Abstract
Muscle-derived stem-cell (MDSC) transplantation presents a promising method for the treatment of muscle injuries. This study investigated the ability of exercise to enhance MDSC transplantation into the injured muscle. Mice were divided into four groups: contusion + phosphate-buffered saline (C + PBS; n = 14 muscles), C + MDSC transplantation (n = 12 muscles), C + PBS + treadmill running (C + PBS + TM; n = 17 muscles), and C + MDSC + TM (n = 13 muscles). One day after injury, the TM groups began running for 1 or 5 weeks. Two days after injury, muscles of C + MDSC and C + MDSC + TM groups were injected with MDSCs. One or 5 weeks later, the number and differentiation of transplanted MDSCs, myofiber regeneration, collagen I formation, and vascularity were assessed histologically. In vitro, MDSCs were subjected to mechanical stimulation, and growth kinetics were quantified. In vitro, mechanical stimulation decreased the MDSC population doubling time (18.6 ± 1.6 h) and cell division time (10.9 ± 0.7 h), compared with the controls (population doubling time: 23.0 ± 3.4 h; cell division time: 13.3 ± 1.1 h) (p = 0.01 and 0.03, respectively). In vivo, 5 weeks of TM increased the myogenic contribution of transplanted MDSCs, compared with the controls (p = 0.02). C + MDSC, C + PBS + TM, and C + MDSC + TM demonstrated decreased fibrosis at 5 weeks, compared with the C + PBS controls (p = 0.00, p = 0.03, and p = 0.02, respectively). Results suggest that the mechanical stimulation favors MDSC proliferation, both in vitro and in vivo, and that exercise enhances MDSC transplantation after injury.
Introduction
Muscle injuries, following military combat, sports, surgery, or even during daily activities, are common. In fact, traumatic muscle contusion and strain injuries make up approximately 90% of all sports-related injuries.1 Although skeletal muscle contains the ability to regenerate after injury, the healing process is slow and often results in incomplete functional recovery and an increased risk for recurrence (reviewed in Huard et al.2). Despite the considerable medical need, there has been relatively little progress in the development of therapeutic approaches to enhance healing after muscle injury. Traditionally, three schools of thought have been pursued: rehabilitative, surgical, and biological. Although the first two have commonly complemented one another, the development of biological approaches to enhance skeletal muscle healing after injury has rarely been integrated into the world of rehabilitation. It may be for this reason that the development of biological therapies for muscle injuries has yet to enjoy significant integration into medical practice.
Biological approaches, including the use of stem-cell transplantation, have been widely investigated as a means to enhance the level of skeletal muscle healing in cases of muscle pathology, such as Duchenne Muscular Dystrophy3–6 and cardiac healing after an infarct.7,8 We have isolated a population of neonatal muscle-derived stem cells (MDSCs) that display the ability to proliferate in vivo for an extended period of time, demonstrate strong capacity for self-renewal, and undergo multipotent differentiation.3 Muscle stem cells are defined as cells that possess the ability to produce both new muscle stem cells as well as myoblast and myofiber progeny, without themselves expressing markers of muscle differentiation. Their clinical application is promising and safe, and MDSCs have the advantage of being isolated from a simple muscle biopsy procedure. The autologous use of MDSCs has already been approved by the Food and Drug Administration and is now undergoing clinical trials for the treatment of urinary incontinence.9 However, to date, few studies have investigated the use of MDSCs for the treatment of acute skeletal muscle injury.
One study demonstrated that transplanted MDSCs predominantly contribute to the formation of scar tissue 5 weeks after skeletal muscle injury,10 thereby limiting the in vivo regenerative capacity. It was discovered that this overwhelming differentiation toward a myofibroblastic lineage was primarily a result of transforming growth factor-β1 secretion accompanying the injured skeletal muscle environment.10 Authors argued that for stem-cell transplantation as a treatment for muscle injury to be a clinical reality, the environmental milieu should be optimized to facilitate the myogenic differentiation of transplanted cells, while concomitantly minimizing their differentiation toward a fibroblastic lineage.
Although methods such as gene therapy and direct growth factor injection have been investigated in the laboratory to optimize the skeletal muscle microenvironment, each is accompanied by drawbacks, such as cost, feasibility, and safety issues, that limit their clinical application. Toward the end of finding a more practical approach, exercise has been shown to enhance the local environment's microvascular supply, increase the secretion of myogenically favorable growth factors, and to decrease the formation of fibrosis after skeletal muscle injury.11–17 This study combines the rehabilitation strategies with regenerative medicine approaches to maximize the overall healing of the injured skeletal muscle. The purpose of this study was to investigate the ability of mechanical stimulation to enhance the MDSC regeneration potential in vitro and in vivo.
Materials and Methods
MDSC isolation
MDSCs were isolated using a modified preplate technique, as previously described.3 Briefly, the hindlimb muscle tissues were harvested from neonatal C57BL/6J mice (The Jackson Laboratories, Bar Harbor, ME). Tissues were immediately placed in saline solution and finely minced in 1× Hank's balanced salt solution (HBSS) (Invitrogen, 24020, Carlsbad, CA). Minced pieces were subsequently digested using 0.2% collagenase, dispase (2.4 U/1 mL HBSS), and 0.1% trypsin. After digestion, the tissue/HBSS was passed through needles ranging in size to dissociate any larger cellular pieces. The solution was then placed into a 25 cm2 collagen-coated culture plate for 2 h and transferred to another 25 cm2 collagen-coated culture plate for 24 h. This step was repeated for an additional four times. The sixth preplate population of cells has been identified by our group as displaying properties of enhanced myogenic potential and immune privileged behavior when compared with the muscle-derived cell populations.3
Transduction to express the nuclear-localized LacZ reporter gene
To enable tracking of the cells after implantation into the muscle, MDSCs were genetically engineered to express the nuclear-localized LacZ (nLacZ) reporter gene.3 Cells were transduced with the retroviral vector MFG-NB containing a modified nLacZ gene (gift from Dr. P. Robbins), which includes a nuclear localization sequence cloned from the simian virus 40 large tumor antigen, and is transcribed from the long terminal repeat. Before injection, transduced MDSCs were assayed for nLacZ expression, as described previously.8
Transduced MDSCs were then cultured in normal growth medium, consisting of Dulbecco's modified Eagle's medium (Invitrogen, 11995-073) supplemented with 10% fetal bovine serum, 10% horse serum (HS), 1% penicillin/streptomycin, and 0.5% chick embryo extract (Gibco-BRL, Carlsbad, CA). MDSCs were maintained in growth medium to approximately 30% confluence and were subsequently passaged. On the day of injection, MDSCs were trypsinized and spun at 2000 rpm for 5 min. The resulting pellet was resuspended in phosphate-buffered saline (PBS) + 0.1% microsphere beads solution at a concentration of 1.0–1.4 × 107 cells/mL. Microsphere beads were used to localize the injection site at the time of histology.
In vitro mechanical stimulation of MDSCs and live automated cell imaging
To investigate the effect of mechanical stimulation on MDSC growth kinetics, we subjected MDSCs to cyclical mechanical strain, in vitro, as previously described.8 Briefly, MDSCs were seeded onto the six-well collagen-coated Bioflex culture plates at a density of 5 × 104/well. Cyclic stretch was applied using a Flexercell Strain Unit (Flexcell International, Export, PA), which biaxially elongates cells (10% average surface elongation), at a frequency of 30 cycles/min over 24 h. Control cells were seeded at an equal density onto the Bioflex culture plates but were not subjected to mechanical stimulation. After 24 h, cells were trypsinized and seeded onto the collagen-coated plates at a density of 800 cells/cm2 for analysis of proliferation using the live automated cell imaging system, as previously described.18 Live automated cell imaging system was used to obtain real-time digital images of cells every 10 min for a total of 60 h. At 0, 12, 24, 36, 48, and 60 h time points, the number of cells/high-power field of view was manually counted using Image J software (National Institutes of Health, Bethesda, MD). From this, the population doubling time (PDT), defined as T/(log2[NF/N0]), where T = time in culture, N0 = initial number of cells, and NF = final number of cells, was determined.19,20 We also used the time-lapsed data to determine (1) the cell division time (DT), or cell cycle time, and (2) the mitotic fraction, or an estimate of the fraction of cells in a population which are actively dividing. We have previously described these methods.18–21 For each group, three independent samples of cells were plated in triplicate and analyzed by a blinded investigator.
In vivo MDSC transplantation into injured animals
Animals
A total of 40 female B6 mice with severe combined immunodeficiency (age range 6–8 weeks; weight 15–17 g) were used. We used severe combined immunodeficiency mice to minimize the immune rejection against transplanted MDSC surface antigens. Animals were either obtained from Jackson Laboratories or were bred in-house. All mice, housed in plastic cages in a room kept at 23 ± 2°C and a 12h:12h dark–light cycle, had free access to water and standard food. Animal protocols used for these experiments were approved by the Institutional Animal Care and Use Committee of Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center (UPMC).
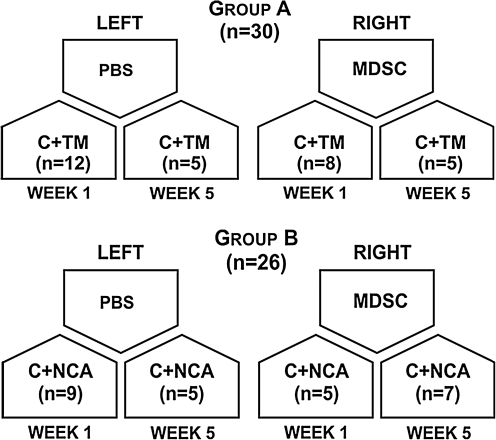
Animals were divided into two main groups: group A: contusion + treadmill running (C + TM) (n = 30 muscles) and group B: contusion + normal cage activity (C + NCA; n = 26 muscles) (Fig. 1). For each animal, both the left and right tibialis anterior (TA) muscles were contused. The right TA muscles were transplanted with MDSCs, whereas the left TA muscles were transplanted with PBS solution and were used as controls. Thus, the four groups compared were as follows: contusion + PBS (C + PBS) (n = 14 muscles); contusion and MDSC transplantation (C + MDSC) (n = 12 muscles); contusion + PBS + TM running (C + PBS + TM) (n = 17 muscles); and contusion, MDSC transplantation, and TM running (C + MDSC + TM) (n = 13 muscles) (Fig. 1). Of a total of 80 muscles harvested, 24 muscles were not considered in the analysis because either there was no evidence of green fluorescent microsphere beads or there was no evidence of skeletal muscle injury.
FIG. 1.
Schematic of experimental groups. PBS, phosphate-buffered saline; MDSC, muscle-derived stem-cell; C, contusion; TM, treadmill running; NCA, normal cage activity.
Contusion injury
Mice were anesthetized with 2% isoflurane (Abbott Laboratories, North Chicago, IL) in 100% O2 gas. The mouse's hindlimb was then extended at the knee and plantarflexed 90° at the ankle. A 16.2 g stainless steel ball (Small Parts, Miami Lakes, FL) was dropped from a height of 100 cm through an impactor positioned on the mouse's TA muscle. The muscle contusion made by this method is a high-energy blunt injury that creates a large hematoma and is followed by a healing cascade very similar to that seen in humans.22 Control muscles (uninjured and uninjected) (n = 2) from age- and sex-matched animals were also analyzed for hematoxylin and eosin staining, collagen area, and vascularity as a measure of baseline structure.
MDSC transplantation
Two days after contusion injury, animals were anesthetized with 2% isofluorane, administered by inhalation. The right TAs were injected intramuscularly with 1.0 × 105 MDSCs, suspended in 7 μL PBS + 0.1% green fluorescent microsphere beads. Left TAs were injected with an equal volume of PBS + 0.1% green fluorescent microsphere beads.
Exercise protocols
Animals in group A (C + TM) ran on a motor-driven TM (AccuScan Instruments, Columbus, OH) for either 1 week (n = 20 muscles) or 5 weeks (n = 10 muscles). The mice in the 1-week TM group started running 1 day after injury and continued running for 7 consecutive days. On the first day, mice ran for 20 min on a 0° incline at a velocity of 15 m/min. After 4 days, animals ran on 10° incline, and animals were progressed at a rate of 2 m/min to a final velocity of 15–20 m/min and total duration of 1 h, based on a protocol as described by Palermo et al.23 and based on the animal tolerance. A mild electric shock was applied from a grid behind the TM if the animals stopped running. Mice were euthanized on the eighth day after injury, at which time the TA muscles were harvested and snap frozen in 2-methylbutane cooled in liquid nitrogen. Muscles were maintained at −80°C for subsequent histological analysis.
Mice in the 5-week intervention group ran 5 days/week with the same training progression as described above for the 1-week intervention group. Animals continued to run for 60 min/day at a 10° incline for the last 4 weeks of the training protocol. Velocity was increased 2 m/min/week to a maximum velocity of 21 m/min on the last week of training. All mice were euthanized 5 weeks after injury, and the TA muscles were harvested and frozen as described above.
Transplanted MDSC tracking through nLacZ detection
Muscle cryosections (10 μm) were fixed with 1% glutaraldehyde (Sigma Chemical, St. Louis, MO) and were incubated with Lac-Z staining solution (0.5 M K4Fe[CN]6, 0.5 M K3Fe[CN]6, and 1.0 M MgCl2) for 2 h at 37°C, as previously described.24 The sections were subsequently stained with eosin. Sections were then viewed under a light microscope. All results were obtained with high power (20× objective) by a regular microscope (Nikon Eclipse E800; Nikon, Tokyo, Japan). Northern Eclipse software (Empix Imaging, Cheektawaga, NY) was used to count the total number of nLacZ+ cells across 12 sections, with approximately 140 μm between sections.
Cross-sectional areas for nLacZ+ muscle fibers were also measured using the National Institutes of Health (NIH)-developed image analysis software, ImageJ. All nLacZ+ muscle fibers were traced using the freehand selection tool within the ImageJ software. Fiber areas were computed and recorded in pixels/mm. The investigator performing the analysis was blinded to the treatment group.
Quantification of collagenous tissue
Trichrome staining was performed to quantify collagen content along the zone of injury. Slides were processed using Masson's Trichrome Stain Kit as per manufacturer's instructions (K7228; IMEB, Chicago, IL). This process stains skeletal muscle fibers red, collagen blue, and nuclei black. For each sample, five sections, approximately 600 μm apart, were analyzed, and the percentage of the total collagen-positive area relative to the total cross-sectional area was calculated. For determination of collagen content, images (10× objective) were obtained from a light microscope (Nikon Eclipse E800; Nikon) and analyzed using Northern Eclipse software (Empix Imaging).
Immunofluorescent staining of skeletal muscle vascularity
For CD31 staining (as a measure of skeletal muscle vascularity), muscle sections were fixed in 4% formalin for 5 min and then washed twice in PBS. Nonspecific binding was then blocked for 1 h using 5% HS. Sections were then incubated for 1 h at room temperature with a rat anti-mouse primary antibody (1:300 dilution in 5% HS; Pharmingen, San Diego, CA). After three PBS washes, sections were treated for 1 h with a 555-labeled goat anti-rat secondary antibody (diluted 1:300 in 5% HS). At the site of greatest MDSC injection, the number of capillaries per high-power field of view (20 × objective) was manually counted using fluorescent microscopy and averaged.
Colocalization of collagen I, vascularity, and nLacz for fate determination of transplanted cells
To determine the terminal differentiation of donor cells, C + MDSC and C + MDSC + TM muscle sections were fixed in 5% formalin for 15 min and then washed three times with PBS. The tissues were then incubated at 37°C for nLacZ staining solution as described above. After incubation, tissues were washed with 0.5% bovine serum albumin (BSA) and blocked using 2% BSA for 1 h, followed by subsequent washes using 0.5% BSA. Rabbit polyclonal to collagen I (1:500 dilution in 0.5% BSA; Abcam, Cambridge, MA) and rat anti-mouse CD31 (as a measure of vascularity) (1:200 dilution in 0.5% BSA; Pharmingen) primaries were applied for 1 h. After five additional washes with 0.5% BSA, goat anti-rat 488 (1:500 dilution in 0.5% BSA; Invitrogen) and sheep anti-goat fragment-CY3 (1:1000 dilution in 0.5% BSA; Sigma, St. Louis, MO) secondary antibodies were applied for 1 h.
Images were captured in 40 × objectives for nLacZ, collagen I, and CD31 at the section with the greatest donor cell engraftment. Images were then merged using Metamorph software (Molecular Devices, Downingtown, PA). nLacZ fluorescent analysis used the invert contrast function and then was assigned the color blue in Metamorph. Image J was used to manually count the number of nLacZ+ cells colocalizing with capillaries, collagenous tissue, or muscle fibers.
Quantification of myofiber regeneration
Hematoxylin and eosin stains were performed to quantify the regeneration index (total number of centrally located nuclei/total number of fibers) for each experimental group. For each sample, three muscle sections were selected to manually count the total number of fibers, and the number of fibers with centrally located nuclei were manually counted using Image J software. The results for each sample were then averaged.
Statistical analysis
All analyses were performed using standard statistical software packages (SPSS v14.0 software, Chicago, IL). Data are expressed as means ± standard deviations. One-way analysis of variance was used to compare across the four groups, with subsequent post hoc Tukey tests, as appropriate. To investigate the relationship between skeletal muscle vascularity and fibrosis formation, a linear regression was used. The effect of mechanical strain on PDT, DT, and mitotic fraction was determined using an unpaired Student's t-test. Statistical significance was established, a priori, at p ≤ 0.05.
Results
In vitro proliferation kinetics
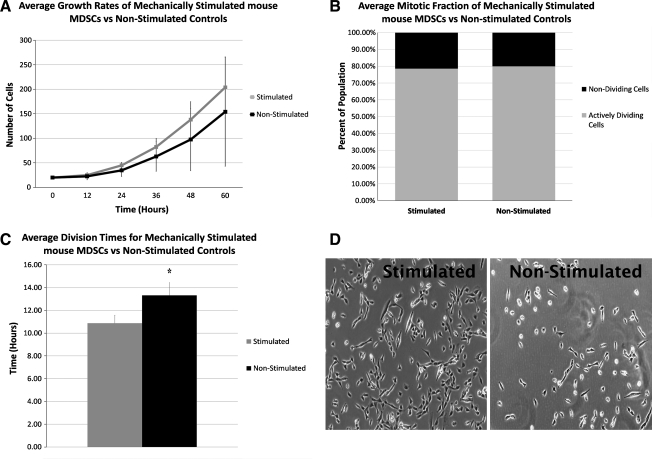
There was no significant increase in the overall growth rates curves of mechanically stimulated MDSCs after 60 h, when compared with the nonstimulated static cultures (Fig. 2A). However, there was a significant decrease in the PDT of MDSCs (18.6 ± 1.6 h) when compared with the nonstimulated controls (PDT: 23.0 ± 3.4, p = 0.01). To further explore if the decrease in PDT after mechanical stimulation was a result of faster cell cycle times, or due to a larger proportion of the cells that were actively dividing, we used the time-lapsed images to examine the mitotic fraction and DT, respectively. There was no difference in the mitotic fraction of stimulated versus nonstimulated controls (80% vs. 79%, p > 0.05, Fig. 2B); there was a significantly decreased DT of MDSCs after mechanical stimulation (10.9 ± 0.7 h) as compared with the nonstimulated controls (DT: 13.3 ± 1.1 h, p = 0.03) (Fig. 2C).
FIG. 2.
In vitro cellular growth kinetics. (A) There was no difference in the cellular growth curves of the two groups over 60 h. (B) No differences were found in mitotic fractions between stimulated and nonstimulated MDSCs. (C) Stimulated MDSCs showed a significantly lower division time when compared with the controls. *Significantly different than nonstimulated controls (p = 0.03). (D) Live automated cell imaging system pictures after 60 h of proliferation. MDSCs, muscle-derived stem cells.
Cell tracking through nLacZ transduction
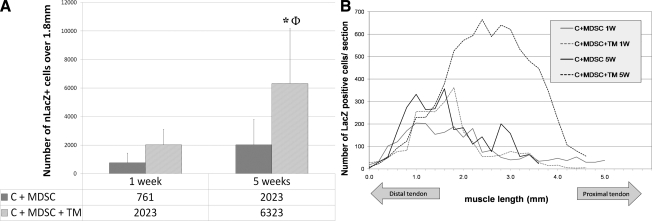
Before injection, nLacZ-transduced cells were confirmed to be 97%–100% positive for nLacZ expression. At 1 week, there was a nonsignificant increase in the number of nLacZ+ cells in the C + MDSC + TM group (2023 ± 1090), compared with the C + MDSC group (761 ± 680, p = 0.60). However, at 5 weeks, there was a significant increase in the number of nLacZ+ cells in the C + MDSC + TM (6323 ± 3862), when compared with the C + MDSC (2178 ± 1787, p = 0.02) (Fig. 3). Although at 5 weeks the total number of nLacZ+ cells was significantly increased after the addition of TM running, there was no significant difference in the average nLacZ + fiber area between the two groups (C + MDSC: 595.6 ± 332.5 pixels/mm and C + MDSC + TM: 582.7 ± 197.5 pixels/mm, p = 0.47).
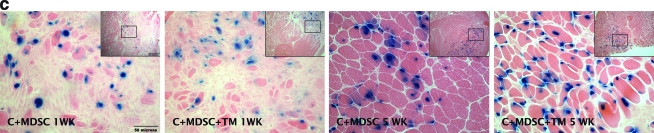
FIG. 3.
nLacZ+ cells among C + MDSC and C + MDSC + TM groups. (A) After 5 weeks, the C + MDSC + TM group showed a significant increase in the number of nLacZ+ cells when compared with the C + MDSC group. *Significantly different when compared with C + MDSC 5 weeks (p = 0.02). ΦSignificantly different when compared with C + MDSC and C + MDSC + TM 1 week (p = 0.00 and 0.01, respectively). (B) Schematic representation of nLacZ+ cell distribution in four MDSC transplanted groups. (C) Histological representation of nLacZ+ cells (blue) in contused muscle tissue (20 × objective). c, contusion; nLacZ, nuclear-localized LacZ; TM, treadmill. (Insets represent 10 × magnification).
Quantification of myofiber regeneration
After both 1 and 5 weeks, there was no difference in myofiber regeneration between any of the groups (Table 1).
Table 1.
Total Number of Muscle Fibers, Centrally Located Nuclei, and Regeneration Indexes (Number of Centrally Located Nuclei/Total Number of Muscle Fibers) Between Experimental Groups
| Time points | Experimental groups | Centrally located nuclei | Number of muscle fibers | Regeneration index |
|---|---|---|---|---|
| Non-injured Control | 2.16 ± 2.12 | 1147 ± 28 | 0.00 ± 0.00 | |
| 1 week | C + PBS | 558 ± 209 | 673 ± 209 | 0.81 ± 0.06 |
| C + MDSC | 419 ± 124 | 680 ± 216 | 0.61 ± 0.03 | |
| C + PBS + TM | 504 ± 195 | 772 ± 251 | 0.66 ± 0.16 | |
| C + MDSC + TM | 796 ± 535 | 1082 ± 560 | 0.73 ± 0.21 | |
| 5 weeks | C + PBS | 508 ± 118 | 861 ± 156 | 0.58 ± 0.08 |
| C + MDSC | 654 ± 194 | 1186 ± 326 | 0.56 ± 0.10 | |
| C + PBS + TM | 481 ± 112 | 976 ± 107 | 0.49 ± 0.12 | |
| C + MDSC + TM | 631 ± 86 | 1188 ± 263 | 0.54 ± 0.12 |
No significant differences were found between any groups.
Quantification of collagenous tissue
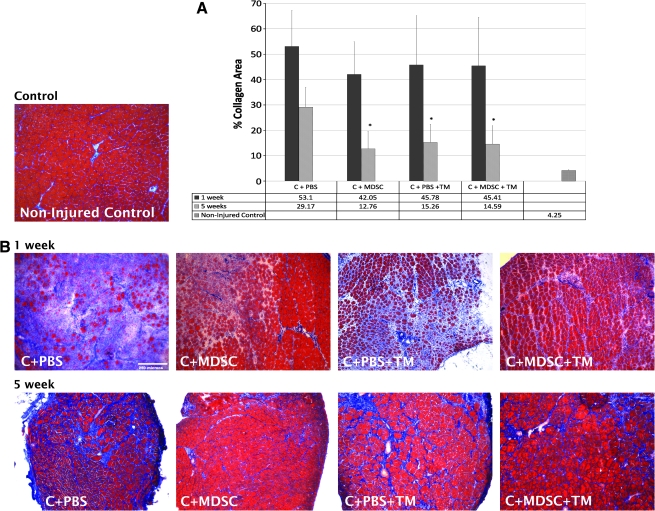
After 1 week, there was no significant difference in the % collagen-positive area between any of the four groups investigated. However, after 5 weeks, C + PBS + TM, C + MDSC, and C + MDSC + TM groups demonstrated significantly less collagen I (15.3 ± 7.2, 12.8 ± 6.9, and 14.6 ± 7.2% collagenous area/cross section, respectively), when compared with the C + PBS counterparts (29.22 ± 7.8%) (p = 0.03, p = 0.00, and p = 0.02, respectively) (Fig. 4).
FIG. 4.
Percentage of collagen I–positive area. (A) After 5 weeks, C + MDSC, C + PBS + TM, and C + MDSC + TM showed a decrease in the percentage of collagenous area when compared with the C + PBS 5 weeks. *Significantly different when compared with C + PBS 5 weeks (p = 0.00, p = 0.03, and p = 0.02, respectively). (B) Masson's trichrome staining shows muscle fibers (red) and collagen (blue) among different groups (10 × objective). PBS, phosphate-buffered saline.
Quantification of vascularity
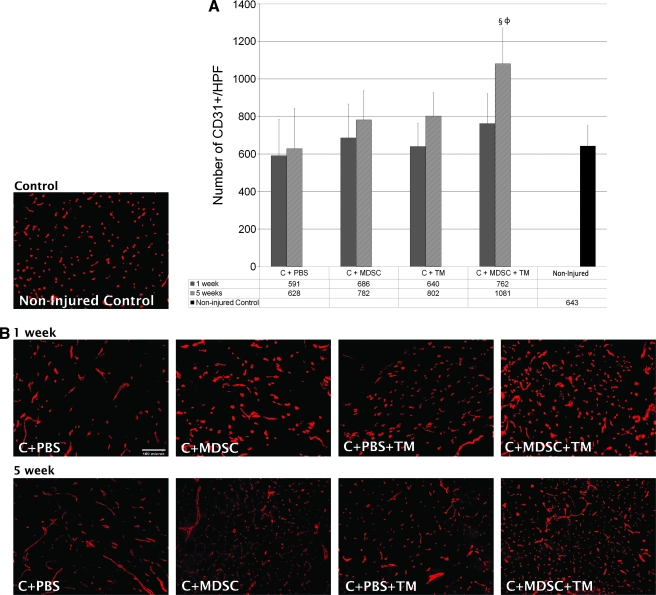
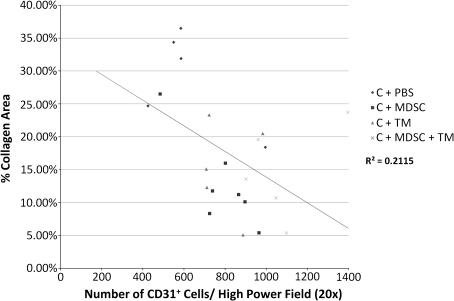
After 1 week of TM running, there was an increase in the number of capillaries, both in the presence and absence of MDSCs (C + PBS + TM = 640 ± 125 capillaries/high-power field of view; C + MDSC + TM = 762 ± 160) as compared with the C + PBS (591 ± 195), although this difference was not significant. When comparing the change in vascularity between the 1-week and 5-week time points, only C + MDSC + TM muscles demonstrated a significant increase in the number of capillaries (p = 0.03). At 5 weeks, C + MDSC + TM muscles also demonstrated an increase in vascularity (1081 ± 192) when compared with the C + PBS (628 ± 216, p = 0.00) group (Fig. 5). There was a significant and inverse relationship between skeletal muscle vascularity and fibrosis formation at both 1 and 5 weeks (R2 = 0.166; p = 0.02 and R2 = 0.211; p = 0.03, respectively) (Fig. 6).
FIG. 5.
CD31 immunofluorescent staining. (A) Comparison of the average number of CD31+ cells/high-powered field between the experimental groups. §Significantly different when compared with C + PBS at 5 weeks (p = 0.00). ΦSignificantly different when compared with C + MDSC + TM at 1 week (p = 0.03). (B) Immunofluorescence of CD31 (red) after 1- and 5-week treatment (20 × objective).
FIG. 6.
Relationship between skeletal muscle vascularity and percent collagen area after 5 weeks (R2 = 0.2115, p = 0.03).
Fate determination of transplanted cells
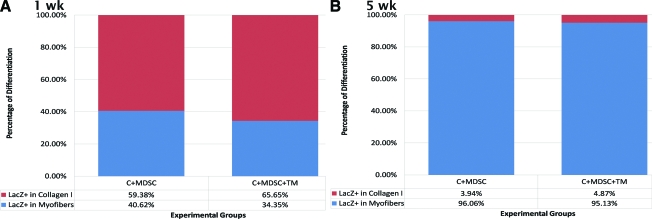
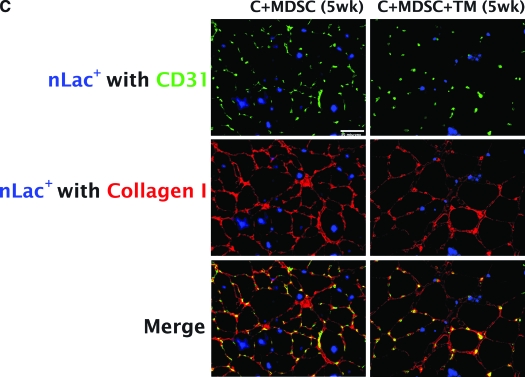
After 1 week, only 40.62 ± 22.73% and 34.35 ± 20.84% of the nLacZ+ cells were colocalized with myofibers for C + MDSC and C + MDSC + TM, respectively (Fig. 7A). However, after 5 weeks, a significant increase in the number of nLacZ+ cells localized within myofibers was seen for both C + MDSC (96.06 ± 3.94%, p = 0.00) and C + MDSC + TM (95.13 ± 4.87%, p = 0.00) groups when compared with the 1-week groups (Fig. 7B), suggesting that the majority of surviving nLacZ + donor cells terminally differentiated toward a myogenic lineage at the later time point. The remainder of the cells were colocalized to collagen I; none were colocalized with CD31, suggesting that donor cells did not terminally differentiate toward an endothelial lineage.
FIG. 7.
Terminal differentiation of nLacZ+ cells. (A) Percentage of nLacZ+ cells differentiated into myofibers and collagen 1 week after transplantation. (B) Percentage of nLacZ+ cells differentiated into myofibers and collagen 5 weeks after transplantation. (C) Immunohistochemistry of collagen I (red), CD31-positive cells (green), and nLacZ (blue).
Discussion
The development of biological therapies offers promise for the treatment of musculoskeletal injuries. However, progress in the development of cellular therapies to improve skeletal muscle healing after injury has been slow and limited by increased scar tissue formation,25 massive cell death after transplantation,26 and clinically less desirable administration methods, such as systemic delivery. It is clear that alongside the development of these therapies, concurrent rehabilitation protocols designed to maximize the efficacy of these biological interventions will become critical to optimize clinical outcomes. This study investigated the effect of mechanical stimulation on the growth kinetics of muscle stem cells and the in vivo myofiber regeneration of transplanted cells after an acute muscle injury. We also investigated the relationship between an exercise-induced increase in skeletal muscle vascularity and the formation of collagen.
Our results demonstrated that 5 weeks of daily TM running significantly increased the number of transplanted cells. These results suggest that exercise, in vivo, can influence the cellular growth kinetics after transplantation and enhance the engraftment efficacy of donor cells. Our findings are in agreement with the results of Palermo et al.,23 who demonstrated the addition of a TM running protocol significantly increased the number of systemically injected bone marrow–derived stem cells recruited to regenerate myofibers in an uninjured TA muscle.23 From this previous study, authors suggested that a specific set of molecular cues associated with physiological stress increases the myogenic contribution of donor bone marrow–derived stem cells. Further, it has been suggested that transplanted stem cells may respond to the skeletal muscle needs for regeneration and/or repair that result from muscle injury or overuse,27 much in the same way that resident muscle stem cells respond to injury or exercise-induced muscle damage.28 This study demonstrates that MDSCs, when delivered immediately after an acute injury, also respond to external mechanical loading cues through an increased participation in myogenic regeneration. This is important, considering it has recently been shown that there is a dose–response relationship between the engraftment of transplanted cells and improvements in skeletal muscle physiological functioning after injury.29 Additionally, 5 weeks after transplantation, greater than 95% of the nLacZ+ cells were localized within the myofibers for both C + MDSC and C + MDSC + TM groups, suggesting that the majority of donor cells terminally differentiated toward a myogenic lineage.
Still unclear are the underlying mechanisms by which exercise enhances the number of transplanted cells that have engrafted into myofibers within the host tissue. Do mechanotransductive pathways enhance cellular survival, or do they enhance the post-transplantation proliferation of cells within the host niche? Since there was no significant difference in the number of nLacZ+ cells present after 1 week of TM running when compared with the sedentary controls, we hypothesize that a comparable number of cells may have survived transplantation between the two groups. Therefore, the difference may lie in the subsequent behavior of surviving cells. In the absence of mechanical stimuli, it appears that transplanted cells fail to rapidly divide, as suggested by the fact that there was no significant difference in the number of transplanted cells 1 week and 5 weeks after transplantation in C + MDSC muscles. On the other hand, in the TM group, the number of donor cells present at 5 weeks was significantly greater than that observed after 1 week, suggesting that mechanotransductive pathways may promote the proliferation of donor cells after transplantation. This hypothesis is supported by our in vitro investigations demonstrating that just 24 h of continuous cyclical mechanical stimulation significantly decreases MDSC PDT and DT. Although the total number of engrafted cells was significantly higher after the addition of an exercise protocol, there was no significant difference in the average single fiber area of nLacZ+ cells between the C + MDSC versus C + MDSC + TM groups. This raises the question as to whether the donor cell-derived myofibers are capable of responding to hypertrophy cues resulting from muscle loading in a manner similar to host myofibers. It is possible that if we had prolonged the total number of training weeks, donor myofibers in the TM group may have demonstrated an increased hypertrophy in comparison to the sedentary controls. Alternatively, TM running may not be the best activity to induce donor cell hypertrophy. In this case, neuromuscular electrical stimulation or a strength training regimen may be a better alternative. Finally, it is possible that the engrafted myofibers fail to become innervated and are, therefore, not subject to the hypertrophy signaling mechanisms after differentiation. These questions should be the topic of future studies.
The formation of fibrosis has been shown to compete with myofiber regeneration for tissue area within damaged skeletal muscle, with one inhibiting the other.2 Fibrosis formation after injury has also been associated with an increased likelihood for recurrent injury.30 The results of this study demonstrated no significant differences in collagen formation between any of the groups 1 week after contusion. However, at 5 weeks, we did observe significantly less collagen formation in the C + PBS + TM, C + MDSC, and C + MDSC + TM, when compared with C + PBS. The fact that differences were only observed 5 weeks after injury is not surprising, given the development of fibrosis peaks the second week after muscle injury and continues for up to 35 days after injury.31,32
Our results suggest that the beneficial effect of muscle loading and/or stem-cell transplantation to inhibit collagen formation may be a result of an increase in skeletal muscle vascularity. In fact, the skeletal muscle environmental milieu and circulating factors have been shown to largely dictate cellular function, and the skeletal muscle vascular niche is nonrandomly associated with progenitor cell localization, activation, and proliferation.33 In this study, we found that the skeletal muscle vascularity was inversely related to collagen composition at both 1 week and 5 weeks. In fact, from the data we obtained, there was clearly one leverage point that, when removed from the regression analysis, strengthened considerably the relationship between CD31 and collagen to an R2 = 0.4871, p = 0.002. However, we did not remove this data point since we did not have a definitive reason to explain this sample's deviance from the mean. We also found that, in severely injured muscle, only a combination of MDSC transplantation and TM running resulted in significantly increased skeletal muscle vascularity at the later time point. These findings are consistent with a previous study from our laboratory which demonstrated that an increased vascularization of infarcted hearts transplanted with MDSCs was associated with a decreased scar tissue formation at 2 and 6 weeks.8 Findings from this study suggest that exercise may play a role in donor cell promotion of angiogenesis. Along these lines, it has been previously shown in vitro that cyclical mechanical stimulation enhances the secretion of vascular endothelial growth factor, the most potent angiogenic stimulator, by MDSCs.8 Moreover, it has been shown that an increased vascular endothelial growth factor is associated with an increased MDSC proliferation.20 The role of skeletal muscle vascularity on MDSC transplantation capacity, and its possible mediation by exercise, deserves further investigation.
Future studies should also investigate dosing and timing protocols designed to optimize the beneficial effect of muscle loading on skeletal muscle healing. Such studies will prove to be valuable not only in the development of autologous stem-cell transplantation to heal muscle injuries but also for providing a greater understanding of mechanotransductive pathways that may be critical in determining muscle precursor cell function.
Conclusions
This study demonstrated that mechanical stimulation favors MDSC proliferation, both in vitro and in vivo, and that exercise enhances MDSC transplantation after injury. Further, TM running, the transplantation of MDSCs, and a combination of the two therapies can prevent fibrosis formation 5 weeks after a muscle injury. This effect appears to be associated with increased skeletal muscle vascularity. These findings have important implications in the autologous use of stem cells for the treatment of musculoskeletal injuries and suggest there may be a synergistic effect between physical therapeutics and stem-cell therapy for the treatment of acute skeletal muscle injury.
Acknowledgments
This study was supported by the National Institutes of Health (NIH) K12 for Physical and Occupational Therapists—Comprehensive Opportunities in Rehabilitation Research Training (K12 HD055931), the Foundation for Physical Therapy, and the Department of Physical Medicine & Rehabilitation of the University of Pittsburgh. Authors are grateful to Mary Synnott for graphical assistance, Christopher Weiss for technical assistance, and the Center for Biological Imaging at the University of Pittsburgh for assistance with immunohistological staining and imaging.
Disclosure Statement
No competing financial interests exist.
References
- 1.Garrett W.E., Jr. Muscle strain injuries: clinical and basic aspects. [Report] Med Sci Sports Exerc. 1990;22:436. [PubMed] [Google Scholar]
- 2.Huard J. Li Y. Fu F.H. Current concepts review—muscle injuries and repair: current trends in research. J Bone Joint Surg. 2002;84A:822. [PubMed] [Google Scholar]
- 3.Qu-Petersen Z. Deasy B. Jankowski R. Ikezawa M. Cummins J. Pruchnic R. Mytinger J. Cao B. Gates C. Wernig A. Huard J. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morgan J.E. Hoffman E.P. Partridge T.A. Normal myogenic cells from newborn mice restore normal histology to degenerating muscles of the mdx mouse. J Cell Biol. 1990;111(6 Pt 1):2437. doi: 10.1083/jcb.111.6.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge T.A. Morgan J.E. Coulton G.R. Hoffman E.P. Kunkel L.M. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 6.Gussoni E. Soneoka Y. Strickland C.D. Buzney E.A. Khan M.K. Flint A.F. Kunkel L.M. Mulligan R.C. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature. 1999;401:390. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 7.Oshima H. Payne T.R. Urish K.L. Sakai T. Ling Y. Gharaibeh B. Tobita K. Keller B.B. Cummins J.H. Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12:1130. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 8.Payne T.R. Oshima H. Okada M. Momoi N. Tobita K. Keller B.B. Peng H. Huard J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts [see comment] J Am Coll Cardiol. 2007;50:1677. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 9.Carr L. Steele D. Steele S. Wagner D. Pruchnic R. Erikson J. Huard J. Chancellor M.B. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J. 2008;19:881. doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 10.Li Y. Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161:895. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brutsaert T.D. Gavin T.P. Fu Z. Breen E.C. Tang K. Mathieu-Costello O. Wagner P.D. Regional differences in expression of VEGF mRNA in rat gastrocnemius following 1 hr exercise or electrical stimulation. BMC Physiol. 2002;2:8. doi: 10.1186/1472-6793-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gavin T.P. Westerkamp L.M. Zwetsloot K.A. Soleus, plantaris and gastrocnemius VEGF mRNA responses to hypoxia and exercise are preserved in aged compared with young female C57BL/6 mice. Acta Physiol. 2006;188:113. doi: 10.1111/j.1748-1716.2006.01609.x. [DOI] [PubMed] [Google Scholar]
- 13.Faria F.E. Ferrari R.J. Distefano G. Ducatti A.C. Soares K.F. Montebelo M.I. Minamoto V.B. The onset and duration of mobilization affect the regeneration in the rat muscle. Histol Histopathol. 2008;23:565. doi: 10.14670/HH-23.565. [DOI] [PubMed] [Google Scholar]
- 14.Gregory T.M. Heckmann R.A. Francis R.S. The effect of exercise on the presence of leukocytes, erythrocytes and collagen fibers in skeletal muscle after contusion. J Manipulative Physiol Ther. 1995;18:72. [PubMed] [Google Scholar]
- 15.Hameed M. Orrell R.W. Cobbold M. Goldspink G. Harridge S.D. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise [see comment] J Physiol. 2003;547(Pt 1):247. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang H. Alnaqeeb M. Simpson H. Goldspink G. Changes in muscle fibre type, muscle mass and IGF-I gene expression in rabbit skeletal muscle subjected to stretch. J Anat. 1997;190:613. doi: 10.1046/j.1469-7580.1997.19040613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa A. Dalloul H. Hegyesi H. Apor P. Csende Z. Racz L. Vaczi M. Tihanyi J. Impact of repeated bouts of eccentric exercise on myogenic gene expression. Eur J Appl Physiol. 2007;101:427. doi: 10.1007/s00421-007-0510-z. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt B.T. Feduska J. Witt A. Deasy B.M. Robotic cell culture system for stem cell assays. Ind Robot. 2008;35:116. [Google Scholar]
- 19.Deasy B.M. Jankowski R.J. Payne T.R. Cao B. Goff J.P. Greenberger J.S. Huard J. Modeling stem cell population growth: incorporating terms for proliferative heterogeneity. Stem Cells. 2003;21:536. doi: 10.1634/stemcells.21-5-536. [DOI] [PubMed] [Google Scholar]
- 20.Deasy B.M. Qu-Peterson Z. Greenberger J.S. Huard J. Mechanisms of muscle stem cell expansion with cytokines. Stem Cells. 2002;20:50. doi: 10.1634/stemcells.20-1-50. [DOI] [PubMed] [Google Scholar]
- 21.Deasy B.M. Lu A. Tebbets J.C. Feduska J.M. Schugar R.C. Pollett J.B. Sun B. Urish K.L. Gharaibeh B.M. Cao B. Rubin R.T. Huard J. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasemkijwattana C. Menetrey J. Somogyi G. Moreland M.S. Fu F.H. Buranapanitkit B. Watkins S.C. Huard J. Development of approaches to improve the healing following muscle contusion. Cell Transplant. 1998;7:585. doi: 10.1177/096368979800700609. [DOI] [PubMed] [Google Scholar]
- 23.Palermo A.T. Labarge M.A. Doyonnas R. Pomerantz J. Blau H.M. Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev Biol. 2005;279:336. doi: 10.1016/j.ydbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Lee J.Y. Qu-Petersen Z. Cao B. Kimura S. Jankowski R. Cummins J. Usas A. Gates C. Robbins P. Wernig A. Huard J. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150:1085. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y. Foster W. Chan Y.S. Horaguchi T. Badlani N. Huard J. Inhibition of skeletal muscle fibrosis by neutralizing TGF-beta1 production. J Neurol Sci. 2002;199:S30. [Google Scholar]
- 26.Fan Y. Maley M. Beilharz M. Grounds M. Rapid death of injected myoblasts in myoblast transfer therapy. Muscle Nerve. 1996;1919:853. doi: 10.1002/(SICI)1097-4598(199607)19:7<853::AID-MUS7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Labarge M.A. Blau H.M. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 28.Grounds M.D. Age-associated changes in the response of skeletal muscle cells to exercise and regeneration. [Review] [110 refs] Ann NY Acad Sci. 1998;854:78. doi: 10.1111/j.1749-6632.1998.tb09894.x. [DOI] [PubMed] [Google Scholar]
- 29.Winkler T. von Roth P. Matziolis G. Mehta M. Perka C. Duda G.N. Dose-response relationship of mesenchymal stem cell transplantation and functional regeneration after severe skeletal muscle injury in rats. Tissue Eng. 2009;15:3. doi: 10.1089/ten.tea.2007.0426. [DOI] [PubMed] [Google Scholar]
- 30.Croisier J.L. Factors associated with recurrent hamstring injuries. [Review] [85 refs] Sports Med. 2004;34:681. doi: 10.2165/00007256-200434100-00005. [DOI] [PubMed] [Google Scholar]
- 31.Sato K. Li Y. Foster W. Fukushima K. Badlani N. Adachi N. Usas A. Fu F.H. Huard J. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- 32.Li Y. Foster W. Deasy B.M. Chan Y.S. Prisk V. Tang Y. Cummins J. Huard J. Transforming growth factor-beta 1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle—a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christov C. Chrétien F. Abou-Khalil R. Bassez G. Vallet G. Authier F.J. Bassaglia Y. Shinin V. Tajbakhsh S. Chazaud B. Gherardi R.K. Muscle satellite cells and endothelial cells: close neighbors and priviledged partners. Mol Biol Cell. 2007;18:4,1397. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]