Abstract
It was hypothesized that previously optimized serum-free culture conditions for juvenile bovine chondrocytes could be adapted to generate engineered cartilage with physiologic mechanical properties in a preclinical, adult canine model. Primary or passaged (using growth factors) adult chondrocytes from three adult dogs were encapsulated in agarose, and cultured in serum-free media with transforming growth factor-β3. After 28 days in culture, engineered cartilage formed by primary chondrocytes exhibited only small increases in glycosaminoglycan content. However, all passaged chondrocytes on day 28 elaborated a cartilage matrix with compressive properties and glycosaminoglycan content in the range of native adult canine cartilage values. A preliminary biocompatibility study utilizing chondral and osteochondral constructs showed no gross or histological signs of rejection, with all implanted constructs showing excellent integration with surrounding cartilage and subchondral bone. This study demonstrates that adult canine chondrocytes can form a mechanically functional, biocompatible engineered cartilage tissue under optimized culture conditions. The encouraging findings of this work highlight the potential for tissue engineering strategies using adult chondrocytes in the clinical treatment of cartilage defects.
Introduction
Osteoarthritis is a common degenerative condition of joints that affects millions of people with associated annual financial costs of more than $100 billion dollars worldwide.1 Loss of functional articular cartilage is considered the hallmark of osteoarthritis and is accompanied by inflammation, degradation, and dysfunction, affecting all tissues that comprise the joint organ. Due to the poor regenerative capacity of adult articular cartilage and the eventual joint pain/disability,2 osteoarthritic patients frequently opt for joint replacement (arthroplasty) surgery, with more than 680,000 arthroplasties performed each year in the United States (Medical Data International. Report # RT-781322, Aug 1998). Although joint replacement provides an operative treatment that generally succeeds in decreasing or eliminating pain and improving joint function, the lifespan of prostheses is limited due to wear, loosening, infection, and fracture of the implant or surrounding bone.3 Total joint replacement is also inappropriate for the treatment of smaller cartilage lesions that may afflict younger patients with active lifestyles. Based on these limitations, there has been increasing interest in developing biologic treatments for articular cartilage repair. Current biologic treatments for joint resurfacing and lesion repair include the transplantation of osteochondral grafts (either autograft4 or allograft5) and the expansion and implantation of chondrocytes by various methods.6 However, these techniques are not free from complications7 and are limited by defect size, geometry, and extent; patient age; tissue availability; donor-site morbidity; and concerns regarding disease transmission (for allografts).8–12
Functional tissue engineering aims to integrate physiologic, mechanical, and biochemical stimuli to create replacement tissues in vitro for the eventual use as an in vivo biologic treatment for ailments in patients.13 The potential for articular cartilage tissue engineering covers all stated limitations, including those of total joint arthroplasty and other biologic treatments. For treating cartilage lesions, the creation of an engineered tissue with sufficient extracellular matrix (ECM) composition, geometry, and material properties before in vivo implantation may be necessary for the construct to maintain its integrity under the rigorous biologic and biomechanical demands of functional weight-bearing joints.14–16 The ECM may shield the implanted cells (especially if from an allogeneic source) and/or scaffold material from host immunogenic, inflammatory, and degradative responses.17 Over the past several years, our laboratory has adopted and refined a chondrocyte-seeded agarose hydrogel system for articular cartilage tissue engineering.18 In this model system, juvenile bovine chondrocytes are isolated and uniformly seeded in an agarose hydrogel scaffold. Agarose was chosen for its characteristics in maintaining chondrocyte phenotype19,20 and transmitting mechanical forces to the embedded cells.18,21 Immature, differentiated chondrocytes were chosen due to their responsiveness to mechanical forces.18,22 Also, as these cells are already differentiated (as opposed to stem cells), research efforts can focus immediately on promoting their biosynthetic activities to produce functional tissue. Recent developments in our laboratory using the application of growth factors and deformational loading in this model system have led to the creation of engineered cartilage with physiologic Young's modulus and glycosaminoglycan (GAG) content.23,24
On the basis of these promising results using juvenile bovine chondrocytes, our laboratories have begun exploring the translation of these results into a clinically relevant, large animal model with the goal of successful in vivo implantation of functional tissue-engineered constructs for cartilage resurfacing. The adult canine animal model was selected based on the availability of vast amounts of historical data; its clinical relevance with respect to anatomy, function, and clinical disorders in veterinary patients; and the ability to readily perform surgical treatments such as arthroplasty, autologous chondrocyte transplantation, and osteochondral grafting, as well as postoperative management protocols, including bandaging, braces, and physical rehabilitation modalities.25–29 These aspects of the canine model are important for future validation and comparison of tissue engineering to the accepted clinical treatments. In addition, whether from allograft or from autograft sources, the cells that will likely be used for human treatment will probably be derived from older adults. Therefore, success in a mature, canine chondrocyte animal model increases the evidence, supporting further studies to develop these techniques for the treatment of humans.
To achieve the successful translation from the juvenile bovine model to the mature canine model, we undertook two studies. In study 1, it was hypothesized that passaged adult canine chondrocytes from multiple donors could reproducibly create an engineered cartilage with functional mechanical properties. This hypothesis was based on the explosive growth seen in the bovine model system23 as well as preliminary experiments with adult canine chondrocytes.30 Then, in study 2, preliminary biocompatibility/rejection of engineered chondral and osteochondral constructs31 was assessed in an in vivo canine femoral-trochlear groove defect model over a 3-month period. Passaged allogeneic canine chondrocytes were chosen for these studies because we believe that they are a clinically relevant cell source for engineered cartilage tissues, as they avoid donor-site morbidity and the need for two surgeries with the associated time, costs, and recovery periods. In addition, by utilizing an allogeneic cell source, implant size and architecture are not limited by available cell numbers derived from the patient.
Materials and Methods
Overview of experimental design
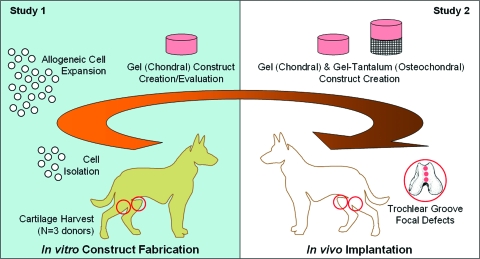
There are two studies presented here (schematically outlined in Fig. 1). In study 1, canine chondrocytes were isolated from three adult mongrel dogs, expanded, encapsulated in agarose to form three-dimensional (3D) constructs, and grown in well-defined serum-free media supplemented with transforming growth factor (TGF)-β3. The expansion and culture protocol was determined from previous experiments.30 Further, to test for variability due to cell source, 3D constructs were formed using cells isolated either from a single donor dog or from multiple dogs. In study 2, mechanically competent canine engineered cartilage constructs (chondral and osteochondral) were cultured for 42 days and were then implanted into the femoral trochlear groove to assess in vivo biocompatibility and performance over a 3-month period.
FIG. 1.
Schematic of experimental design. In study 1, adult chondrocytes were harvested from canines, expanded, and then encapsulated in a three-dimensional agarose gel scaffold, with tissue properties monitored over a 28-day culture period. In study 2, gel-alone (chondral) and gel-tantalum (osteochondral) constructs were created from passaged chondrocytes, cultured until reaching native Young's moduli, and then implanted in to the knee joints of adult dogs to study biocompatibility and immune response to the implanted constructs. Color images available online at www.liebertonline.com/ten.
Isolation of canine chondrocytes
Cartilage was harvested from the patellas, femoral trochlear grooves, femoral condyles, and humeral heads of 2–5-year-old mongrel canine cadavers (n = 3, male, 50–60 lbs (22.7–27.2 kg); Central Missouri Humane Society, Columbia, MO) after euthanasia was performed for reasons unrelated to this study. Cartilage flakes were then digested for 8 h using combined pronase (0.0005 g/mL) and collagenase (390 U/mL, type V; Sigma Aldrich, St. Louis, MO) in 7.5 mL/g tissue of high-glucose Dulbecco's Modified Eagle's Medium (DMEM) (Invitrogen, Carlsbad, CA) with sodium bicarbonate buffer and 5% fetal bovine serum (FBS; Atlanta Biologics, Atlanta, GA). Isolated cells from each donor were filtered, resuspended, and expanded in monolayer culture using a growth factor cocktail (see below). Cells from each donor were either kept separate to observe donor-to-donor variability or mixed together (similar to previous bovine chondrocyte protocols18).
Expansion of canine chondrocytes
For expansion, canine chondrocytes were plated in T-150 culture flasks at an initial density of 4 × 106 cells/flask in 50 mL of DMEM with 5% FBS and 1 ng/mL TGF-β1, 5 ng/mL fibroblast growth factor-2, and 10 ng/mL platelet-derived growth factor-ββ,32 with media changed every 2 days. When cells were ∼90% confluent, the passage 1 (P1) chondrocytes were released using 100 U/mL collagenase and 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid (Invitrogen), washed, and resuspended in chondrogenic media, composed of high-glucose DMEM, 1% ITS + (BD, Franklin Lakes, NJ), 0.1 μM dexamethasone (Sigma Aldrich), 110 μg/mL sodium pyruvate (Invitrogen), 50 μg/mL L-proline (Sigma Aldrich), sodium bicarbonate, and antibiotics.23
Creation and culture of engineered cartilage constructs
Primary or passage 1 (P1) canine chondrocytes were resuspended at a concentration of 60 × 106 cells/mL and mixed with equal parts molten 4% agarose (type VII; Sigma Aldrich) at 45°C to form a 2% agarose suspension with 30 × 106 cells/mL. Chondral constructs were created by pouring this suspension between two glass plates, cooling at room temperature for 20 min, and then coring out disks (Ø4.0 × 2.3 mm) using a sterile biopsy punch. Osteochondral constructs (Ø4.0 × 6 mm, with ∼2 mm chondral region, for study 2 only) were created by pouring the gel suspension using custom molds over porous trabecular metal cylinders (tantalum metal; Zimmer, Warsaw, IN). Tantalum trabecular metal was utilized for the bony substrate based on previous studies indicating a detrimental effect of devitalized bone.31 Constructs were cultured in chondrogenic media with 50 μg/mL ascorbate-2-phosphate (Sigma Aldrich) and 10 ng/mL of TGF-β3 (Invitrogen), with media changed every other day. Constructs were cultured for 2 days before the initiation of mechanical testing, designated as day 0.
Whole-construct mechanical testing
Mechanical testing was performed in unconfined compression between two impermeable platens in a custom material testing device as previously described.33 Constructs (n = 4–5 per group, per time point) were first equilibrated under a creep tare load of ∼0.02 N followed by a stress relaxation test with a ramp displacement of 0.05% strain/sec to 10% strain (based on the measured postcreep thickness). The compressive Young's modulus (EY) was determined from the equilibrium response of the stress relaxation test by dividing the equilibrium stress (minus the tare stress) by the applied static strain. Dynamic compressive modulus (G*) was determined by analyzing the stress–strain response of the tissue to an applied sinusoidal deformation of 1% strain at 1 Hz. Posttesting, all constructs were frozen and stored at −80°C for biochemical analysis.
Biochemical analysis
The samples were thawed, weighed wet, and digested for 16 h at 56°C with 0.5 mg/mL proteinase K (EMD Biosciences, San Diego, CA) in 50 mM Tris buffered saline containing 1 mM ethylenediaminetetraacetic acid, 1 mM iodoacetamide, and 10 μg/mL pepstatin A (Sigma Aldrich).34 These digests were used to determine sample GAG content via the 1,9 dimethylmethylene blue (Sigma Aldrich) dye-binding assay35 and for DNA content via the PicoGreen assay (Invitrogen). The overall collagen content was measured using orthohydroxyproline colorimetric assay.36 Collagen content was calculated by assuming a 1:10 orthohydroxyproline-to-collagen mass ratio.37 Assays were adapted for use in 96-well microtiter plates. GAG and collagen content was normalized to the construct wet weight (% ww).
Gene expression
RNA extraction
Total RNA was extracted using a modified TRIspin method.38 Construct samples were homogenized in 1 mL of Trizol (Invitrogen). Homogenates were extracted with chloroform and separated by centrifugation. The RNA was precipitated using isopropanol and the pellet was suspended in 100 μL of diethylpyrocarbonate (DEPC) water containing DNase. The DNA was digested at 37°C for 15 min. The RNA was further processed using the RNeasy Minelute cleanup kit (Qiagen, Valencia, CA) following the manufacture's protocol. Total RNA was eluted with 14 μL of water. Concentration and purity were determined by absorbance and stored at −80°C.
Reverse transcription
Total RNA (500 ng) was reverse converted to cDNA in 20 μL reactions using 500 ng of oligo(dT) primers and Superscript III reverse transcriptase (Invitrogen) according to the manufacturer's instructions. The reverse transcription profile was as follows: 50°C for 60 min, 70°C for 15 min, and 4°C hold. The cDNA was diluted with 180 μL of water, and 4 μL of the diluted cDNA was used for subsequent real-time polymerase chain reaction (PCR).
Polymerase chain reaction
Relative gene expression levels were determined by real-time PCR using species-specific primers for collagen type I, collagen type II, and aggrecan, with the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase to normalize the data (Table 1). Real-Time PCR was performed using the Roche LightCycler® 480 (Roche, Basal, Germany) using the Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) following the manufacturer's guidelines. The PCR profile for all tests consisted of an initial incubation of 94°C for 15 min, followed by 50 cycles of 5 s at 94°C, 5 s at 60°C, and 10 s at 72°C. After the PCR profile, a melt curve analysis was done to ensure specific amplification for each sample. SYBR green fluorescence was monitored during the extension step of the PCR profile, and take off values were determined using the LightCycler 480 software. Target gene expression was normalized to glyceraldehyde 3-phosphate dehydrogenase expression and determined using Q-gene.39 No-reverse transcription controls were tested for each primer set utilized to ensure that there was no contamination genomic DNA in the sample.
Table 1.
Real-Time Polymerase Chain Reaction Primers Utilized for Gene Expression Analysis
| Gene | Orientation | Primer sequence | Amplicon size (bp) |
|---|---|---|---|
| GAPDH | Forward | GTGACTTCAACAGTGACACC | 152 |
| Reverse | CCTTGGAGGCCATGTAGACC | ||
| COL 1 | Forward | TGCACGAGTCACACTGGAGC | 124 |
| Reverse | ATGCCGAATTCCTGGTCTGG | ||
| COL 2 | Forward | GGCCTGTCTGCTTCTTGTAA | 127 |
| Reverse | ATCAGGTCAGGTCAGCCATT | ||
| Aggrecan | Forward | ATCGAAGGGGACTTCCGCTG | 106 |
| Reverse | ATCACCACACAGTCCTCTCCG |
In vivo biocompatibility
Biocompatibility studies were performed with implantation of chondral and osteochondral (gel-tantalum) constructs in focal cartilage defects created in the canine stifle (knee) joint. The latter represents an engineered tissue more similar to clinically used autologous or allogeneic osteochondral grafts.4,5 All procedures were approved by the University of Missouri–Columbia Institutional Animal Care and Use Committee. Mixed chondrocyte, chondral, and osteochondral constructs were cultured until they reached native canine Young's modulus (EY) values (∼200 kPa, determined via unconfined compression in study 1). Adult (2–4 years of age) mongrel research dogs (50–60 lbs, i.e., 22.7–27.2 kg) with clinically and radiographically normal knee joints were premedicated, anesthetized, and prepared for aseptic surgery of one hind limb. After lateral parapatellar incision and arthrotomy of the knee, 4.0-mm-diameter full-thickness (completely through subchondral bone plate) defects were created in the center of the femoral trochlear groove using a 3.9-mm drill bit followed by a 4.0-mm biopsy punch. Chondral and osteochondral constructs were then press-fit into the defects (n = 3 constructs per animal, n = 4 animals per group) such that they were visibly and palpably flush with the articular surface of the trochlear groove. The unoperated contralateral knee joints served as normal controls. Empty defects (n = 3) were used as an in vivo negative control (n = 1 animal). Joint capsule, fascia, subcutaneous tissues, and skin were closed routinely. Postoperative radiographs of the operated limbs were obtained. Postoperative analgesics were provided for a minimum of 48 h postoperatively. Animals were allowed unrestricted kennel activity after an initial soft-casting period of 1 week. Six weeks after surgery, aseptic arthrocentesis was performed for subsequent cytologic assessment of the synovial fluid followed by arthroscopic evaluation of the treated knee joints. At 12 weeks after surgery, categorical lameness scoring, radiographic assessment, synovial fluid cytology, and arthroscopic assessment were performed before euthanasia of the dogs.40,41 All dogs survived surgery and for the intended study duration. No gross evidence of infection, rejection, or systemic inflammatory responses was noted.
Statistical Methods and Analysis
At least 4–5 samples in each group were analyzed for each data point, with data reported as the mean and standard deviation. This sample size was chosen based on a power analysis of preliminary results30 to determine the number of specimens required for a power greater than 0.8 (the generally accepted power for life sciences42) for α = 0.05. For mechanical and biochemical data, groups were examined using multivariate analysis of variance (ANOVA) with EY, G*, GAG, or collagen as the dependent variables, and culture time, growth factor group (study 1 only), donor animal (study 1 only), or implantation group (study 2 only) as the independent variables. ANOVA for the gene expression was performed on the logarithmically transformed data to account for unequal variances. Tukey honestly significant difference (HSD) post hoc tests were carried out with statistical significance set at α = 0.05.
Results
Study 1: Multiple donors
The results from the three donor dogs (dog 1, dog 2, and dog 3) between primary and passaged chondrocytes were similar across animals. Passaging led to ∼8–10 × increases in cell number over 10 days in monolayer culture, and all donor cell lines had sufficient viable cell numbers for encapsulation. With prolonged culture to day 28, P0 constructs from all three donor animals exhibited small, but significant, increases in GAG content (∼0.6–0.75%ww, p < 0.05, Table 2) from day 0 values, with no other significant changes in mechanical or biochemical measures. In contrast, all constructs seeded with P1 chondrocytes possessed significant ∼10–20-fold increases in measured mechanical properties and biochemical content over time in culture (Table 2). The highest values were attained by dog 1, with measured EY of 191.9 ± 26.7 kPa, G* 1.70 ± 0.25 MPa, GAG 2.75 ± 0.41%ww, and collagen 3.09 ± 0.44%ww. These values compare favorably with the values of native canine patellofemoral groove cartilage obtained from each donor (average values: EY 244.8 ± 17.1 kPa, G* 2.56 ± 0.67 MPa, GAG 4.79 ± 0.57%ww, collagen 15.82 ± 2.89%ww; Table 2).
Table 2.
Mechanical and Biochemical Properties of Engineered Canine Cartilage Constructs
| Group | Day | EY (kPa) | G* 0.1 Hz (MPa) | GAG (%ww) | Collagen (%ww) |
|---|---|---|---|---|---|
| Dog 1 P0 | 0 | 14.2 ± 2.0 | 0.1 ± 0.1 | 0.10 ± 0.04 | 0.11 ± 0.21 |
| 14 | 16.2 ± 1.7 | 0.1 ± 0.1 | 0.25 ± 0.03 | 0.21 ± 0.35 | |
| 28 | 20.3 ± 3.2 | 0.1 ± 0.1 | 0.70 ± 0.08a,b | 0.16 ± 0.25 | |
| Dog 2 P0 | 0 | 18.5 ± 2.9 | 0.1 ± 0.1 | 0.15 ± 0.05 | 0.20 ± 0.27 |
| 14 | 18.9 ± 3.5 | 0.1 ± 0.1 | 0.18 ± 0.03 | 0.19 ± 0.04 | |
| 28 | 24.0 ± 3.7 | 0.1 ± 0.1 | 0.74 ± 0.13a,b | 0.16 ± 0.28 | |
| Dog 3 P0 | 0 | 18.4 ± 3.6 | 0.1 ± 0.1 | 0.12 ± 0.02 | 0.18 ± 0.14 |
| 14 | 19.6 ± 2.1 | 0.1 ± 0.0 | 0.35 ± 0.04 | 0.37 ± 0.21 | |
| 28 | 25.2 ± 3.3 | 0.1 ± 0.1 | 0.60 ± 0.21a,b | 0.25 ± 0.39 | |
| Dog 1 P1 | 0 | 17.1 ± 2.0 | 0.1 ± 0.1 | 0.10 ± 0.04 | 0.14 ± 0.06 |
| 14 | 80.6 ± 10.5a,c | 0.8 ± 0.2a,c | 1.10 ± 0.10a,c | 1.49 ± 0.11a,c | |
| 28 | 191.9 ± 26.7a–d | 1.7 ± 0.4a–c | 2.75 ± 0.41a–c | 3.09 ± 0.44a–d | |
| Dog 2 P1 | 0 | 15.8 ± 1.7 | 0.1 ± 0.1 | 0.10 ± 0.01 | 0.11 ± 0.18 |
| 14 | 65.6 ± 7.8a,c | 0.5 ± 0.1a,c | 1.21 ± 0.16a,c | 1.08 ± 0.19a,c | |
| 28 | 135.4 ± 24.0a–c | 1.5 ± 0.6a–c | 2.40 ± 0.25a–c | 2.32 ± 0.41a–c | |
| Dog 3 P1 | 0 | 15.1 ± 1.8 | 0.1 ± 0.1 | 0.05 ± 0.04 | 0.04 ± 0.20 |
| 14 | 101.0 ± 9.7a,c | 0.6 ± 0.3a,c | 0.93 ± 0.09a,c | 1.21 ± 0.23a,c | |
| 28 | 151.1 ± 5.4a–c | 1.5 ± 0.3a–c | 2.25 ± 0.20a–c | 2.28 ± 0.22a–c | |
| Mixed P1 | 0 | 9.9 ± 0.4 | 0.0 ± 0.0 | 0.23 ± 0.14 | 0.00 ± 0.00 |
| 14 | 78.9 ± 5.1a | 0.5 ± 0.1a | 2.55 ± 0.08a | 0.65 ± 0.44 | |
| 28 | 193.6 ± 11.1a,b | 1.3 ± 0.1a,b | 4.27 ± 0.19a,b | 1.06 ± 0.15a | |
| Dog 1 cart | 304.0 ± 10.8 | 2.4 ± 0.7 | 4.32 ± 0.17 | 15.78 ± 2.16 | |
| Dog 2 cart | 205.2 ± 18.9 | 2.8 ± 0.4 | 5.21 ± 0.87 | 14.12 ± 3.54 | |
| Dog 3 cart | 225.3 ± 23.4 | 2.5 ± 0.6 | 4.84 ± 0.66 | 17.46 ± 2.98 |
Primary (P0) adult canine chondrocytes exhibited a significant increase in GAG content only after 28 days in culture, with no significant increases over time in all other mechanical and biochemical measures. All passaged (P1) chondrocytes elaborated a cartilage matrix after 28 days that possessed a Young's modulus (EY), dynamic modulus (G*), and GAG content in the range of native adult canine cartilage values.
p < 0.05 versus d0.
p < 0.05 versus d14.
p < 0.05 versus respective P0.
p < 0.05 versus all groups at all time points.
GAG, glycosaminoglycan.
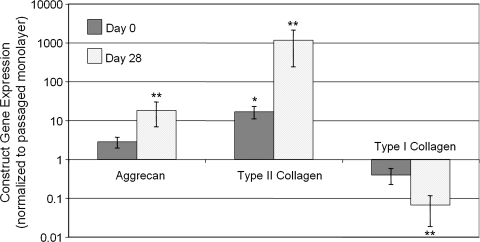
Mixed chondrocyte constructs increased in properties similarly to each individual donor animal (Table 2). As all donor cell populations responded similarly, gene expression analysis was performed only on mixed chondrocyte constructs. ANOVA for the gene expression was performed on the logarithmically transformed data to account for unequal variances (Fig. 2). Aggrecan expression and type II collagen expression were the lowest for monolayer cultures, and increased with culture time after encapsulation in agarose hydrogel constructs. In contrast, type I collagen expression exhibited the opposite trend. These results are consistent with an increase in chondrogenic phenotype expected from 3D culture19 and are complementary to the increases of cartilage-relevant ECM measured from other constructs.
FIG. 2.
Construct gene expression of aggrecan, type II collagen, and type I collagen. With three-dimensional encapsulation on day 0 and with continued culture to day 28, passaged adult canine chondrocytes exhibited significantly increased aggrecan and type II collagen gene expression with significantly decreased type I collagen gene expression, indicating a chondrogenic phenotype. Note: Y-axis is logarithmic scale. *p < 0.05 versus monolayer, **p versus respective day 0 group.
Study 2: In vivo biocompatibility
At 12 weeks, implanted dogs had normal gait (grade 0/5 lameness) with no palpable joint effusion on orthopedic examination. The dog with empty control defects showed grade 2/5 lameness at 12 weeks and had palpable joint effusion in the operated knee.
Radiographic assessment of operated knees performed 12 weeks after surgery suggested that chondral and osteochondral constructs were associated with no overt complications or morbidity. For knees receiving chondral or osteochondral implants, no radiographic evidence for effusion or soft tissue proliferation (synovitis), sclerosis, or osteophytosis was seen. For defects treated with chondral grafts, the defect sites could be identified radiographically with variable degrees of reestablishment of radiographic subchondral bone plate architecture. For defects treated with osteochondral grafts, the tantalum substrates were all in place with no evidence for migration, subsidence, or periimplant lucency or sclerosis. The radiographic appearance of the knee with empty defects included mild effusion/soft tissue proliferation, clearly visible defect sites, and associated sclerosis of the trochlear groove.
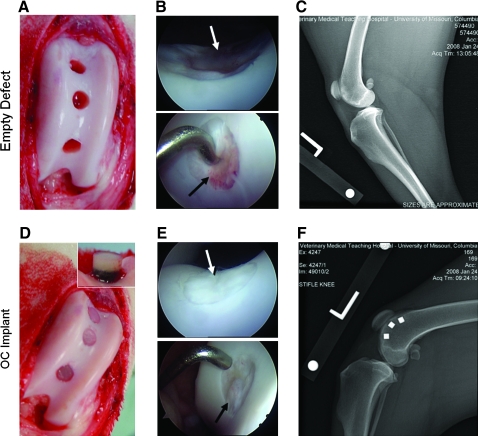
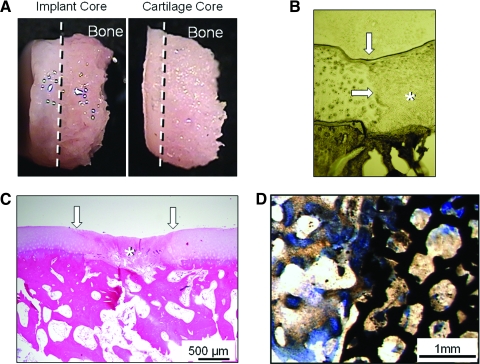
Repair tissue in empty defects observed at 6- and 12-week arthroscopies was graded poor and was fibrous (granulation tissue) and/or fibrocartilaginous in appearance and did not restore surface congruity or cartilage volume in the defect site (Fig. 3B, top and bottom). In contrast, the sites receiving engineered cartilage constructs were graded from excellent (>90% of defect filled with opaque white tissue [hyaline-like] with a smooth surface matching the contour of the host articular surface; Fig. 3E, top) to good (>75% of defect filled with opaque white tissue [hyaline-like], surface fibrillations/irregularities may be present; Fig. 3E, bottom) filling with hyaline-like cartilage. No constructs migrated (Fig. 3F, osteochondral construct shown) or failed under in vivo loading. Osteochondral constructs displayed less subsidence than chondral constructs. Apposing patellar surfaces were normal in all cases.
FIG. 3.
Perioperative gross appearance of empty defects (A) and implanted osteochondral constructs (D). Animal knees (stifles) were monitored arthroscopically during the 3-month experimental period to nondestructively study animal healing and response to implants (B, E; arrows indicate defect sites). Empty defects showed generally poor filling of the defect site with fibrous/fibrocartilage appearance (B, top and bottom), whereas sites receiving engineered cartilage ranged from good (E, bottom) to excellent (E, top) filling. Digital radiographic imaging was also used to confirm that implanted osteochondral constructs remained in place in the defect sites based on location and appearance of the tantalum trabecular metal (F). The empty defect radiograph is shown as a technical control (C). Color images available online at www.liebertonline.com/ten.
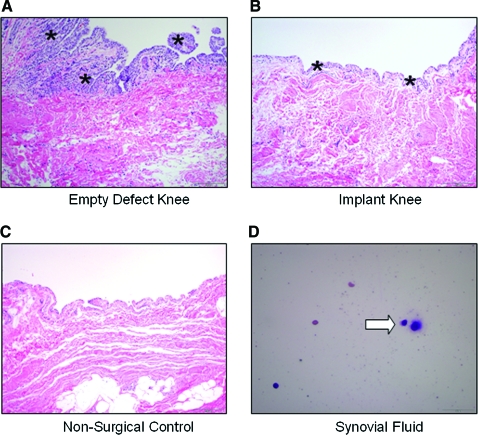
In histological sections of harvested synovium (Fig. 4), lymphoid aggregates, giant cells, neutrophils, and macrophages were not observed in any samples. Infiltrates of small numbers of lymphocytes and plasma cells along with marked hyperplasia were observed for the empty defect knee and considered the only significant lesion of all samples. The lack of neutrophils in any of the sections suggests no suppurative inflammation. As expected, the synovium in knees undergoing arthrotomy for implant placement exhibited mild hyperplasia relative to the nonsurgical control specimen. No clinical evidence of joint effusion was present in any dog, and cytologic examination revealed normal synovial fluid in all cases (e.g., few mononuclear cells [synovial lining cells] in proteinaceous background, no neutrophils, lymphocytes, or plasma cells), with few cells introduced by aspiration. Chondral constructs showed integration with underlying bone (Fig. 5A), and both chondral and osteochondral constructs showed no gap formation with surrounding cartilage tissue (Fig. 5B, C, chondral construct shown only). Osteochondral constructs showed bony ingrowth into the trabecular metal base (Fig. 5D), consistent with clinical use of the metal.43
FIG. 4.
Representative histology (hematoxylin and eosin) of synovium (A–C, hyperplasia: asterisks). As expected, the synovium in implanted knees exhibited mild hyperplasia due to surgery relative to nonsurgical controls. Empty defect knees exhibited prominent hyperplasia, with multiple layers of swollen synovial cells and fronds of papillary projection. For synovial cytology, plasma cell (D, white arrow) and some red blood cells were present, likely introduced during aspiration. Color images available online at www.liebertonline.com/ten.
FIG. 5.
Gross pictures of implanted chondral constructs cored from defect site compared with an osteochondral core from normal contralateral canine knee (A) show excellent integration with underlying bone. Unstained (B) and hematoxylin and eosin–stained (C) sections of the chondral construct repair site reveal no gap between the margins of the defect (white arrows) and implant (*). Osteochondral constructs exhibited all of the aspects of chondral constructs and good bone–tantalum integration after 12 weeks of culture (D). Color images available online at www.liebertonline.com/ten.
Discussion
The results of the presented studies demonstrate the successful expansion of mature canine chondrocytes and the formation of engineered cartilage tissue with a compressive Young's modulus in the physiologic range for native canine cartilage as verified by cartilage testing and previous reports.44,45 The measured compressive stiffness and GAG content represent the highest reported values for engineered cartilage formed from mature chondrocytes.46,47 Preliminary experiments established that, contrary to published juvenile bovine results,24,48 a temporal application of growth factors did not encourage rapid matrix development.30 Therefore, continuous TGF-β3 treatment was adopted for the presented studies using adult cells, consistent with other preliminary work performed by our laboratory on mature bovine cells.46 Differences in mature and immature chondrocyte response to the continuous/temporal application of TGF-β3 are likely due to age-related issues in TGF-β signal transduction such as decreases in TGF-β receptors and changes in Smad signaling within the cell.49 The results of study 1 indicate that the in vitro culture process utilized is consistent and translatable between adult cells of different donor animals. This is a promising finding for future clinical application given that a mixed population of chondrocytes, as used in past bovine studies, may not be feasible due to possible biocompatibility and other unforeseeable postoperative complications.
The use of a growth factor cocktail during passaging appears to be necessary to invigorate the chondrocytes to activate rapid matrix synthesis. This protocol was adopted from techniques applied to human chondrocytes for future use in conjunction with autologous chondrocyte implantation,32 and the results of this paper are the first to show that this can directly lead to an engineered cartilage tissue with functional mechanical properties. The function of this growth factor cocktail appears to be in rapidly expanding cell numbers (∼10 days for 10 × increase in cells), priming the expanded chondrocytes for rapid matrix synthesis in the 3D scaffold environment, and preventing phenotype dedifferentiation observed in mammalian chondrocytes in monolayer culture, including the canine model used in this paper.19,50 Gene expression results confirmed that the adult canine chondrocytes possessed a synthetically active phenotype following passaging and encapsulation in the agarose matrix. This change in phenotype may also be dependent on the scaffold as agarose has been shown to maintain chondrocyte phenotype19 as well as allow for the assembly and synthesis of biomimetic GAG molecules51 and type II and type VI collagens.48,52 It is well known that mature chondrocytes are not as functionally active as juvenile cells and express a phenotype preferring homeostasis rather than rapid matrix development.53 Indeed, the freshly harvested adult canine chondrocytes in study 1 did not develop a functional matrix in an agarose hydrogel scaffold, contrary to well-established published results with juvenile bovine cells.18,20 As the cells utilized for human cartilage repair treatments are likely to be from older adults, this growth factor expansion step is of crucial importance in the development of a mechanically competent engineered tissue. In addition, the chondrocyte passaging procedure for humans will likely utilize autologous human serum (as opposed to the FBS used in this study) or require the development of serum-free passaging media to remove any risk of an immunogenic response.
The in vivo experiments were performed to assess the biocompatibility and immune response of a host animal to the implanted engineered cartilage tissue. A focal defect model using dogs was chosen for this purpose, as the canine model provides for assessment in a clinically relevant species in which the anatomy, physiology, and biomechanics of the knee are similar to humans. Importantly, the feasibility of surgery, postoperative management, and outcome assessments are similar to humans such that experiments can yield clinically applicable data. The ability to obtain multiple matched assessments from the same joint, utilize nondestructive assessments (e.g., arthroscopy), and perform controlled longitudinal studies using this model enhances our ability to adhere to the “refine, reduce, and, to some degree, replace” effort in animal research advocated by the NIH. Chondral and osteochondral constructs were utilized for this as it was unknown a priori whether a chondral-only construct would integrate with the surrounding tissues and remain in the defect site.
The lack of any fulminate joint effusion or infiltrates of inflammatory cells on cytologic and histologic assessments suggests that intraarticular implantation of allogeneic chondrocyte-seeded agarose constructs were well tolerated such that healing/integration occurred with no clinical, radiographic, histologic, or cytologic evidence for untoward inflammatory or immune responses. This is consistent with preliminary human trials utilizing an agarose-alginate hydrogel.54 Although agarose can elicit a severe rejection response in areas of rich blood supply, such as in a subcutaneous pouch,55 the lack of blood vessels and immune cells leads to the immuno-privileged nature of the articular joint. In addition, after in vitro culture, the agarose scaffold represents only a small fraction of the construct wet weight (at most the initial 2%), whereas elaborated matrix represents a much larger fraction of the construct solid mass (∼2–3 times that of the agarose in this study, ∼3–5 times in bovine constructs24), and this elaborated matrix will therefore help prevent any host–scaffold or host–allogeneic cell interactions.17 Given the observed lack of biocompatibility problems, changes in implanted tissue properties and related functional outcomes other than biocompatibility are planned for extensive study in future work.
In conclusion, the use of a growth factor cocktail during passaging and expansion of adult canine chondrocytes coupled with the use of a well-defined culture medium supplemented with TGF-β3 led to the formation of an engineered cartilage tissue with physiologic mechanical properties and no apparent biocompatibility problems. These findings advance the knowledge that has been compiled by our laboratories with a juvenile bovine animal model and translate it into a clinically relevant model for potential future use in humans. Future studies are planned to examine the effects of dynamic deformational loading in vitro before in vivo implantation along with better understanding the changes in implanted constructs and host tissues over time postimplantation, as well as any benefits of osteochondral constructs. Eventually, the ultimate goal is to scale up these findings into anatomically shaped constructs and allow for total joint resurfacing.56,57
Acknowledgments
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (AR46568 and AR52871) and by the Musculoskeletal Transplant Foundation (CU07-194).
Disclosure Statement
No competing financial interests exist.
References
- 1.Felson D.T. Stepping away from OA: a scientific conference on the prevention, onset, progression, and disability of osteoarthritis. National Institutes of Health; Bethesda, Maryland: Jul 23–24, 1999. [Google Scholar]
- 2.Mankin H.J. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460. [PubMed] [Google Scholar]
- 3.Buckwalter J.A. Lohmander S. Operative treatment of osteoarthrosis. Current practice and future development. J Bone Joint Surg Am. 1994;76:1405. doi: 10.2106/00004623-199409000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Hangody L. Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A Suppl 2:25. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 5.Shasha N. Krywulak S. Backstein D. Pressman A. Gross A.E. Long-term follow-up of fresh tibial osteochondral allografts for failed tibial plateau fractures. J Bone Joint Surg Am. 2003;85-A Suppl 2:33. doi: 10.2106/00004623-200300002-00005. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 7.Niemeyer P. Pestka J.M. Kreuz P.C. Erggelet C. Schmal H. Suedkamp N.P. Steinwachs M. Characteristic complications after autologous chondrocyte implantation for cartilage defects of the knee joint. Am J Sports Med. 2008;36:2091. doi: 10.1177/0363546508322131. [DOI] [PubMed] [Google Scholar]
- 8.Mandelbaum B. Browne J.E. Fu F. Micheli L.J. Moseley J.B., Jr. Erggelet C. Anderson A.F. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35:915. doi: 10.1177/0363546507299528. [DOI] [PubMed] [Google Scholar]
- 9.International Cartilage Repair Society Cartilage Evaluation Package, ICRS Standards. Workshop, Schloss; Munchenwiler, Switzerland: Jan 27–30, 2000. [Google Scholar]
- 10.LaPrade R.F. Botker J.C. Donor-site morbidity after osteochondral autograft transfer procedures. Arthroscopy. 2004;20:e69. doi: 10.1016/j.arthro.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Reddy S. Pedowitz D.I. Parekh S.G. Sennett B.J. Okereke E. The morbidity associated with osteochondral harvest from asymptomatic knees for the treatment of osteochondral lesions of the talus. Am J Sports Med. 2007;35:80. doi: 10.1177/0363546506290986. [DOI] [PubMed] [Google Scholar]
- 12.Whittaker J.P. Smith G. Makwana N. Roberts S. Harrison P.E. Laing P. Richardson J.B. Early results of autologous chondrocyte implantation in the talus. J Bone Joint Surg Br. 2005;87:179. doi: 10.1302/0301-620x.87b2.15376. [DOI] [PubMed] [Google Scholar]
- 13.Butler D.L. Goldstein S.A. Guilak F. Functional tissue engineering: the role of biomechanics. J Biomech Eng. 2000;122:570. doi: 10.1115/1.1318906. [DOI] [PubMed] [Google Scholar]
- 14.Brown T.D. Shaw D.T. In vitro contact stress distributions in the natural human hip. J Biomech. 1983;16:373. doi: 10.1016/0021-9290(83)90071-4. [DOI] [PubMed] [Google Scholar]
- 15.Brown T.D. Shaw D.T. In vitro contact stress distribution on the femoral condyles. J Orthop Res. 1984;2:190. doi: 10.1002/jor.1100020210. [DOI] [PubMed] [Google Scholar]
- 16.Macirowski T. Tepic S. Mann R.W. Cartilage stresses in the human hip joint. J Biomech Eng. 1994;116:10. doi: 10.1115/1.2895693. [DOI] [PubMed] [Google Scholar]
- 17.Arnoczky S.P. The biology of allograft incorporation. J Knee Surg. 2006;19:207. doi: 10.1055/s-0030-1248109. [DOI] [PubMed] [Google Scholar]
- 18.Mauck R.L. Soltz M.A. Wang C.C. Wong D.D. Chao P.H. Valhmu W.B. Hung C.T. Ateshian G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 19.Benya P.D. Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 20.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Kimura J.H. Hunziker E.B. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;10:745. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- 21.Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Hunziker E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108 (Pt 4):1497. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 22.Lee D.A. Bader D.L. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 23.Byers B.A. Mauck R.L. Chiang I.E. Tuan R.S. Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage. Tissue Eng Part A. 2008;14:1821. doi: 10.1089/ten.tea.2007.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lima E.G. Bian L. Ng K.W. Mauck R.L. Byers B.A. Tuan R.S. Ateshian G.A. Hung C.T. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15:1025. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pond M.J. Nuki G. Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis. 1973;32:387. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mastbergen S.C. Marijnissen A.C. Vianen M.E. van Roermund P.M. Bijlsma J.W. Lafeber F.P. The canine groove model of osteoarthritis is more than simply the expression of surgically applied damage. Osteoarthritis Cartilage. 2006;14:39. doi: 10.1016/j.joca.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Conzemius M.G. Aper R.L. Corti L.B. Short-term outcome after total elbow arthroplasty in dogs with severe, naturally occurring osteoarthritis. Vet Surg. 2003;32:545. doi: 10.1111/j.1532-950x.2003.00545.x. [DOI] [PubMed] [Google Scholar]
- 28.Olmstead M.L. Canine cemented total hip replacements: state of the art. J Small Anim Pract. 1995;36:395. doi: 10.1111/j.1748-5827.1995.tb02962.x. [DOI] [PubMed] [Google Scholar]
- 29.Breinan H.A. Minas T. Hsu H.P. Nehrer S. Shortkroff S. Spector M. Autologous chondrocyte implantation in a canine model: change in composition of reparative tissue with time. J Orthop Res. 2001;19:482. doi: 10.1016/S0736-0266(00)90015-9. [DOI] [PubMed] [Google Scholar]
- 30.Ng K.W. O'Conor C.J. Lima E.G. Lo S.B. Ateshian G.A. Cook J.L. Hung C.T. Primed mature canine chondrocytes can develop an engineered cartilage tissue with physiologic properties. Trans Orthop Res. 2008;31:599. [Google Scholar]
- 31.Lima E.G. Grace Chao P.H. Ateshian G.A. Bal B.S. Cook J.L. Vunjak-Novakovic G. Hung C.T. The effect of devitalized trabecular bone on the formation of osteochondral tissue-engineered constructs. Biomaterials. 2008;29:4292. doi: 10.1016/j.biomaterials.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Francioli S.E. Martin I. Sie C.P. Hagg R. Tommasini R. Candrian C. Heberer M. Barbero A. Growth factors for clinical-scale expansion of human articular chondrocytes: relevance for automated bioreactor systems. Tissue Eng. 2007;13:1227. doi: 10.1089/ten.2006.0342. [DOI] [PubMed] [Google Scholar]
- 33.Mauck R.L. Wang C.C. Oswald E.S. Ateshian G.A. Hung C.T. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Riesle J. Hollander A.P. Langer R. Freed L.E. Vunjak-Novakovic G. Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J Cell Biochem. 1998;71:313. doi: 10.1002/(sici)1097-4644(19981201)71:3<313::aid-jcb1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 35.Farndale R.W. Sayers C.A. Barrett A.J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 36.Stegemann H. Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 37.Hollander A.P. Heathfield T.F. Webber C. Iwata Y. Bourne R. Rorabeck C. Poole A.R. Increased damage to type II collagen in osteoarthritic articular cartilage detected by a new immunoassay. J Clin Invest. 1994;93:1722. doi: 10.1172/JCI117156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reno C. Marchuk L. Sciore P. Frank C.B. Hart D.A. Rapid isolation of total RNA from small samples of hypocellular, dense connective tissues. Biotechniques. 1997;22:1082. doi: 10.2144/97226bm16. [DOI] [PubMed] [Google Scholar]
- 39.Muller P.Y. Janovjak H. Miserez A.R. Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32:1372. [PubMed] [Google Scholar]
- 40.Cook J.L. Tomlinson J.L. Kreeger J.M. Cook C.R. Induction of meniscal regeneration in dogs using a novel biomaterial. Am J Sports Med. 1999;27:658. doi: 10.1177/03635465990270051901. [DOI] [PubMed] [Google Scholar]
- 41.Cook J.L. Hudson C.C. Kuroki K. Autogenous osteochondral grafting for treatment of stifle osteochondrosis in dogs. Vet Surg. 2008;37:311. doi: 10.1111/j.1532-950X.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- 42.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 43.Levine B.R. Sporer S. Poggie R.A. Della Valle C.J. Jacobs J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials. 2006;27:4671. doi: 10.1016/j.biomaterials.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 44.Athanasiou K.A. Rosenwasser M.P. Buckwalter J.A. Malinin T.I. Mow V.C. Interspecies comparisons of in situ intrinsic mechanical properties of distal femoral cartilage. J Orthop Res. 1991;9:330. doi: 10.1002/jor.1100090304. [DOI] [PubMed] [Google Scholar]
- 45.LeRoux M.A. Arokoski J. Vail T.P. Guilak F. Hyttinen M.M. Kiviranta I. Setton L.A. Simultaneous changes in the mechanical properties, quantitative collagen organization, and proteoglycan concentration of articular cartilage following canine meniscectomy. J Orthop Res. 2000;18:383. doi: 10.1002/jor.1100180309. [DOI] [PubMed] [Google Scholar]
- 46.Bian L.M. Angione S.L. Lima E.G. Ng K.W. Ateshian G.A. Hung C.T. Tissue-engineered cartilage constructs using mature bovine chondrocytes: effectes of temporal exposure to growth factors and dynamic deformational loading. Trans Orthop Res. 2006;32:1480. [Google Scholar]
- 47.Chen A.C. Masuda K. Nakagawa K. Wong V.W. Pfister B.E. Thonar E.J. Sah R.L. Tissue engineered cartilage from adult human chondrocytes: Biomechanical properties and function-composition relationships. Trans Orthop Res. 2003;28:945. [Google Scholar]
- 48.Ng K.W. DeFrancis J.G. Kugler L.E. Kelly T.A. Ho M.M. O'Conor C.J. Ateshian G.A. Hung C.T. Amino acids supply in culture media is not a limiting factor in the matrix synthesis of engineered cartilage tissue. Amino Acids. 2008;35:433. doi: 10.1007/s00726-007-0583-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blaney Davidson E.N. Scharstuhl A. Vitters E.L. van der Kraan P.M. van den Berg W.B. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giannoni P. Crovace A. Malpeli M. Maggi E. Arbico R. Cancedda R. Dozin B. Species variability in the differentiation potential of in vitro-expanded articular chondrocytes restricts predictive studies on cartilage repair using animal models. Tissue Eng. 2005;11:237. doi: 10.1089/ten.2005.11.237. [DOI] [PubMed] [Google Scholar]
- 51.Mouw J.K. Case N.D. Guldberg R.E. Plaas A.H. Levenston M.E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13:828. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 52.Kelly T.A. Ng K.W. Wang C.C. Ateshian G.A. Hung C.T. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2006;39:1489. doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 53.Barbero A. Grogan S. Schafer D. Heberer M. Mainil-Varlet P. Martin I. Age related changes in human articular chondrocyte yield, proliferation and post-expansion chondrogenic capacity. Osteoarthritis Cartilage. 2004;12:476. doi: 10.1016/j.joca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Selmi T.A. Verdonk P. Chambat P. Dubrana F. Potel J.F. Barnouin L. Neyret P. Autologous chondrocyte implantation in a novel alginate-agarose hydrogel: outcome at two years. J Bone Joint Surg Br. 2008;90:597. doi: 10.1302/0301-620X.90B5.20360. [DOI] [PubMed] [Google Scholar]
- 55.Xu X.L. Lou J. Tang T. Ng K.W. Zhang J. Yu C. Dai K. Evaluation of different scaffolds for BMP-2 genetic orthopedic tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;75:289. doi: 10.1002/jbm.b.30299. [DOI] [PubMed] [Google Scholar]
- 56.Hung C.T. Lima E.G. Mauck R.L. Takai E. LeRoux M.A. Lu H.H. Stark R.G. Guo X.E. Ateshian G.A. Anatomically shaped osteochondral constructs for articular cartilage repair. J Biomech. 2003;36:1853. doi: 10.1016/s0021-9290(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 57.Cook J.L. Lima E.G. Ng K.W. Kuroki K. Stoker A.M. Bal B.S. Ateshian G.A. Hung C.T. Towards biologic osteochondral resurfacing of the canine patella using tissue engineered anatomic constructs. Trans Orthop Res. 2009;34:1355. [Google Scholar]