. It is preferred that chimeric NVCP fusion polypeptides efficiently assemble VLPs, in order to benefit from the immunogenicity-enhancing features conferred by the repeating structure and particulate form. Thus, it is useful to consider what is known about the NVLP assembly process.
Prasad et al. suggested a model for assembly of the 38-nm T = 3 icosahedral NVLP, in which monomer subunits must conformationally adapt to three quasi-equivalent positions termed A, B and C [11,12]. In the first step of assembly, dimers of two types (‘bent’ A/B and ‘flat’ C/C) form and may reversibly interconvert. Five A/B dimers then form a pentamer that constitutes a capsomer, which are then joined together by the binding of C/C dimers to form the icosahedral VLP [11]. Deletion studies showed that the removal of at least 34 N-terminal amino acids of NVCP abolished VLP formation, and deletion of 20 C-terminal residues affected the size and stability of VLPs [55]. Complete removal of the surface protruding (P) domain, leaving the internal shell (S) domain, produced ‘smooth’ 30 nm VLP.
Expression of ‘P particles’ in E. coli was achieved by using fusion protein technology [56]. The NoV GII.4 strain VA387 capsid protein P domain was fused to glutathione-S-transferase and proteolytically cleaved to release P domains, which formed 5-nm VLPs termed P particles, thought to be T = 1 icosahedrons. Interestingly, previous studies that had included the hinge region between the S and P domains resulted in the formation of dimers, but not particles [57]. These workers found that including end-linked cysteine residues allowed the formation of intermolecular disulfide bonds that stabilize the P particle [56]. Although this material has not been tested for immunogenicity, P particles have been used to evaluate binding to human HBGA [18,58] and determine the structure of the P particle bound to HBGA carbohydrate molecules [20,59]. The crystal structure identified amino acids in the P domain that participate in carbohydrate binding.
With the available structural information, one can design possible insertion sites for heterologous peptides that avoid disruption of dimer contacts, which could affect the efficiency of VLP assembly, and receptor-binding residues, which might affect the efficiency of VLP uptake at mucosal sites. We have used these data to predict potentially effective sites for peptide insertions that would allow display of heterologous antigens on the VLP surface. The NVLP crystal structure [11] shows several potential surface-exposed loops that could tolerate insertions or substitutions for epitope display (Table 2). Most of these lie in the P2 subdomain, and constitute loops that link sequential β-strands of the secondary structure [11,12]. Some loops contain residues that are intimately involved in the associations between individual subunits that constitute dimer contacts (Table 2) [11]. Seven residues in the NoV VA387 capsid protein are involved in HBGA carbohydrate binding, and these amino acids could be mapped to the NVCP sequence via structure-based sequence alignment [59]. We indicate such residues of the NVCP sequence that occur in surface loops in Table 2.
Table 2.
Surface loops in Norwalk virus capsid protein.
| Loop | Sequence | Dimer/sugar contact? |
|---|---|---|
| P2/β1–β2 | G296TV-I299 | No |
| P2/β3–β4 | Q334FGHSSQT341 | Yes/yes |
| P2/β4–β5 | T347TPDT351 | No |
| P2/β5–β6 | A361NGIGS366 | No/yes |
| P2/β6–P1b | S377PPSHP382 | No |
| P1/β4–β5 | P426GPGA430 | Yes/yes |
From these considerations, the surface loops that do not contain either dimer or receptor contacts are P2/β1–β 2, P2/β4–β5 and P2/β6–P1b. In addition, loop P2/β5–β6 contains no dimer contacts, and only a single residue (G363) that is predicted to participate in receptor binding, although this is based on sequence alignment and has not been confirmed by structural data. We have constructed NVCP fusions that insert peptides of nine or 15 amino acids at G363 (P2/β5–β6), P378 (P2/β6–P1b) and G427 (P1/β4–β5), expressed them in N. benthamiana leaf, and showed that they all assemble VLPs that display the peptides [Mason et al., Manuscript in Preparation]. Although immunogenicity testing will more definitively demonstrate the utility of chimeric NVLP as carriers of heterologous antigens, current data indicate substantial potential for this strategy.
There may also be potential for NVLP to carry substantially larger protein antigens fused to the N terminus, which would be sequestered within the hollow center of the VLP. Although these antigens would not be expected to act as potent B-cell epitopes, since the antigens would not be displayed on the surface of the VLP, they could potentially be effective T-cell epitopes by virtue of efficient uptake of VLP by APCs. Thus, NVLP might be used to generate cytotoxic T-cell responses to fused antigens.
Obstacles in NoV vaccine development are multifactorial
Norovirus utilizes a human reservoir, requires a very low infectious dose, is stable in the environment (the virus can withstand conditions ranging from freezing to 60°C), lacks long-term immunity, rapidly evolves new variant strains and has multiple routes of transmission – all factors that contribute to the persistence of this virus in the population [21]. Furthermore, an incomplete understanding of the immune correlates of protection due to the lack of a small, convenient animal model and the lack of in vitro assays to cultivate the virus give rise to difficulties in evaluating the level of protection afforded by a candidate vaccine. Limited cross-protection to NoV genotypes within the same genogroup is observed, however there is very little cross-protection between genogroups; as such, multivalent vaccines will probably be necessary to protect susceptible individuals. There is some debate as to what molecular and antigenic changes confer a novel variant strain. Each of these factors contributes to the challenges in the development of robust, safe and efficacious NoV vaccines.
Conclusions & expert commentary
Norovirus VLPs have been shown to elicit potent systemic and mucosal antibody responses when delivered orally or intranasally in mice and humans. Human testing by oral delivery of NVLP indicates robust production of anti-NV serum and intestinal antibodies, as well as IFN-γ in peripheral blood mononuclear cells. In addition, intranasal delivery of NVLP in humans appears to be safe and immunogenic [60,61]. Owing to the highly variable capsid proteins among different NoV strains, NoV vaccine production must employ vigilant epidemiological surveillance in order to select the most prevalent strains to include in the formulation, akin to the practice already used for influenza virus vaccines. The CDC has recently launched the CaliciNet, a database of NoV genetic sequences identified from outbreaks of NoV that could potentially aid in establishing links between outbreaks and perhaps ultimately predict circulating strains season to season, similar to influenza. Currently, NoV genogroup II.4 strains are the most prevalent [4,7], and thus vaccine work should focus on the production of VLPs for this group.
There appears to be some potential for the use of NoVLP as platforms to display and present heterologous peptide antigens via genetic fusions. Exposed surface loops have been identified, and structural data are available to facilitate the selection of sites that will minimize adverse effects on VLP assembly and HBGA binding. Preliminary data on such fusions in the Mason laboratory indicate that at least three of the NVCP loops can tolerate insertions of up to 15 amino acid residues and still efficiently assemble VLPs that display the epitope upon expression in plant leaves. Presumably, such chimeric NVLPs can also be expressed in insect cells, and capsid proteins from other strains of NoV could be similarly utilized. Studies to determine the immunogenicity of these chimeric NVLP constructs are ongoing.
Five-year view
Norwalk virus-like particle-based vaccines have been studied in Phase I trials and have been shown to be safe and immunogenic; however, the question remains as to whether these vaccines can protect individuals against a live virus challenge. In the next 5 years, there should be data from planned and ongoing Phase I/II challenge trials that will address whether the use of recombinant NVLP can prevent or limit NV infection. These studies will be pivotal in providing insights into the immune correlates of protection, a question that has evaded researchers since the discovery of the virus. Several caveats to the efficacy of a challenge trial versus natural infection include the selection of viral challenge dose, genetic host susceptibility, route of immunization and baseline seropositivity. If this strategy is efficacious, then the next step will be to identify the optimal formulation and most cost-effective production system for addressing the antigenic drift associated with NoV that will most likely necessitate annual evaluation/adaptation to new vaccine strains as they emerge in the population. In this regard, the plant transient expression systems will provide a valuable resource for rapid and robust VLP production.
Key issues
Key challenges to norovirus (NoV) vaccine development include the lack of a small animal model of NoV pathogenesis or in vitro NoV cell culture system, an incomplete understanding of the immune correlates of protection, the lack of long-term immunity, the lack of complete cross-protection, and antigenic drift of the capsid protein resulting in evolution of new strains that can infect susceptible hosts.
The immune correlates of protection need to be established for NoV to accelerate vaccine development.
The route of administration (oral, intranasal or parenteral) could impact the level of protection afforded by NoV-like particle-based vaccines, depending on the correlates of immunity and/or the amount of virus to which the individual is exposed.
Vaccine efficacy has not been determined for Norwalk virus-like particle-based vaccines.
Future human challenge studies must pay careful attention to several key factors: host genetic susceptibility with regard to expression of the major histo-blood group antigens (ABO, Lewis and secretor families) to the strain being tested, the viral dose with which volunteers are challenged (as there may be a threshold at which infection will occur), and the consideration of baseline seropositivity.
Epidemiological studies have indicated that NoV transmission is similar to that of the influenza virus in that it can occur both rapidly in a local area and globally owing to the emergence of new variant strains.
There is a need for accurate and timely epidemiological information that includes disease burden, genetic diversity and geographical distribution.
A multivalent NoV vaccine approach will most likely be required to provide optimal protection against circulating strains.
Epidemic surveillance will be key in predicting predominant strains and identifying a potential reference vaccine strain each year, similar to influenza.
Cost-effective and efficient production systems to manufacture Norwalk virus-like particle-based vaccines will be crucial to addressing the requirement to quickly adapt to novel, emerging strains.
Footnotes
Financial & competing interests disclosure
This work was supported in part by NIH grant NIH U19-AI062150. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Melissa Herbst-Kralovetz, Center for Infectious Diseases and Vaccinology, Biodesign Institute at Arizona State University, Tempe, AZ 85287, USA and Dept of Basic Medical Sciences, The University of Arizona College of Medicine-Phoenix in Partnership with Arizona State University, Phoenix, AZ 85004, USA, melissa.herbst-kralovetz@asu.edu.
Hugh S Mason, Center for Infectious Diseases and Vaccinology, Biodesign Institute at Arizona State University, PO Box 875401, Tempe, AZ 85287-5401, USA and School of Life Sciences, Arizona State University, Tempe, AZ, USA, Tel.: +1 480 727 8228, Fax: +1 480 727 6194, hugh.mason@asu.edu.
Qiang Chen, Center for Infectious Diseases and Vaccinology, Biodesign Institute at Arizona State University, Tempe, AZ 85287, USA and Department of Applied Biological Sciences, Arizona State University, Mesa, AZ 85212, USA, shawn.chen@asu.edu.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1. Fankhauser RL, Noel JS, Monroe SS, Ando T, Glass RI. Molecular epidemiology of “Norwalk-like viruses” in outbreaks of gastroenteritis in the United States. J. Infect. Dis. 1998;178(6):1571–1578. doi: 10.1086/314525. • Documents the prevalence of viral gastroenteritis caused by noroviruses (NoV) in the USA.
- 2.Patel MM, Hall AJ, Vinje J, Parashar UD. Noroviruses: a comprehensive review. J. Clin. Virol. 2009;44(1):1–8. doi: 10.1016/j.jcv.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Koopmans M. Progress in understanding norovirus epidemiology. Curr. Opin Infect. Dis. 2008;21(5):544–552. doi: 10.1097/QCO.0b013e3283108965. [DOI] [PubMed] [Google Scholar]
- 4. Lopman B, Vennema H, Kohli E, et al. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363(9410):682–688. doi: 10.1016/S0140-6736(04)15641-9. • Documents the prevalence of viral gastroenteritis caused by NoV in Europe.
- 5.Patel MM, Widdowson MA, Glass RI, Akazawa K, Vinje J, Parashar UD. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Emerg. Infect. Dis. 2008;14(8):1224–1231. doi: 10.3201/eid1408.071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, Monroe SS. Norovirus classification and proposed strain nomenclature. Virology. 2006;346(2):312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 7. Lindesmith LC, Donaldson EF, Lobue AD, et al. Mechanisms of GII.4 norovirus Norwalk virus-like particles as vaccines persistence in human populations. PLoS Med. 2008;5(2):e31. doi: 10.1371/journal.pmed.0050031. •• Demonstrates that as NoV evolves, the variants bind differently to receptor molecules.
- 8.Siebenga J, Kroneman A, Vennema H, Duizer E, Koopmans M. Food-borne viruses in Europe network report: the norovirus GII.4 2006b (for US named Minerva-like, for Japan Kobe034-like, for UK V6) variant now dominant in early seasonal surveillance. Euro Surveill. 2008;13(2):8009. [PubMed] [Google Scholar]
- 9. Siebenga JJ, Vennema H, Renckens B, et al. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J. Virol. 2007;81(18):9932–9941. doi: 10.1128/JVI.00674-07. •• Reported epochal NoV evolution similar to influenza.
- 10.Jiang X, Wang M, Wang K, Estes MK. Sequence and genomic organization of Norwalk virus. Virology. 1993;195(1):51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- 11. Prasad BVV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. •• Pivotal study that elucidated Norwalk virus (NV) capsid structure.
- 12.Prasad BVV, Hardy ME, Estes MK. Structural studies of recombinant norwalk capsids. J. Infect. Dis. 2000;181(s2):S317–S321. doi: 10.1086/315576. [DOI] [PubMed] [Google Scholar]
- 13.Hansman GS, Natori K, Shirato-Horikoshi H, et al. Genetic and antigenic diversity among noroviruses. J. Gen. Virol. 2006;87(Pt 4):909–919. doi: 10.1099/vir.0.81532-0. [DOI] [PubMed] [Google Scholar]
- 14. Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 1992;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. • First study to show virus-like particle (VLP) assembly of recombinant NV capsid protein (NVCP).
- 15.Santi L, Batchelor L, Huang Z, et al. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine. 2008;26(15):1846–1854. doi: 10.1016/j.vaccine.2008.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang Z, Chen Q, Hjelm B, Arntzen C, Mason H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol. Bioeng. 2009;103(4):706–714. doi: 10.1002/bit.22299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutson AM, Atmar RL, Marcus DM, Estes MK. Norwalk virus-like particle hemagglutination by binding to h histo-blood group antigens. J. Virol. 2003;77(1):405–415. doi: 10.1128/JVI.77.1.405-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13(6):285–293. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Huang P, Farkas T, Marionneau S, et al. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J. Infect. Dis. 2003;188(1):19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 20. Choi JM, Hutson AM, Estes MK, Prasad BV. Atomic resolution structural characterization of recognition of histo-blood group antigens by Norwalk virus. Proc. Natl Acad. Sci. USA. 2008;105(27):9175–9180. doi: 10.1073/pnas.0803275105. •• Demonstrated that histo-blood group antigens bind to the same surface-exposed and highly conserved capsid site in genogroup I noroviruses.
- 21.Glass RI, Parashar UD, Estes MK. Norovirus gastroenteritis. N. Engl. J. Med. 2009;361(18):1776–1785. doi: 10.1056/NEJMra0804575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barr E, Sings HL. Prophylactic HPV vaccines: new interventions for cancer control. Vaccine. 2008;26(49):6244–6257. doi: 10.1016/j.vaccine.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 23.Harper DM. Currently approved prophylactic HPV vaccines. Expert Rev. Vaccines. 2009;8(12):1663–1679. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 24.Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol. Chem. 2008;389(5):521–536. doi: 10.1515/bc.2008.064. [DOI] [PubMed] [Google Scholar]
- 25. Ball JM, Hardy ME, Atmar RL, Conner ME, Estes MK. Oral immunization with recombinant Norwalk virus-like particles induces a systemic and mucosal immune response in mice. J. Virol. 1998;72(2):1345–1353. doi: 10.1128/jvi.72.2.1345-1353.1998. • First report showing that the oral route of administration elicits mucosal immunity to Norwalk VLPs in mice.
- 26.Guerrero RA, Ball JM, Krater SS, Pacheco SE, Clements JD, Estes MK. Recombinant Norwalk virus-like particles administered intranasally to mice induce systemic and mucosal (fecal and vaginal) immune responses. J. Virol. 2001;75(20):9713–9722. doi: 10.1128/JVI.75.20.9713-9722.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mason HS, Ball JM, Shi JJ, Jiang X, Estes MK, Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl Acad. Sci. USA. 1996;93(11):5335–5340. doi: 10.1073/pnas.93.11.5335. •• First report demonstrating that NVCP assembles orally immunogenic VLP when expressed in transgenic tobacco and potato.
- 28.Zhang X, Buehner NA, Hutson AM, Estes MK, Mason HS. Tomato is a highly effective vehicle for expression and oral immunization with Norwalk virus capsid protein. Plant Biotechnol. J. 2006;4(4):419–432. doi: 10.1111/j.1467-7652.2006.00191.x. [DOI] [PubMed] [Google Scholar]
- 29.Nilsson M, Hedlund KO, Thorhagen M, et al. Evolution of human calicivirus RNA in vivo: accumulation of mutations in the protruding P2 domain of the capsid leads to structural changes and possibly a new phenotype. J. Virol. 2003;77(24):13117–13124. doi: 10.1128/JVI.77.24.13117-13124.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LoBue AD, Thompson JM, Lindesmith L, Johnston RE, Baric RS. Alphavirus-adjuvanted norovirus-like particle vaccines: heterologous, humoral, and mucosal immune responses protect against murine norovirus challenge. J. Virol. 2009;83(7):3212–3227. doi: 10.1128/JVI.01650-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ausar SF, Foubert TR, Hudson MH, Vedvick TS, Middaugh CR. Conformational stability and disassembly of norwalk virus like particles: effect of pH and temperature. J. Biol. Chem. 2006;281:19478–19488. doi: 10.1074/jbc.M603313200. [DOI] [PubMed] [Google Scholar]
- 32.Mason HS, Ball JM, Shi J-J, Estes MK, Arntzen CJ. Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity in mice. Proc. Natl Acad. Sci. USA. 1996;93:5335–5340. doi: 10.1073/pnas.93.11.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rybicki EP. Plant-produced vaccines: promise and reality. Drug Discov. Today. 2009;14(1–2):16–24. doi: 10.1016/j.drudis.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Santi L, Huang Z, Mason H. Virus-like particles production in green plants. Methods. 2006;40(1):66–76. doi: 10.1016/j.ymeth.2006.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gleba Y, Klimyuk V, Marillonnet S. Viral vectors for the expression of proteins in plants. Curr. Opin Biotechnol. 2007;18(2):134–141. doi: 10.1016/j.copbio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Baric RS, Yount B, Lindesmith L, et al. Expression and self-assembly of Norwalk virus capsid protein from venezuelan equine encephalitis virus replicons. J. Virol. 2002;76(6):3023–3030. doi: 10.1128/JVI.76.6.3023-3030.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrington PR, Yount B, Johnston RE, Davis N, Moe C, Baric RS. Systemic, mucosal, and heterotypic immune induction in mice inoculated with Venezuelan equine encephalitis replicons expressing Norwalk virus-like particles. J. Virol. 2002;76(2):730–742. doi: 10.1128/JVI.76.2.730-742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. LoBue AD, Lindesmith L, Yount B, et al. Multivalent norovirus vaccines induce strong mucosal and systemic blocking antibodies against multiple strains. Vaccine. 2006;24(24):5220–5234. doi: 10.1016/j.vaccine.2006.03.080. • Presents data supporting the use of multivalent vaccines for NoV.
- 39. Ball JM, Graham DY, Opekun AR, Gilger MA, Guerrero RA, Estes MK. Recombinant Norwalk virus-like particles given orally to volunteers: Phase I study. Gastroenterology. 1999;117(1):40–48. doi: 10.1016/s0016-5085(99)70548-2. •• First study to demonstrate that baculovirus-derived Norwalk VLPs are immunogenic in humans when administered orally.
- 40.Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J. Infect. Dis. 2000;182(1):302–305. doi: 10.1086/315653. [DOI] [PubMed] [Google Scholar]
- 41.Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin. Immunol. 2003;108:241–247. doi: 10.1016/s1521-6616(03)00120-7. [DOI] [PubMed] [Google Scholar]
- 42.Sakaguchi S, Yamaguchi T, Nomura T. Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Lico C, Chen Q, Santi L. Viral vectors for production of recombinant proteins in plants. J. Cell. Physiol. 2008;216(2):366–377. doi: 10.1002/jcp.21423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Z, Phoolcharoen W, Lai H, et al. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol. Bioeng. 2010 doi: 10.1002/bit.22652. DOI: 10.1002/bit.22652 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Q. Expression and purification of pharmaceutical proteins in plants. Biol. Eng. 2008;1(4):291–321. [Google Scholar]
- 46.Platis D, Labrou NE. Affinity chromatography for the purification of therapeutic proteins from transgenic maize using immobilized histamine. J. Sep. Sci. 2008;31(4):636–645. doi: 10.1002/jssc.200700481. [DOI] [PubMed] [Google Scholar]
- 47.Roque ACA, Lowe CR, Taipa MA. Antibodies and genetically engineered related molecules: production and purification. Biotechnol. Prog. 2004;20(3):639–654. doi: 10.1021/bp030070k. [DOI] [PubMed] [Google Scholar]
- 48.Estes MK. Virus like particle (VLP) vaccines. In: Levine MM, editor. New Generation Vaccines. NY, USA: Academic Press; 2004. pp. 283–294. [Google Scholar]
- 49.Rolland D, Gauthier M, Dugua JM, et al. Purification of recombinant HBc antigen expressed in Escherichia coli and Pichia pastoris: comparison of size-exclusion chromatography and ultracentrifugation. J. Chromatog. B Biomed. Sci. App. 2001;753(1):51–65. doi: 10.1016/s0378-4347(00)00538-7. [DOI] [PubMed] [Google Scholar]
- 50.Cook JC, Joyce JG, George HA, et al. Purification of virus-like particles of recombinant human papillomavirus type 11 major capsid protein L1 from Saccharomyces cerevisiae. Protein Expr. Purif. 1999;17(3):477–484. doi: 10.1006/prep.1999.1155. [DOI] [PubMed] [Google Scholar]
- 51.Pattenden LK, Middelberg APJ, Niebert M, Lipin DI. Towards the preparative and large-scale precision manufacture of virus-like particles. Trends Biotechnol. 2005;23(10):523–529. doi: 10.1016/j.tibtech.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Palomares LA, Ramírez OT. Challenges for the production of virus-like particles in insect cells: the case of rotavirus-like particles. Biochem. Eng. J. 2009;45(3):158–167. [Google Scholar]
- 53.Vicente T, Sousa MFQ, Peixoto C, Mota JPB, Alves PM, Carrondo MJT. Anion-exchange membrane chromatography for purification of rotavirus-like particles. J. Mem. Sci. 2008;311(1–2):270–283. [Google Scholar]
- 54.Goodridge L, Goodridge C, Wu J, Griffiths M, Pawliszyn J. Isoelectric point determination of norovirus virus-like particles by capillary isoelectric focusing with whole column imaging detection. Anal. Chem. 2004;76(1):48–52. doi: 10.1021/ac034848s. [DOI] [PubMed] [Google Scholar]
- 55.Bertolotti-Ciarlet A, White LJ, Chen R, Prasad BV, Estes MK. Structural requirements for the assembly of Norwalk virus-like particles. J. Virol. 2002;76(8):4044–4055. doi: 10.1128/JVI.76.8.4044-4055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan M, Jiang X. The P domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J. Virol. 2005;79(22):14017–14030. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan M, Hegde RS, Jiang X. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J. Virol. 2004;78(12):6233–6242. doi: 10.1128/JVI.78.12.6233-6242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen R, Neill JD, Estes MK, Prasad BV. X-ray structure of a native calicivirus: structural insights into antigenic diversity and host specificity. Proc. Natl Acad. Sci. USA. 2006;103(21):8048–8053. doi: 10.1073/pnas.0600421103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cao S, Lou Z, Tan M, et al. Structural basis for the recognition of blood group trisaccharides by norovirus. J. Virol. 2007;81(11):5949–5957. doi: 10.1128/JVI.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Kamary S, Pasetti M, Tacket CO, et al. Phase 1 dose escalation, safety and immunogenicity of intranasal dry powder Norovirus vaccine. Presented at: 3rd International Calicivirus Conference; 10–13 November 2007; Cancun, Mexico. (Abstract S5-3). [Google Scholar]
- 61.Tacket CO, Frey S, Bernstein DI, et al. Phase 1 dose-comparison, safety and immunogenicity of intranasal dry powder Norwalk VLP vaccine. Presented at: 5th International Conference on Vaccines for Enteric Diseases; 9–11 September 2009; Malaga, Spain. (Session 4). [Google Scholar]
Website
- 101.Norwalk Vaccine Study. http://clinicaltrials.gov/ct2/show/NCT00973284?term=ligocyte&rank=2.