Abstract
Limited autologous vascular graft availability and poor patency rates of synthetic grafts for bypass or replacement of small-diameter arteries remain a concern in the surgical community. These limitations could potentially be improved by a tissue engineering approach. We report here our progress in the development and in vivo testing of a stem-cell-based tissue-engineered vascular graft for arterial applications. Poly(ester urethane)urea scaffolds (length = 10 mm; inner diameter = 1.2 mm) were created by thermally induced phase separation (TIPS). Compound scaffolds were generated by reinforcing TIPS scaffolds with an outer electrospun layer of the same biomaterial (ES-TIPS). Both TIPS and ES-TIPS scaffolds were bulk-seeded with 10 × 106 allogeneic, LacZ-transfected, muscle-derived stem cells (MDSCs), and then placed in spinner flask culture for 48 h. Constructs were implanted as interposition grafts in the abdominal aorta of rats for 8 weeks. Angiograms and histological assessment were performed at the time of explant. Cell-seeded constructs showed a higher patency rate than the unseeded controls: 65% (ES-TIPS) and 53% (TIPS) versus 10% (acellular TIPS). TIPS scaffolds had a 50% mechanical failure rate with aneurysmal formation, whereas no dilation was observed in the hybrid scaffolds. A smooth-muscle-like layer of cells was observed near the luminal surface of the constructs that stained positive for smooth muscle α-actin and calponin. LacZ+ cells were shown to be engrafted in the remodeled construct. A confluent layer of von Willebrand Factor–positive cells was observed in the lumen of MDSC-seeded constructs, whereas acellular controls showed platelet and fibrin deposition. This is the first evidence that MDSCs improve patency and contribute to the remodeling of a tissue-engineered vascular graft for arterial applications.
Introduction
According to the American Heart Association, cardiovascular disease claims almost 1 million lives every year in the United States alone, and more than 50% of those are caused by coronary artery disease.1 Attempts to alleviate this disease include surgical procedures such as coronary artery by-pass for myocardial revascularization, peripheral by-pass of the limbs, and arteriovenous fistulae for dialysis. Inadequate performance of synthetic grafts in small-diameter (<5 mm) vascular replacement and limited availability of autologous vessels make current alternatives suboptimal.2–5 The fabrication of a tissue-engineered vascular graft (TEVG) appears to hold great promise for future treatments of cardiovascular disease, where the development of new conduits is clearly needed.6,7
Various approaches to achieve a fully functional TEVG have been studied, including a completely cellular approach,8,9 decellularized matrices,10,11 and a combination of cells and either natural or synthetic scaffolds.6,12 Two clinical studies showed the successful implantation of a TEVG as total cavopulmonary connection in pediatric patients using a biodegradable scaffold and bone marrow progenitor cells13 and in end-stage renal disease patients as a graft for arteriovenous fistula preparation.9
One approach in the fabrication of a TEVG is to incorporate some form of scaffold to provide mechanical integrity upon implantation to the arterial circulation.14,15 Synthetic biodegradable polymers can regulate the graft mechanical properties, facilitate cell incorporation, and at the same time be readily available, making them promising for vascular tissue engineering applications.7,16
Most TEVG approaches have relied on one type of cell source to achieve the desired biological function. Although many previous approaches have prompted the use of terminally differentiated smooth muscle and/or endothelial cells, these have often shown an inability to reconstitute tissues.17 Multipotential progenitor cells that have been identified in adult tissues have shown promise to overcome these limitations.18–24 Efficient and rapid incorporation of cells inside the scaffolds can also be an important determinant for the feasibility of constructing a clinically viable TEVG.25 We have recently reported a vacuum seeding method to incorporate a large number of cells in a short period of time with an even distribution throughout the length and thickness of porous tubular scaffolds.26
In this study, we evaluate the in vivo remodeling of a porous biodegradable elastomeric scaffold seeded with muscle-derived stem cells (MDSCs) in a rat model after 8 weeks. The constructs were shown to be mechanically suitable to the systemic circulation, incorporated cells participated in the remodeling, and patency rates were improved compared with acellular grafts. These findings might contribute to successful clinical translation of a TEVG to treat cardiovascular disease.
Materials and Methods
Cell source
Lewis rat MDSCs were isolated by means of an established preplating technique, and transfected with the LacZ reporter gene as previously described.22,27 Cells were then plated at low density (200 cells/cm2) on a 175 cm2 tissue culture polystyrene flask and cultured at 37°C and 5% CO2 with complete Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (Atlanta Biologicals, Norcross, GA), 10% horse serum (Invitrogen, Carlsbad, CA), 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were expanded to the desired number and were used between passages 10 and 15. Medium changes were performed every 48 h during culture. Before use, MDSC monolayers were washed three times in Dulbecco's modified phosphate-buffered saline (PBS) and then incubated with 0.1% trypsin for 5 min to remove them from the flasks. MDSCs were centrifuged at 1200 rpm for 5 min to form a pellet, and then resuspended in DMEM to the desired concentration in preparation for seeding.
Polymer synthesis
Poly(ester urethane)urea (PEUU) based on polycaprolactone diol (molecular weight = 2000), 1,4-diisocyanatobutane (BDI; Fluka, Milwaukee, WI), and putrescine was synthesized as described previously using a two-step solution polymerization.16 PEUU tubular scaffolds were fabricated by combining two previously described methods.28 Constructs were formed via thermally induced phase separation (TIPS) within a tubular mold consisting of an outer glass tube (inner diameter 1.5 mm) and a solid inner polytetrafluoroethylene cylinder (outer diameter 1.3 mm). Bilayered constructs were prepared by electrospinning an outer layer of PEUU on the surface of the TIPS constructs (ES-TIPS).29 Construct length was approximately 15 mm.
Cell seeding
To incorporate the MDSCs within the scaffold, a previously described rotational vacuum seeding device was utilized.26 The constructs were mounted inside the chamber between the two tees and simultaneously perfused with 5 mL of cell suspension (2 × 106 cells/mL) by means of a precision syringe pump at a constant rate of 5 mL/min. The constructs were then flushed with 5 mL of plain DMEM to wash residual cells from the lumen of the scaffolds. The constructs were then removed and incubated in a Petri dish for 1 h. After seeding, the TEVGs were placed in 500 mL spinner flasks (196580575; Bellco Glass, Vineland, NJ) with 100 mL of culture media and stirred at 15 rpm for 48 h.
In vivo study design
A total of 42 adult Lewis rats (weight ∼300 g) were divided in three groups and received an aortic interposition by-pass graft. The first group (n = 15) received a TIPS PEUU construct seeded with MDSCs. The second group (n = 17) received an ES-TIPS PEUU construct seeded with MDSCs, whereas the third group (n = 10) served as an acellular TIPS PEUU control. Animals were electively sacrificed at 8 weeks or euthanized earlier if ambulatory impairment or distress was evident due to construct occlusion.
Surgical procedure
Rats were anesthetized with isofluorane (2% for induction and 1% for maintenance) and a single dose of 5 mg/100 g ketamine intramuscular (IM), and placed on a warming pad (37°C) in the supine position.
The skin of the ventral abdomen was aseptically prepped with povidone–iodine solution. All animal procedures were performed in compliance with the 1996 “Guide for The Care and Use of Laboratory Animals.” Briefly, a midline laparotomy incision was made and the abdominal aorta exposed below the renal arteries. About 40 IU of sodium heparin was given intravenous (IV) through a tributary vein to the inferior vena cava. Microclamps were applied to the infrarenal aorta, proximally and distally, and the vessel was sectioned between the clamps creating a gap of approximately 1 cm. The TEVG graft to be implanted was trimmed on both edges to obtain a 1-cm-long construct and then sutured in place to the native aorta under 10 × magnification in an end-to-end interrupted anastomotic pattern with 10.0 prolene. An average of six stitches per anastomosis were utilized. After the graft was anastomosed, the clamps were released and patency was verified by direct observation. Clamping time was approximately 25 min. Finally, the muscle layer and skin were closed with 3-0 polyglactin absorbable suture (Vicryl; Ethicon, Boston, MA). The rats were observed in the surgical suite until fully recovered from anesthesia and then returned to the housing area. The first 3 days after surgery, buprenorphine (0.5 mg/kg b.i.d.) and cefuroxime (100 mg/kg b.i.d.) were administered subcutaneously. Antiaggregation therapy was started after the surgery with aspirin and dypiridamole (200 mg PO daily during the first week and 100 mg PO daily after the first week until sacrifice).
Angiography and gross morphology evaluation
After 8 weeks, animals were heparinized (40 IU) and subsequently euthanized with a lethal intracardiac injection of KCl (5 mL). After death, an 18-gauge ABOcath was placed in the thoracic descending aorta and secured with a 2.0 silk Dynamic angiography was performed by injecting a contrast agent (Renograf, Glenwood, London, United Kingdom) and images were acquired with a fluoroscopic C-arm. Patency rate was calculated as number of animals with positive contrast flow through the construct over total number of animals within each group. Mechanical failure was considered when obvious dilation could be observed at the time of fluoroscopy. After angiography, constructs were dissected and explanted from the animal and subsequently assessed for gross macroscopic remodeling, abscess formation, presence of intraluminal thrombus, and vessel wall thickening.
Microscopic evaluation
Before implantation, the trimmed edges of each construct were fixed in paraformaldehyde and 10 μm cryosections were incubated with Alexa 488–conjugated phalloidin (dilution 1:250; Sigma-Aldrich, St. Louis, MO) as previously described to assess seeding efficacy.30
For histological assessment of explanted constructs, separate ring segments were embedded in paraffin blocks and 5 μm sections were cut using a microtome (Thermo Shandon, Pittsburgh, PA). Sections were mounted on slides, stained with Masson's trichrome and hematoxylin and eosin, and viewed under bright light optics using a Nikon Eclipse E600 microscope.
Phenotypic assessment of newly formed tissue was assessed via immunofluorescence using a previously described protocol.30 Sections were incubated for 60 min at room temperature with the monoclonal antibodies for the following antigens: smooth muscle alpha-actin (1:500; Sigma-Aldrich), Calponin (1:200; Sigma-Aldrich), myosin heavy chain (1:500; Sigma-Aldrich), and von Willebrand Factor (1:100; Dako, Carpinteria, CA). Unbound primary antibody was removed by subsequent washes in PBS. Next, the samples were incubated with a Cy3-conjugated secondary antibody (1:500; Sigma-Aldrich) for 1 h at room temperature and then rinsed three times for 15 min with PBS. For nuclear observation, cells were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI). The samples were then mounted in gelvatol and viewed under fluorescent microscopy using an Olympus Fluoview 1000 microscope.
Cell tracking was done by transducing MDSCs with a retrovirus containing a nuclear LacZ reporter gene (a gift from Dr. Paul Robbins, University of Pittsburgh) for 24 h and following the fate of the transduced cells after engraftment using B-gal substrate (x-gal). The staining was performed on 5 μm frozen sections fixed in 2% glutaraldehyde, followed by PBS rinse 2×, and subsequent incubation with x-gal solution at 37°C overnight according to previously described protocols. Slides were stained with eosin to facilitate imaging of LacZ+ nuclei, which stained blue.22,31
Scanning electron microscopy
Explanted constructs were fixed in 2.5% glutaraldehyde, dehydrated in a graded series of ethanol/water solutions, dried, and then sputter coated with gold. The surface of the constructs was examined under a scanning electron microscope operated at 3 kV.
Statistical analysis
Fisher's exact test for nonparametric variables was used to compare patency rates between groups. p-Values < 0.05 were considered significant.
Results
After 48 h of dynamic culture, the constructs in group one and two appeared completely populated with cells that were spread inside the scaffolds (Fig. 1A, B). Surgical implantation was feasible in all cases and no differences were noted between the groups in the surgical features of the constructs (i.e., tissue handling and suturability) (Fig. 1C, D). All animals recovered well from the surgery and showed no immediate signs of postoperative distress. In group one (TIPS-PEUU), two animals developed signs of lower limb impairment, absence of pulses, and foot necrosis within the first 72 h that required early sacrifice. One animal had a fatal aneurysmal rupture at day 4. In group 2 (ES-TIPS PEUU), three animals were sacrificed before the planned time due to ischemia on the lower limbs but no aneurysmal failures were detected. In group 3 (acellular TIPS-PEUU), five animals showed evidence of lower limb ischemia that required early sacrifice within the first week. Four animals (two in group 1 and two in group 2) that had a self-limited necrotic plaque in the distal part of the tail but had positive femoral pulses were treated with a partial resection of the tail and sacrificed at the same time as the others. All remaining animals in the three groups had a successful clinical outcome and were electively sacrificed at 8 weeks.
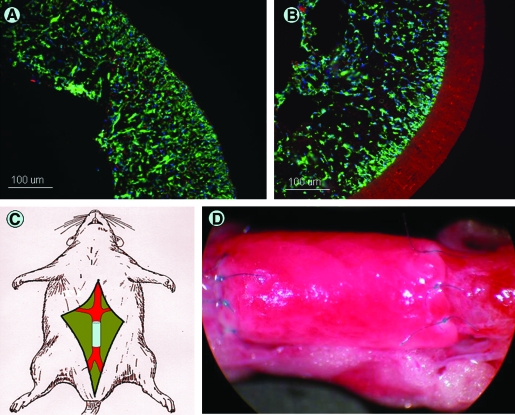
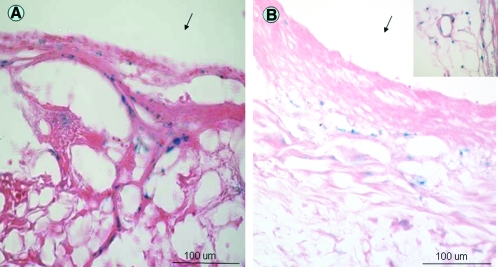
FIG. 1.
In vivo implantation of a TEVG. TIPS (A) and ES-TIPS (B) preimplant sections showing MDSCs populating the scaffolds throughout the entire thickness. (C) Schematic of surgical procedure. The TEVG is implanted in the abdominal aorta of the rat. (D) TEVG after implant sutured to the native aorta with 10.0 prolene. TEVG, tissue-engineered vascular graft; TIPS, thermally induced phase separation; ES-TIPS, bilayered constructs prepared by electrospinning an outer layer of poly(ester urethane)urea on the surface of the TIPS constructs; MDSCs, muscle-derived stem cells. Color images available online at www.liebertonline.com/ten.
Angiographic findings
The angiograms performed to evaluate patency rate (Fig. 2A–C) showed that 8 out of 15 animals (53%) in group 1 had a patent construct with no signs of stenosis. However, four of these animals (50%) showed aneurysmal dilation. In group 2, 11 out of 17 animals (65%) were patent with no signs of stricture and no aneurysms were detected. In contrast, 9 out of 10 animals in group 3 showed a clear obstruction proximal to the graft and only 1 (10%) remained patent. There were no significant differences in patency rate between groups 1 and 2 (p = 0.22), but when compared with the acellular controls in group 3, both groups had significantly higher patency rate (p = 0.03 for group 1 vs. group 3; p = 0.007 for group 2 vs. group 3).
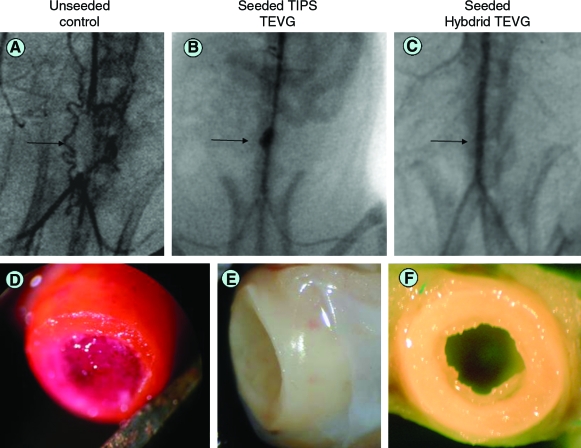
FIG. 2.
Angiographic findings and gross morphological aspect of TEVG. (A) Unseeded TEVG control shows obstruction of the flow at the level of the graft (arrow). (B) Seeded TIPS TEVG shows aneurysmal dilation (arrow). (C) Seeded ES-TIPS TEVG shows patent and no dilated graft after 8 weeks (arrow). (D) Intraluminal thrombus in unseeded control. Tissue-like aspect of explants for TIPS (E) and ES-TIPS (F) TEVG, respectively. Color images available online at www.liebertonline.com/ten.
Gross morphology findings
All patent constructs in groups 1 and 2 showed tissue-like appearance with clear remodeling of the scaffold without signs of scar tissue at the level of the anastomoses (Fig. 2E, F). A smooth shiny surface was noticed in the luminal side with almost unnoticeable transition from the native aorta. Nonpatent constructs in these groups had white organized tissue in the lumen, mainly at the anastomoses, suggestive of intimal hyperplasia formation. In group 3, all constructs but one showed a clear intraluminal thrombus formation with little or no remodeling of the scaffold (Fig. 2D).
Scanning electron microscopy
Surface analysis of the explanted constructs showed an endothelial-like layer on the luminal side in groups 1 and 2 (Fig. 3) with a smooth transition from the native endothelium to the tissue-engineered graft. There were no signs of stricture at the anastomosis level in the patent constructs. There was a clear integration of the native vessel wall with the implanted polymer. In group 3, the inner layer showed the surface completely covered by deposited platelets.
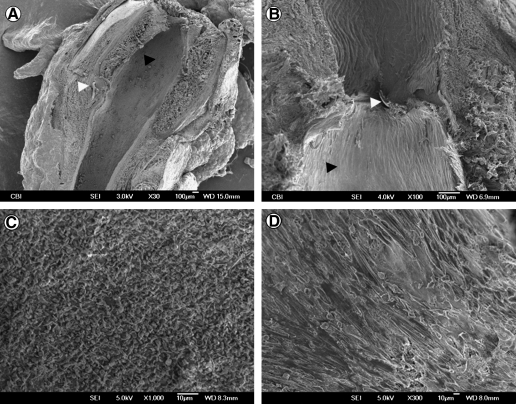
FIG. 3.
Scanning electron microscopy results. (A) ES-TIPS TEVG (black arrowhead) shows a smooth transition between the aorta and the graft, with no signs of stenosis. Note the 10.0 prolene suture at the level of the anastomosis (white arrow head). (B) Higher magnification at the anastomosis level for a TIPS TEVG. (C) Unseeded control showing platelet adhesion on the luminal side. (D) New endothelial layer in the center of a seeded ES-TIPS TEVG.
Histological findings
Histological analysis of the TEVGs in groups 1 and 2 showed formation of an external capsule with aligned collagen fibers (Fig. 4). It also showed an internal tissue layer with aligned collagen fibers and cellular components characteristic of smooth muscle exposed to the cyclical stretching caused by the systemic circulation. There were no signs of intimal hyperplasia in the patent constructs consistent with the angiographic findings. Three of the occluded constructs in group 2 had signs of intimal thickening at the anastomosis level, whereas the remaining three had organized scar tissue inside the lumen, suggesting chronic thrombosis. The scaffold showed intensive remodeling on the TIPS layer in both groups and little or no remodeling in the electrospun layer in the case of group 2. There was evidence of giant cells' characteristic of foreign body response together with a population of mononuclear cells inside the scaffold, suggesting an active degradation process (Fig. 4B, D, F). Although not quantified, the cell density inside the polymer appeared to be lower than the preimplant analysis, suggesting cell migration to both luminal and abluminal sides. In group 3, there were almost no cellular components inside the scaffold with scarce remodeling and a clear organized fibrin structure in the lumen characteristic of thrombus formation (Fig. 4A, B). X-gal staining of the sections in groups 1 and 2 showed LacZ+ cells that participated in the remodeling corroborating the presence of the implanted MDSCs at 8 weeks (Fig. 5A, B).
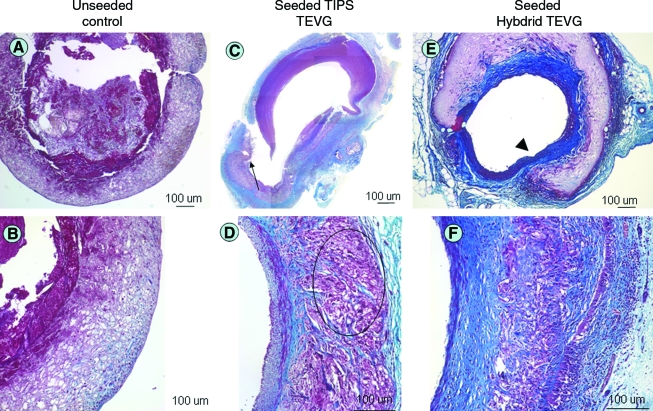
FIG. 4.
Histological findings of TEVG explants. (A) Unseeded controls show intraluminal thrombus with no remodeling of the scaffold. (B) Higher magnification of a different section of the same scaffold as in (A). (C) TIPS TEVG showing newly formed tissue in the inner layer with extensive cell infiltrate and degradation of the scaffold leading to aneurysmal dilation and rupture (arrow). (D) Higher magnification of a different section of the same TIPS scaffold as in (C): giant cells and mononuclear infiltrates are evident within the scaffold (circled area). (E) ES-TIPS scaffold at the anastomosis level showing the transition between native aorta (arrowhead) and the TEVG. (F) Higher magnification at the center of the ES-TIPS TEVG. Color images available online at www.liebertonline.com/ten.
FIG. 5.
LacZ+ staining showing engraftment of MDSCs. (A) TIPS construct. (B) ES-TIPS TEVG. Inset shows MDSCs participating in new capillary formation. Red = eosin, blue = LacZ+ transfected nuclei. Arrows indicate luminal side of sections. Color images available online at www.liebertonline.com/ten.
Immunohistochemistry findings
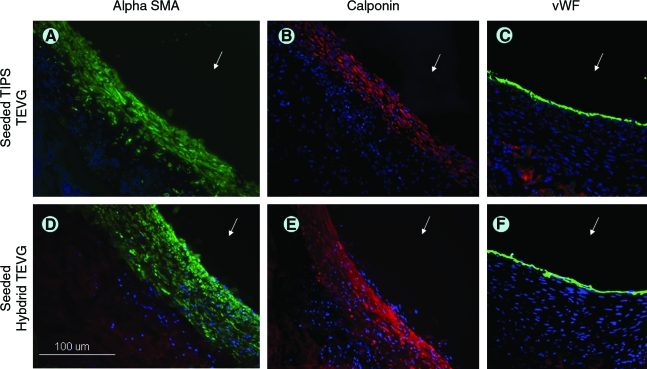
The immunohistochemical analysis showed that the inner tissue layer noted in groups 1 and 2 was positive for both smooth-muscle α-actin and calponin, suggesting a contractile phenotype. The lumen of the constructs also stained positive for von Willebrand factor, indicating the presence of endothelial cells (Fig. 6). Myosin heavy chain was not positive in any of the samples (data not shown).
FIG. 6.
Immunohistochemistry of TEVG. Inner new tissue layer in seeded TIPS stains positive for smooth muscle α-actin (A; green), calponin (B; red), and Von Willebrand factor (C; green). Blue = nuclei. (D–F) Similar findings for seeded ES-TIPS. Arrow indicates lumen. Color images available online at www.liebertonline.com/ten.
Discussion
We have shown in this study that the combination of two different processing techniques of PEUU scaffolds seeded with MDSCs and dynamically cultured for 48 h can serve as an arterial substitute in a rat model where the cellular component significantly improves the patency rate when compared with the unseeded constructs. The newly remodeled tissue consists of aligned collagen fibers, smooth muscle cells, and an endothelial layer. The configuration of the ES-TIPS scaffolds consisting of an outer electrospun layer prevents aneurysm formation in all cases, which is essential for arterial applications.
For cardiovascular applications a polymer must be elastic to be amenable to mechanical conditioning. Porosity and pore connectivity are also important to achieve successful cellular incorporation. PEUU biodegradable scaffolds meet all of these requirements and are currently being studied in several tissue engineering applications.28,29,32,33 Although most porous scaffold processing techniques greatly reduce the mechanical properties relative to the bulk polymer, electrospinning retains a great deal of the strength and distensibility of the bulk PEUU.29,32 In our study, the combination of two processing techniques to obtain an ES-TIPS scaffold provides the necessary porosity for cell support and tissue ingrowth while maintaining the mechanical properties to withstand systemic circulation. Moreover, there was no difference in the remodeling of the TIPS layer and the performance of the grafts when comparing groups 1 and 2, suggesting that the electrospun layer does not affect either the cellular activity or diffusion of nutrients.
Many of the previously described cellularized approaches for vascular tissue engineering have relied on a long-term culture period to reach the desired cell density and mechanical strength.8,34,35 We have previously shown that vacuum cell seeding of MDSCs within PEUU scaffolds, followed by a short-term culture period in a dynamic environment, effectively provides the initial in vitro conditions to obtain a TEVG in a clinically relevant period of time without affecting the stem cell phenotype of the cellular component.30 In this study, we show in vivo evidence that such an approach is a suitable alternative to obtain an arterial substitute in a short period of time that is mechanically sound and biologically active in the long term.
A similar approach to ours was described by Shin'oka and colleagues, where a copolymer of L-lactide and e-caprolactone was used to create a TEVG that was successfully implanted in human patients as a pulmonary artery replacement.13,36,37 Although the constructs were implanted in the low pressure pulmonary circulation, they have clearly shown the relevance of a cellular component on the patency rate and tissue remodeling and the feasibility of clinical application of this type of approach. Further proof of clinical translation of a TEVG, but for the arterial circulation, has been described by L'Heureux et al.9 with a completely cellularized approach without the use of a scaffold. Although this represents a major breakthrough in the field of tissue engineering, the length of the fabrication process is still a limitation with this approach.
Progenitor cells show great potential for use in tissue engineering applications and may circumvent many of the shortcomings associated with other options in cell sourcing. In a recent study by Hashi et al. bone marrow mesenchymal stem cells have shown antithrombogenic properties when used in vascular applications and they reduced the amount of intimal hyperplasia formation.38 MDSCs have previously shown the ability to retain their phenotype for more than 30 passages with normal karyotype and were able to differentiate into muscle, neural, and endothelial lineages both in vivo and in vitro.22,23 The engraftment of MDSCs in the vascular tissue described in this study is the first evidence of the contribution of this cell line to the performance of an arterial substitute. Although this study shows a clear benefit of incorporating a cellular component in the TEVGs, further studies are required to elucidate the specific advantages of MDSCs to other cell lines. However, the advantages of availability and proliferation during the fabrication process would make the MDSCs attractive when compared with terminally differentiated cells, even if they would be found to have similar contributions to TEVG patency rate. Although colocalization was not an endpoint of this study, direct comparison between histology and LacZ+ images did not show a spatial relationship between the engrafted cells and the new tissue formation. The mechanism of action by which the cells prevent thrombosis of the grafts remains to be elucidated, but there is evidence that stem cells and endothelial cells release a variety of growth factors (e.g., vascular endothelial growth factor and Ang-1) that play an important role in angiogenesis and vasculogenesis.39 It has been hypothesized that stem cells contributing to formation of vascular tissue-engineered tissues act with an autocrine/paracrine mechanism interacting with surrounding structures and recruiting circulating cells to remodel the TEVG.38,39
A potential limitation of this study is the amount of polymer degradation observed at 8 weeks. Although the TIPS layer appears heavily remodeled and infiltrated, the electrospun layer remains intact, making it difficult to evaluate when the newly formed tissue would replace all of the polymer and whether it would then be mechanically sound. However, we have observed on a separate pilot study (data not shown) that electrospun constructs can persist in vivo for more than 6 months without failing mechanically when implanted as a vascular graft. It is estimated that, by that time, the TIPS layer will be sufficiently remodeled and the newly formed tissue will be strong enough to withstand systemic circulation. However, the degradation rate needs to be optimized in future studies.
Conclusion
We describe here the in vivo results of an arterial TEVG fabricated with biodegradable tubular scaffolds and seeded with MDSCs where the cellularized constructs had a significant improvement in patency rate when compared with unseeded controls. The ES-TIPS configuration (combination of two different processing techniques) of the scaffolds was essential to maintain adequate mechanical properties to withstand systemic circulation while still allowing rapid cell seeding and tissue remodeling. Although longer-term endpoints and large animal studies are required, this first evidence of the role of MDSCs in the in vivo performance of an arterial TEVG could be an important milestone for future clinical translation.
Acknowledgment
The authors would like to acknowledge funding from NIH BRP #R01 HL069368 (to W.R.W. and D.A.V.) and American Heart Association Postdoctoral Fellowship 0525585U (to A.N.), and a Ruth L. Kirschstein National Research Service Award EB004791 (to T.M.M.)
Disclosure Statement
Dr. Johnny Huard serves as a consultant for Cook MyoSite (Pittsburgh, PA).
References
- 1.American Heart Association. Heart Disease and Stroke Statistics-2004 Update. Dallas, TX: 2004. [Google Scholar]
- 2.Grigioni M. Daniele C. D'Avenio G. Barbaro V. Biomechanics and hemodynamics of grafting. In: Tura A., editor. Vascular Grafts: Experiment and Modeling. Boston: WIT Press; 2003. pp. 41–82. [Google Scholar]
- 3.Davids L. Dower T. Zilla P. The lack of healing in conventional vascular grafts. In: Zilla P., editor; Greisler H.P., editor. Tissue Engineering of Vascular Prosthetic Grafts. Austin: R.G. Landes; 1999. pp. 3–45. [Google Scholar]
- 4.Williams S.K. Rose D.G. Jarrell B.E. Microvascular endothelial cell sodding of ePTFE vascular grafts: improved patency and stability of the cellular lining. J Biomed Mater Res. 1994;28:203. doi: 10.1002/jbm.820280210. [DOI] [PubMed] [Google Scholar]
- 5.Weintraub W.S. Jones E.L. Craver J.M. Guyton R.A. Frequency of repeat coronary bypass or coronary angioplasty after coronary artery bypass surgery using saphenous venous grafts. Am J Cardiol. 1994;73:103. doi: 10.1016/0002-9149(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 6.Nerem R.M. Seliktar D. Vascular tissue engineering. Annu Rev Biomed Eng. 2001;3:225. doi: 10.1146/annurev.bioeng.3.1.225. [DOI] [PubMed] [Google Scholar]
- 7.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 8.L'Heureux N. Paquet S. Labbe R. Germain L. Auger F.A. A completely biological tissue engineered human blood vessel. FASEB J. 1998;12:47. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 9.L'Heureux N. Dusserre N. Konig G. Victor B. Keire P. Wight T.N. Chronos N.A. Kyles A.E. Gregory C.R. Hoyt G. Robbins R.C. McAllister T.N. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12:361. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho S.W. Lim S.H. Kim I.K. Hong Y.S. Kim S.S. Yoo K.J. Park H.Y. Jang Y. Chang B.C. Choi C.Y. Hwang K.C. Kim B.S. Small-diameter blood vessels engineered with bone marrow-derived cells. Ann Surg. 2005;241:506. doi: 10.1097/01.sla.0000154268.12239.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaner P.J. Martin N.D. Tulenko T.N. Shapiro I.M. Tarola N.A. Leichter R.F. Carabasi R.A. Dimuzio P.J. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40:146. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Niklason L.E. Abbott W. Gao J. Klagges B. Hirschi K.K. Ulubayram K. Conroy N. Jones R. Vasanawala A. Sanzgiri S. Langer R. Morphologic and mechanical characteristics of engineered bovine arteries. J Vasc Surg. 2001;33:628. doi: 10.1067/mva.2001.111747. [DOI] [PubMed] [Google Scholar]
- 13.Shin'oka T. Imai Y. Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 14.Vorp D.A. Maul T.M. Nieponice A. Molecular aspects of vascular tissue engineering. Front Biosci. 2005;10:768. doi: 10.2741/1571. [DOI] [PubMed] [Google Scholar]
- 15.Nieponice A. Maul T. Soletti L. Vorp D. Encyclopedia of Biomaterials and Biomedical Engineering. Taylor and Francis; 2006. Vascular tissue engineering; pp. 1–14. [Google Scholar]
- 16.Guan J. Sacks M.S. Beckman E.J. Wagner W.R. Synthesis, characterization, and cytocompatibility of elastomeric, biodegradable poly(ester-urethane)ureas based on poly(caprolactone) and putrescine. J Biomed Mater Res. 2002;61:493. doi: 10.1002/jbm.10204. [DOI] [PubMed] [Google Scholar]
- 17.Niklason L.E. Langer R.S. Advances in tissue engineering of blood vessels and other tissues. Transpl Immunol. 1997;5:303. doi: 10.1016/s0966-3274(97)80013-5. [DOI] [PubMed] [Google Scholar]
- 18.Nieponice A. Maul T. Cumer J. Soletti L. Vorp D. Mechanical stimulation induces morphological and phenotypic changes in bone marrow-derived progenitor cells within a three-dimensional fibrin matrix. J Biomed Mater Res. 2007;81:523. doi: 10.1002/jbm.a.31041. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton D.W. Maul T.M. Vorp D.A. Characterization of the response of bone marrow derived progenitor cells to cyclic strain: implications for vascular tissue engineering applications. Tissue Eng. 2004;10:361. doi: 10.1089/107632704323061726. [DOI] [PubMed] [Google Scholar]
- 20.Deasy B.M. Jankowski R.J. Huard J. Muscle-derived stem cells: characterization and potential for cell-mediated therapy. Blood Cells Mol Dis. 2001;27:924. doi: 10.1006/bcmd.2001.0463. [DOI] [PubMed] [Google Scholar]
- 21.Lee J.Y. Qu Petersen Z. Cao B. Kimura S. Jankowski R. Cummins J. Usas A. Gates C. Robbins P. Wernig A. Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qu-Petersen Z. Deasy B. Jankowski R. Ikezawa M. Cummins J. Pruchnic R. Mytinger J. Cao B. Gates C. Wernig A. Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deasy B.M. Li Y. Huard J. Tissue engineering with muscle-derived stem cells. Curr Opin Biotechnol. 2004;15:419. doi: 10.1016/j.copbio.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Cao Y. Sun Z. Liao L. Meng Y. Han Q. Zhao R.C. Martinez-Estrada O.M. Munoz-Santos Y. Julve J. Reina M. Vilaro S. Human adipose tissue-derived stem cells differentiate into endothelial cells in vitro and improve postnatal neovascularization in vivo. Biochem Biophys Res Commun. 2005;332:370. doi: 10.1016/j.bbrc.2005.04.135. [DOI] [PubMed] [Google Scholar]
- 25.Burg K.J. Holder W.D., Jr. Culberson C.R. Beiler R.J. Greene K.G. Loebsack A.B. Roland W.D. Eiselt P. Mooney D.J. Halberstadt C.R. Comparative study of seeding methods for three-dimensional polymeric scaffolds. J Biomed Mater Res. 2000;51:642. doi: 10.1002/1097-4636(20000915)51:4<642::aid-jbm12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 26.Soletti L. Nieponice A. Guan J. Stankus J. Wagner W. Vorp D.A. A novel seeding device for tissue engineered tubular structures. Biomaterials. 2006;27:4863. doi: 10.1016/j.biomaterials.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 27.Gharaibeh B. Lu A. Tebbets J. Zheng B. Feduska J. Crisan M. Peault B. Cummins J. Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 28.Guan J. Fujimoto K.L. Sacks M.S. Wagner W.R. Preparation and characterization of highly porous, biodegradable polyurethane scaffolds for soft tissue applications. Biomaterials. 2005;26:3961. doi: 10.1016/j.biomaterials.2004.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong Y. Ye S.H. Nieponice A. Soletti L. Vorp D.A. Wagner W.R. A small diameter, fibrous vascular conduit generated from a poly(ester urethane)urea and phospholipid polymer blend. Biomaterials. 2009;30:2457. doi: 10.1016/j.biomaterials.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nieponice A. Soletti L. Guan J. Deasy B.M. Huard J. Wagner W.R. Vorp D.A. Development of a tissue-engineered vascular graft combining a biodegradable scaffold, muscle-derived stem cells and a rotational vacuum seeding technique. Biomaterials. 2008;29:825. doi: 10.1016/j.biomaterials.2007.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Payne T.R. Oshima H. Sakai T. Ling Y. Gharaibeh B. Cummins J. Huard J. Regeneration of dystrophin-expressing myocytes in the mdx heart by skeletal muscle stem cells. Gene Ther. 2005;12:1264. doi: 10.1038/sj.gt.3302521. [DOI] [PubMed] [Google Scholar]
- 32.Stankus J.J. Guan J. Wagner W.R. Fabrication of biodegradable, elastomeric scaffolds with sub-micron morphologies. J Biomed Mater Res. 2004;70:603. doi: 10.1002/jbm.a.30122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimoto K.L. Tobita K. Merryman W.D. Guan J. Momoi N. Stolz D.B. Sacks M.S. Keller B.B. Wagner W.R. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol. 2007;49:2292. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.L'Heureux N. Germain L. Labbe R. Auger F.A. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J Vasc Surg. 1993;17:499. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 35.Niklason L.E. Gao J. Abbott W.M. Hirschi K.K. Houser S. Marini R. Langer R. Functional arteries grown in vitro. Science. 1999;284:489. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 36.Naito Y. Imai Y. Shin'oka T. Kashiwagi J. Aoki M. Watanabe M. Matsumura G. Kosaka Y. Konuma T. Hibino N. Murata A. Miyake T. Kurosawa H. Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J Thorac Cardiovasc Surg. 2003;125:419. doi: 10.1067/mtc.2003.134. [DOI] [PubMed] [Google Scholar]
- 37.Shin'oka T. Matsumura G. Hibino N. Naito Y. Watanabe M. Konuma T. Sakamoto T. Nagatsu M. Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 38.Hashi C.K. Zhu Y. Yang G.Y. Young W.L. Hsiao B.S. Wang K. Chu B. Li S. Antithrombogenic property of bone marrow mesenchymal stem cells in nanofibrous vascular grafts. Proc Natl Acad Sci USA. 2007;104:11915. doi: 10.1073/pnas.0704581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumura G. Miyagawa-Tomita S. Shin-oka T. Ikada Y. Kurosawa H. First evidence that bone marrow cells contribute to the construction of tissue-engineered vascular autografts in vivo. Circulation. 2003;108:1729. doi: 10.1161/01.CIR.0000092165.32213.61. [DOI] [PubMed] [Google Scholar]