Abstract
Vocal fold diseases and disorders are difficult to treat surgically or therapeutically. Tissue engineering offers an alternative strategy for the restoration of functional vocal folds. As a first step toward vocal fold tissue engineering, we investigated the responses of primary vocal fold fibroblasts (PVFFs) to two types of collagen and hyaluronic acid (HA)-based hydrogels that are compositionally similar, but structurally variable and mechanically different. Type A hydrogels were composed of mature collagen fibers reinforced by oxidized HA, whereas type B hydrogels contained immature collagen fibrils interpenetrated in an amorphous, covalently cross-linked HA matrix. PVFFs encapsulated in either matrix adopted a fibroblastic morphology and expressed genes related to important extracellular matrix proteins. DNA analysis indicated a linear growth profile for cells encapsulated in type B gels from day 0 to 21, in contrast to an initial dormant, nonproliferative period from day 0 to 3 experienced by cells in type A gels. At the end of the culture, similar DNA content was detected in both types of constructs. A reduction in collagen content was observed for both types of constructs after 28 days of culture, with type A constructs generally retaining higher amounts of collagen than type B constructs. The HA content in the constructs decreased steadily throughout the culture, with type A constructs consistently exhibiting less HA than type B constructs. Using the torsional wave analysis, we found that the elastic moduli for type A constructs decreased sharply during the first week of culture, followed by 2 weeks of matrix stabilization without significant changes in matrix stiffness. Conversely, the elastic modulus for type B constructs increased moderately over time. It is postulated that PVFFs residing in gels alter the matrix organization, chemical compositions, and viscoelasticity through cell-mediated remodeling processes.
Introduction
Vocal folds, when driven into a wave-like motion by the air from the lung, produce a great variety of sounds that color our lives. The biomechanical function of the vocal fold is a direct result of its unique, laminated structure consisting of a squamous epithelium, the lamina propria (LP), and the vocalis muscle. The superficial layer of LP, combined with epithelium, is also known as the mucosa.1,2 Many stimuli can lead to vocal fold dysfunction and damage, including excessive mechanical stress, smoke inhalation, acid reflux, allergies, inflammation, surgery, and cancer.3 Scarred vocal folds exhibit increased stiffness and reduced flexibility that compromise the vocal fold functions.4 More severe vocal fold diseases, such as vocal fold paralysis and tumors, require drastic surgical procedures to restore minimum sound production capability. The development of a tissue engineering methodology for the reconstruction of the vocal fold will not only provide an in vitro platform for the investigation of vocal fold diseases but also offer alternative treatments for vocal fold disorders.
The viscoelastic properties of the vocal fold LP depend on structure and composition of its extracellular matrix (ECM). Type I collagen fibers not only provide tensile strength to the vocal fold LP but also maintain its organization during vibration.5,6 Interestingly, these collagen fibers exhibit an intertwined network arrangement, giving rise to a collagenous mesh that allows for the phonation frequency to be readily modulated by the intrinsic musculature of the larynx.7,8 On the other hand, studies indicate that hyaluronic acid (HA) contributes to the maintenance of optimal tissue viscosity to facilitate phonation, and an optimal tissue stiffness that may also be important for vocal fundamental frequency control.9 HA has been proposed to act as a shock absorber for the tissue during normal phonation,10 and a promoter for scarless healing in the fetal vocal folds.11
These studies suggest that biomaterial composites based on collagen and HA may provide useful tissue engineering strategies for vocal fold repair or replacement. Although collagen alone has been used for three-dimensional (3D) culture of fibroblasts, the reconstituted collagen gels suffer from low mechanical strength and rapid degradation.12,13 The incorporation of virgin HA into collagen gels does not lead to hybrid matrices with enhanced stability and improved mechanical strength that could be used in long-term culture of vocal fold fibroblasts.13 In fact, high molecular weight (HMW) virgin HA is reported to facilitate fibroblast-mediated collagen gel compaction,14 a process that contributes to a myofibroblast phenotype and scar formation.15
In the natural ECM, collagen is covalently cross-linked by lysyl oxidase during fibril association. The cross-linking process is believed to play a key role in the maturation of macroassemblies and is a determinant of the mechanical properties of connective tissues.16 Similarly, HA is not simply physically entangled in the interstitial of the natural ECM; it locally stabilizes and is stabilized by other ECM through specific HA binding proteins.17,18 Consequently, HA not only helps to create a lattice that regulates cell adhesion and migration19 but also gives rise to defined tissue viscoelasticity.20,21
Abundant studies have demonstrated that, in addition to the biological cues, substrate microstructures and stiffness have profound effects on cell phenotype, cell adhesion, cell motility, cytoskeleton condensation, gene expression, and intracellular signaling pathways.22–26 For vocal fold tissue engineering to be successful, the chemical composition, structural characteristics, and mechanical properties of the vocal fold LP need to be recaptured in vitro. The combination of chemically modified, covalently cross-linkable HA derivatives with self-assembling collagen monomers offers an attractive strategy to provide custom-designed, conducive matrices that overcome the limitations associated with matrices based on HA or collagen alone.
The goal of this study was to assess the effects of matrix composition, microstructure, and viscoelasticity on the behaviors of vocal fold fibroblasts cultured in hydrogel networks. To this end, collagen (type I) monomers were combined with HA derivatives carrying aldehyde (CHO) groups (HACHO) and hydrazide groups (via reaction with adipic acid dihydrazide, ADH) (HAADH) to form chemically defined composite hydrogels. Two types of collagen–HA gels were used for in situ encapsulation and prolonged, static culture of primary vocal fold fibroblasts (PVFFs). Type A hydrogels are composed of collagen and HACHO, whereas type B gels contain collagen interpenetrated in an amorphous interstitial formed by HAADH and HACHO. The functions of PVFFs in terms of cell morphology, cell proliferation, gene expression, and matrix remodeling within both types of matrices were systematically investigated. The viscoelastic properties of the cell-populated scaffolds were monitored at different time points using a torsional wave apparatus (TWA) at frequencies close to human phonation.
Materials and Methods
Materials
HA (sodium salt) of high and low molecular weight (HMW: 1.3 MDa; LMW: 490 kDa) was generously donated by Genzyme Corporation (Cambridge, MA). Rat tail collagen (type I) was purchased from BD Biosciences (San Jose, CA). Paraformaldehyde (2% in DPBS), Triton X-100 (0.2% in DPBS), chloramine-T aqueous solution, p-dimethylaminobenzaldehyde, concentrated sulfuric acid, sodium hydroxide, hydrochloric acid, and absolute ethanol were purchased from Thermo Fisher Scientific (Waltham, MA). All cell culture reagents, staining reagents, including propidium iodide (1:2000 in Dulbecco's phosphate-buffered saline [DPBS]), SYTO 13 (1:1000 in DPBS), and Alexa Fluor 488 Phalloidin (1:1000 in DPBS), digestion enzyme (proteinase K 67 μg/mL in DPBS), picogreen DNA assay kit, and TRIzol reagent were obtained from Invitrogen (Carlsbad, CA). Mouse anti-vimentin primary antibody (1:100) was purchased from Genway Biotech (San Diego, CA). Mouse anti-muscle-specific actin (MSA) primary antibody (ab-4 clone HHF35, 1:50) was obtained from Thermo Fisher Scientific. Alexa Fluor 488–labeled secondary antibody (goat anti-mouse IgG, 1:50) was obtained from Invitrogen, and Draq-5 (1:1000) was purchased from Axxora LLC (San Diego, CA). All antibodies were diluted in PBS containing 1% bovine serum albumin (Jackson Immunoresearch, West Grove, PA). Tissue Tek O.C.T.™ was purchased from Sakura Finetek USA (Torrance, CA). The antifading medium, Gel/Mount, was purchased from Biomeda (Foster City, CA).
Synthesis and characterization of HA derivatives
Complementary functional groups, aldehyde or hydrazide, were introduced to HA via sodium periodate oxidation using HMW HA and carbodiimide-mediated coupling with adipic acid dehydrazide using LMW HA, respectively. The resulting derivatives (HACHO and HAADH) were characterized as previously described.27–29 The sterile filtered, freeze-dried products were stored at 4°C before use.
Cell culture
Fresh porcine (2–3 year-old male) larynxes were collected from Salem Packing (Salem, NJ) and were used within 4 h of slaughtering. Under sterile conditions, the larynx was cut open longitudinally, and the vocal folds were dissected with a 5 mm dermal biopsy punch. The underlying muscle layer was carefully removed using scalpels. The PVFFs were isolated by collagenase disaggregation following an established protocol.30 The isolated cells were cultured on tissue culture polystyrene (TCPS) at 37°C with 5% CO2 in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 1% penicillin–streptomycin. PVFFs were used between passages 3 and 8 in all experiments.
Cell encapsulation and 3D culture
HACHO and HAADH were separately dissolved in PBS solution at a concentration of 20 mg/mL. A high concentration of type I collagen was neutralized with NaOH (1 M), 10 × DPBS, and deionized (DI) H2O, and brought to a concentration of 8.33 mg/mL. Phenol red was used as a pH indicator. PVFFs were trypsinized, counted, centrifuged, and resuspended in regular media at a desired cell density. An aliquot of the cell suspension was then added to the HACHO solution to a final cell density of 1 × 105 cells/mL. The ice-cold, neutralized collagen solution was mixed with the HACHO–cell solution to form the type A construct.13 To prepare type B constructs, the HACHO–cell suspension was premixed with the collagen solution before adding the HAADH component. The final concentrations of collagen and functionalized HA were, respectively, 5 and 8 mg/mL. Aliquots of 0.2 mL of the above mixture were placed into cell culture inserts (Cellulose Membrane, 0.4 μm, 12 mm diameter; Millipore, Billerica, MA) in 24-well plates. The plates were placed in an incubator (37°C and 5% CO2) for 45 min to allow for complete gelation, after which the medium was added into the wells. The constructs were cultured for up to 28 days, and the medium was changed every other day.
Hydrogel microstructure
Cryogenic scanning electron microscopy (CryoSEM) was applied to analyze the microstructure of collagen–HA composite hydrogels. The control samples included collagen alone and collagen mixed with LMW HA. For CryoSEM, samples were pressurized (≥2000 bar) and rapidly frozen (≥10,000 degrees/s) before being transferred to the preparation chamber under vacuum, where they were freeze-fractured in liquid nitrogen. After water was sublimed, samples were brought back to a temperature of −130°C, coated with gold-palladium (10 mA current), and viewed in a Hitachi S-4700 field emission scanning electron microscope (FESEM) at 1 kV (emission current 30 μA) with a working distance of approximately 3–6 mm.27
Cell viability and cytoskeleton organization
To assess the cell viability, cell–gel constructs were rinsed with DPBS, and stained with propidium iodide and SYTO 13. To observe the cytoskeletal organization, the constructs were fixed in 2% paraformaldehyde, permeabilized with 0.2% Triton X-100, treated with image-iTFX signal enhancer, and stained with Alexa Fluor 488 Phalloidin and Draq-5. The stained constructs were inspected using a Zeiss LSM510 Axiovert confocal microscope.
Cell proliferation
The 3D constructs were harvested at predetermined time points and digested in proteinase K (67 μg/mL) at 55°C overnight.13,31 The total DNA content was quantified using a picogreen DNA assay following the manufacturer's protocol. Briefly, 10 μL of each digested sample was diluted in a Tris-HCl–EDTA buffer to a final volume of 1.0 mL. One milliliter of picogreen working solution was added to each sample, and incubated for 2–5 min at room temperature in the dark. Using a PerkinElmer microplate reader, the samples were excited at 485 nm and the intensity of the fluorescence emission was measured at 530 nm. The standard curve was prepared using double stranded DNA (dsDNA) provided in the assay.
Gene expression
Reverse transcription–polymerase chain reaction (PCR) was employed to analyze the expression of ECM-related genes for PVFFs. Once harvested, the constructs were immediately snap-frozen and stored in liquid nitrogen. RNA was extracted from the constructs using TRIzol reagent according to the manufacturer's protocol. The RNA concentration was determined at 260 nm using a Nanodrop ND-1000 spectrophotometer (Nanodrop Technologies, Wilmington, DE). Reverse transcription of RNA into cDNA was implemented using the QuantiTect Reverse Transcription kit from Qiagen following the manufacturer's instructions. PCR amplification was performed for 35 cycles using GoTaq Green master mix (Promega, Madison, WI), and a MyGene Series Peltier thermal cycler (MG96G; Long Gene, Hangzhou, China). The cycling conditions were: initial denaturation at 94°C for 3 min, additional denaturation at 94°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 1 min, and a final extension at 72°C for 10 min. PCR products were electrophoresed on 1.2% agarose gels with ethidium bromide, and the images were captured with an Alpha Imager (Alpha Innotech, San Leandro, CA). Genes32–34 for hyaluronidase 2, matrix metalloproteinase 1, procollagen I, HA synthase 2, and fibronectin were analyzed. Glyceraldehyde-3-phosphate dehydrogenase was included as a control. All primers were synthesized by Integrated DNA Technologies (Coralville, IA). The primer sequences and optimal conditions are summarized in Table 1.
Table 1.
Primer Sequences and Optimal Polymerase Chain Reaction Conditions Used in Reverse Transcription–Polymerase Chain Reaction
| Gene | GenBank number | Forward (5′–3′) | Reverse (5′–3′) | PS (bp) | AT (°C) |
|---|---|---|---|---|---|
| HYAL2 | NM214440.1 | AGGGCTTAGCGAGATGGATCT | TGTCAGGTAATCCTTGAGGTATTGG | 136 | 55 |
| MMP1 | X54724.1 | CGTGCCATTGAGAAAGCCTT | GTCTGCTTGACCCTCGGAGA | 81 | 55 |
| ProCOLI | NM000088.3 | GTTGGTGCTAAGGGTGAAGC | ACCAGTGTCTCCTTTGCTGC | 294 | 55 |
| HAS2 | NM005328.2 | GTTGGCTACCAGTTTATCCAAACG | CTTTATGTGACTCATCTGTCTCAC | 403 | 55 |
| FN | NG012196.1 | TACCAACCTACGGATGACTCG | CATCATCGTAACACGTTGCC | 300 | 55 |
| GAPDH | NG007073.2 | ACCCAGAAGACTGTGGATGG | TGAGCTTGACAAAGTGGTCG | 382 | 55 |
PS, product size; AT, annealing temperature; HYAL2, hyaluronidase 2; MMP1, matrix metalloproteinase 1; ProCOLI, procollagen I; HAS2, hyaluronic acid synthase 2; FN, fibronectin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Collagen content
The total collagen content was quantified using an established hydroxyproline assay.31,35 Briefly, 50 μL digested samples was hydrolyzed with 60 μL 12 N HCl in glass vials fitted with Teflon-lined caps at 110°C for 18 h. As a pH indicator, 2 μL of methyl red was added to the hydrolyzed samples; the solutions were neutralized with sodium hydroxide and hydrochloric acid, and brought to the same volume with distilled water. One hundred microliters of the neutralized solution was added in a 96-well plate, and reacted with 50 μL of chloramine-T solution for 15 min at room temperature. Fifty microliters of p-dimethylaminobenzaldehyde solution was then added, and the plate was incubated at 37°C for 30 min. The absorbance of the final solution was read at 550 nm using a PerkinElmer plate reader. The standards used were different dilutions of type I collagen in 0.02 N acetic acid.
HA content
The total HA content was quantified using the carbazole assay.36 Briefly, sodium tetraborate decahydrate (Na2B4O7 · 10H2O, 1.0 g) was dissolved in 200 mL concentrated H2SO4 and the solution was cooled to 0°C before 25 mL of distilled water was slowly added. The Na2B4O7 solution (3.5 mL each) was aliquoted to glass vials fitted with Teflon-lined caps and was cooled to 0°C before the addition of 100 μL of sample solutions. The reaction mixture was heated at 100°C for 10 min to ensure complete degradation of HA. A freshly prepared carbazole solution (1.25 mg/mL in absolute ethanol, 100 μL) was added to the ice-cold mixture and the resulting solution was again heated at 100°C for color development. The final solutions were cooled and transferred to disposable UV cuvettes for HA quantification at 530 nm using a PerkinElmer Lambda 35 UV–Vis spectrophotometer. The standard curve was obtained using HA solutions of varying concentrations.
Immunohistochemistry and histology
At a predetermined time, the constructs were harvested, embedded in Tissue-Tek OCT, cryosectionned with a Leica CM3050S cryostat, and stored at −80°C until all sections were collected. For immunohistochemical analysis, the sections (20 μm thick) were then fixed in cold methanol for 10 min, rehydrated in PBS for 5 min, blocked with 1% bovine serum albumin–PBS for 1 h, and incubated with the primary antibody (mouse Vimentin, or mouse MSA) for 1 h. After rinsing in PBS for 5 min, the sections were incubated with the secondary antibody and were counterstained with Alexa Fluor 488 anti-mouse IgG and Draq-5 for 1 h, respectively. The resulting sections were rinsed in PBS for 5 min, mounted with Gel/Mount, and imaged using an LSM510 Axiovert confocal microscope. Freshly isolated PVFFs cultured as a monolayer were subjected to the same staining protocols as the controls. For histological analysis, the cryosections (20 μm) were mounted on microscope slides, stained with hematoxylin and eosin (H&E) and Movat's pentachrome following standard protocols,37 and observed on a Nikon TS100-F phase-contrast microscope.
Mechanical testing
At each time point, the cell–gel constructs, along with the cell-free hydrogels incubated under the same conditions, were harvested, snap frozen in liquid nitrogen, and shipped to Brown University (Providence, RI) for mechanical testing. Samples were individually thawed in a 37°C water bath for ∼10 min before being sandwiched between two vertically aligned hexagonal plates. The plates containing the sample were enclosed in an environmental chamber with controlled temperature (34–37°C) and humidity (>94%). The bottom plate was driven into motion (at a rotational angle of <0.2°) by a function generator with frequencies ranging from 10 to 200 Hz. As the bottom plate oscillates, a torsional wave propagates through the sample, driving the top plate into oscillation. The rotation amplitudes of the top and bottom plates were measured by an optical lever technique. The amplification factor, defined as the ratio of the amplitude of the rotation of the top plate to that of the bottom plate, reached a peak value at the resonance frequency of the sample. The average amplification factors (obtained from three consecutive measurements with the same sample geometry) were compared with those predicted by a linear viscoelastic model in terms of the amplitude of the complex shear modulus and the loss angle. For each test, a constant modulus and loss angle were obtained that provided the best fit between the model and the experimental results over a range of frequencies spanning the resonance frequency. Details regarding the design and data reduction for TWA can be found in our previous publications.27,28,38
Statistical analysis
All quantitative measurements were performed on three to five repeats. Data reported are mean values ± standard error of the mean. Statistical significance was analyzed using a two-tailed, equal variance Student's t-test. A p-value of less than 0.05 was considered to be statistically different.
Results
Hydrogel microstructure
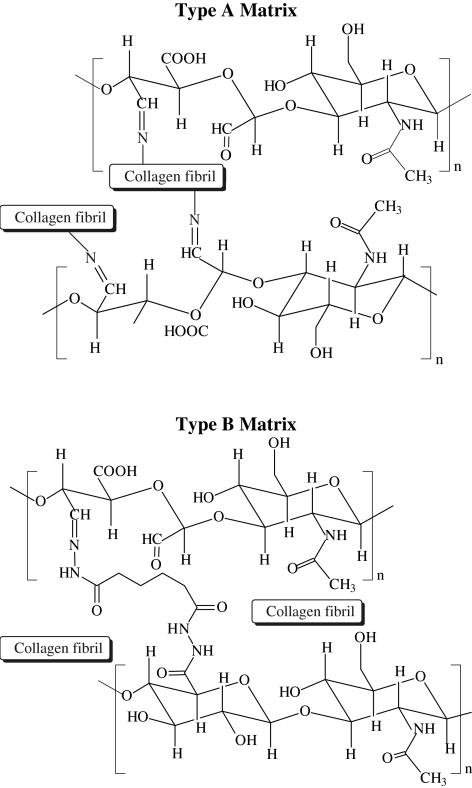
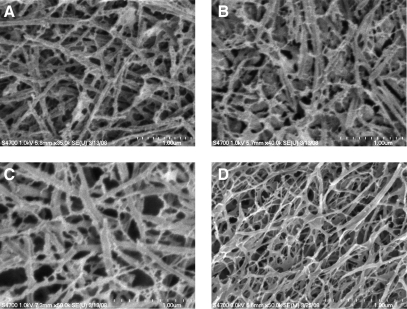
Composite matrices based on self-assembled collagen and HA derivatives containing mutually reactive functional groups (HACHO and HAADH) offer the opportunity to fine-tune the matrix properties. Two types of composite hydrogels were assessed in this study. Type A gels were formed by mixing HACHO with self-assembling collagen monomers, whereas type B gels were prepared by addition of HAADH to the premixed HACHO–collagen solution (Fig. 1). Hydrogels based on collagen and LMW virgin HA, as well as collagen only, were included as the controls. CryoSEM images (Fig. 2) show that all four types of hydrogels are composed of interdigitated fibrillar structures with pores in the nanometer scale. The microstructure of collagen–HA and collagen–HACHO gels closely resembles that of the collagen gels, consisting mainly of entangled fibrils of ∼50–100 nm in diameter and several microns long. A close inspection of the CryoSEM image for type B gels (Fig. 2D) reveals subtle differences in its microstructure from other composite hydrogels investigated. Although fibrillar entities are clearly present, they are less defined and exhibit a smaller overall fiber diameter. The distinct interstitial phase resembles the microstructure of the amorphous gels formed by direct mixing of HACHO and HAADH.27Although the fibers in other gels follow straight, strut-like pathways, the fibrils in type B gels are readily bent and appeared more flexible. Individual fibrils seem to be interconnected by an amorphous matrix with smaller pore sizes. Small-angle neutron scattering (SANS) analysis (Supplemental Fig. S1; supplemental material available online at www.liebertonline.com/ten), on the other hand, did not reveal significant structural differences for all matrices investigated.
FIG. 1.
Schematic representations of the chemical reactions involved in the formation of composite collagen–hyaluronic acid (HA) matrices. Type A matrices are composed of collagen and HACHO (top), whereas type B gels are derived from collagen/HACHO/HAADH (bottom). HACHO, HA derivatives carrying aldehyde groups; HAADH, hydrazide groups via reaction with adipic acid dihydrazide.
FIG. 2.
Representative cryogenic scanning electron microscopy images of a collagen gel (A); a collagen gel containing low molecular weight virgin HA (B); type A composite gels based on collagen and HACHO (C); and type B composite gels based on collagen, HACHO, and HAADH (D). Scale bar (dotted line): 1.00 μm.
Cell viability, phenotype, and proliferation
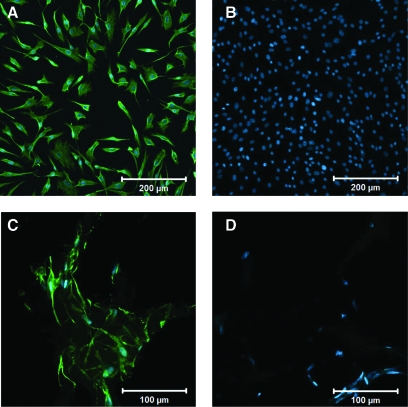
Freshly isolated PVFFs exhibited an elongated spindle-shaped morphology as revealed by the phase contrast microscopy (data not shown). The fibroblastic phenotype of PVFFs before the encapsulation was confirmed by a positive staining for vimentin, an intermediate filament present in fibroblastic cells and absent in epithelial cells (Fig. 3A), and a negative staining for MSA (Fig. 3B), a cytoplasmic marker for myofibroblasts and smooth muscle cells. In situ encapsulation of PVFFs in collagen–HA gels was achieved by mixing PVFFs with soluble HA derivatives and collagen followed by 45-min incubation at 37°C. The encapsulation process was highly compatible since the majority of cells remained viable immediately after cell encapsulation as assessed by the live/dead assay (Fig. 4). PVFFs cultured in both types of composite matrices exhibit the same staining patterns for vimentin (positive) and MSA (negative) as those freshly isolated from the tissue (Fig. 3C, D). No gel compaction could be visually detected for both types of constructs until the end of culture when a minor reduction of construct diameter was observed.
FIG. 3.
Representative fluorescence images showing PVFFs stained positive for vimentin (green, A, C) and negative for muscle-specific actin (no green, B, D). The nuclei were stained blue. (A, B) PVFFs cultured on tissue culture polystyrene (scale bar: 200 μm); (C, D) PVFFs cultured in type A gels for 28 days (scale bar: 100 μm). PVFF, primary vocal fold fibroblasts. Color images available online at www.liebertonline.com/ten.
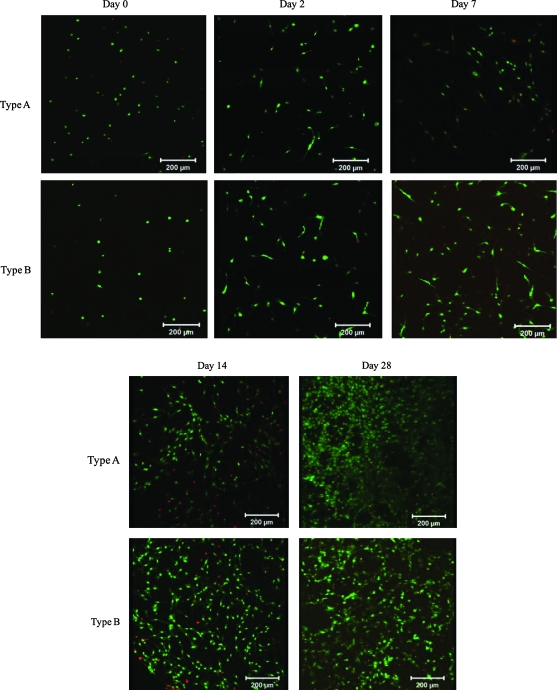
FIG. 4.
Representative live/dead staining of the cell–gel constructs cultured under static conditions for 0, 2, 7, 14, and 28 days. Live (green) and dead (red) cells were stained with SYTO 13 and propidium iodide, respectively. Scale bar: 200 μm. Color images available online at www.liebertonline.com/ten.
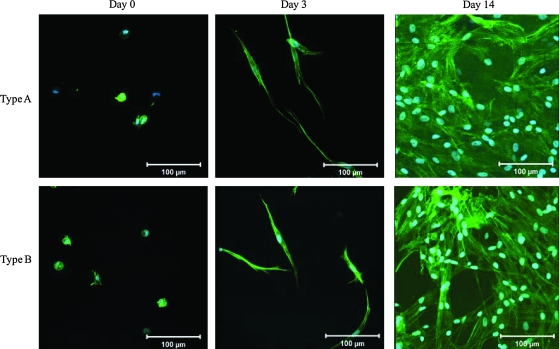
Cells maintain their ability to proliferate within the 3D hydrogels as indicated by the increase in cell number with the days of culture (Fig. 4). By day 28, both types of gels have been densely populated by the proliferating PVFFs. Occasionally, isolated dead cells (stained red) were detected in the gels. F-actin staining was applied to cells encapsulated in 3D hydrogels to assess the cell morphology and cytoskeleton organization (Fig. 5). Several hours after encapsulation (day 0, Fig. 5), actin filaments remained clustered around the nuclei, giving rise to a rounded morphology. The majority of the cells remained rounded after 1 day of culture (data not shown) and an additional day of culture is necessary for most cells to attach and spread within the matrices (Fig. 4). By day 3, PVFFs have already developed distinctly long and organized cytoskeleton processes, suggesting their involvement in cell attachment (Fig. 5). The proliferating PVFFs maintained their elongated morphology throughout the matrix during the course of culture. Comparable cell morphology and cell distribution patterns were observed in both types of hydrogels. PVFFs cultured in 3D composite hydrogels seem to be more elongated than those cultured on 2D plastic surfaces.
FIG. 5.
Cytoskeleton staining of the cell–gel constructs cultured under static conditions for 0, 3, and 14 days. F-actin (green) and nuclei (blue) were stained with phalloidin Alexa Fluor 488 and Draq-5, respectively. Scale bar: 100 μm. Color images available online at www.liebertonline.com/ten.
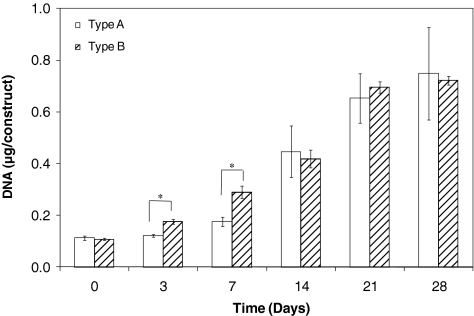
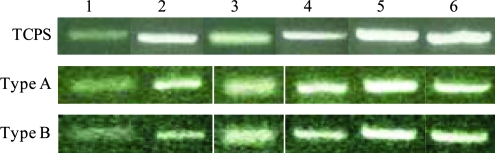
Cell proliferation was quantified by analyzing the DNA content per construct (Fig. 6). For type A construct, an initial dormant, nonproliferative period from day 0 to 3 was followed by a linear growth phase from day 7 to 21, reaching a plateau after 21 days of culture. On the other hand, the DNA content in type B gels continues to increase after encapsulation, reaching a maximum value at day 21, with no further changes during the last week of culture. The DNA content per construct increased by 6.8-fold for type A constructs and 6.5-fold for type B matrices over 28 days of culture, suggesting an active cell proliferation within the constructs. This proliferative trend reflects the confocal observation after live/dead staining (Fig. 4). To gain further insight into the functions of PVFFs cultured in the hydrogel matrices, gene expression related to several ECM proteins, including hyaluronidase 2, HA synthase 2, ProCOL1, and fibronectin, as well as an enzyme that degrades collagen (matrix metalloproteinase 1), was examined (Fig. 7). Freshly isolated PVFFs cultured on TCPS were included as the controls. Our results show that genes related to these ECM proteins were consistently and robustly expressed for cells cultured under all conditions. The gene expression level, however, was not quantified.
FIG. 6.
DNA content per construct as a function of culture time. Type A constructs, day x (x = 7, 14, 21, and 28) versus day 0: values are significantly different (p ≤ 0.01); type B constructs, day x (x = 3, 7, 14, 21, and 28) versus day 0: all values are significantly different (p < 0.001). *Significant difference between type A and type B gel (p ≤ 0.01).
FIG. 7.
mRNA expression patterns for extracellular-matrix-related genes analyzed by reverse transcription–polymerase chain reaction. 1, hyaluronidase 2; 2, HA synthase 2; 3, matrix metalloproteinase 1; 4, ProCOL1; 5, fibronectin; 6, glyceraldehyde-3-phosphate dehydrogenase. RNA was isolated from PVFFs cultured on tissue culture polystyrene (TCPS) (top) as well as those cultured in type A gels (middle) and type B gels (bottom) for 28 days. Color images available online at www.liebertonline.com/ten.
Matrix composition
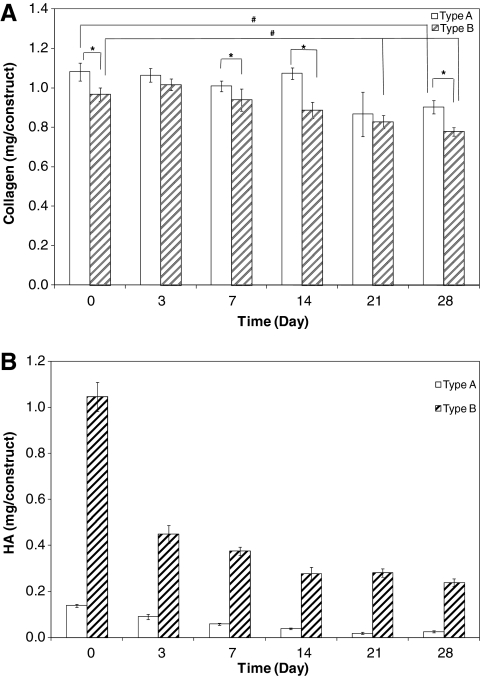
Hydroxyproline assay was utilized to quantify the overall collagen content in the cell–gel constructs. In general, type A constructs contained statistically (p < 0.05) higher amounts of collagen than type B constructs at all times except day 3 and 21. For type A constructs, the collagen content remained relatively stable until day 21 when an approximately 20% decrease was detected. For type B constructs, the collagen content gradually decreased over the entire course of culture. Overall, collagen content in both types of constructs at the end of the culture was distinctly (p < 0.01) lower than at day 0; a 16% and 20% decrease in collagen content was detected for type A and type B constructs, respectively (Fig. 8A). For type A matrices incubated under the same conditions in the absence of the encapsulated cells, a similar amount of collagen was retained over the entire course of culture. At the end of the culture, the amount of collagen in cell-free hydrogels (1.22 mg/gel) was statistically (p < 0.01) higher than that in the cell–gel constructs (0.92 mg/construct). For type B matrices, hydroxyproline analysis did not reveal any statistical differences between the cell–gel constructs and the cell-free matrices incubated for the same days.
FIG. 8.
Quantification of collagen (A) and HA (B) content as a function of culture time. (A) Collagen content quantified by hydroxyproline assay; #Significant difference from day 0, p ≤ 0.05. *Significant difference between type A and type B gels, p ≤ 0.05. (B) HA content analyzed by carbazole assay; type A versus type B: statistically different at all times (p < 0.001); type A, day x (x = 3, 7, 14, 21, and 28) versus day 0: all values were significantly different; day 7 versus day 14: significantly different (p ≤ 0.01); day 14 versus day 21: significantly different (p ≤ 0.01); type B, day x (x = 3, 7, 14, 21, and 28) versus day 0: all values were significantly different (p ≤ 0.01); day 7 versus day 14: significantly different (p ≤ 0.05).
Separately, carbazole assay was applied to monitor the overall HA content in each constructs (Fig. 8B). For type A constructs, the total HA content decreased steadily from 0.14 mg at day 0 to 0.02 mg at day 21 and remained essentially unchanged thereafter. For type B constructs, a rapid decrease (from 1.05 mg at day 0 to 0.45 mg at day 3) was observed during the initial 3 days of culture and a relatively steady level of HA (0.24–0.28 mg/construct) was maintained from day 14 to 28. HA content remained significantly higher (p < 0.001) in type B gels than in type A gels at all times. Control experiments with cell-free hydrogels showed similar profiles of HA retention as a function of culture time for both types of matrices. For type A gels, the total HA content decreased from 0.23 mg/construct at day 0 to 0.06 mg/construct at day 28. For type B gels, the total HA content decreased from 1.07 mg at day 0 to 0.34 mg per constructs at day 28. At the end of culture, the overall HA content was slightly higher in cell-free hydrogels than in the corresponding cell–gel constructs for both types of matrices.
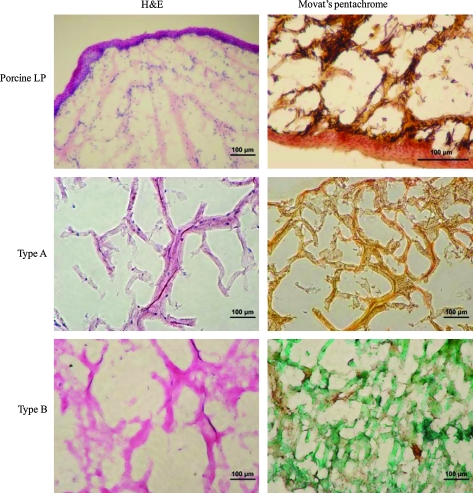
The matrix composition was also analyzed by histological staining after 28 days of culture. Histology images of the cell–gel constructs (Fig. 9) revealed a typical loose connective tissue morphology, containing abundant open, fluid-filled spaces similar to those observed for porcine vocal fold LP. The dense epithelial layer on top of the LP is clearly visible in the porcine tissue. H&E staining shows the presence of scattered PVFFs in the constructs. The seemingly low cellularity observed in the histology samples does not contradict the high cell density suggested by the live/dead assay since the former was an image of a 20-μm-thick section, whereas the latter is a stacked 3D image. Collagen and HA seem to be colocalized in both types of constructs. Type A gels were stained brownish yellow by Movat pentachrome staining, whereas type B gels were stained green due to the relatively higher HA content in type B gels. Noticeably, both H&E and Movat pentachrome staining revealed that type B constructs are more diffuse and amorphous in nature than type A constructs. The presence of elastin in the tissue is confirmed by the dark purple color in the Movat staining. No elastin was detected histologically in the cell–gel constructs.
FIG. 9.
Histological comparison of porcine vocal fold lamina propria (LP; top row) with type A (middle row) and type B (bottom row) constructs after 28 days of culture. Color images available online at www.liebertonline.com/ten.
Matrix viscoelasticity
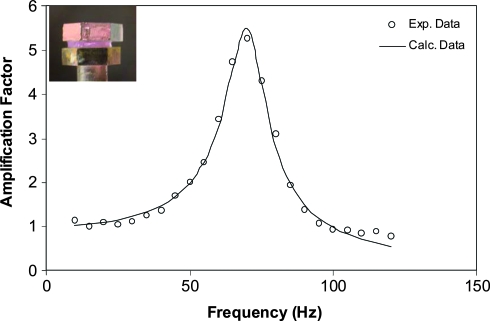
The viscoelasticity of the composite hydrogels as well as the cell–gel constructs was evaluated using a custom-designed TWA at frequencies close to human phonation.38 All samples were rapidly frozen in liquid nitrogen and were stored and shipped under liquid nitrogen atmosphere before the measurements. Good agreement between predicted linear viscoelastic response and experimental data (Fig. 10) was observed for all samples tested. Two important parameters that can be deduced from the TWA experiments are elastic modulus (G′) and the loss tangent [tan (δ): ratio between the loss modulus, G" and the elastic modulus, G′].
FIG. 10.
Frequency-dependant amplification factor for a representative cell–gel construct (type B). The inset shows the sample sandwiched between two parallel plates. Three consecutive measurements were conducted on the same sample and the experimental data reported are an average of three tests. Color images available online at www.liebertonline.com/ten.
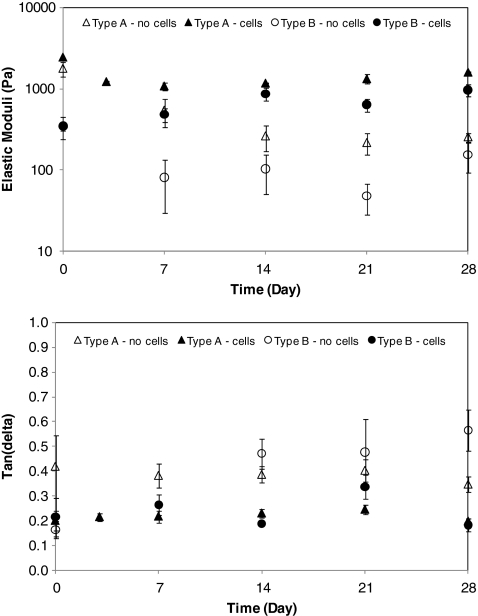
At day 0, the cell–gel constructs and cell-free matrices exhibited similar elastic modulus (Fig. 11), for both type A and type B gels, respectively. The average G′ and tan (δ) for type A gels in the absence of PVFFs at a resonance frequency of 65–205 Hz were 1768 Pa and 0.17, respectively. Type B gels, however, were much softer, exhibiting an elastic modulus of 308 Pa and a tan (δ) value of 0.16 at the resonance frequency of 26–60 Hz. The viscoelastic properties of type A and type B gels are similar to that of mature and newborn porcine vocal fold LP (data not shown), respectively. Type A hydrogels without the encapsulated PVFFs exhibited a steady decrease in elastic modulus until day 14, reaching a plateau value of 200 Pa after day 21. On the other hand, type B gels experienced a dramatic decrease in G′ from day 0 (345 Pa) to day 7 (81 Pa). For each type of matrices, the cell-free hydrogels are significantly (p < 0.05) softer than their cell-laden counterparts at all times except day 0.
FIG. 11.
Evolution of matrix elastic modulus (top) and tan (δ) as a function of culture time. Data reported are an average of results obtained from three different samples. Open symbols, cell-free hydrogels; closed symbols, cell–gel constructs.
The evolution of matrix elasticity in the presence of PVFFs as a function of culture time follows distinctly different trends for the types of constructs investigated. Type A constructs were significantly stiffer than type B constructs at day 0. The stiffness of type A constructs decreased sharply during the first week of culture, followed by a week of matrix stabilization without dramatic changes in the observed elastic modulus. At the end of the culture, the elastic modulus increased slightly, reaching an average value of 1600 Pa at day 28 (Fig. 11). Contrarily, the elastic modulus for type B constructs increased monotonically overtime, ranging from an initial value of 345 Pa at day 0 to 970 Pa at day 28 (Fig. 11). The storage moduli of both constructs appear to be converging to a similar end value. The tan (δ) values remained essentially unchanged over the course of culture for both types of constructs, with or without cells. However, a relatively higher tan (δ) value was observed for the cell-free hydrogels compared with the same type of constructs with cells and cultured for the same duration (Fig. 11).
Discussion
Regeneration of a functional vocal fold LP is essential for the reintroduction of patients into society after vocal fold trauma or exposed to toxic stimuli. Using multipoint mesenchymal stem cells, 3D biomimetic matrices, and defined biological and biomechanical cues in vitro, we work to engineer such functional vocal fold replacement, which is the main goal of our lab. Here, we focus on the fundamental understanding of responses of PVFFs to engineered matrices under static culture conditions. An ideal matrix for in vitro vocal fold tissue engineering should mimic the native ECM of the LP in terms of its chemical composition, structural organization, and biological and biomechanical functions. Collagen and HA, two of the most abundant ECM molecules in vocal fold LP, when strategically combined, can recapitulate the native microenvironment of vocal fold LP. The incorporation of covalently cross-linkable HA derivatives into self-assembled collagen fibers not only enhances the integrity of composite gels but also allows for fine-tuning of their structure and mechanics. Elastic, macroscopic gels formed in less than 30 s upon mixing of 1wt% aqueous solutions of HACHO and HAADH, and formed stable hydrazone bonds and released water as the only by-product.39 The crosslinking chemistry, combined with the biocompatible nature of HA constituents,28 makes these derivatives attractive candidates for in situ cell encapsulation. Two types of collagen–HA matrices have been explored in the current study. Type A gels consisted of self-assembled collagen fibers reinforced by HACHO, whereas type B gels are based on covalently cross-linked HA matrix interpenetrated with self-assembled collagen fibers. To determine the biologic effects of matrix composition, microstructure, and viscoelasticity on PVFF cells, alterations in cell morphology, proliferation, ECM production, and matrix remodeling on the different matrices were systematically analyzed.
Matrix microstructure
Based on the microscopic length scale attainable by CryoSEM, one can see that type A gels exhibit similar structural features as gels prepared from collagen alone, suggesting that HACHO did not significantly alter the collagen assembly process. Type B gels, on the other hand, are composed of two levels of networks: one via the physical entanglement of collagen fibers and the other by interfibrillar cross-linking via HA derivatives. Our observations do not contradict with the abundant literature demonstrating the effects of glycosaminoglycans on collagen fibrillogenesis.40–43 Collagen assembly can be affected by the structure, chemical identity, and concentration of the glycosaminoglycan molecules. It can be further complicated by the collagen concentration, solution temperature, pH, and ionic strength as well as the assembly kinetics. Depending on the analytical tools employed, certain morphological differences may not be detected. Therefore, a direct comparison between various assembly systems that are quantified with different characterization methods can be challenging.
The subtle structural differences between type A and type B hydrogels can be explained in terms of the assembly kinetics and the cross-linking chemistry. In type A gels, exogenous HACHO resulted in the formation of Schiff base between the collagen fibers and HACHO.44 The aqueous solution of HACHO is a nonviscous liquid, and its addition to the collagen monomer solution did not alter the structure of the resulting collagen fibers significantly, as indicated by the CryoSEM results. When HAADH was added to the collagen–HACHO mixture, a competing chemical reaction between HACHO and HAADH occurred. Due to the high nucleophilicity, low pKa value of hydrazide groups, and the high solubility of HA derivatives,45,46 HACHO is likely to react with HAADH much more readily than with the lysine amine in collagen. Once cross-linking occurs, the solution viscosity increases dramatically, inhibiting the mobility and the assembly process of collagen within the matrix. Thus, type B gels are composed of interstitial, amorphous gels derived from HAADH and HACHO interpenetrated with relatively immature collagen fibrils with smaller diameter. The insensitivity of SANS to such subtle structural changes can be attributed to the presence of the hydrated mesh-like structure that dominates the overall features in the gels.
Cell viability, phenotype, and proliferation
Essential to the development of any biomimetic matrix is its biocompatibility and its ability to support cell attachment, promote cell proliferation, maintain cell phenotype, and induce the production of tissue-specific ECM. Our cell isolation procedure resulted in primary PVFFs with fibroblastic phenotype when grown on a 2D TCPS, as demonstrated by the immunostaining and reverse transcription PCR analyses. Prolonged culture of PVFFs in the 3D environment created by both types of matrices did not alter the fibroblastic phenotype, confirming the biocompatible and biomimetic nature of the matrices.
The encapsulation process is well tolerated by PVFFs as indicated by live/dead staining. Once encapsulated, the PVFFs begin to attach to the matrix by day 2 and adopt a spindle-shaped morphology. It is well known that integrin α2β1 mediates cell adhesion to and spreading on fibrillar collagen (not collagen monomer),47 promoting the formation of long cellular projections not seen for cells cultured on 2D plastic surfaces. Cell viability is not compromised during long-term culture, suggesting that the degradation products from the gels do not adversely affect cell functions. The introduction of chemically modified HA to self-assembled collagen effectively reinforced the matrix, preventing fibroblast-mediated gel compaction. Collectively, our results demonstrate that the 3D culture environment did not induce phenotypic or functional changes to the cultured PVFFs.
The composite matrices containing the amorphous, interstitial HA phase and fibrous collagen meshes not only support cell adhesion and spreading within the matrix but also facilitate cell proliferation. The number of PVFFs in type A constructs remained unchanged from day 0 to 3 since cells were confined in their rounded morphology after encapsulation; additional time was needed for them to establish proper connection with the surrounding matrix before their growth.48 Interestingly, cells residing in type B gels grew faster than those in type A gels during the first week of culture. Such difference was due, in part, to the enhanced stimulatory effects49 exerted on the cells by the diffusible HA component in type B constructs (see below). Statistical analysis revealed no difference in DNA content at day 21 and 28 for each type of construct, respectively. We speculate that during the last week of culture, PVFFs started actively remodeling the matrices rather than continuing to grow. The similarity in DNA content between the two types of constructs at the end of the culture implies that cell proliferation is not a simple function of HA content in the constructs; many other factors, including the crosslinking chemistry, the microstructure, and the viscoelasticity of the matrix, also affect cell proliferation. It is difficult to decouple the contributions of the individual factors to cell proliferation in the composite matrices investigated here.
Matrix composition
We monitored the collagen and HA content in the construct throughout the culture to gain an insight into the evolution of matrix composition. Relatively higher amounts of collagen were retained in type A constructs than type B immediately upon hydrogel construction. This observation is in good agreement with the CryoSEM result and further confirms our speculation that the collagen assembly was hindered in type B gel due to the presence of gelling HA building blocks (HACHO and HAADH). As a result, a relatively large portion of collagen monomers were not incorporated (washed away) in type B gels. The increase in solution viscosity sterically hindered the fibril aggregation and growth, giving rise to immature fibers with smaller fiber diameter and loosely packed bundles that do not contribute to the overall structure of the final product. The improved collagen retention in both types of matrices is in sharp contrast to previously reported results on collagen–HA gels where collagen was lost continuously throughout the culture due partially to the absence of covalent reinforcement.13 Interestingly, the reduction in collagen content in type A constructs coincides with the switch from the linear growth phase to the plateau phase as suggested by DNA quantification. Such correlation suggests the alteration of PVFFs from proliferative phenotype to a more synthetic phenotype during the last week of culture.
The overall higher HA retention in type B gels than in type A gels is a direct consequence of the higher stability of the hydrazone bond in type B gels than the imine bond in type A gels. On the other hand, the relatively higher percentage (56.7%) of HA reduction in type B gels compared with type A gels (35.7%) during the initial 3 days of culture can be attributed to a higher sol fraction in the former. This is not surprising since fast crosslinking kinetics is frequently associated with higher sol faction in the resulting gels.27,50 The physically trapped, uncrosslinked HA components (sol fraction) in type B gels readily diffuse out of the matrix, causing a dramatic decrease in HA retention within 3 days of culture. The rapid loss of large amount of soluble HA in type B gels coincided with the facile cell proliferation in the same matrix from day 0 to 3.
It is well known that VFFs are the dominant cells that are responsible for maintaining the ECM compartment in functional condition by actively producing and degrading ECM components.51,52 The analytical methods employed in the current study do not differentiate collagen and HA original in the matrix from those produced by the cells, and may not be sensitive enough to track the small amount collagen and HA produced by the entrapped cells. However, the ability of cells to express genes related to structural proteins and their corresponding enzymes suggests that PVFFs residing in these composite hydrogels actively produce new ECM components and, at the same time, degrade the old ones.
Matrix viscoelasticity
We are interested in understanding whether the entrapped PVFFs are capable of altering the mechanical properties of their scaffolds during the in vitro culture. This is important since the mechanical properties of a tissue's ECM are optimized for proper organ function, and these same properties strongly influence the functional behaviors of resident cells. We have recently designed and validated a novel instrument that is capable of measuring viscoelastic responses of both vocal fold tissue and hydrogels at phonation frequencies.27,28,38 A thin cylindrical sample is sandwiched between two hexagonal plates and is driven into motion by the bottom plate. At each frequency, measured ratios of the top and bottom plates are used to determine the ratio of the amplitude of rotation of the two plates. The amplification factor increases when the driving frequency approaches the resonance frequency of the sample, reaching a peak value at the resonance frequency, and subsequently decreases. Comparison of the frequency dependence of this ratio with that predicted for torsional waves in a linear viscoelastic material allows for the storage modulus and the loss angle, in shear, to be calculated by a best-fit procedure (Fig. 10). The reliability of this apparatus has been extensively validated.27,28,38 To minimize undesirable alteration of material properties during shipping and storage, care was taken to ensure that all samples, including the cell–gel constructs and the cell-free matrices, were subjected to the same freezing and thawing protocol without repetitive freezing–thawing cycles.53
Our TWA analyses clearly indicate differences in the viscoelastic properties between type A and type B hydrogels. In the absence of PVFFs at day 0, type A gels were stiffer and more viscous than type B gels. Such differences in matrix viscoelasticity reflect the nanoscale organization that is a result of different assembly and cross-linking kinetics, as discussed above. The relatively higher collagen–HA ratio agrees well with the higher elastic modulus for type A gels throughout the course of incubation, as the mature collagen fibers are the major contributing factor to the overall viscoelasticity. On the other hand, the covalently crosslinked HA interstitial dominates the initial matrix elasticity for type B gels, giving rise to a softer and less viscous [lower tan (δ) value] composite scaffold at day 0. Both types of cell-free matrices witnessed a dramatic decrease in elastic modulus within the first week of incubation due to the rapid loss of the constituent matrix components. The lack of change in hydrogel stiffness from day 7 to 28 reflects the relative stable levels of HA and collagen present in the gels. PVFFs residing in the HA–collagen matrices are likely to exhibit different cellular responses that are direct results of the cellular perception of the apparent stiffness of local physical environment.54
PVFFs did not contribute significantly to the matrix viscoelasticity when initially encapsulated in the hydrogels as evidenced by the similar G′ and tan (δ) values for the respective cell–gel constructs and the cell-free hydrogels. It is highly possible that cells in their rounded morphology cannot link the ECM molecules together nor can they exert traction forces to their matrix. After 2 days of culture, a majority of the PVFFs became attached to both types of matrices, thus capable of connecting to and reinforcing the matrix. The loss of HACHO from the covalent crosslinks in type A constructs (through hydrolysis of the imine bond at the crosslinking points) manifested itself in the reduction of matrix stiffness. The reinforcing mechanism provided by the entrapped cells was not strong enough to compensate for such loss. On the other hand, the cross-linked interstitial HA matrix generated by HAADH and HACHO essentially serves as the “glue” to maintain the overall structural integrity for type B gels. The rapid loss of diffusible HA (sol fraction) within the initial 3 days of culture for type B gels did not result in the drop in G′ value since the uncrosslinked HA components did not contribute significantly to the overall mechanical integrity of the gels. With the cells pulling the matrix together, it is no surprise that type B construct became slightly stiffer. Overall, both the elastic modulus and the tan (δ) values are statistically lower for gels without cells than the cell–gel constructs cultured for the same duration of time. Therefore, we conclude that the encapsulated cells actively remodel and reinforce the matrices, contributing significantly to the construct viscoelasticity.
After the initial decrease in elastic modulus within the first week of culture, type A constructs became moderately stiffer as the culture time increased, reaching a G′ value of 1602 Pa at day 28. The evolution of cell–matrix interactions may provide a mechanism for cells to reinforce their matrix. On the other hand, the elastic modulus for the type B construct steadily increased over the course of culture (from 284 Pa at day 0 to 1108 Pa at day 28, representing close to a three fold increase in modulus) even though both HA and collagen loss followed the similar patterns to those for type A constructs. It is highly possible that the collagen fibers in type B constructs were rendered more mature by the cells over the course of culture. This speculation is supported by previous studies that demonstrate the ability of fibroblasts to transport and remodel collagen matrix.55,56
Although a loss tangent close to 0 indicates a perfect elastic solid, a high tan (δ) value implies the presence of energy dissipative elements in the gels. The consistently lower tan (δ) values for the cell–gel constructs compared with their cell-free counterparts originate from the cells connecting the matrix, acting as the reinforcing element to reduce the construct viscosity. In contrast to the continuous changes in the construct stiffness, the loss tangent (or the damping factor) remained relatively flat over the entire course of culture. Similar observations have been reported previously for covalently stabilized, self-assembled peptide hydrogels57 as well as HA-based, doubly crosslinked networks.27,28 Chan and Titze58 discovered that while the elastic modulus of human vocal fold mucosa varies from subject to subject with two order of magnitude difference, the loss tangent was flat function for most subjects. In fact, the loss tangent is within a very small range of values for most subjects, approximately 0.2–0.5 at frequencies up to 10 Hz. We speculate that the insensitivity of loss tangent to the culture time may be a direct consequence of the minute changes in the overall network connectivity in the collagen–HA composite gels.
The evolution of matrix stiffness can be attributed to a number of concerted processes that include cellular proliferation, migration and traction force generation, matrix reorganization, and cross-linking (multiple integrins on one cell may connect adjacent collagen fibers), as well as the synthesis or destruction of matrix constituents. As suggested above, the lack of cell proliferation during the last week of culture implies that cells may be engaged in matrix synthesis and degradation. Although these changes are not readily detected by histology staining, we believe that such subtle changes in matrix structure and composition may contribute to the increase in the measured elastic modulus for both types of constructs. In our system, the production of new matrix components, although minute in overall amount, may also contribute to the alteration of the mechanical properties of the cell–gel constructs.
Conclusion
In this study, we evaluated the applicability of chemically defined collagen–HA composite hydrogels for use as conducive, 3D matrices for in vitro static culture of vocal fold fibroblasts. Two types of hydrogels were synthesized using soluble collagen and HA derivatives carrying mutually reactive functional groups (HACHO and HAADH) as the starting materials. Type A gels consist of self-assembled, mature collagen fibers stabilized by HACHO and type B gels contain immature collagen fibrils interpenetrated with an amorphous matrix formed by HAADH and HACHO. Type B gels contained lower amounts of collagen but consistently higher amounts of HA than those in type A gels. Both types of hydrogels allowed for in situ cell encapsulation and supported cell attachment and cell proliferation in 3D. PVFFs residing in both matrices robustly expressed genes related to several ECM proteins. Our torsional wave analysis showed that when encapsulated in matrices with distinctly different stiffness, PVFFs were capable of altering the matrices' modulus to a similar end value. The collagen–HA composite hydrogels, when properly formulated, are attractive candidates for prolonged 3D culture of vocal fold fibroblasts. We are currently investigating the effects of physiologically relevant mechanical stimulations on PVFFs cultured in collagen–HA matrices in terms of cell proliferation, cell signaling, gene expression, and ECM production.
Supplementary Material
Acknowledgments
We thank Dr. Mary C. Farach-Carson for the stimulating discussions. We also thank Dr. Kirk Czymmek for his training and advice on confocal imaging, and Debbie Powell and David Scheiblin for their help with the CryoSEM. This work is funded by NIH/NIDCD R01 008965 (to X.J.). The SANS studies are supported by the SANS on Polymers and Complex Fluids Award (U.S. Department of Commerce, # 70NANB7H6178, to D.P.P.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Hirano M. Structure of the vocal fold in normal and diesease states: anatomical and physical studies. ASHA Rep. 1981;11:11. [Google Scholar]
- 2.Gray S.D. Cellular physiology of vocal folds. Otolaryngol Clin North Am. 2000;33:679. doi: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 3.Rosen C.A. Vocal fold scar: Evaluation and treatment. Otolaryngol Clin North Am. 2000;33:1081. doi: 10.1016/s0030-6665(05)70266-8. [DOI] [PubMed] [Google Scholar]
- 4.Hirano S. Current treatment of vocal fold scarring. Curr Opin Otolaryngol Head Neck Surg. 2005;13:143. doi: 10.1097/01.moo.0000162261.49739.b7. [DOI] [PubMed] [Google Scholar]
- 5.Gray S.D. Cellular physiology of the vocal folds. Otolaryngol Clin North Am. 2000;33:679. doi: 10.1016/s0030-6665(05)70237-1. [DOI] [PubMed] [Google Scholar]
- 6.Ishii K. Zhai W.G. Akita M. Hirose H. Ultrastructure of the lamina propria of the human vocal fold. Acta Otolaryngol. 1996;116:778. doi: 10.3109/00016489609137924. [DOI] [PubMed] [Google Scholar]
- 7.de Melo E.C.M. Lemos M. Ximenes J.A. Sennes L.U. Saldiva P.H.N. Tsuji D.H. Distribution of collagen in the lamina propria of the human vocal fold. Laryngoscope. 2003;113:2187. doi: 10.1097/00005537-200312000-00027. [DOI] [PubMed] [Google Scholar]
- 8.Buhler R.B. Sennes L.U. Mauad T. Melo E.C.M. Silva L.F.F. Saldiva P.H.N. Collagen fiber and versican distribution within the lamina propria of fetal vocal folds. Laryngoscope. 2008;118:371. doi: 10.1097/MLG.0b013e318159aa0d. [DOI] [PubMed] [Google Scholar]
- 9.Chan R.W. Gray S.D. Titze I.R. The importance of hyaluronic acid in vocal fold biomechanics. Otolaryngol Head Neck Surg. 2001;124:607. doi: 10.1177/019459980112400602. [DOI] [PubMed] [Google Scholar]
- 10.Butler J.E. Hammond T.H. Gray S.D. Gender-related differences of hyaluronic acid distribution in the human vocal fold. Laryngoscope. 2001;11:907. doi: 10.1097/00005537-200105000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Savani R. Bagli D.J. Harrison R.E. The role of hyaluronan-recpetor interactions in wound repair. In: Garg H.G., editor; Longaker M.T., editor. Scarless Wound Healing. New York: Marcol Dekker; 2000. pp. 115–143. [Google Scholar]
- 12.Brigham M.D. Bick A. Lo E. Bendali A. Burdick J.A. Khademhosseini A. Mechanically robust and bioadhesive collagen and photocrosslinkable hyaluronic acid semi-interpenetrating networks. Tissue Eng Part A. 2009;15:1645. doi: 10.1089/ten.tea.2008.0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hahn M.S. Teply B.A. Stevens M.M. Zeitels S.M. Langer R. Collagen composite hydrogels for vocal fold lamina propria restoration. Biomaterials. 2006;27:1104. doi: 10.1016/j.biomaterials.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Travis J.A. Hughes M.G. Wong J.M. Wagner W.D. Geary R.L. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts—role of cd44 and implications for constrictive remodeling. Circ Res. 2001;88:77. doi: 10.1161/01.res.88.1.77. [DOI] [PubMed] [Google Scholar]
- 15.Dallon J.C. Ehrlich H.P. A review of fibroblast-populated collagen lattices. Wound Repair Regen. 2008;16:472. doi: 10.1111/j.1524-475X.2008.00392.x. [DOI] [PubMed] [Google Scholar]
- 16.Graham J.S. Vomund A.N. Phillips C.L. Grandbois M. Structural changes in human type I collagen fibrils investigated by force spectroscopy. Exp Cell Res. 2004;299:335. doi: 10.1016/j.yexcr.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 17.Toole B.P. Hyaluronan and its binding proteins: the hyaladherins. Curr Opin Cell Biol. 1990;2:839. doi: 10.1016/0955-0674(90)90081-o. [DOI] [PubMed] [Google Scholar]
- 18.Knudson C.B. Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233. [PubMed] [Google Scholar]
- 19.Wu Y.J. La Pierre D.P. Wu J. Yee A.J. Yang B.B. The interaction of versican with its binding partners. Cell Res. 2005;15:483. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 20.Mehra T.D. Ghosh K. Shu X.Z. Prestwich G.D. Clark R.A.F. Molecular stenting with a crosslinked hyaluronan derivative inhibits collagen gel contraction. J Invest Dermatol. 2006;126:2202. doi: 10.1038/sj.jid.5700380. [DOI] [PubMed] [Google Scholar]
- 21.Sorrell J.M. Carrino D.A. Baber M.A. Caplan A.I. Versican in human fetal skin development. Anat Embryol. 1999;199:45. doi: 10.1007/s004290050208. [DOI] [PubMed] [Google Scholar]
- 22.Stevens M.M. George J.H. Exploring and engineering the cell surface interface. Science. 2005;310:1135. doi: 10.1126/science.1106587. [DOI] [PubMed] [Google Scholar]
- 23.Goldberg M. Langer R. Jia X.Q. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18:241. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh K. Pan Z. Guan E. Ge S.R. Liu Y.J. Nakamura T. Ren X.D. Rafailovich M. Clark R.A.F. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Discher D.E. Janmey P. Wang Y.-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 26.Engler A.J. Griffin M.A. Sen S. Bonnetnann C.G. Sweeney H.L. Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jha A.K. Hule R.A. Jiao T. Teller S.S. Clifton R.J. Duncan R.L. Pochan D.J. Jia X.Q. Structural analysis and mechanical characterization of hyaluronic acid-based doubly cross-linked networks. Macromolecules. 2009;42:537. doi: 10.1021/ma8019442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia X.Q. Yeo Y. Clifton R.J. Jiao T. Kohane D.S. Kobler J.B. Zeitels S.M. Langer R. Hyaluronic acid-based microgels and microgel networks for vocal fold regeneration. Biomacromolecules. 2006;7:3336. doi: 10.1021/bm0604956. [DOI] [PubMed] [Google Scholar]
- 29.Gurski L. Jha A.K. Zhang C. Jia X.Q. Farach-Carson M.C. Hyaluronic acid hydrogel as a scaffold for chemotherapeutic drug selection with poorly adherent cells. Biomaterials. 2009;30:6076. doi: 10.1016/j.biomaterials.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freshney R.I. Culture of Animal Cells: A Manual of Basic Techniques. New York: J. Wiley; 2000. [Google Scholar]
- 31.Luo Y. Kobler J.B. Zeitels S.M. Langer R. Effects of growth factors on extracellular matrix production by vocal fold fibroblasts in 3-dimensional culture. Tissue Eng Part A. 2006;12:3365. doi: 10.1089/ten.2006.12.3365. [DOI] [PubMed] [Google Scholar]
- 32.Chung C. Burdick J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng Part A. 2009;15:243. doi: 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X. Thibeault S.L. Novel isolation and biochemical characterization of immortalized fibroblasts for tissue engineering vocal fold lamina propria. Tissue Eng Part C Methods. 2009;15:201. doi: 10.1089/ten.tec.2008.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Titze I.R. Hitchcock R.W. Broadhead K. Webb K. Li W.H. Gray S.D. Tresco P.A. Design and validation of a bioreactor for engineering vocal fold tissues under combined tensile and vibrational stresses. J Biomech. 2004;37:1521. doi: 10.1016/j.jbiomech.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Ross J.J. Tranquillo R. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol. 2003;22:477. doi: 10.1016/s0945-053x(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 36.Bitter T. Muir H.M. A modified uronic acid carbazole reaction. Anal Biochem. 1962;4:330. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- 37.Bancroft J.D. Gamble M. Theory and Practice of Histological Techniques. London: Harcourt Publishers Limited; 2002. [Google Scholar]
- 38.Jiao T. Farran A. Jia X. Clifton R.J. High frequency measurements of viscoelastic properties of hydrogels for vocal fold regeneration. Exp Mech. 2009;49:235. doi: 10.1007/s11340-008-9126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jia X.Q. Colombo G. Padera R. Langer R. Kohane D.S. Prolongation of sciatic nerve blockade by in situ cross-linked hyaluronic acid. Biomaterials. 2004;25:4797. doi: 10.1016/j.biomaterials.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y.L. Kaufman L.J. Rheology and confocal reflectance microscopy as probes of mechanical properties and structure during collagen and collagen/hyaluronan self-assembly. Biophys J. 2009;96:1566. doi: 10.1016/j.bpj.2008.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brightman A.O. Rajwa B.P. Sturgis J.E. McCallister M.E. Robinson J.P. Voytik-Harbin S.L. Time-lapse confocal reflection microscopy of collagen fibrillogenesis and extracellular matrix assembly in vitro. Biopolymers. 2000;54:222. doi: 10.1002/1097-0282(200009)54:3<222::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 42.Gobeaux F. Mosser G. Anglo A. Panine P. Davidson P. Giraud-Guille M.M. Belamie E. Fibrillogenesis in dense collagen solutions: a physicochemical study. J Mol Biol. 2008;376:1509. doi: 10.1016/j.jmb.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 43.Silver F.H. Type I collagen fibrillogenesis in vitro. J Biol Chem. 1981;256:4973. [PubMed] [Google Scholar]
- 44.Weng L.H. Pan H. Chen W.L. Self-crosslinkable hydrogels composed of partially oxidized hyaluronan and gelatin: in vitro and in vivo responses. J Biomed Mater Res A. 2008;85A:352. doi: 10.1002/jbm.a.31491. [DOI] [PubMed] [Google Scholar]
- 45.Shu X.Z. Liu Y.C. Palumbo F.S. Lu Y. Prestwich G.D. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Bulpitt P. Aeschlimann D. New strategy for chemical modification of hyaluronic acid: preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J Biomed Mater Res. 1999;47:152. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 47.Jokinen J. Dadu E. Nykvist P. Kapyla J. White D.J. Ivaska J. Vehvilainen P. Reunanen H. Larjava H. Hakkinen L. Heino J. Integrin-mediated cell adhesion to type I collagen fibrils. J Biol Chem. 2004;279:31956. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- 48.Radisic M. Park H. Shing H. Consi T. Schoen F.J. Langer R. Freed L.E. Vunjak-Novakovic G. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc Natl Acad Sci USA. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greco R.M. Iocono J.A. Ehrlich H.P. Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J Cell Physiol. 1998;177:465. doi: 10.1002/(SICI)1097-4652(199812)177:3<465::AID-JCP9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 50.Kaihara S. Matsumura S. Fisher J.P. Synthesis and properties of poly[poly(ethylene glycol)-co-cyclic acetal] based hydrogels. Macromolecules. 2007;40:7625. [Google Scholar]
- 51.Catten M. Gray S.D. Hammond T.H. Zhou R. Hammond E. Analysis of cellular location and concentration in vocal fold lamina propria. Otolaryngol Head Neck Surg. 1998;118:663. doi: 10.1177/019459989811800516. [DOI] [PubMed] [Google Scholar]
- 52.Fuja T.J. Ostrem E.M. Probst-Fuja M.N. Titze I.R. Differential cell adhesion to vocal fold extracellular matrix constituents. Matrix Biol. 2006;25:240. doi: 10.1016/j.matbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 53.Chan R.W. Titze I.R. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann Biomed Eng. 2003;31:482. doi: 10.1114/1.1561287. [DOI] [PubMed] [Google Scholar]
- 54.Discher D.E. Janmey P. Wang Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 55.Leung L.Y. Tian D. Brangwynne C.P. Weitz D.A. Tschumperlin D.J. A new microrheometric approach reveals individual and cooperative roles for tgf-beta 1 and il-1 beta in fibroblast-mediated stiffening of collagen gels. FASEB J. 2007;21:2064. doi: 10.1096/fj.06-7510com. [DOI] [PubMed] [Google Scholar]
- 56.Meshel A.S. Wei Q. Adelstein R.S. Sheetz M.P. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7:157. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- 57.Jung J.P. Jones J.L. Cronier S.A. Collier J.H. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials. 2008;29:2143. doi: 10.1016/j.biomaterials.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chan R.W. Titze I.R. Viscoelastic shear properties of human vocal fold mucosa: measurement methodology and empirical results. J Acoust Soc Am. 1999;106:2008. doi: 10.1121/1.427947. [DOI] [PubMed] [Google Scholar]
- 59.Glinka C.J. Barker J.G. Hammouda B. Krueger S. Moyer J.J. Orts W.J. The 30 m small-angle neutron scattering instruments at the National Institute of Standards and Technology. J Appl Crystallogr. 1998;31:430. [Google Scholar]
- 60.Kline S.R. Reduction and analysis of SANS and USANS data using IGOR Pro. J Appl Crystallogr. 2006;39:895. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.