Abstract
Enzymatically degradable semi-interpenetrating polymer networks (edsIPNs) were explored for their biocompatibility and ability to promote new scleral tissue growth, as a means of reinforcing the posterior wall of the eye. The edsIPNs comprised thermoresponsive poly(N-isopropylacrylamide-co-acrylic acid), customizable peptide crosslinkers cleavable by matrix metalloproteinases, and interpenetrating linear poly(acrylic acid)-graft-peptide chains to engage with cell surface receptors. Rheological studies revealed an increase in stiffness at body temperature; the complex shear modulus |G*| was 14.13 ± 6.13 Pa at 22°C and 63.18 ± 12.24 Pa at 37°C, compatible with injection at room temperature. Primary chick scleral fibroblasts and chondrocytes cultured on edsIPN increased by 15.1- and 11.1-fold, respectively, over 11 days; both exhibited delayed onset of exponential growth compared with the cells plated on tissue culture polystyrene. The edsIPN was delivered by retrobulbar injection (100 μL) to nine 2-week-old chicks to assess biocompatibility in vivo. Ocular axial dimensions were assessed using A-scan ultrasonography over 28 days, after which eyes were processed for histological analysis. Although edsIPN injections did not affect the rate of ocular elongation, the outer fibrous sclera showed significant thickening. The demonstration that injectable biomimetic edsIPNs stimulate scleral fibrous tissue growth represents proof-of-principle for a novel approach for scleral reinforcement and a potential therapy for high myopia.
Introduction
High myopia, sometimes also referred to as pathological myopia, represents a significant public health burden due to the associated ocular complications, such as retinal detachment and macular degeneration, both of which may result in substantial vision loss and even blindness.1 In Taiwan, one of many Asian countries showing epidemic levels of myopia, myopic macular degeneration is now the second highest cause of visual impairment in the over 65-year age group.2 Such complications reflect the stretching and thinning of the retina, a product of the excessive axial ocular elongation underlying myopia. The sclera, the outer wall of the eye, plays a fundamental role in axial ocular elongation, undergoing increased remodeling, thinning, and in some cases progressive biomechanical failure in the region of the posterior pole, leading to the development of staphylomas.
The sclera represents the outer mechanical support layer of the eye (Fig. 1A), and in mammalian and primate eyes is comprised of avascular fibrous connective tissue containing various collagens and proteoglycans.3,4 In mature sclera, the collagen fibers are layered anisotropically and show a gradient in fiber diameter, with the smallest fibers found innermost toward the retina. This gradient is lost in high myopia,5,6 and the myopic sclera shows additional ultrastructural and biomechanical changes. For example, there are significant decreases in the amounts of glycosaminoglycans and proteoglycans in myopic eyes.7–9 These changes likely contribute to the scleral thinning reported in high myopia in humans and animal models of the same,7,10–13 contributing to observed increases in scleral creep rates and rendering such eyes more vulnerable to the stretching influence of intraocular pressure.14–16 The fibrous sclera of the avian eye shares many of the same features of the mammalian sclera, as well as changes with myopia, but the avian sclera also includes an additional inner cartilaginous layer, making it much more rigid.
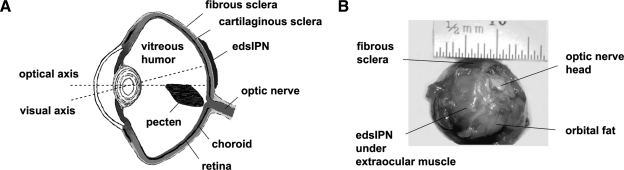
FIG. 1.
(A) Transverse cross-sectional diagram of a chick eye showing the location of the injected enzymatically degradable semiinterpenetrating polymer network (edsIPN) on the outer surface of fibrous sclera with respect to the visual and optical axes, and (B) posterior view of an enucleated chick eye showing injection site.
Various interventions have been explored to strengthen the myopic sclera, including chemical- and irradiation-induced scleral collagen crosslinking,17 systemic delivery of methylxanthines to increase scleral collagen concentration and fiber diameters,18 and scleroplasty involving the attachment over the posterior sclera of allografts or synthetic materials to provide direct mechanical support.19–21 A sub-Tenon's injection of a polymeric gel formulation comprised mainly of polyvinylpyrrolidone has been used to control myopia progression, but testing has been limited to a Moscow-based small human study and a mechanistic animal study.20,22 These options have yet to gain wide clinical acceptance because of high toxicity (e.g., glutaraldehyde), lack of complete understanding of the mechanism of action (methylxanthines), limited access to clinical trial data (polyvinylpyrrolidone injections), and for scleroplasty, limited supply of allograft material, its invasive nature, and limited data supporting its long-term efficacy.23
As an alternative, a minimally invasive scleral reinforcement strategy that takes advantage of the biochemical properties of host sclera was developed. We have begun testing injectable and highly tunable biomimetic hydrogels based on poly(N-isopropylacrylamide). The merits of these synthetic hydrogels include environmental responsiveness and independent tunability with respect to the mechanical properties, customizable biological ligands that promote cell and tissue adhesion, and controllable protease degradation of the hydrogel to mediate cellular infiltration.24 In particular, hydrogels based on poly(N-isopropylacrylamide) are known to be injectable at room temperature, while forming viscoelastic solids in situ at higher physiological (body) temperatures.24 In this study, we used enzymatically degradable semiinterpenetrating polymer networks (edsIPNs) composed of poly(N-isopropylacrylamide-co-acrylic acid) with proteolytically degradable peptide crosslinkers and physically entangled peptide-functionalized linear poly(acrylic acid) [p(AAc)] chains that interpenetrate the synthetic hydrogel matrix.25,26 Enzymatically degradable hydrogels, used as biomimetic extracellular matrix (ECM), have been reported to support human embryonic stem-cell self-renewal,27 act as vehicles for cell transplantation by sustaining early cellular remodeling and growth,28 aid myocardium regeneration,29 and promote mesenchymal stem-cell proliferation and bone formation in vivo.25
Based on the known biochemistry of the fibrous sclera and characterization of various edsIPNs, one formulation was selected for evaluation. Rheological testing was initially performed to establish the injectability of the edsIPN material and its likely stabilization after injection in vivo. In vitro cellular proliferation studies, using chick scleral fibroblasts and chondrocytes, were conducted to establish the cytocompatibility of the edsIPN. In vivo biocompatibility testing assessed the effects on ocular growth and scleral histology of a one-time retrobulbar injection of edsIPN, applied against the external scleral surface at the posterior pole of normal chick eyes. We found that the injected hydrogels preserved the phenotypes and allowed proliferation of both cell types in vitro and stimulated the addition of fibrous tissue to the native fibrous sclera when injected in vivo.
Materials and Methods
Synthesis and chemical composition of edsIPNs
All chemicals were purchased from Aldrich (St. Louis, MO) and used without further purification unless otherwise specified. All water used was ultrapure American Society for Testing and Materials (ASTM) type I reagent grade (18.2 MΩ · cm, pyrogen-free, endotoxin <0.03 endotoxin unit (EU)/mL). The edsIPN was synthesized by redox radical addition polymerization at room temperature with molar ratios of 95:5:0.3 [N-isopropylacrylamide (NIPAAm): acrylic acid:crosslinker] in 1 × Dulbecco's phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA) without calcium and magnesium as previously described (Fig. 2A).25,26,30 The peptide crosslinker, Gln-Pro-Gln-Gly-Leu-Ala-Lys-NH2 (QPQGLAK-NH2; American Peptide, Sunnyvale, CA), was cleavable by matrix metalloproteinase (MMP)-13 and other collagenases,30 and included a glutamine residue to promote solubility and a lysine residue to provide amine functional groups for modification. Reacting the peptide with acryloyl chloride in the presence of triethylamine generated amide linkages between the peptide and acrylic group, thereby introducing bifunctional acryl groups amenable to addition polymerization. The inclusion of MMP degradable peptide crosslinkers offers potential control over the rate of scleral remodeling, given that MMPs are known to be involved in scleral remodeling.31–33 The poly(N-isopropylacrylamide-co-acrylic acid) polymer network was interpenetrated by p(AAc)-graft-Ac-CGGNGEPRGDTYRAY-NH2 [p(AAc)-g-RGD] linear polymer chains to promote cell adhesion.26 Linear p(AAc) chains (MW 450,000; Polysciences; Warrington, PA) were first grafted with maleimide side groups, then the synthetic peptide, Ac-CGGNGEPRGDTYRAY-NH2 derived from bone sialoprotein [bsp-RGD(15); American Peptide], was grafted to the maleimide side groups to produce p(AAc)-g-RGD linear chains. The selection of the bsp-RGD(15) motif was based on its known binding activity to several integrin receptors, including α2, αVβ3, β1, and α2β1 and known angiogenic activity.34–36 Further, bsp-RGD(15) has been shown to enhance the attachment of fibroblasts,37 and associated integrin receptors have been implicated in the growth of both normal and myopic mammalian sclera.38
FIG. 2.
(A) Schematic diagram illustrating the reaction steps involved in the synthesis of edsIPNs. (B) The thermoresponsive edsIPN acts as a synthetic extracellular matrix containing proteolytically degradable peptide crosslinkers and peptide-functionalized cell adhesion signals. (C) Scanning electron microscopic image showing the three-dimensional structure of the edsIPN. 1000 × magnification, scale bar = 20 μm.
The synthesis of the edsIPNs was achieved by first bubbling nitrogen gas through a solution of NIPAAm, acrylic acid, acrylated peptide crosslinker, and p(AAc)-g-RGD in calcium and magnesium-free PBS for 30 min to remove dissolved oxygen. Following nitrogen purging, 0.8 wt% of ammonium persulfate (Polysciences) and 4% v/v of N,N,N′,N′-tetramethylenediamine (Polysciences) were added as initiator and accelerator, respectively. The mixture was stirred vigorously for 10 s and allowed to polymerize for 24 h. After polymerization, edsIPNs were washed three times in excess water to remove unreacted reagents, sterilized with 70% ethanol, and again washed three times in water to remove the ethanol. This technique was previously shown to be effective in sterilizing peptide-modified hydrogels without peptide degradation.39 The resulting edsIPN structure is depicted in Figure 2B. All edsIPNs were stored in sterile PBS at room temperature before use.
Determination of concentration of grafted RGD
The degree of substitution of bsp-RGD(15) on the p(AAc) chains was assessed using previously developed methods.34,40 Briefly, fluorescein isothiocyanate (FITC)–labeled bsp-RGD(15) peptide [Ac-CGGNGEPRGDTYRAYK(FITC)GG-NH2; American Peptide] was grafted to the p(AAc) chains as described above, and fluorescence was measured in a SpectraMax Gemini XS fluorometer (Molecular Devices, Sunnyvale, CA) (excitation 485 nm, emission 438 nm, cut-off 530 nm). A digestion buffer containing 100 μL of 10 mM Tris–HCl (pH = 8.0, 100 mM CaCl2) and 1546 U/mL bovine chymotrypsin was added to each well. Control solution standards (100 μL/well) containing 0.001–10 μM of peptide were digested in parallel. The fluorescence of the wells was monitored in situ at 10-min intervals over 2-h period at 25°C. The concentration of the grafted RGD was then determined and normalized to the weight of the linear p(AAc) chains. In this study, 300 μM bsp-RGD(15) was chosen to present significant RGD motifs to the scleral cells. Previous studiesi ndicated that this concentration was sufficient for cell adhesion, migration, and proliferation.25,26,29 The grafted bsp-RGD(15) peptide chains have a statistical bulk distribution within the edsIPN, because the linear p(AAc) chains interpenetrate the synthetic network.26,41
Electron microscopy imaging of edsIPN
The general surface features of the edsIPN were studied by scanning electron microscopy. Samples were lyophilized to constant weight. Small thin samples were then broken off the lyophilized edsIPN with forceps, placed on adhesive carbon conductive tape, and sputter coated with 1–2 nm of gold. Samples were viewed by cold field emission electron microscopy (S-5000; Hitachi, Toronto, Canada) using an accelerating voltage of 10 kV.
Rheological characterization of edsIPN
To characterize the viscoelastic properties of the edsIPN, dynamic oscillatory shear measurements were undertaken using a parallel plate rheometer (Paar Physica MCR 300; Anton Paar, Ashland, VA) with sanded 50 mm parallel plates at a gap height of 1.0 mm. A humidity chamber was placed around samples during testing to prevent dehydration. The lower plate temperature was regulated with a Peltier heating element connected to a recirculating water bath. The complex shear modulus |G*| was determined by measuring the storage modulus |G′| and loss modulus |G″| over a temperature range of 22–40°C and a frequency range of 0.001–14 Hz at 5% strain, which is within linear elastic region for the set gels. Samples were heated at a rate of 1°C/min. The lower critical solution temperature phase transition was determined using an UV–vis spectrophotometer to monitor transmittance at λ = 500 nm, as a function of temperature. All rheological measurements were repeated five times for each of the five samples of the synthesized edsIPN.
In vitro cytocompatibility testing of edsIPNs using cultures of chick scleral cells
The presence in the chick sclera of an outer fibrous layer similar in structure to the mammalian sclera, combined with the fast eye growth and the practical advantages of working with chicks, provided the rationale for using the chick model in this study. White Leghorn chicks (Gallus gallus domesticus) were obtained as hatchlings from a commercial hatchery (Privett Hatchery, Portales, NM) and reared in 12/12-h light/dark cycle, with food and water available ad libitum. Care and use of the animals were in compliance with the animal use protocol approved by the Animal Care and Use Committee of the University of California, Berkeley.
Primary chick scleral fibroblasts and chondrocytes were isolated from the eyes of a 1-week-old chick. Immediately after sacrifice, both eyes were enucleated and their outer scleral surface cleared of extraocular muscles and other adherent orbital tissue. Circumferential incisions at the equator were performed to remove the anterior ocular segments, and subsequently the vitreous, retina, and choroid were removed from the remaining scleral cups. The scleral cups were then put into Ringer's buffer and the outer fibrous sclera separated from the inner cartilaginous sclera using sharp (fine-pointed) forceps. The separated tissues were cut into small, 1 × 1 mm pieces, and digested by incubation with 0.3% w/v dispase (Bacillus polymyxa; Calbiochem, La Jolla, CA) and 0.2% w/v collagenase (Clostridium histolyticum; Calbiochem) in Dulbecco's modified Eagle's medium (DMEM) and Ham's F-12 media (1:1) with 10% fetal bovine serum (Invitrogen) supplemented with 1% penicillin–streptomycin antibiotic (Invitrogen) at 37°C. In each case, the medium was removed by centrifugation (1200 g for 1 min) after a 48-h enzymatic digestion period, and the softened tissue was gently pipetted three times in additional 1:1 DMEM/F-12 media to physically dissociate the cells. The cell-containing medium was then filtered through a 40-μm sieve (Falcon cell strainer nylon; Becton Dickinson, Franklin Lakes, NJ) to remove undissociated cell and tissue aggregates. The filtered media with dissociated cells was centrifuged and then replaced with fresh media before cell counting with a hemacytometer.
Primary chick fibroblasts and chondrocytes were seeded at a cell density of 2.0 × 103 cells/cm2 onto 48-well tissue culture-treated polystyrene (TCPS) plates, either directly or on the top of the edsIPN, which uniformly covered the bottom of the wells. Cells were subsequently cultured in 1:1 DMEM/F-12 media with 1% penicillin–streptomycin and 10% fetal bovine serum and placed in 5% CO2 incubator at 37°C. Phase-contrast microscopic images of the cell cultures were taken at 1, 6, and 11 days after seeding. A CyQUANT® cell proliferation assay kit (Molecular Probes, Eugene, OR) was used, following the manufacturer's instructions, to quantify cell proliferation on a daily basis over an 11-day period. A hemacytometer was also used to confirm the cell numbers separately. Five samples were used for each time period and measured in triplicate.
In vivo ocular biocompatibility assessment of edsIPNs
To assess the effect of the designed edsIPN on the ocular sclera, an in vivo experiment using 2-week-old White Leghorn chicks (G. gallus domesticus; n = 9) was undertaken. The chicks received a retrobulbar injection of 100 μL edsIPN. All polymer injections were monocular, assigned randomly to the left or the right eye; the untreated fellow eye served as controls. Before implantation, chicks were first anesthetized with 2% isoflurane in oxygen. Access to the orbit was gained through a small temporal incision. A 7-0 silk anchoring suture was placed in the anterior sclera to rotate and fixate the eye to allow easier access to the posterior pole. The polymer was delivered over the posterior pole, between the sclera and the dorsal oblique extraocular muscle using a curved, blunt-end 19-gauge needle (sub-Tenon's anesthesia cannula needle; BD Ophthalmic Systems, Franklin Lakes, NJ) with the aid of a surgical microscope, which also allowed unwanted anterior diffusion of the injected edsIPN to be ruled out. An antibiotic ointment was applied prophylactically, after suturing closed the incision site. The anatomical position of the injected edsIPN is shown with respect to the visual axis (Fig. 1A) and for an enucleated eye (Fig. 1B).
Measurement and analyses of ocular dimensions and refractions
Axial eye growth was monitored in vivo using a custom high-frequency (30 MHz) A-scan ultrasonography setup that offers measurement precision down to 10 μm.42 The thickness of the sclera and all other axial ocular components (anterior chamber depth, lens thickness, vitreous chamber depth, retina, and choroid) were obtained by averaging data from a minimum of 12 measurements per eye per time point. Chicks were anesthetized with 1.5% isoflurane in oxygen for all measurements. Data were collected for all chicks on the day of but before the injection (day 0; baseline), as well as at weekly intervals out to 28 days. Only data from the six birds that completed the 4-week monitoring period were included in ultrasonography data analyses. Data for treated and fellow eyes were normalized so that their baseline means matched the overall baseline mean for all eyes. Scleral cup depth data were obtained by adding together the vitreous chamber depth, retinal thickness, and choroidal thickness, as an index of scleral surface area. Scleral thickness was not included in this parameter to allow for the possibility that it changed independently of scleral surface area. It was also not included in the derived axial length parameter, which was calculated as the sum of anterior chamber depth, lens thickness, and scleral cup depth. Neither anterior chamber depth nor lens thickness was reported separately as they were not affected by the treatment. Axial length was used as an index of ocular elongation. In addition to ultrasonography, endpoint refractions were measured by streak retinoscopy on the eyes of the same birds at the end of the monitoring period. Treatment-induced changes in refraction can be expected from effects on either or both the curvature of the optical components of the eye, and ocular growth. Normalized data from treated and fellow control eyes were compared statistically using two-way repeated measures analysis of variance and paired t-test (Statview, Version 4.0; SAS Institute, Cary, NC). A p-value of less than 0.05 was used for statistical significance.
Histological analyses of in vivo effects of injected edsIPN
To further investigate the scleral effects of the edsIPN implants, one chick was randomly picked for sacrifice at weekly intervals after injection for histological analysis. Eyes were carefully enucleated immediately after sacrifice, leaving the implants and any associated connective tissue in place, and visually inspected under a surgical microscope. The anterior segments of the eyes were then removed and the posterior eyecups fixed overnight at 4°C in 4% paraformaldehyde in 0.1 M Sorensen's buffer with 3% sucrose. The fixed eyecups were then rinsed three times in 0.1 M Sorensen's buffer for 10 min each, before being cryoprotected overnight with 30% sucrose in 0.1 M Sorensen's buffer at 4°C. Afterward, eyecups were embedded in Tissue-Tek optimal cutting temperature (OCT) compound at −25°C using a dry, ice-chilled alcohol slurry. The frozen blocks were clearly marked to allow localization of the posterior pole of the eyecups in subsequent sectioning. Cryostat sections, 10 μm in thickness, were cut in a temporal to nasal direction, air dried, and stained by hematoxylin and eosin and Masson's trichrome (American MasterTech Scientific, Lodi, CA). Histological findings were qualitatively described for all time points, and thickness data for the band of new fibrous tissue generated by cellular infiltration of the implants, that is, the scleral integration layer, were also included for the 4-week time point.
Results
Characterization of the edsIPNs
At room temperature, the synthesized edsIPNs were optically clear and easily deformable. Scanning electron microscopic images revealed the edsIPN to have an overall porous structure with interconnected pores and rough surface (Fig. 2C). The concentration of the grafted bsp-RGD(15) was 36.5 μmol/g of p(AAc), established using a FITC-conjugated bsp-RGD(15) peptide.
The viscoelastic properties of edsIPN, shown as plots of the complex shear modulus |G*| as functions of frequency and temperature (Fig. 3A, B), were similar to the previously reported data.26,30 The edsIPN had a lower |G*| at 22°C than at 37°C (Fig. 3A). The temperature-dependent viscoelastic behavior was also consistent with the measured onset of the phase transition, starting at 35°C (Fig. 3B). The phase angle varied from 5° to 10° during the experiments. The mean |G*| was 14.13 ± 6.13 Pa at 22°C and 1 Hz, and was 63.18 ± 12.24 Pa at 37°C at the same frequency, representing a 4.5-fold increase in |G*| due to the lower critical solution temperature transition. These properties were compatible with our requirement of a polymer that can be easily injected through a 19-gauge needle at room temperature, and yet will set and so remain localized to the site of injection at body temperature.
FIG. 3.
Results of rheological measurements of 95:5:0.3 [N-isopropylacrylamide (NIPAAm):acrylic acid:crosslinker] edsIPN with 300 μM bsp-RGD(15). The complex modulus plotted against frequency at 22°C and 37°C (A), and plotted against temperature at frequency setting of 1 Hz (B).
In vitro cytocompatibility assay using chick scleral fibroblast and chondrocyte cultures
The chick scleral fibroblasts and chondrocytes exhibited different morphology, depending on whether they were cultured on the edsIPNs or TCPS (Fig. 4A). On TCPS, the fibroblasts spread and possessed a spindle-shaped morphology throughout the growth period (ScF TCPS, Fig. 4A), whereas in contrast, scleral fibroblasts cultured on edsIPN had a rounded morphology from the outset and maintained this morphology throughout the 11-day culture period (ScF edsIPN, Fig. 4A). On edsIPN, the scleral chondrocytes also exhibited on day 1 after seeding a phenotypic rounded morphology, which was maintained throughout the growth period (ScC edsIPN, Fig. 4A). Although the chondrocytes cultured on TCPS initially also exhibited a rounded morphology, they started to loose this morphology by day 6, exhibiting a fibroblast-like morphology by day 11, suggesting dedifferentiation into fibroblasts (ScC TCPS, Fig. 4A).
FIG. 4.
Phase-contrast images of scleral fibroblasts (ScF) and scleral chondrocytes (ScC) cultured on edsIPN and tissue culture polystyrene (TCPS) at 1, 6, and 11 days after seeding (A) (10 × magnification, scale bar = 100 μm); cells retained normal phenotypic appearance longer on edsIPN but grew more slowly. Cell density plotted against days in culture for ScF and ScC on either edsIPN or TCPS over 11 days (B); both cell types proliferated slower on edsIPN than on TCPS. Bar chart shows percent increase of cells over initial attached number of cells (C); lower growth rate recorded for ScC on edsIPN compared to all other groups.
In addition to the above morphological differences observed with the two different culture surfaces, the scleral cells also exhibited related differences in their rates of proliferation. Scleral fibroblasts proliferated more rapidly over the 11-day period when cultured on TCPS compared with the edsIPN (Fig. 4B). On TCPS, the exponential growth phase for scleral fibroblasts began around day 3 and ended around day 7, after which the cells continued to proliferate at a slower pace, reaching confluence at 7.98 ± 0.48 × 104 cells/cm2 (filled circles, Fig. 4B), a 38.9-fold increase over seeding density. On the other hand, the exponential growth phase for scleral fibroblasts cultured on edsIPN was delayed until day 7, and lasted until around day 10, with a cell count at that time of 3.22 ± 0.39 × 104 cells/cm2 (open circles, Fig. 4B), a 15.1-fold increase over their initial seeding density. Similarly, scleral chondrocytes exhibited delayed growth profiles on the edsIPN. For these cells, the exponential growth phase started on day 7 when cultured on edsIPN, compared with day 3 for TCPS. On TCPS, scleral chondrocyte density reached confluence at 7.78 ± 0.64 × 104 cells/cm2, a 37.9-fold increase over seeding density, similar to the density achieved by scleral fibroblasts on TCPS (filled squares, Fig. 4B). On the edsIPN, scleral chondrocytes grew exponentially from days 7 to 11, when the cell density reached 2.42 ± 0.37 × 104 cells/cm2 (open squares, Fig. 4B), an 11.1-fold increase over seeding density. In addition to the delayed growth responses seen with edsIPN, both cell types showed significant early losses over the first day in culture. On the edsIPN, fibroblast density decreased by 67.5% from their seeding density to 650 cells/cm2, with comparable figures for chondrocytes of 46.5% and 1070 cells/cm2. Early losses were much smaller for the TCPS, 17.5% to 1650 cells/cm2 of the seeded density for scleral fibroblasts, and 20.0% to 1600 cells/cm2 for scleral chondrocytes.
Cell density data were further analyzed with Verhulst's logistic growth model, which assumes exponential growth with contact inhibition, and contains the following parameters: effective growth rate (r), time in days (t), maximal substrate carrying capacity (K), and initial cell number (N0).34,43
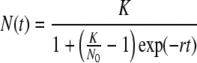 |
(1) |
For each culture surface and cell type, both the initial cell number and maximum carrying capacity were determined experimentally, by counting cells 2 h after seeding as well as after reaching confluence or near confluence, respectively. For scleral chondrocytes and fibroblasts cultured on edsIPN, effective growth rates (r) were 0.19 and 0.27 per day, corresponding to cell population doubling times (1/r) of 5.3 and 3.7 days, respectively. The effective growth rates of scleral chondrocytes and fibroblasts cultured on TCPS were both higher than those recorded for edsIPN, that is, 0.51 and 0.60 per day, corresponding to cell population doubling times of 2.0 and 1.7 days, respectively. The calculated maximum carrying capacity was 8.0 × 104 cells/cm2 for TCPS; approximately double the capacity of the edsIPN of 3.9 × 104 cells/cm2. To normalize the effect of initial cell adhesion on cell proliferation, the percent cell number increase was calculated using the following equation:
 |
(2) |
where Nf is the maximum cell density at day 11 and Ni the initial attached cell density after day 1. The ScF edsIPN, ScF TCPS, and ScC TCPS groups all recorded similar percent increases, that is, 4853.8%, 4736.4%, and 4762.5%, respectively. However, ScC edsIPN group increased just 2272.5%, slightly less than half of the values recorded by the other groups (Fig. 4C).
Effect of injected edsIPN on in vivo axial ocular dimensions and refractions
Sample ultrasonography traces obtained from an eye 28 days after the edsIPN injection is shown in Figure 5A. The injected edsIPN had no negative effects on either ocular growth or refractions. Only parameters likely to be affected by the treatment are described here. Comparison of the normalized axial length data for edsIPN-treated eyes and their fellows revealed no significant difference between them (Fig. 5C). Over the 28-day monitoring period, the axial lengths of edsIPN-injected eyes grew by 1.450 to 12.464 ±0.052 mm, compared with 1.444 to 12.458 ± 0.051 mm for fellow eyes. Scleral cup depths, which served as an index of scleral surface area, also did not show any significant differences between treatment and fellow eyes (Fig. 5C). Over the 28-day monitoring period, the scleral cup depth of edsIPN-injected eyes grew by 0.705 to 7.403 ± 0.042 mm, compared with 0.703 to 7.401 ± 0.038 mm for fellow eyes. Normalized scleral thickness increased for edsIPN-injected eyes from 124 ± 3 μm at baseline to 142 ± 6 μm by day 28, compared with 148 ± 4 μm for fellow eyes (Fig. 5B). Additional peaks were observed in some of the ultrasonography traces behind the peak corresponding to the external sclera, perhaps originating from the injected edsIPN and/or added fibrous tissue, but such peaks were not consistently observed and thus were not analyzed in this study. Endpoint refractions (measured in diopters, D) and number of eyes measured at 28 days are summarized in Table 1. The refractions of the edsIPN-injected and fellow control eyes were near zero (emmetropic) and not statistically different from each other.
FIG. 5.
Sample trace recorded using high-frequency A-scan ultrasonography (A), showing ocular components of interest: (1) anterior chamber, (2) lens, (3) vitreous chamber, (4) retina, (5) choroid, and (6) sclera. Scleral thickness (B) as well as scleral cup depth and axial length (C) plotted against time for edsIPN-injected or fellow chick eyes, which show similar growth patterns, confirming the biocompatibility of the edsIPN.
Table 1.
Mean Endpoint Refraction ± Standard Error (in Diopters, D) of Chick Eyes at 28 Days Post-Injection
| Eye | Refraction (D) | Number of eyes |
|---|---|---|
| Control (fellow) | 0.3 ± 1.1 | 6 |
| edsIPN* injected | 0.2 ± 1.9 | 6 |
edsIPN, enzymatically degradable semi-interpenetrating polymer network.
Scleral histology
With careful enucleation and subsequent removal of adhering fatty tissues from enucleated eyes, it proved possible to view the injected edsIPNs at their expected location, attached to the sclera at the posterior pole of eyes (Fig. 1B). However, their location was more readily viewed in the frozen sections, which were prepared from edsIPN-treated and untreated fellow eyes, collected at weekly intervals over the 4-week treatment period (Fig. 6). One week after its injection, the edsIPN was seen positioned between the posterior outer scleral surface and the inner surface of extraocular muscle (week 1, Fig. 6), bringing the edsIPN in close proximity to the blood vessels in the latter case (red arrow, week 1, Fig. 6). The numerous small clear vesicles within the edsIPN-injected space were most likely fractured gels due to injection and/or histological processing. Over the remainder of the treatment period, progressively more collagen was deposited and cell infiltration increased (weeks 2, 3, and 4, Fig. 6). By week 4, the edsIPN was largely replaced by dense fibrous connective tissue (red box, week 4, Fig. 6), and there was a clear indication of cell migration from the fibrous sclera into the edsIPN matrix (Fig. 7A–C), demonstrating the integration of the edsIPN with the host fibrous layer. The thickness of the scleral integration layer adjacent to the host fibrous layer ranged approximately between 20 and 180 μm at 4 weeks. The cells observed in the edsIPN matrix are likely to be fibroblasts originating from the host sclera, monocytes originating from blood vessels in extraocular muscles, or both, although no specific markers were used to identify the cells in this study. In addition, at 4 weeks, there was evidence of deposition of collagen-like fibers within the edsIPN matrix (Fig. 7D, E); the orientation of these fibers was more disorderly compared with those of the native fibrous sclera. None of the chick eyes showed any evidence of inflammation, that is, redness or swelling, over the course of the treatment period.
FIG. 6.
Representative hematoxylin and eosin–stained histological sections of scleras from eyes treated with edsIPN compared with the fellow untreated eyes, collected at weekly intervals from 1 to 4 weeks (10 × magnification, scale bar = 100 μm). Red arrow points to blood vessel in 1-week edsIPN-injected sample. Red box indicates region where substantial fibrous tissue integration with edsIPN has occurred in 4-week edsIPN-injected sample. CS, cartilage sclera; FS, fibrous sclera; EM, extraocular muscle. Color images available online at www.liebertonline.com/ten.
FIG. 7.
Histology section of posterior pole region of sclera from eye injected with edsIPN, sampled at 4 weeks, stained with hematoxylin and eosin (A–C) or Masson's trichrome (D, E), and photographed at increasing magnification. EdsIPN partly integrated with fibrous sclera (A, 10× magnification, scale bar = 100 μm); red arrows in (B) points to nests of cells within the edsIPN matrix (40× magnification, scale bar = 50 μm); red arrows in (C) indicate presumed fibroblasts in fibrous sclera and fibrotic edsIPN matrix (100 × magnification, scale bar = 20 μm); light-blue stained collagen fibers within edsIPN (E, 63 × magnification, scale bar = 50 μm); section from posterior sclera from fellow untreated eye shown at same magnification for comparison (D).
Discussion
This study aimed to establish the in vitro cytocompatibility of a biomimetic edsIPN with ocular scleral cells and to investigate its ocular effects in vivo when applied to the outer scleral surface of the posterior pole. Although edsIPN of a specific composition and modulus was used in this study, as a class of materials, edsIPNs are mechanically and biologically tunable and degrade when exposed to MMPs.25,26,30 The edsIPN preserved the phenotypes of the scleral cells in vitro, and the injected edsIPN increased the thickness of the outer wall of the eye by promoting cellular infiltration and subsequent deposition of collagen into the edsIPN. In addition, normal eye growth rate was not affected by the injected edsIPN. These findings suggest that a novel approach to the treatment of high myopia using injectable biomimetic hydrogels, for example, edsIPNs, is possible.
For our application, we required the edsIPN to be sufficiently fluid as to be injectible but to stiffen in the body, to facilitate its retention at the injection site, where its primary role was to support attachment, migration, and proliferation of scleral cells. In vitro measurements of the edsIPN and experience from in vivo studies confirmed the suitability of the tested hydrogel. Its phase transition was compatible with easy passage through the 19-gauge needle used for retrobulbar injections, and the stiffening of the edsIPN on exposure to body temperature stabilized the edsIPN at the posterior sclera. In vitro measurements indicated an approximately 4.5-fold increase in the complex shear modulus with an increase from room to body temperature. Its modulus never reached the native stiffness of the sclera. The shear modulus for chick sclera is around 5.2 MPa (approximated by 15.38 MPa elastic modulus and 0.48 as the Poisson ratio)8,44 and for human sclera is on the order of 0.6–1 MPa (approximated by 1.8–2.9 MPa elastic modulus and 0.48 as the Poisson ratio).44,45 Thus, this edsIPN would not have provided significant mechanical support to the sclera. The low substrate stiffness of our edsIPN likely also explains why treated eyes showed normal, rather than slowed, rates of scleral cup and overall axial elongation.
The influence of ECM mechanical properties on cell growth is well established, with relevant observations reported for several cell types including fibroblasts and chondrocytes.46–48 Specifically, soft substrates tend to inhibit cell spreading and slow migration compared to their stiffer counterparts49,50; they also induce differentiation of mesenchymal stem cells to specific phenotypes.51 In this study, a mechanical influence could explain both the delayed proliferation and slower overall rates of proliferation for cells cultured on edsIPN compared with TCPS. After initial attachment, both scleral cell types began proliferating on TCPS on day 3, while their proliferation was delayed on edsIPN to day 7. From our calculated percent increase in cell numbers, it is clear that proliferation was slowest for scleral chondrocytes cultured on edsIPN. Specifically, scleral chondrocytes cultured on edsIPN did not proliferate as much as the other groups, independent of their low initial cell attachment. It is likely that their lack of initial attachment was primarily due to the low complex modulus of the edsIPN, given that scleral chondrocytes naturally reside within a more rigid environment. Further, it was shown previously that adult human dermal fibroblasts did not proliferate much, if at all, over 3 days when cultured on gels of similar stiffness (95 Pa in that study compared with 63 Pa for our edsIPN at body temperature), but did proliferate significantly when cultured on higher stiffness gels.52 Thus, our results for chick scleral fibroblasts and chondrocytes were consistent with the published findings. In follow-up studies we will optimize the mechanical properties of edsIPN for our intended therapeutic application for human myopia, employing a mammalian in vitro scleral cell culture model, which does not have a chondrocyte population.
The inclusion of MMP degradable peptide crosslinkers allows controlled cellular migration for in-growth into the synthetic ECM.26 Without the MMP degradable peptide crosslinkers, the synthetic ECM would not be degradable and would loose the control mechanism for cellular infiltration based on cell-mediated degradation. The testing of a material containing biodegradable peptide crosslinkers is in line with our long-term goal to develop a hydrogel for use in myopia control, because MMP-2 and MMP-13 are known to be upregulated during myopia progression.31–33 Support for cell attachment was provided in the design of our edsIPN by grafting of the bsp-RGD(15) peptide sequences onto the linear interpenetrating p(AAc) chains.34 The 300 μM bsp-RGD(15) concentration for the edsIPN was chosen to provide adequate support for scleral cell adhesion, and this was confirmed in our cell culture study. The statistical bulk distribution of the grafted bsp-RGD(15) was expected to aid the in vivo performance of the material by allowing cells to continually attach, proliferate, and infiltrate the synthetic ECM throughout the remodeling process. However, we observed significantly more early cell loss for scleral cells cultured on the edsIPN compared with the TCPS. It is possible that our choice of the peptide ligand was suboptimal or the modulus was too low for in vitro scleral cell culture. It is also known that ligand presentation and density can affect the phenotypic expression of differentiated cells.53,54 Thus, the effect of substituting alternative peptide ligands and varying ligand density on initial attachment and growth rates of cells cultured on the edsIPN will be explored in the follow-up studies.
Having established the in vitro cytocompatibility of the edsIPN for chick scleral cells, we undertook in vivo biocompatibility testing of the same edsIPN. Based on the ultrasonography data, ocular axial dimensions, including scleral cup depth and axial length, were unaffected by the presence of the edsIPN on its outer surface, suggesting good biocompatibility with scleral tissue. Note that for both treated and fellow eyes, increase in scleral cup depth over the monitoring period was slower than that of the axial length increase, reflecting the contribution to axial lengths of the anterior chamber segments, which grew faster than the posterior segment (Fig. 5C). That treated and fellow eyes showed no difference in endpoint refractions is consistent with the lack of treatment effects on the axial dimensions of the anterior chamber and lens, and rules out even subtle effects on the curvature of the cornea and lens, confirming the safety of the edsIPN injections.
Over the 4-week experimental period, there was a progressive cellular invasion of the edsIPN with subsequent collagen deposition, as evidenced in histology sections, which revealed collagen-like fibers highlighted with Masson's trichrome stain. Remnants of nondegraded edsIPN were likely present at the 4-week time point because there were small clear regions within the newly formed fibrous tissue at the posterior pole. This interpretation is also consistent with the relatively slow degradation rate for the degradable peptide crosslinker used in our study, quantified in a separate in vitro study.55 Unlike native, untreated fibrous sclera, the collagen fibers within the edsIPN did not appear to have any organized fiber orientation. Although the cellular infiltration of the implants resulted in an apparent thickening of the outer fibrous layer of the sclera, this increase in thickness was not detected by high-frequency ultrasonography, suggesting either that this scleral integration layer had altered impedance compared with the natural sclera or that the combined attenuating effect of all the preceding intervening ocular surfaces rendered our device insufficiently sensitive to detect the boundaries of this most posterior layer. Although these changes contributed an extrafibrous layer to the posterior sclera, they did not retard the rate of elongation of treated eyes compared with the untreated fellow eyes. This lack of effect on ocular elongation is similar to that reported by our group for previously tested HEMA and PVP implants in chick22 and point to complex interactions between scleral tissue growth and intraocular pressure as determinants of eye enlargement. It is likely that the same edsIPN-induced changes will have different effects on the elongation of mammalian eyes, which lack a rigid inner cartilaginous layer, and also on myopic eyes whose scleras show increased remodeling and subsequent thinning.7
In conclusion, exploiting the unique properties of the edsIPN has enabled us to design and synthesize a biomimetic ECM that supports the growth of chick scleral fibroblast and chondrocyte in vitro, that is compatible with its delivery by retrobulbar injection, and that is able to support fibrous connective tissue in growth in vivo. Future work will emphasize design strategies for scleral strengthening in a mammalian model for myopia through the application of edsIPNs, used on their own or as a vehicle for controlled delivery of myopia retarding agents.
Acknowledgments
The authors would like to acknowledge the funding support from William C. Ezell Fellowship from the American Optometric Foundation, and National Institutes of Health grants from National Eye Institute (NEI) (R01-EY012392, R21-EY019628), and National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (R01-AR47304).
Disclosure Statement
No competing financial interests exist.
References
- 1.Saw S.-M. Gazzard G. Shih-Yen E.C. Chua W.-H. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Hsu W.-M. Cheng C.-Y. Liu J.-H. Tsai S.-Y. Chou P. Prevalence and causes of visual impairment in an elderly chinese population in taiwan: the shihpai eye study. Ophthalmology. 2004;111:62. doi: 10.1016/j.ophtha.2003.05.011. [DOI] [PubMed] [Google Scholar]
- 3.McBrien N.A. Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22:307. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 4.Ihanamaki T. Pelliniemi L.J. Vuorio E. Collagens and collagen-related matrix components in the human and mouse eye. Prog Retin Eye Res. 2004;23:403. doi: 10.1016/j.preteyeres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Curtin B.J. Iwamoto T. Renaldo D.P. Normal and staphylomatous sclera of high myopia: an electron microscopic study. Arch Ophthalmol. 1979;97:912. doi: 10.1001/archopht.1979.01020010470017. [DOI] [PubMed] [Google Scholar]
- 6.McBrien N.A. Cornell L.M. Gentle A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Invest Ophthalmol Vis Sci. 2001;42:2179. [PubMed] [Google Scholar]
- 7.Norton T.T. Rada J.A. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 8.Phillips J.R. Khalaj M. McBrien N.A. Induced myopia associated with increased scleral creep in chick and tree shrew eyes. Invest Ophthalmol Vis Sci. 2000;41:2028. [PubMed] [Google Scholar]
- 9.Avetisov E.S. Savitskaya N.F. Vinetskaya M.I. Iomdina E.N. A study of biochemical and biomechanical qualities of normal and myopic eye sclera in humans of different age groups. Metab Pediatr Syst Ophthalmol. 1983;7:183. [PubMed] [Google Scholar]
- 10.Marzani D. Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci. 1997;38:1726. [PubMed] [Google Scholar]
- 11.Rada J.A. Nickla D.L. Troilo D. Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest Ophthalmol Vis Sci. 2000;41:2050. [PubMed] [Google Scholar]
- 12.Nickla D. Rada J. Wallman J. Isolated chick sclera shows a circadian rhythm in proteoglycan synthesis perhaps associated with the rhythm in ocular elongation. J Comp Physiol A. 1999;185:81. doi: 10.1007/s003590050368. [DOI] [PubMed] [Google Scholar]
- 13.Rada J.A. Matthews A.L. Brenza H. Regional proteoglycan synthesis in the sclera of experimentally myopic chicks. Exp Eye Res. 1994;59:747. doi: 10.1006/exer.1994.1161. [DOI] [PubMed] [Google Scholar]
- 14.Friedman B. Stress upon the ocular coats: effects of scleral curvature, scleral thickness, and intraocular pressure. Eye Ear Nose Throat Mon. 1966;45:59. [PubMed] [Google Scholar]
- 15.Wildsoet C. Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995;35:1175. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 16.Phillips J.R. McBrien N.A. Pressure-induced changes in axial eye length of chick and tree shrew: significance of myofibroblasts in the sclera. Invest Ophthalmol Vis Sci. 2004;45:758. doi: 10.1167/iovs.03-0732. [DOI] [PubMed] [Google Scholar]
- 17.Wollensak G. Spoerl E. Collagen crosslinking of human and porcine sclera. J Cataract Refract Surg. 2004;30:689. doi: 10.1016/j.jcrs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Trier K. Olsen E.B. Kobayashi T. Ribel-Madsen S.M. Biochemical and ultrastructural changes in rabbit sclera after treatment with 7-methylxanthine, theobromine, acetazolamide, or l-ornithine. Br J Ophthalmol. 1999;83:1370. doi: 10.1136/bjo.83.12.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerinec A. Slezakova G. Posterior scleroplasty in children with severe myopia. Bratisl Lek Listy. 2001;102:73. [PubMed] [Google Scholar]
- 20.Avetisov E.S. Tarutta E.P. Iomdina E.N. Vinetskaya M.I. Andreyeva L.D. Nonsurgical and surgical methods of sclera reinforcement in progressive myopia. Acta Ophthalmol Scand. 1997;75:618. doi: 10.1111/j.1600-0420.1997.tb00617.x. [DOI] [PubMed] [Google Scholar]
- 21.Tarutta E.P. Andreeva L.D. Markosian G.A. Iomdina E.N. Lazuk A.V. Kruzhkova G.V. Reinforcement of the sclera with new types of synthetic materials in progressive myopia. Vestn Oftalmol. 1999;115:8. [PubMed] [Google Scholar]
- 22.Su J. Iomdina E. Tarutta E. Ward B. Song J. Wildsoet C.F. Effects of poly(2-hydroxyethyl methacrylate) and poly(vinyl-pyrrolidone) hydrogel implants on myopic and normal chick sclera. Exp Eye Res. 2009;88:445. doi: 10.1016/j.exer.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ward B. Tarutta E.P. Mayer M.J. The efficacy and safety of posterior pole buckles in the control of progressive high myopia. Eye. 2009 doi: 10.1038/eye.2008.433. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Stile R.A. Burghardt W.R. Healy K.E. Synthesis and characterization of injectable poly(n-isopropylacrylamide)-based hydrogels that support tissue formation in vitro. Macromolecules. 1999;32:7370. [Google Scholar]
- 25.Chung E.H. Gilbert M. Virdi A.S. Sena K. Sumner D.R. Healy K.E. Biomimetic artificial ECMs stimulate bone regeneration. J Biomed Mater Res A. 2006;79A:815. doi: 10.1002/jbm.a.30809. [DOI] [PubMed] [Google Scholar]
- 26.Kim S. Chung E.H. Gilbert M. Healy K.E. Synthetic MMP-13 degradable ECMs based on poly(n-isopropylacrylamide-co-acrylic acid) semi-interpenetrating polymer networks. I. Degradation and cell migration. J Biomed Mater Res A. 2005;75A:73. doi: 10.1002/jbm.a.30375. [DOI] [PubMed] [Google Scholar]
- 27.Li Y.J. Chung E.H. Rodriguez R.T. Firpo M.T. Healy K.E. Hydrogels as artificial matrices for human embryonic stem cell self-renewal. J Biomed Mater Res A. 2006;79A:1. doi: 10.1002/jbm.a.30732. [DOI] [PubMed] [Google Scholar]
- 28.Kutty J.K. Cho E. Soo Lee J. Vyavahare N.R. Webb K. The effect of hyaluronic acid incorporation on fibroblast spreading and proliferation within PEG-diacrylate based semi-interpenetrating networks. Biomaterials. 2007;28:4928. doi: 10.1016/j.biomaterials.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Wall S. Department of Bioengineering, University of California–Berkeley and the University of California–San Francisco; Berkeley, CA: 2008. Bioactive polymers for cardiac tissue engineering [Ph.D. Dissertation] [Google Scholar]
- 30.Kim S. Healy K.E. Synthesis and characterization of injectable poly(n-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable cross-links. Biomacromolecules. 2003;4:1214. doi: 10.1021/bm0340467. [DOI] [PubMed] [Google Scholar]
- 31.Siegwart J.T., Jr. Norton T.T. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46:3484. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schippert R. Brand C. Schaeffel F. Feldkaemper M.P. Changes in scleral MMP-2, TIMP-2 and tgfβ-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp Eye Res. 2006;82:710. doi: 10.1016/j.exer.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Su J. Zhan Z. Wildsoet C.F. Myopic and hyperopic defocus effects on chick scleral MMP-2, MMP-13, TGFb-2, and TIMP-2 mRNA levels. Invest Ophthalmol Vis Sci; Abstract presented at ARVO; Ft. Lauderdale, FL. 2008. E-abstract 1740. [Google Scholar]
- 34.Harbers G.M. Healy K.E. The effect of ligand type and density on osteoblast adhesion, proliferation, and matrix mineralization. J Biomed Mater Res A. 2005;75A:855. doi: 10.1002/jbm.a.30482. [DOI] [PubMed] [Google Scholar]
- 35.Rezania A. Healy K.E. Integrin subunits responsible for adhesion of human osteoblast-like cells to biomimetic peptide surfaces. J Orthop Res. 1999;17:615. doi: 10.1002/jor.1100170423. [DOI] [PubMed] [Google Scholar]
- 36.Bellahcene A. Bonjean K. Fohr B. Fedarko N.S. Robey F.A. Young M.F. Fisher L.W. Castronovo V. Bone sialoprotein mediates human endothelial cell attachment and migration and promotes angiogenesis. Circ Res. 2000;86:885. doi: 10.1161/01.res.86.8.885. [DOI] [PubMed] [Google Scholar]
- 37.Somerman M.J. Fisher L.W. Foster R.A. Sauk J.J. Human bone sialoprotein I and II enhance fibroblast attachment in vitro. Calcif Tissue Int. 1988;43:50. doi: 10.1007/BF02555169. [DOI] [PubMed] [Google Scholar]
- 38.Metlapally R. Jobling A.I. Gentle A. McBrien N.A. Characterization of the integrin receptor subunit profile in the mammalian sclera. Mol Vis. 2006;6:725. [PubMed] [Google Scholar]
- 39.Huebsch N. Gilbert M. Healy K.E. Analysis of sterilization protocols for peptide-modified hydrogels. J Biomed Mater Res Part B Appl Biomater. 2005;74B:440. doi: 10.1002/jbm.b.30155. [DOI] [PubMed] [Google Scholar]
- 40.Barber T.A. Harbers G.M. Park S. Gilbert M. Healy K.E. Ligand density characterization of peptide-modified biomaterials. Biomaterials. 2005;26:6897. doi: 10.1016/j.biomaterials.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 41.Stile R.A. Chung E. Burghardt W.R. Healy K.E. Poly(n-isopropylacrylamide)-based semi-interpenetrating polymer networks for tissue engineering applications. Effects of linear poly(acrylic acid) chains on rheology. J Biomater Sci Polym Ed. 2004;15:865. doi: 10.1163/1568562041271129. [DOI] [PubMed] [Google Scholar]
- 42.Nickla D.L. Wildsoet C. Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998;66:163. doi: 10.1006/exer.1997.0420. [DOI] [PubMed] [Google Scholar]
- 43.Thieme H. Mathematics in Population Biology. Princeton, NJ: Princeton University Press; 2003. Classic Models of Density-Dependent Population Growth for Single Species. [Google Scholar]
- 44.Battaglioli J.L. Kamm R.D. Measurements of the compressive properties of scleral tissue. Invest Ophthalmol Vis Sci. 1984;25:59. [PubMed] [Google Scholar]
- 45.Friberg T.R. Lace J.W. A comparison of the elastic properties of human choroid and sclera. Exp Eye Res. 1988;47:429. doi: 10.1016/0014-4835(88)90053-x. [DOI] [PubMed] [Google Scholar]
- 46.Eckes B. Zigrino P. Kessler D. Holtkotter O. Shephard P. Mauch C. Krieg T. Fibroblast-matrix interactions in wound healing and fibrosis. Matrix Biol. 2000;19:325. doi: 10.1016/s0945-053x(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 47.Nakagawa S. Pawelek P. Grinnell F. Extracellular matrix organization modulates fibroblast growth and growth factor responsiveness. Exp Cell Res. 1989;182:572. doi: 10.1016/0014-4827(89)90260-7. [DOI] [PubMed] [Google Scholar]
- 48.Shieh A.C. Athanasiou K.A. Principles of cell mechanics for cartilage tissue engineering. Ann Biomed Eng. 2003;31:1. doi: 10.1114/1.1535415. [DOI] [PubMed] [Google Scholar]
- 49.Saha K. Keung A.J. Irwin E.F. Li Y. Little L. Schaffer D.V. Healy K.E. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Irwin E.F. Saha K. Rosenbluth M. Gamble L.J. Castner D.G. Healy K.E. Modulus-dependent macrophage adhesion and behavior. J Biomater Sci Polym Ed. 2008;19:1363. doi: 10.1163/156856208786052407. [DOI] [PubMed] [Google Scholar]
- 51.Engler A.J. Sen S. Sweeney H.L. Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 52.Ghosh K. Pan Z. Guan E. Ge S. Liu Y. Nakamura T. Ren X.-D. Rafailovich M. Clark R.A.F. Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials. 2007;28:671. doi: 10.1016/j.biomaterials.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rezania A. Healy K.E. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J Biomed Mater Res. 2000;52:595. doi: 10.1002/1097-4636(20001215)52:4<595::aid-jbm3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 54.Hsiong S.X. Huebsch N. Fischbach C. Kong H.J. Mooney D.J. Integrin-adhesion ligand bond formation of preosteoblasts and stem cells in three-dimensional RGD presenting matrices. Biomacromolecules. 2008;9:1843. doi: 10.1021/bm8000606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wall S.T. Yeh C.-C. Tu R.Y.K. Mann M.J. Healy K.E. Biomimetic matrices for myocardial stabilization and stem cell transplantation. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32904. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]