Abstract
Cytokine regulation of synovial fluid (SF) lubricants, hyaluronan (HA), and proteoglycan 4 (PRG4) is important in health, injury, and disease of synovial joints, and may also provide powerful regulation of lubricant secretion in bioreactors for articulating tissues. This study assessed lubricant secretion rates by human synoviocytes and the molecular weight (MW) of secreted lubricants in response to interleukin (IL)-1β, IL-17, IL-32, transforming growth factor-beta 1 (TGF-β1), and tumor necrosis factor-alpha (TNF-α), applied individually and in all combinations. Lubricant secretion rates were assessed using ELISA and binding assays, and lubricant MW was assessed using gel electrophoresis and Western blotting. HA secretion rates were increased ∼40-fold by IL-1β, and increased synergistically to ∼80-fold by the combination of IL-1β + TGF-β1 or TNF-α + IL-17. PRG4 secretion rates were increased ∼80-fold by TGF-β1, and this effect was counterbalanced by IL-1β and TNF-α. HA MW was predominantly <1 MDa for controls and individual cytokine stimulation, but was concentrated at >3 MDa after stimulation by IL-1β + TGF-β1 + TNF-α to resemble the distribution in human SF. PRG4 MW was unaffected by cytokines and similar to that in human SF. These results contribute to an understanding of the relationship between SF cytokine and lubricant content in health, injury, and disease, and provide approaches for using cytokines to modulate lubricant secretion rates and MW to help achieve desired lubricant composition of fluid in bioreactors.
Introduction
The synovial joint is a low-friction, low-wear load-bearing system that includes articular cartilage, synovial fluid (SF), and synovium components. Articular cartilage is composed of chondrocytes within a dense extracellular matrix, and bears load and slides relative to an apposing surface with low-friction and low-wear properties. The SF acts as a biomechanical lubricant for articular cartilage, as it contains a number of lubricating molecules as well as regulatory cytokines, nutrients, and other components. The SF lubricant molecule hyaluronan (HA) is produced predominantly by fibroblast-like synoviocytes in synovium (abbreviated here as synoviocytes),1,2 whereas the lubricating molecule proteoglycan 4 (PRG4) is secreted by synoviocytes as well as chondrocytes in the superficial layer of articular cartilage.3–6 The synthesis rate of these lubricating molecules and the molecular weight (MW) of molecules synthesized are important because the balance between the rate of synthesis and the rate of loss of these molecules, by degradation and transport through the semipermeable synovium, dictates their concentration in SF.7,8
In naturally occurring or animal models of osteoarthritis (OA), rheumatoid arthritis (RA), and injury, the concentrations of HA and PRG4 in SF are often decreased, as is the MW of HA.9–13 However, the volume of SF is often increased to an even greater extent,9,14,15 resulting in an overall increase in lubricant content in SF, and complicating assessments of lubricant production and degradation. These changes in lubricant content and volume in SF are often accompanied by increases in the concentrations of certain cytokines in SF, including interleukin (IL)-1β, IL-17, IL-32, transforming growth factor-beta 1 (TGF-β1), and tumor necrosis factor-alpha (TNF-α).16–25 The individual effects of some of these cytokines on lubricant secretion by synoviocytes have been studied,1,26–28 but interactive effects that likely occur in vivo remain to be established. A combinatorial approach to evaluate the interactive effects of cytokines in vitro could provide a better understanding of cytokine-regulated lubricant secretion in vivo.
To gain further understanding of such in vivo regulation, and to generate lubricious fluid using a biomimetic approach, we developed a bioreactor system for evaluating the production and regulation of SF lubricants29 (Fig. 1). Other experimental culture systems have been developed to include certain features of the synovial joint to examine the biology and mechanobiology of synovium and cartilage30,31; however, such systems have not attempted to generate or study a fluid with lubricant properties similar to that of native SF. In the biomimetic bioreactor system, application of cytokines may provide insight into in vivo regulation of SF that occurs in healthy and diseased joints, and may also provide modulation of fluid composition by powerfully regulating lubricant secretion. The ability to generate a lubricating fluid in such a bioreactor system could help to develop arthritis therapies, such as novel viscosupplements.32
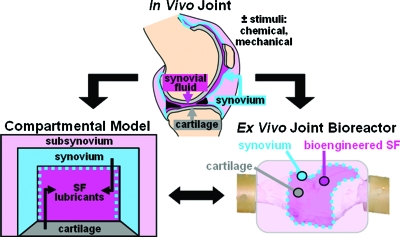
FIG. 1.
Synovial joints may be modeled theoretically with a compartmental approach, and experimentally in a bioreactor with key components that include lubricant-secreting cell types, a SF compartment, and a lubricant-retaining semipermeable membrane. Chemical and mechanical stimuli are key parameters that may regulate lubricant secretion and MW, and affect lubricant composition in native and bioengineered SF. MW, molecular weight; SF, synovial fluid. Color images available online at www.liebertonline.com/ten.
As a step toward developing such a bioreactor to evaluate the production and regulation of lubricants in SF, we evaluated here the regulatory effects of cytokines commonly implicated in arthritis.16–25 Specifically, we examined the secretion rates of HA and PRG4 by human synoviocytes, as well as the MW of these secreted molecules, as regulated by the cytokines IL-1β, IL-17, IL-32, TGF-β1, and TNF-α applied individually and in combination. Using this information, we can tailor the cytokine milieu in future bioreactor studies to mimic normal synovium and arthritis.
Materials and Methods
Synoviocyte and synovial fluid isolation
Human synovial tissue and SF samples were collected with IRB approval and informed donor consent. All studies were approved by the Human Subjects Research Protection Program of the University of California–San Diego.
Synovial tissue was obtained from patients with OA or RA at the time of joint replacement, and synoviocytes were isolated from the tissue. The diagnosis of RA conformed to the 1987 revised American College of Rheumatology criteria. As described previously,20 synovial tissues from both RA and OA sources were minced and incubated with 1 mg/mL collagenase in serum-free Dulbecco's Modified Eagle Medium (DMEM) with additives (100 U/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL Fungizone, 0.1 mM Modified Eagle Medium (MEM) nonessential amino acids, 10 mM HEPES, 0.4 mM L-proline, and 2 mM L-glutamine) for 2 h at 37°C, filtered through a nylon mesh, extensively washed, and cultured in DMEM supplemented with 10% fetal bovine serum (FBS). As native synovium and synovial tissue digests are generally a heterogeneous mixture of cell types (fibroblast-like synoviocytes, macrophage-like synoviocytes, blood cells, etc.), fibroblast-like lubricant-secreting synoviocytes were enriched by removing nonadherent cells after overnight culture, and by subsequent trypsinization and passaging of cells (split at a 1:3 ratio and cultured in DMEM + 10% FBS). Synoviocytes were used from passages 3 through 9 when they are a homogeneous population of fibroblast-like synoviocytes (<1% CD11b, <1% phagocytic, and <1% FcgRII positive).
Normal human SF was obtained from the normal knee (as assessed by X-ray) of subjects with intra-articular fractures in the contralateral knee or ankle, with informed consent. Collected SF was clarified of cells and debris by centrifugation (3000 g, 30 min, 4°C), and resultant samples were stored at −70°C before analysis.
Synoviocyte culture and cytokine treatment
For all experiments, synoviocytes were seeded at 10,000 cells/cm2 in standard multiwell tissue culture plates and cultured as two-dimensional monolayers in DMEM + 10% FBS until confluent. Confluent synoviocytes were subsequently incubated under basal conditions of DMEM + 0.5% FBS for 2 days. This period of serum starvation suppresses proliferation and basal mediator production while permitting a robust response to exogenous cytokines. Cells were then cultured for an additional 3 days in DMEM + 0.5% FBS (control) ± cytokines, applied either individually or in different combinations. All subsequent analyses were performed on either cell layers or conditioned media during the 3 day cytokine treatment. Donor tissue for each experiment consisted of six different donors, with three OA sources (66 ± 20 years, six donors) and three RA sources (60 ± 11 years, six donors). As the effects of individual and combinations of cytokines did not statistically differ for OA and RA groups in the analyses, data are presented and discussed collectively.
Treatment with individual cytokines
Individual cytokines were applied over a range of concentrations to assess dose responses: IL-1β, IL-17, IL-32, TGF-β1 (0.1, 1, and 10 ng/mL), and TNF-α (1, 10, and 100 ng/mL). Concentrations in these ranges have been previously shown to regulate aspects of synoviocyte biosynthesis, such as production of IL-6, IL-8, and granulocyte macrophage colony stimulating factor (GM-CSF).16–21,25 IL-1β, IL-17 (isoform IL-17A), TGF-β1, and TNF-α were purchased from Peprotech (Rocky Hill, NJ), and IL-32 (isoform not assessed) was purchased from Abnova (Taipei City, Taiwan). Endotoxin levels for cytokines were <0.1 ng/μg (1 EU/μg).
Treatment with combinations of cytokines
A fully factorial experimental design of cytokine combinations (32 groups total) was used to elucidate additive and/or synergistic effects of IL-1β, IL-17, IL-32, TGF-β1 (10 ng/mL), and TNF-α (100 ng/mL). In preliminary experiments, certain combinations of cytokines at high concentrations (10 ng/mL for IL-1β, IL-17, IL-32, and TGF-β1, and 100 ng/mL for TNF-α), but not low concentrations (0.1 ng/mL for IL-1β, IL-17, IL-32, and TGF-β1, and 1 ng/mL for TNF-α), resulted in marked synergistic regulation of lubricant secretion; thus, all experiments in this study were carried out using the high cytokine concentrations. Additionally, as different donor tissue was utilized for the different experiments (i.e., individual cytokine dose response and combinations of cytokines), individual cytokine conditions at the high cytokine concentrations were included in both experiments.
DNA content
Net cell proliferation was assessed as the ratio of DNA on day 3 of cytokine treatment to DNA on day 0 of cytokine treatment. Cell layers were solubilized on days 0 and 3 with 0.5 mg/mL proteinase K (Roche, Indianapolis, IN), harvested, and analyzed for DNA using PicoGreen® (Molecular Probes, Eugene, OR).33
Lubricant secretion rates
Secretion of HA and PRG4 was assessed after the 3 day cytokine treatment by analyzing conditioned medium for PRG4 by ELISA using mAb GW4.23 (gift from Dr. Klaus Kuettner),34 and for HA by an enzyme-linked binding assay using HA binding protein.35 Secretion rates (rHA and rPRG4) were determined by normalizing total mass of secreted lubricant to cell number on day 3 as estimated from DNA, and to culture duration.
Molecular weight distribution of secreted HA
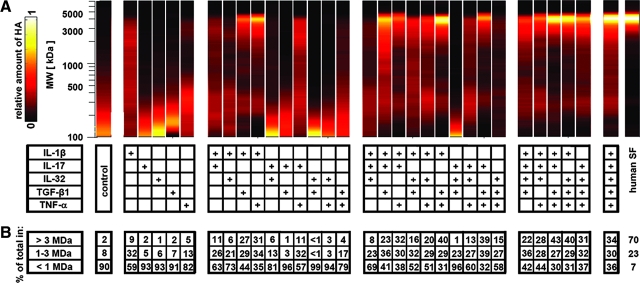
The MW distribution of HA secreted by synoviocytes was performed using an agarose gel electrophoresis and Stainsall detection technique,36 with several modifications. Conditioned medium samples (pooled from six donors for each treatment condition, and subsequently from individual donors for conditions that exhibited a marked regulatory effect to allow statistical analysis), normal human SF, and HA standards in the MW range 0.16–4.0 MDa (Associates of Cape Cod, East Falmouth, MA) were all treated with or without Streptomyces hyaluronidase (10 U/mL) (Seikagaku, Tokyo, Japan) overnight at 37°C, and then subsequently with proteinase K (0.5 mg/mL) overnight at 37°C to eliminate interference of protein in the assay. Samples with HA mass of 200–500 ng were applied to 1% agarose gels (Lonza, Rockland, ME), separated by horizontal electrophoresis at 100 V for 110 min in TAE buffer (0.4 M Tris–acetate and 0.01 M ethylenediaminetetraacetic acid, pH 8.3), and observed after incubation with 0.1% Stainsall reagent (Sigma, St. Louis, MO). Gel images were digitized with a D80 digital camera (Nikon, Melville, NY) and also processed to determine HA distribution by subtracting the intensities of hyaluronidase-treated samples from that of nonhyaluronidase-treated samples, and then displaying on a relative scale (0 to 1) to show relative HA content between 0.1 and 7.0 MDa. Also, the distribution of HA within the MW ranges of 0.1–1, 1–3, and >3 MDa was calculated.
Molecular weight of PRG4
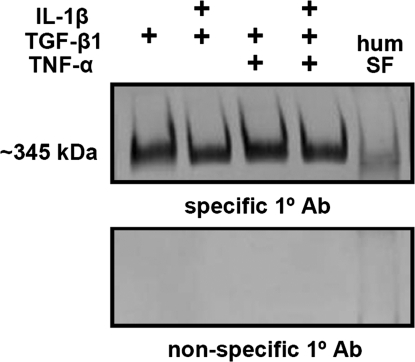
The MW of PRG4 secreted by synoviocytes was determined for samples showing upregulation by Western blot utilizing mAb GW4.23 to PRG434 or nonspecific mouse IgG as a control. Conditioned medium was concentrated with a 30 kDa MWCO centrifugal filtration device (Millipore, Billerica, MA), and then these samples (0.15 μg PRG4 equivalent) and also normal human SF (0.5 μL) were treated with 10 U/mL Streptomyces hyaluronidase (Seikagaku) overnight at 37°C. Samples were then applied to precast 3–8% acrylamide Tris–acetate gels, and separated by electrophoresis at 150 V for 1 h in Tris–acetate sodium dodecyl sulfate running buffer (50 mM Tricine, 50 mM Tris base, and 0.1% sodium dodecyl sulfate, pH 8.24), blotted onto polyvinylidene fluoride (PVDF) membranes (Amersham Biosciences, Piscataway, NJ) at 30 V for 1 h in transfer buffer (25 mM Bicine, 25 mM Bis–Tris, and 1 mM ethylenediaminetetraacetic acid, pH 7.20). The membranes were then blocked for 1 h in 5% normal goat serum in phosphate-buffered saline + 0.1% Tween 20 (pH 7.4), reacted with mAb GW4.23 to PRG4 (or nonspecific mouse IgG as a control [Pierce, Rockford, IL]) at 0.5 μg/mL in 1% bovine serum albumin in phosphate-buffered saline + 0.1% Tween 20 for 1 h, and then a goat anti-mouse secondary antibody conjugated to horseradish peroxidase. Immunoreactivity was detected by ECL-Plus chemiluminescence (Amersham Biosciences), which was recorded with a Storm Imager (GMI, Ramsey, MN).
Statistical analysis
Data are expressed as mean ± standard error of the mean. Data were log transformed to improve normality. For individual cytokines, effects on DNA and secretion rates were analyzed by one-way analysis of variance (ANOVA) and Dunnett's post hoc test to assess differences from controls. For combinations of cytokines, a five-way ANOVA (factors: IL-1β, IL-17, IL-32, TGF-β1, and TNF-α) was used to assess interactive (nonadditive) effects of cytokines on DNA and secretion rates. Tukey post hoc tests assessing the differences between each cytokine combination were also performed (see Supplemental Materials, available online at www.liebertonline.com/ten). For analysis of HA MW, effects of cytokines were analyzed by one-way ANOVA, and Tukey post hoc test to assess differences among samples in the percentage of total HA present in MW ranges of <1, 1–3, and >3 MDa.
Results
DNA analysis
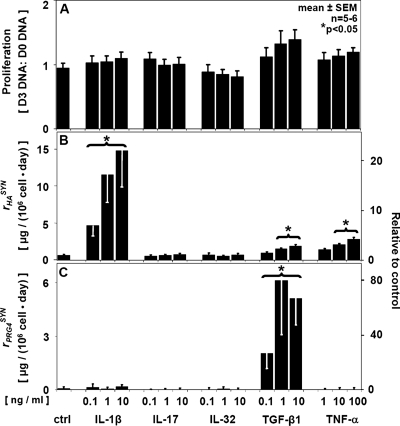
Overall synoviocyte proliferation was low during the 3 day cytokine treatment. The minimal relative proliferation was consistent with the high cell density of monolayers on the initial day of cytokine treatment and the short culture duration. There was a slight stimulatory effect of TGF-β1, IL-1β, and TNF-α (p < 0.05) (Figs. 2A and 3A).
FIG. 2.
Regulatory effects of the individual cytokines IL-1β, IL-17, IL-32, TGF-β1, and TNF-α at a range of concentrations on (A) proliferation, (B) HA secretion, and (C) PRG4 secretion by human synoviocytes. Mean ± SEM, n = 5–6; *p < 0.05. HA, hyaluronan; PRG4, proteoglycan 4; IL, interleukin; TGF-β1, transforming growth factor-beta 1; TNF-α, tumor necrosis factor-alpha; SEM, standard error of the mean.
FIG. 3.
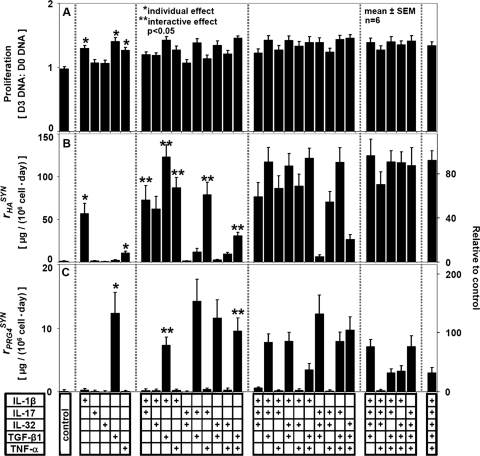
Regulatory effects of different combinations of the cytokines IL-1β, IL-17, IL-32, TGF-β1, and TNF-α (applied at maximum individual stimulation doses) on (A) proliferation, (B) HA secretion, and (C) PRG4 secretion by human synoviocytes. Mean ± SEM, n = 6; *p < 0.05 for individual effect; **p < 0.05 for interactive effect. (+) signs indicate cytokines present in the culture media.
Lubricant secretion rates
rHA and rPRG4 were differentially regulated by individually applied cytokines in a dose-dependent manner (Fig. 2B, C). The rHA of control cultures was increased ∼22-fold by IL-1β (p < 0.05). Less stimulatory to rHA were TNF-α and TGF-β1, causing increases to ∼4.2- and ∼2.8-fold, respectively (each, p < 0.05) (Fig. 2B). In contrast, rPRG4 was affected only by TGF-β1, being increased to ∼78-fold over the control rate (p < 0.05) (Fig. 2C). rHA and rPRG4 were not significantly affected by the addition of IL-17 or IL-32 individually.
Synergistic interactions of certain combinations of cytokines increased rHA markedly over the additive effects of individual cytokines (Fig. 3B). TNF-α acted synergistically with several other cytokines, including IL-1β, IL-17, and TGF-β1, increasing rHA to ∼1.3-, ∼6.3-, and ∼2.3-fold over the predicted additive effects of these cytokines, respectively (each, p < 0.05). IL-1β also acted synergistically with IL-17 and with TGF-β1, increasing rHA ∼1.2- and ∼2.1-fold over the predicted additive effects (each, p < 0.05). rHA was not significantly affected by addition of IL-32 to any other individual cytokine or combination of cytokines.
PRG4 secretion was modulated by cytokines, with counterbalancing effects of certain combinations (Fig. 3C). The stimulatory effect of individually applied TGF-β1 was counterbalanced by addition of either IL-1β or TNF-α, decreasing rPRG4 significantly (p < 0.05). When applied together with TGF-β1, IL-1β and TNF-α further decreased rPRG4. rPRG4 was unchanged by addition of IL-17 or IL-32 to any condition.
Effect of cytokines on lubricant molecular weight
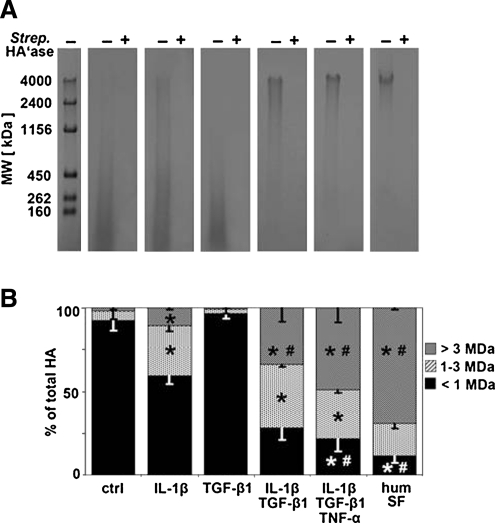
The MW distribution of secreted HA was markedly regulated by individual and combinations of cytokines, and resembled that of normal human SF in some cases. For all samples, hyaluronidase treatment eliminated detection of signal in agarose gels, indicating that staining of the protease-digested samples was specific for HA (Fig. 4A). The percentage of total HA present in the MW ranges of >3, 1–3, and <1 MDa for all cytokine conditions using samples pooled from multiple donors revealed an overall regulation by the cytokines IL-1β, TGF-β1, and TNF-α, applied individually and in combination (Fig. 5). Selected regulatory conditions that were further analyzed from individual donors (Fig. 4B) showed that for control and TGF-β1 conditions, >90% of HA had a MW below 1 MDa. It should be noted that as IL-17 and IL-32 appeared to induce a similar low-MW profile for HA, these conditions were not statistically analyzed. For individually applied IL-1β, the MW profile of HA was more diffuse, with a larger percentage of HA in the 1–3 MDa range (∼30% for IL-1β vs. ∼5% for control and ∼3% for TGF-β1, p < 0.05) and also in the >3 MDa range (∼11% for IL-1β vs. ∼2% for control and ∼1% for TGF-β1, p < 0.05), although the majority of HA was still present at <1 MDa. The effect of TGF-β1 in combination with IL-1β shifted the MW distribution of HA to a higher percentage in the >3 MDa range (∼34% vs. ∼11% for IL-1β, p < 0.05). The effect of TNF-α in combination with TGF-β1 and IL-1β did not significantly alter the MW distribution from that of TGF-β1 + IL-1β, but showed a trend of increasing percentage of HA in the >3 MDa range. This trio combination of cytokines generated a MW profile that most closely resembled that of normal human SF (∼70% in the >3 MDa range).
FIG. 4.
(A) Observation of HA in conditioned medium samples from selected regulatory cytokine treatments in the MW range of 0.1–7.0 MDa, using agarose gel electrophoresis technique. Plus (+) signs indicate the negative control samples that were subjected to HA digestion with Streptomyces hyaluronidase. (B) Percentage of total HA in the MW ranges of >3, 1–3, and <1 MDa, averaged from relative intensities of samples in (A), mean ± SEM, n = 2–4; *p < 0.05 versus both control and TGF-β1; #p < 0.05 versus IL-1β.
FIG. 5.
(A) Relative HA intensity profiles in the MW range of 0.1–7.0 MDa for all conditioned medium samples (pooled from n = 6) and normal human SF. (B) Percentage of total HA in the MW ranges of >3, 1–3, and <1 MDa, calculated from relative intensities of samples in (A). (+) signs indicate cytokines present in the culture media. Color images available online at www.liebertonline.com/ten.
The secreted PRG4 had a high MW that did not appear to be differentially regulated by any combination of cytokines analyzed, and also appeared similar to that present in normal human SF (Fig. 6).
FIG. 6.
Observation of PRG4 from conditioned medium with selected individual and combination cytokine treatments and also human SF, using techniques of gel electrophoresis, Western blotting, and chemiluminescent detection. (+) signs indicate cytokines present in the culture media.
Discussion
This study examined the regulatory effects of selected cytokines, individually and in combination, on synoviocyte HA and PRG4 secretion. HA secretion rates were increased ∼40-fold by IL-1β and to a lesser extent by TNF-α, and increased synergistically to ∼80-fold by the combination of IL-1β + TGF-β1 or TNF-α + IL-17. PRG4 secretion rates were increased ∼80-fold by TGF-β1, with counterbalancing effects by IL-1β and TNF-α. The MW of secreted HA was distributed primarily in the <1 MDa range for controls and after stimulation by individual cytokines, but was distributed in the >1 MDa range and concentrated at >3 MDa after stimulation by IL-1β + TGF-β1 + TNF-α and resembled the distribution in normal human SF. The MW of secreted PRG4 was unaffected by cytokines and similar to that present in normal human SF. The results of this study contribute to an understanding of the relationship between SF cytokine and lubricant content in health, injury, and disease, and provide approaches for using cytokines to modulate lubricant secretion rates and MW to achieve desired lubricant composition of fluid generated in joint bioreactors.
The interpretation of the data from the present study may apply to synoviocytes isolated from a normal joint, although the synoviocytes utilized in this study were isolated from synovium of patients undergoing joint replacement due to either OA or RA. In using these cells, we note that most functions of late passage synoviocytes isolated from normal, OA, and RA synovium have similar responses to cytokines, including proliferation, metalloproteinase production, adhesion molecule expression, and activation of intracellular signaling pathways.37 Although some modest differences have been observed in the invasion into cartilage in vivo, these properties were not examined in the present studies. These considerations, along with difficulty obtaining well-characterized fibroblast-like synoviocytes from normal individuals, led us to focus on cells derived from total joint replacement samples.
The effects of individually applied cytokines extend previous studies on regulation of synoviocyte HA and PRG4 secretion and provide key information for development of an ex vivo bioreactor to study SF biology. The observed stimulatory effects on HA secretion of individually applied IL-1β, TGF-β1, and TNF-α, and the synergistic effects of IL-1β and TGF-β1 have been previously demonstrated.1,26,27 Similarly, the observed stimulatory effects of TGF-β1 on PRG4 secretion, and the counterbalancing effects of IL-1β and TNF-α are consistent with the reported individual effects of these cytokines.4,38,39 The demonstrated effect of IL-1β to induce secreted HA to be distributed at higher MWs over basal controls also confirms previous reports,1,28 as does the observed high MW of PRG4 secreted with TGF-β1 stimulation.4
The current study provides new information on the synergistic effects of TNF-α and multiple cytokines on HA secretion. Although synoviocyte synthesis of certain molecules, including IL-6, IL-8, and GM-CSF, is synergistically stimulated by interaction of TNF-α with other cytokines,16,19,21 this study is the first report of such interactive regulation of lubricant secretion. The synergistic effects of cytokines on HA secretion would likely lead to an increase in total HA content in native SF, with effects on concentration that are dependent on fluid volume. The volume of SF in normal knee joints is ∼1–2 mL, but increased ∼10–25-fold in inflamed or diseased joints9,14,15; in the latter case, HA concentration is decreased, but only to 30–50% of normal levels.9,11,13 Thus, the total mass of HA in SF (volume multiplied by concentration) is typically substantially increased with joint inflammation or disease, and could be a result of upregulation of synoviocyte secretion, mediated by certain cytokines. The synergistic effect of IL-17 with TNF-α suggests that T cells secreting IL-17 could play a role in the regulation of SF lubricant composition, whereas the interactive effects of TNF-α with not only IL-17, but also IL-1β and TGF-β1 suggest that therapies targeting TNF-α40,41 might be more potent than expected based on the individual effects of TNF-α.
There are a number of mechanisms by which cytokines could have regulated HA and PRG4 secretion as observed in this study. Marked increases in mRNA expression of a specific HA synthase (HAS) enzyme, HAS1 (rather than HAS2 or HAS3), occurs in response to IL-1β, TGF-β1, or TNF-α, applied individually,42 and certain intracellular signaling molecules, including MAP kinases, have been identified in the regulation of such expression.42,43 The HAS enzymes might also underlie the regulation of HA size, as HAS3-transfected fibroblasts synthesized HA of lower MW than HAS2- and HAS1-transfected cells.44 Other potential mechanisms of cytokine regulation of HA include catabolism mediated by HA-degrading hyaluronidases,45 cellular trafficking of HAS proteins, activation of sugar nucleotide HA building blocks, interactions between HAS enzymes and the nascent growing HA chains as they are extruded from the cell,46,47 and interactions between HA and binding proteins such as TSG-618 that stabilize HA and prevent its depolymerization in SF.48 Signaling mechanisms behind PRG4 regulation have been studied less extensively than that of HA, but have suggested the role of Smad2/3 in mediating TGF-β1 signaling.49 Although these regulatory mechanisms of HA and PRG4 secretion by individual cytokines have been identified, it is unknown whether regulation by cytokines in combination occurs through signaling pathways that are distinct or involve crosstalk. Further analyses of such signaling pathways may reveal whether certain combinations of cytokines have counterbalancing effects.
The size distribution of HA in SF is dependent not only upon synthesis and degradation processes, but also upon HA retention. As transport of HA out of SF occurs through the voids of the synovium extracellular matrix,8,50 alterations in the tissue structure or in the MW of secreted HA might affect the rate that HA permeates the tissue and is lost from SF. The effect of cytokine-regulated alterations in the size distribution of HA may be magnified by size-dependent retention properties of the synovium, with low MW forms of HA being lost quickly from SF and high MW forms being selectively retained.
The ability of cytokines acting individually or synergistically to regulate synoviocyte HA and PRG4 secretion likely affects the health of articulating joint surfaces in vivo, and will be particularly useful in in vitro synovial joint bioreactors to modulate the lubricity of the fluid component.30 For example, a ∼100-fold increase in HA concentration with certain combinations of cytokines would likely result in increased fluid viscosity, as well as increased binding of lubricants to tissue surfaces, thus enhancing the friction-reducing ability of the fluid. Our subsequent studies have incorporated the information of the current study into experiments to evaluate the proper membranes for synoviocyte adherence and lubricant flux, and to generate a lubricating fluid in a biomimetic bioreactor.29 Although the effects of cytokine combinations on chondrocyte PRG4 secretion and matrix metabolism were not assessed in this study, the reported negative effects of cytokines such as IL-1β and TNF-α on these processes may be balanced by the positive effects of TGF-β1. Cytokine combinations that result in imbalances and lubricant deficiencies in fluid generated in synovial joint bioreactors may be useful in mimicking aspects of injury and disease and gaining insight into in vivo regulation of SF that may occur in pathological conditions.
The results of this study provide information on cytokine regulation of HA and PRG4 secretion rates and MW, and contribute to an understanding of the relationship between alterations in SF cytokine and lubricant content that occur in injury and disease. The results also provide approaches for using cytokines in bioreactors to modulate lubricant secretion rates and MW over a range of magnitudes. Application of this knowledge may allow achievement of desired lubricant composition of fluid generated in bioreactors with articulating tissues. The ability to generate a fluid with modulated lubricant composition could also facilitate development of arthritis therapies, such as viscosupplements or molecules that regulate lubricant secretion.
Supplementary Material
Acknowledgments
The authors thank Brooke Ballard, M.D., Prof. Paul Girard, M.D., Barbara Schumacher, and Prof. Alexandra Schwartz, M.D., for providing normal human SF, Josh Hillman for maintaining the human synoviocyte lines, and David Boyle for managing the laboratory. This work was supported by research grants from the AO Foundation, the National Institutes of Health (R01 AR051565, P01 AG007996, and R01 AI070555), an award to UCSD under the HHMI Professor Program (R.L.S.), and by University of California Systemwide Biotechnology Research & Education Program Graduate Research Education and Training Grant 2006-17 (M.E.B.).
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.Haubeck H.-D. Kock R. Fischer D.-C. van de Leur E. Hoffmeister K. Greiling H. Transforming growth factor β1, a major stimulator of hyaluronan synthesis in human synovial lining cells. Arthritis Rheum. 1995;38:669. doi: 10.1002/art.1780380515. [DOI] [PubMed] [Google Scholar]
- 2.Coleman P.J. Scott D. Ray J. Mason R.M. Levick J.R. Hyaluronan secretion into the synovial cavity of rabbit knees and comparison with albumin turnover. J Physiol. 1997;503(Pt 3):645. doi: 10.1111/j.1469-7793.1997.645bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jay G.D. Britt D.E. Cha D.-J. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27:594. [PubMed] [Google Scholar]
- 4.Jones A.R. Flannery C.R. Bioregulation of lubricin expression by growth factors and cytokines. Eur Cell Mater. 13:40. doi: 10.22203/ecm.v013a04. discussion 45, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher B.L. Block J.A. Schmid T.M. Aydelotte M.B. Kuettner K.E. A novel proteoglycan synthesized and secreted by chondrocytes of the superficial zone of articular cartilage. Arch Biochem Biophys. 1994;311:144. doi: 10.1006/abbi.1994.1219. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher B.L. Hughes C.E. Kuettner K.E. Caterson B. Aydelotte M.B. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. J Orthop Res. 1999;17:110. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 7.Levick J.R. A method for estimating macromolecular reflection by human synovium, using measurements of intra-articular half-lives. Ann Rheum Dis. 1998;57:339. doi: 10.1136/ard.57.6.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blewis M.E. Nugent-Derfus G.E. Schmidt T.A. Schumacher B.L. Sah R.L. A model of synovial fluid lubricant composition in normal and injured joints. Eur Cell Mater. 2007;13:26. doi: 10.22203/ecm.v013a03. [DOI] [PubMed] [Google Scholar]
- 9.Asari A. Miyauchi S. Sekiguchi T. Machida A. Kuriyama S. Miyazaki K. Namiki O. Hyaluronan, cartilage destruction and hydrarthrosis in traumatic arthritis. Osteoarthritis Cartilage. 1994;2:79. doi: 10.1016/s1063-4584(05)80058-5. [DOI] [PubMed] [Google Scholar]
- 10.Elsaid K.A. Jay G.D. Warman M.L. Rhee D.K. Chichester C.O. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 11.Belcher C. Yaqub R. Fawthrop F. Bayliss M. Doherty M. Synovial fluid chondroitin and keratan sulphate epitopes, glycosaminoglycans, and hyaluronan in arthritic and normal knees. Ann Rheum Dis. 1997;56:299. doi: 10.1136/ard.56.5.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahl L.B. Dahl I.M. Engstrom-Laurent A. Granath K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann Rheum Dis. 1985;44:817. doi: 10.1136/ard.44.12.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balazs E.A. The Physical Properties of Synovial Fluid and the Special Role of Hyaluronic Acid. Philadelphia: Lippincott Co.; 1974. pp. 63–75. [Google Scholar]
- 14.Ropes M.W. Rossmeisl E.C. Bauer W. The origin and nature of normal human synovial fluid. J Clin Invest. 1940;19:795. doi: 10.1172/JCI101182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jawed S. Gaffney K. Blake D.R. Intra-articular pressure profile of the knee joint in a spectrum of inflammatory arthropathies. Ann Rheum Dis. 1997;56:686. doi: 10.1136/ard.56.11.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz Y. Nadiv O. Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 2001;44:2176. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Joosten L.A. Netea M.G. Kim S.H. Yoon D.Y. Oppers-Walgreen B. Radstake T.R. Barrera P. van de Loo F.A. Dinarello C.A. van den Berg W.B. IL-32, a proinflammatory cytokine in rheumatoid arthritis. Proc Natl Acad Sci USA. 2006;103:3298. doi: 10.1073/pnas.0511233103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehlen A. Pachnio A. Thiele K. Langner J. Gene expression induced by interleukin-17 in fibroblast-like synoviocytes of patients with rheumatoid arthritis: upregulation of hyaluronan-binding protein TSG-6. Arthritis Res Ther. 2003;5:R186. doi: 10.1186/ar762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granet C. Maslinski W. Miossec P. Increased AP-1 and NF-kappaB activation and recruitment with the combination of the proinflammatory cytokines IL-1beta, tumor necrosis factor alpha and IL-17 in rheumatoid synoviocytes. Arthritis Res Ther. 2004;6:R190. doi: 10.1186/ar1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvaro-Gracia J.M. Zvaifler N.J. Firestein G.S. Cytokines in chronic inflammatory arthritis. V. Mutual antagonism between interferon-gamma and tumor necrosis factor-alpha on HLA-DR expression, proliferation, collagenase production, and granulocyte macrophage colony-stimulating factor production by rheumatoid arthritis synoviocytes. J Clin Invest. 1990;86:1790. doi: 10.1172/JCI114908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvaro-Gracia J.M. Zvaifler N.J. Brown C.B. Kaushansky K. Firestein G.S. Cytokines in chronic inflammatory arthritis. VI. Analysis of the synovial cells involved in granulocyte-macrophage colony-stimulating factor production and gene expression in rheumatoid arthritis and its regulation by IL-1 and tumor necrosis factor-alpha. J Immunol. 1991;146:3365. [PubMed] [Google Scholar]
- 22.Marks P.H. Donaldson M.L. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;21:1342. doi: 10.1016/j.arthro.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Bertone A.L. Palmer J.L. Jones J. Synovial fluid cytokines and eicosanoids as markers of joint disease in horses. Vet Surg. 2001;30:528. doi: 10.1053/jvet.2001.28430. [DOI] [PubMed] [Google Scholar]
- 24.Wei X. Messner K. Age- and injury-dependent concentrations of transforming growth factor-beta 1 and proteoglycan fragments in rabbit knee joint fluid. Osteoarthritis Cartilage. 1998;6:10. doi: 10.1053/joca.1997.0087. [DOI] [PubMed] [Google Scholar]
- 25.Kotake S. Udagawa N. Takahashi N. Matsuzaki K. Itoh K. Ishiyama S. Saito S. Inoue K. Kamatani N. Gillespie M.T. Martin T.J. Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chenevier-Gobeaux C. Morin-Robinet S. Lemarechal H. Poiraudeau S. Ekindjian J.C. Borderie D. Effects of pro- and anti-inflammatory cytokines and nitric oxide donors on hyaluronic acid synthesis by synovial cells from patients with rheumatoid arthritis. Clin Sci (Lond) 2004;107:291. doi: 10.1042/CS20040104. [DOI] [PubMed] [Google Scholar]
- 27.Recklies A.D. White C. Melching L. Roughley P.J. Differential regulation and expression of hyaluronan synthases in human articular chondrocytes, synovial cells and osteosarcoma cells. Biochem J. 2001;354:17. doi: 10.1042/0264-6021:3540017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawakami M. Suzuki K. Matsuki Y. Ishizuka T. Hidaka T. Konishi T. Matsumoto M. Kataharada K. Nakamura H. Hyaluronan production in human rheumatoid fibroblastic synovial lining cells is increased by interleukin 1 beta but inhibited by transforming growth factor beta 1. Ann Rheum Dis. 1998;57:602. doi: 10.1136/ard.57.10.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blewis M.E. Lao B.J. Jadin K.D. McCarty W.J. Antonacci J.M. Bugbee W.D. Firestein G.S. Sah R.L. Biomimetic bioengineering of synovial fluid: a bioreactor for a functional lubricant solution. Trans Orthop Res Soc. 2009;34:121. [Google Scholar]
- 30.Nugent-Derfus G.E. Takara T. O'Neill J.K. Cahill S.B. Gortz S. Pong T. Inoue H. Aneloski N.M. Wang W.W. Vega K.I. Klein T.J. Hsieh-Bonassera N.D. Bae W.C. Burke J.D. Bugbee W.D. Sah R.L. Continuous passive motion applied to whole joints stimulates chondrocyte biosynthesis of PRG4. Osteoarthritis Cartilage. 2007;15:566. doi: 10.1016/j.joca.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grad S. Gogolewski S. Alini M. Wimmer M.A. Effects of simple and complex motion patterns on gene expression of chondrocytes seeded in 3D scaffolds. Tissue Eng. 2006;12:3171. doi: 10.1089/ten.2006.12.3171. [DOI] [PubMed] [Google Scholar]
- 32.Tehranzadeh J. Booya F. Root J. Cartilage metabolism in osteoarthritis and the influence of viscosupplementation and steroid: a review. Acta Radiol. 2005;46:288. doi: 10.1080/02841850510016027. [DOI] [PubMed] [Google Scholar]
- 33.McGowan K.B. Kurtis M.S. Lottman L.M. Watson D. Sah R.L. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 34.Su J.-L. Schumacher B.L. Lindley K.M. Soloveychik V. Burkhart W. Triantafillou J.A. Kuettner K.E. Schmid T.M. Detection of superficial zone protein in human and animal body fluids by cross-species monoclonal antibodies specific to superficial zone protein. Hybridoma. 2001;20:149. doi: 10.1089/027245701750293475. [DOI] [PubMed] [Google Scholar]
- 35.Afify A. Lynne L.C. Howell L. Correlation of cytologic examination with ELISA assays for hyaluronan and soluble CD44v6 levels in evaluation of effusions. Diagn Cytopathol. 2007;35:105. doi: 10.1002/dc.20585. [DOI] [PubMed] [Google Scholar]
- 36.Lee H.G. Cowman M.K. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal Biochem. 1994;219:278. doi: 10.1006/abio.1994.1267. [DOI] [PubMed] [Google Scholar]
- 37.Firestein G.S. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 38.Khalafi A. Schmid T.M. Neu C. Reddi A.H. Increased accumulation of superficial zone protein (SZP) in articular cartilage in response to bone morphogenetic protein-7 and growth factors. J Orthop Res. 2007;25:293. doi: 10.1002/jor.20329. [DOI] [PubMed] [Google Scholar]
- 39.Niikura T. Reddi A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor beta/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 40.Edwards C.J. Immunological therapies for rheumatoid arthritis. Br Med Bull. 2005;73:71. doi: 10.1093/bmb/ldh051. [DOI] [PubMed] [Google Scholar]
- 41.Christodoulou C. Choy E.H. Joint inflammation and cytokine inhibition in rheumatoid arthritis. Clin Exp Med. 2006;6:13. doi: 10.1007/s10238-006-0088-5. [DOI] [PubMed] [Google Scholar]
- 42.Stuhlmeier K.M. Pollaschek C. Differential effect of transforming growth factor beta (TGF-beta) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-beta-induced hyaluronan synthase 1 activation. J Biol Chem. 2004;279:8753. doi: 10.1074/jbc.M303945200. [DOI] [PubMed] [Google Scholar]
- 43.Stuhlmeier K.M. Prostaglandin E2: a potent activator of hyaluronan synthase 1 in type-B-synoviocytes. Biochim Biophys Acta. 2007;1770:121. doi: 10.1016/j.bbagen.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 44.Itano N. Sawai T. Yoshida M. Lenas P. Yamada Y. Imagawa M. Shinomura T. Hamaguchi M. Yoshida Y. Ohnuki Y. Miyauchi S. Spicer A.P. McDonald J.A. Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 45.El Hajjaji H. Cole A.A. Manicourt D.H. Chondrocytes, synoviocytes and dermal fibroblasts all express PH-20, a hyaluronidase active at neutral pH. Arthritis Res Ther. 2005;7:R756. doi: 10.1186/ar1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hascall V.C. Majors A.K. De La Motte C.A. Evanko S.P. Wang A. Drazba J.A. Strong S.A. Wight T.N. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673:3. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Pummill P.E. DeAngelis P.L. Alteration of polysaccharide size distribution of a vertebrate hyaluronan synthase by mutation. J Biol Chem. 2003;278:19808. doi: 10.1074/jbc.M301097200. [DOI] [PubMed] [Google Scholar]
- 48.Hutadilok N. Ghosh P. Brooks P.M. Binding of haptoglobin, inter-alpha-trypsin inhibitor, and alpha 1 proteinase inhibitor to synovial fluid hyaluronate and the influence of these proteins on its degradation by oxygen derived free radicals. Ann Rheum Dis. 1988;47:377. doi: 10.1136/ard.47.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neu C.P. Khalafi A. Komvopoulos K. Schmid T.M. Reddi A.H. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor beta signaling. Arthritis Rheum. 2007;56:3706. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 50.Levick J.R. An analysis of the interaction between interstitial plasma protein, interstitial flow, and fenestral filtration and its application to synovium. Microvasc Res. 1994;47:90. doi: 10.1006/mvre.1994.1007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.