Abstract
The efficient culture of stem cells from epithelial tissues such as skin and corneas is important for both experimental studies and clinical applications of tissue engineering. We now demonstrate that treatment of human-skin-derived keratinocytes with a Rho-associated protein kinase inhibitor Y-27632 for the initial 6 days of primary culture can increase the number of keratinocytes that possess stem cell properties to form colonies during in vitro culture of freshly isolated cells and subsequent passage (50-fold). Further, we show that Y-27632 treatment can increase the total number of prostate epithelial cells derived from human prostate specimens. Therefore, the use of Y-27632 during primary cultures offers a simple and effective way to prepare a large number of epithelial stem cells from various human epithelial tissues.
Introduction
Autologous primary keratinocytes have been used to treat injuries to the skin1 and corneas.2 Primary keratinocytes can also be genetically modified and grafted to treat a genetic skin disease.3 In such applications, prompt and effective procurement of primary keratinocytes from skin biopsies can accelerate the generation of autografts, and shorten the time to treat patients. However, when keratinocytes are prepared from tissue specimens and placed in culture, the number of keratinocytes that can grow out and behave as keratinocyte stem cells (KSC) is limited. Although the estimated KSC frequency in human epidermis ranges form 1% to 10%, based on a variety of experimental assessments, including label retention studies,4–6 only 0.1–1% of epidermal cells freshly isolated from tissues can behave as KSC and form colonies when assessed by colony formation assay,7–10 which can accurately predict the relative enrichment of KSC as previously demonstrated in our in vivo competitive repopulation assay using human-skin-equivalent xenografts.9,11 This low colony-forming efficiency (CFE) of keratinocytes implies that many of the KSC in human skin are lost or not growing during the course of primary culture. Better approaches for establishing primary keratinocyte culture are required to increase KSC numbers and to improve cell-based therapies. Hence, we began to assess the effects of different experimental manipulations, including treatments with pharmacologic agents, that might improve CFE and KSC growth.
Materials and Methods
Tissues and cells
Human neonatal foreskin specimens and prostate tissues were collected with informed consent of patients, parents, or guardians, and institutional approval. Keratinocyte suspension was prepared by step-wise processing with dispase (BD Biosciences, San Jose, CA) and trypsin (USB Corporation, Cleveland, OH) as previously described.12 Prostate cell suspension was prepared by digestion with a mixture of dispase and collagenase Type 1A (Sigma-Aldrich, St. Louis, MO) followed by trypsin (Invitrogen, Carlsbad, CA).
Cell culture assays
CFE was measured as described13 substituting gamma-ray irradiation (6000 rad) of 3T3 cells for mitomycin C treatment. The medium was prepared by mixing Dulbecco's modified Eagle's medium and Ham's F12 medium at 3:1, and adding 1.8 × 10−4 M adenine, 5 μg/mL insulin, 0.5 μg/mL hydrocortisone, 10−10 cholera toxin (Sigma-Aldrich), 10 ng/mL epidermal growth factor (Invitrogen), and 10% fetal bovine serum. Cells were incubated at 37°C in a humidified 5% CO2 atmosphere. Cell outputs were assessed as described by Li and colleagues.8 InSolution® Y-27632 and z-VAD-fmk were purchased from EMD Chemicals (Gibbstown, NJ). Other culture media components were purchased from Gibco (Invitrogen). Prostate cells were cultured in keratinocyte-SFM supplemented with 25 μg/mL bovine pituitary extract and 0.2 ng/mL recombinant epidermal growth factor (Invitrogen). Cells were incubated at 37°C in a humidified 5% CO2 atmosphere. The epithelial identity of cultured prostate cells was determined by positive staining for EpCAM using mouse mAb (clone VU-1D9; Stem Cell Technologies, Vancouver, Canada) and for epithelial keratins using mouse mAb (clone AE3; Millipore, Temecula, CA).
Results
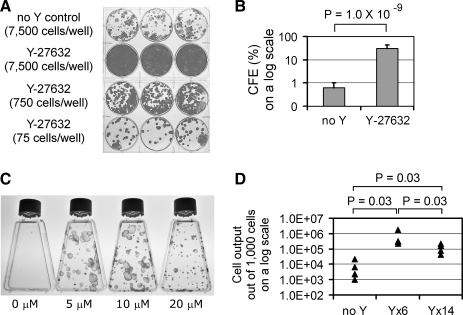
To analyze primary keratinocytes, epidermal cells were isolated from neonatal foreskins as a single-cell suspension and seeded onto a feeder layer of irradiated 3T3 cells. Because tissue processing and subsequent cell culture can stress the cells and induce cell death, one explanation for the lower than predicted CFE results could be that keratinocytes in the epidermal cell suspension are dying before they can form colonies. Therefore, a broad-spectrum caspase inhibitor z-VAD-fmk was tested by treating cells throughout the course of tissue processing and subsequent cell culture. But z-VAD-fmk (10 or 30 μM) did not improve CFE (Supplemental Fig. S1, available online at www.liebertonline.com/ten). We then investigated the effects of a Rho-associated protein kinase (ROCK) inhibitor Y-27632, which had previously been shown to improve the survival of dissociated human embryonic stem cells.14 Amazingly, Y-27632 (10 μM) dramatically increased CFE by 50-fold: from 0.6% ± 0.4% to 30.2% ± 11.8% (mean ± standard deviation; n = 8; p = 1.0 × 10−9; two-tailed t-test; Fig. 1A, B). Because lower (5 μM) and higher (20 μM) concentrations of Y-27632 had similar effects on CFE (although colony sizes were smaller at 20 μM as discussed later; Fig. 1C), all subsequent experiments were performed with 10 μM Y-27632. The dramatic increase in CFE resulting from Y-27632 treatment did not associate with the increase of adherent cells during initial plating (Supplemental Fig. S2, available online at www.liebertonline.com/ten), or with the decrease of apoptotic cells during early phase of culture (Supplemental Fig. S3, available online at www.liebertonline.com/ten). Cell proliferation, as determined by KI-67 expression, was not changed by Y-27632 on day 3 of primary culture (Supplemental Fig. S4, available online at www.liebertonline.com/ten). The percentage of cells expressing α6 integrin, a marker for a proliferating population of keratinocytes, on day 3 was also not changed by Y-27632 (Supplemental Fig. S5, available online at www.liebertonline.com/ten). Therefore, the relative percentages of proliferating and differentiating keratinocytes were not altered by Y-27632 treatment. The growth potential was also assessed by measuring the total number of cells produced during 14 days of culture. Keratinocytes were treated with Y-27632 for 0, 6, or 14 days, and cell output was measured on day 14. Although 1000 cells of untreated control generated 1000–22,000 cells, the same number of cells treated with Y-27632 for 6 or 14 days generated 226,000–1,730,000 and 42,000–213,000 cells, respectively (n = 4; p = 0.03; Mann–Whitney U-test; Fig. 1D). Thus, consistent with the increase in CFE, Y-27632 treatment boosted the cell output of primary keratinocytes.
FIG. 1.
Y-27632 treatment increases colony-forming efficiency (CFE) and cell output of epidermal keratinocytes freshly isolated from skin specimens. (A) Keratinocyte colonies formed in 14 days of culture either without (top row) or with 10 μM of Y-27632 (second to fourth rows). This is a representative experiment of eight independent sets. Three wells in each row represent triplicates within the single set. (B) Fifty times higher CFE induced by Y-27632 treatment for 14 days (p = 1.0 × 10−9; n = 8; two-tailed t-test). Error bars indicate standard deviation. (C) Keratinocyte colonies after treatment with Y-27632 at 0, 5, 10, and 20 μM for 14 days. (D) The number of cells generated out of 1000 cells by 0, 6, or 14 days of Y-27632 treatment during 14 days of culture (n = 4; Mann–Whitney U-test).
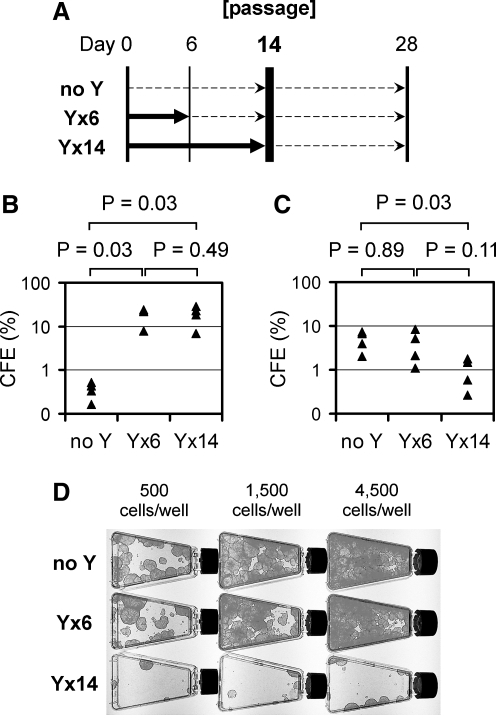
To determine whether Y-27632-treated keratinocytes in increased colonies preserve the growth potential of KSC, cells were subjected to a serial passage. Keratinocytes from human foreskins were exposed to Y-27632 for 0, 6, or 14 days of the initial 14-day culture, harvested, and passaged onto a fresh feeder layer for a second round of culture for additional 14 days without Y-27632 (Fig. 2A). In the initial culture, cells treated with Y-27632 for either 6 or 14 days showed significantly higher CFE (7–29%) than the untreated control cells (0.2–0.5%; n = 4; p = 0.03; Mann–Whitney U-test; Fig. 2B). Importantly, the increased colonies that resulted from the 6-day Y-27632 treatment in the initial culture maintained their colony-forming ability in the second round after passage (CFE of 1.1–8.2%) and formed as many large healthy colonies in the second round as the normal KSC of the untreated control (CFE of 2.1–7.4%) when equivalent numbers of keratinocytes were passaged (n = 4; p = 0.89; Mann–Whitney U-test; Fig. 2C, D). Thus, once treated with Y-27632 for 6 days, up to 29% of keratinocytes became highly clonogenic, and maintained the growth potential of KSC even after cessation of Y-27632 treatment in the second round. On the other hand, we noted that prolonged Y-27632 treatment for 14 days of the initial culture resulted in significantly lower CFE (0.3–1.8%) than the untreated control in the second round following passage (n = 4; p = 0.03; Mann–Whitney U-test; Fig. 2C, D). Therefore, transient Y-27632 treatment for up to 6 days causes many keratinocytes to behave like highly clonogenic KSC during serial passage, but this effect is counteracted by prolonged 14-day Y-27632 treatment. The adverse effect of prolonged Y-27632 treatment was already evident in the initial culture, in which 14-day treatment resulted in small and thick colonies (Fig. 1A), indicative of accelerated differentiation, especially at a higher concentration (20 μM) of Y-27632 (Fig. 1C).
FIG. 2.
Keratinocytes treated with Y-27632 maintain the growth potential after cessation of Y-27632 at serial passage. (A) Schematic depiction of Y-27632 treatment during keratinocyte serial passage. Thick solid arrows indicate time frames when Y-27632 was used. Thin dashed-line arrows indicate time frames when cells were cultured in the absence of Y-27632. (B) CFE in the first round (days 0–14) in the presence or absence of Y-27632; measured on day 14 (n = 4; Mann–Whitney U-test). (C) CFE in the second round (days 14–28) in the absence of Y-27632 for all samples of “no Y,” “Y × 6” and “Y × 14”; measured on day 28 (n = 4; Mann–Whitney U-test). (D) Colonies of the second round (days 14–28, in the absence of Y-27632); fixed and stained on day 28. A representative set of four independent experiments summarized in (C) is presented. Numbers of cells seeded at the passage on day 14 are shown on the top.
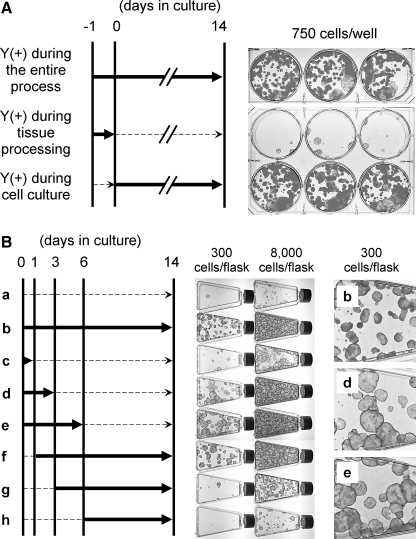
Since the timing of Y-27632 treatment seemed critical for a high CFE, we assessed the influence of Y-27632 treatment during tissue processing and during the 14-day culture. CFE did not increase when specimens were treated with Y-27632 only during tissue processing (Fig. 3A; middle row). Additionally, Y-27632 treatment during tissue processing did not further enhance the increased CFE that results from Y-27632 treatment during cell culture (Fig. 3A; top and bottom rows). Next, we assessed the effect of Y-27632 on CFE when keratinocytes were treated for different time periods during the 2-week culture (Fig. 3B). Compared to cells that were never treated with Y-27632 (Fig. 3B, sample a), cells treated during the first 1, 3, or 6 days (Fig. 3B, samples c, d, and e, respectively) demonstrated progressive enhancement of CFE. In contrast, when Y-27632 treatment was initiated on day 3 or 6 of culture (Fig. 3B, samples g and h), the CFE was only minimally improved. Thus, this time-course study demonstrates that the timing of Y-27632 treatment plays a critical role in increasing the number of clonogenic keratinocytes during the first 3–6 days of culture. Notably, epidermal cells treated for the initial 3 or 6 days of culture not only had a high CFE but also formed large and round colonies (Fig. 3B, samples d and e) reminiscent of those formed by highly clonogenic holoclones that are believed to represent KSC.15
FIG. 3.
Y-27632 treatment during the first 6 days of primary culture is critical for increased CFE. (A) Human skin specimens and primary keratinocytes were treated with Y-27632 during tissue processing, subsequent 14-day culture, or both. Thick solid arrows indicate time frames when Y-27632 was used. Thin dashed-line arrows indicate time frames when specimens or cells were not exposed to Y-27632. CFE is increased only if Y-27632 is present during the cell culture period. Wells in each row are triplicates. (B) Primary keratinocytes were treated with Y-27632 over various time frames during the 14-day culture. Thick solid arrows indicate time frames when Y-27632 was used. Thin dashed-line arrows indicate time frames when cells were cultured in the absence of Y-27632. Y-27632 treatment for the first 6 days (sample e) increases CFE equally well as the treatment for the entire culture period (sample b). Enlarged images of colonies in samples b, d, and e are shown on the right. Note larger colony sizes in samples d and e compared to those in sample b.
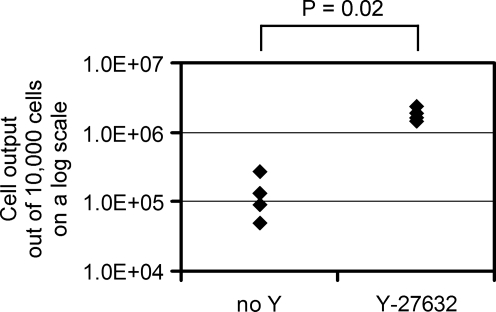
To determine whether ROCK inhibition by Y-27632 could increase the growth of other human epithelial cells in addition to keratinocytes, we prepared single-cell suspensions from fresh human prostate specimens and cultured them for 10 days with or without Y-27632. Y-27632 treatment increased the number of prostate epithelial cells by 19-fold (n = 4; p = 0.02; Mann–Whitney U-test; Fig. 4). Accordingly, Y-27632 can increase the growth potential of two distinct types of human epithelial cells.
FIG. 4.
Y-27632 treatment improves the procurement of primary prostate epithelial cells. Cells from human prostate specimens were cultured in the presence or absence of Y-27632. Numbers of epithelial cells generated from 10,000 cells during 10 days of culture are shown (n = 4, Mann–Whitney U-test).
Discussion
A ROCK inhibitor Y-27632 increased CFE (50-fold) when used during the initial 6 days of primary culture. One possible explanation for this remarkable result could be that Y-27632 is transiently boosting the growth of non-KSC that are close to a postmitotic stage and cannot form large colonies in the absence of Y-27632. If this was the case, serial passage of these non-KSC colonies in the absence of Y-27632 could not be successful because, without Y-27632, those boosted cells should lose growth potential and would not form large colonies. But this scenario is unlikely because, as shown in Figure 2, serial passage experiments demonstrated that keratinocytes resulting from a 6-day Y-27632 treatment in the initial culture maintained their clonogenic phenotype in the second round after passage, and formed as many and large colonies in the absence of Y-27632 as the normal KSC of the untreated control did. Therefore, Y-27632 is not transiently boosting the growth of non-KSC. Y-27632 rather seems to be increasing the number of KSC that can survive and grow to form healthy colonies in the primary culture. Additionally, Y-27632-treated cells did not show any signs of uncontrolled growth before or after passage. The benign behavior of the cells is consistent with the safety history of Y-27632 in preclinical studies.
The effect of Y-27632 on apoptosis depends on the cell types and experimental conditions utilized. Y-27632 has been reported to exert antiapoptotic effect in endothelial cells,16,17 myocytes,18,19 and embryonic stem cells,14 whereas others have reported its proapoptotic effect in endothelial cells,20 myocytes,21 and airway and intestinal epithelial cells.22,23 However, our observation of increased number of colony-forming keratinocytes cannot be attributed to antiapoptotic effect of Y-27632 because the percentage of apoptotic cells determined by Annexin V staining was not decreased by Y-27632 treatment (Supplemental Fig. S3), and because treatment of cells with a broad-spectrum caspase inhibitor z-VAD-fmk did not improve CFE (Supplemental Fig. S1). However, since specific markers are not available to prospectively identify pure populations of KSC for biological assays, it is not feasible to specifically monitor the behavior of KSC, which would represent a minor population in epidermal cell suspensions. Consequently, we cannot experimentally exclude the possibility that Y-27632 selectively affects KSC to improve their survival without affecting the predominant non-KSC population. Additionally, Y-27632 seems to be most effective on cells freshly isolated from tissues rather than cells that have adapted to cell culture environments because passaged keratinocytes demonstrated only a twofold increase in CFE by Y-27632 treatment in an earlier report investigating the regulation of keratinocyte differentiation by Rho/ROCK signaling.24 Further research efforts are required to understand the molecular mechanisms responsible for the effect of Y-27632 on keratinocytes and KSC.
Since only 1–10% of keratinocytes in the basal layer of epidermis are thought to represent KSC,4–6 the CFE of 30% observed in Y-27632-treated keratinocytes was substantially higher than the percentage predicted from the number of stem cells, suggesting that Y-27632 increases the number of cells exhibiting stem cell behavior. Extra stem cells might be generated by increasing the frequency of symmetric division of stem cells, reprogramming of non-stem cells into stem cells, or the combination of both. The mechanisms can be better understood by tracking the fate of individual cells in the primary culture once specific stem cell markers become available for the prospective identification of KSC.
The dramatic effect of Y-27632 to increase the cell output during culture was not limited to primary keratinocytes; it was observed also in primary epithelial culture from prostate specimens. These results imply that Y-27632 may increase the growth potential of various human epithelial cells placed in culture. Such a feature would be particularly beneficial when preparing primary cells from a very limited size of donor tissue such as cornea. To further assess the effects of Y-27632 on the growth of primary cells from epithelial tissues, the use of standardized in vivo assays would be useful. Since standardized in vivo stem cell assays for human keratinocytes stem cells exist, human epidermis can be a good model to determine the effects of Y-27632 on epithelial cells under conditions closely simulating the in vivo environments.9,11 The experimental approach described here generates large numbers of clonogenic primary cells from human epithelia for clinical applications, and offers a valuable experimental tool for stem cell research.
Supplementary Material
Acknowledgments
The authors thank Mark Udey for valuable suggestions. This study was supported by Intramural Research Program of the National Institutes of Health, National Cancer Institute, and Center for Cancer Research.
Disclosure Statement
No competing financial interests exist.
References
- 1.O'Connor N. Mulliken J.B. Banks-Schlegel S. Kehinde O. Green H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981;1:75. [PubMed] [Google Scholar]
- 2.Pellegrini G. Traverso C.E. Franzi A.T. Zingirian M. Cancedda R. De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 3.Mavilio F. Pellegrini G. Ferrari S. Di Nunzio F. Di Iorio E. Recchia A. Maruggi G. Ferrari G. Provasi E. Bonini C. Capurro S. Conti A. Magnoni C. Giannetti A. De Luca M. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- 4.Heenen M. Galand P. The growth fraction of normal human epidermis. Dermatology. 1997;194:313. doi: 10.1159/000246122. [DOI] [PubMed] [Google Scholar]
- 5.Cotsarelis G. Kaur P. Dhouailly D. Hengge U. Bickenbach J. Epithelial stem cells in the skin: definition, markers, localization and functions. Exp Dermatol. 1999;8:80. doi: 10.1111/j.1600-0625.1999.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 6.Terunuma A. Jackson K.L. Kapoor V. Telford W.G. Vogel J.C. Side population keratinocytes resembling bone marrow side population stem cells are distinct from label-retaining keratinocyte stem cells. J Invest Dermatol. 2003;121:1095. doi: 10.1046/j.1523-1747.2003.12531.x. [DOI] [PubMed] [Google Scholar]
- 7.Rheinwald J.G. Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 8.Li A. Simmons P.J. Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA. 1998;95:3902. doi: 10.1073/pnas.95.7.3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terunuma A. Kapoor V. Yee C. Telford W.G. Udey M.C. Vogel J.C. Stem cell activity of human side population and alpha6 integrin-bright keratinocytes defined by a quantitative in vivo assay. Stem Cells. 2007;25:664. doi: 10.1634/stemcells.2006-0434. [DOI] [PubMed] [Google Scholar]
- 10.Terunuma A. Cross J.W. Dyke M. Kapoor V. Telford W.G. Vogel J.C. Behavior of human foreskin keratinocytes expressing a hair follicle stem cell marker CD200. J Invest Dermatol. 2008;128:1332. doi: 10.1038/sj.jid.5701154. [DOI] [PubMed] [Google Scholar]
- 11.Terunuma A. Shaya M.B. Udey M.C. Vogel J.C. An in vivo competitive repopulation assay system to evaluate human keratinocyte stem cells. J Invest Dermatol. 2004;123:993. doi: 10.1111/j.0022-202X.2004.23456.x. [DOI] [PubMed] [Google Scholar]
- 12.Pfutzner W. Hengge U.R. Joari M.A. Foster R.A. Vogel J.C. Selection of keratinocytes transduced with the multidrug resistance gene in an in vitro skin model presents a strategy for enhancing gene expression in vivo. Hum Gene Ther. 1999;10:2811. doi: 10.1089/10430349950016546. [DOI] [PubMed] [Google Scholar]
- 13.Jones P.H. Watt F.M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe K. Ueno M. Kamiya D. Nishiyama A. Matsumura M. Wataya T. Takahashi J.B. Nishikawa S. Nishikawa S. Muguruma K. Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 15.Barrandon Y. Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrache I. Verin A.D. Crow M.T. Birukova A. Liu F. Garcia J.G. Differential effect of MLC kinase in TNF-alpha-induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1168. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- 17.Petrache I. Crow M.T. Neuss M. Garcia J.G. Central involvement of Rho family GTPases in TNF-alpha-mediated bovine pulmonary endothelial cell apoptosis. Biochem Biophys Res Commun. 2003;306:244. doi: 10.1016/s0006-291x(03)00945-8. [DOI] [PubMed] [Google Scholar]
- 18.Del Re D.P. Miyamoto S. Brown J.H. RhoA/Rho kinase up-regulate Bax to activate a mitochondrial death pathway and induce cardiomyocyte apoptosis. J Biol Chem. 2007;282:8069. doi: 10.1074/jbc.M604298200. [DOI] [PubMed] [Google Scholar]
- 19.Bao W. Hu E. Tao L. Boyce R. Mirabile R. Thudium D.T. Ma X.L. Willette R.N. Yue T.L. Inhibition of Rho-kinase protects the heart against ischemia/reperfusion injury. Cardiovasc Res. 2004;61:548. doi: 10.1016/j.cardiores.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Li X. Liu L. Tupper J.C. Bannerman D.D. Winn R.K. Sebti S.M. Hamilton A.D. Harlan J.M. Inhibition of protein geranylgeranylation and RhoA/RhoA kinase pathway induces apoptosis in human endothelial cells. J Biol Chem. 2002;277:15309. doi: 10.1074/jbc.M201253200. [DOI] [PubMed] [Google Scholar]
- 21.Ogata Y. Takahashi M. Takeuchi K. Ueno S. Mano H. Ookawara S. Kobayashi E. Ikeda U. Shimada K. Fluvastatin induces apoptosis in rat neonatal cardiac myocytes: a possible mechanism of statin-attenuated cardiac hypertrophy. J Cardiovasc Pharmacol. 2002;40:907. doi: 10.1097/00005344-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Moore M. Marroquin B.A. Gugliotta W. Tse R. White S.R. Rho kinase inhibition initiates apoptosis in human airway epithelial cells. Am J Respir Cell Mol Biol. 2004;30:379. doi: 10.1165/rcmb.2003-0019OC. [DOI] [PubMed] [Google Scholar]
- 23.Song J. Li J. Lulla A. Evers B.M. Chung D.H. Protein kinase D protects against oxidative stress-induced intestinal epithelial cell injury via Rho/ROK/PKC-delta pathway activation. Am J Physiol Cell Physiol. 2006;290:C1469. doi: 10.1152/ajpcell.00486.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMullan R. Lax S. Robertson V.H. Radford D.J. Broad S. Watt F.M. Rowles A. Croft D.R. Olson M.F. Hotchin N.A. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol. 2003;13:2185. doi: 10.1016/j.cub.2003.11.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.