Abstract
Adipose-derived stromal cells (ASCs) constitute a promising source of cells for regenerative medicine applications. Previous studies of osteogenic potential in ASCs have focused on chemicals, growth factors, and mechanical stimuli. Citing the demonstrated role electric fields play in enhancing healing in bone fractures and defects, we investigated the ability of pulsed direct current electric fields to drive osteogenic differentiation in mouse ASCs. Employing 50 Hz direct current electric fields in concert with and without osteogenic factors, we demonstrated increased early osteoblast-specific markers. We were also able to establish that commonly reported artifacts of electric field stimulation are not the primary mediators of the observed effects. The electric fields caused marked changes in the cytoskeleton. We used atomic force microscopy–based force spectroscopy to record an increase in the cytoskeletal tension after treatment with electric fields. We abolished the increased cytoskeletal stresses with the rho-associated protein kinase inhibitor, Y27632, and did not see any decrease in osteogenic gene expression, suggesting that the pro-osteogenic effects of the electric fields are not transduced via cytoskeletal tension. Electric fields may show promise as candidate enhancers of osteogenesis of ASCs and may be incorporated into cell-based strategies for skeletal regeneration.
Introduction
Craniofacial abnormalities such as cleft palate as well as other bone defects are a significant clinical problem.1,2 Patients undergo extensive surgical intervention often requiring bone grafting. This creates a substantial need for bone or a viable substitute. The ideal source for donor material for craniofacial reconstruction is autogenous bone from the iliac crest, the rib, or split calvarial grafts. The local delivery of multipotent cells to a defect site where they could form the foundation of newly regenerating tissue may constitute a viable alternative to autogenous bone and the problems associated with donor site morbidity. However, multipotent donor cells often require growth factor and small molecule conditioning for increased rates of differentiation into useful grafts.3–5 The goal of this work was to determine if an alternative, noninvasive, technique using electric fields can be used to enhance the differentiation of adult mesenchymal stromal cells ex vivo for faster bone formation in vivo. An additional motivation for exploring the noninvasive techniques relates to the Federal Drug Administration guidelines for clinical therapies with regards to the “minimally manipulated” cell sources to be used at the point of care.6 Such a therapy could, in theory, be exempt from regulation under the same surgical procedure provision, using cells that are autologous and used within a short period of time.
Adipose-derived stromal cells (ASCs) are an attractive autologous, multipotent cell source for minimally manipulated clinical therapies not only because of their capacity for osteogenic differentiation but also for their abundance, accessibility, and ease of culture.7–9 Our laboratory has worked toward characterizing the nature and capacity for differentiation of the ASC population.10 Clinical applications are beginning to emerge from a better understanding of ASC biology. We have previously demonstrated the ability of mouse ASCs (mASCs), without further manipulation, to regenerate bone in critical-sized calvarial defects.11 Rapid progress has even yielded a successful application of ASCs in humans.12 Factors to differentiate ASCs include chemicals, growth factors, and mechanical stimuli.13,14 However, little attention has been paid to electromagnetic stimulation as a viable modulator of cell differentiation.
In light of the important role electric fields play in organisms during embryogenesis and regeneration, it is surprising that electric fields have not been pursued more intensively as potential mitogenic or osteogenic factors.15,16 Electric fields impact the biology of orthopedic tissues17; chick tibiae and mouse calvaria have shown increased bone matrix formation in response to the capacitively coupled electric fields.18 Pulsed electric fields have also been used therapeutically in humans to increase union rates in refractory fractures.19 The osteogenic properties of pulsed electric fields suggest a possible therapy involving the treatment of ASCs with electric fields ex vivo to affect the increased bone formation rates after implantation. Evidence to substantiate this proposed therapy comes from current studies on electromagnetic stimulation driving differentiation in cells.20–22 These studies are often limited in scope to electrophysiologically active cell types such as cardiomyocytes.23 The effects of electric fields on the differentiation of stem or multipotent cells toward osteogenic lineages in general is not well known. We specifically investigated the effects of pulsed direct current (DC) electric fields on ASCs with an eye toward potential clinical therapies.
Materials and Methods
Chemicals and media
Dulbecco's modified Eagle's medium (DMEM) (Cat. No. 10569-010), Fura-2 AM (Cat. No. F-1221), 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein (Cat. No. C368), rhodamine phalloidin (Cat. No. R415), and Alexa Fluor 488–conjugated actin (Cat. No. A12373) were purchased from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Gemini Bioproducts (Woodland, CA). Osteogenic components, ascorbic acid 2-phosphate (Cat. No. A8960) and β-glycerophosphate (Cat. No. G6251), were purchased from Sigma-Aldrich (St. Louis, MO) as was collagenase A (Cat. No. C9891). Primary antibodies against osteopontin (OPN; Cat. No. sc-10593), type I collagen (Col I, Cat. No. sc-8784), and osteocalcin (OCN, Cat. No. sc-18319) were all obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-runt-related transcription factor 2 (runx2) primary antibody was obtained from Cemines (Golden, CO; Cat. No. AB/RNT20).
Tissue harvest and primary cell culture
All experiments were performed in accordance with the Stanford University Animal Care and Use Committee guidelines. CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA). The inguinal fat pads from 3-week-old CD-1 mice were carefully dissected and washed sequentially in a betadine and phosphate-buffered saline (PBS) (Fisher Scientific, Fair Lawn, NJ) solution.10 The fat pads were finely minced and digested with 0.075% collagenase A with vigorous shaking, 150 rpm, in a 37°C water bath for 30 min. Collagenase was neutralized with an equal volume of standard growth media containing DMEM with 10% FBS and 1% penicillin/streptomycin. The cell suspension was filtered through a cell strainer (Falcon, BD Biosciences, San Jose, CA; 100 μm pore size) to remove larger, undigested fat tissue. After filtration, the cells were centrifuged at 1000 rpm for 5 min. The cell pellet was resuspended in fresh growth media and plated in culture dishes. After 24 h, nonadherent cells and debris were washed from the plate, and the cells were cultured until 80% confluence and then passaged. Cells up to the second passage were used for experiments at densities of 104 cell/cm2.
Electrical stimulation and chamber design
A chamber was fabricated to expose adherent cells to a uniform pulsed DC electric field. Commercial stimulation systems designed to increase healing rates in fractures have shown osteogenic effects with frequencies ranging from 2 to 123 Hz.24 The applied frequency was chosen within this range to reflect attributes of those successful commercial systems. The amplitude was chosen such that it was great enough to possibly bias voltage-gated ion channels to impinge on as many signaling pathways as possible. To create a defined cell stimulation area, optically clear plastic and mylar were machined into chambers (Fig. 1). Each device was constructed of a 100 mm culture dish, 100-μm-thick Melinex sheet with two-sided adhesive (Fralock Division Lockwood Industries, Valencia, CA), a machined acrylic chamber, and four 15 cm agarose salt bridges. The Melinex sheet forms the sidewalls of the culture area, while the acrylic chamber bounds the top and the tissue culture dish serving as the cell substrate. For rapid prototyping, we used a CO2 laser cutter (Universal Laser Systems, Scottsdale, AZ) to machine the acrylic and the Melinex. The cell culture area was 5 cm long, 5 cm wide, and 100 μm deep. The acrylic chamber has a 3 mm wide, 5 cm long vent cut in the center, allowing access to the culture area for gas exchange and uniform cell loading. The chamber layout was designed using the software AutoCAD (AutoDESK, San Rafael, CA). For assembly, the Melinex channel structure was aligned and laminated to a laser cut acrylic part, and then the assembly was adhered to a 100 mm polystyrene culture dish (Nunclon; Thermo Fisher Scientific, Waltham, MA), creating a uniform cell culture chamber (Fig. 1A). The chamber was flooded with sterile filtered 70% ethanol and incubated overnight for sterilization. The ethanol was washed out multiple times with FBS containing DMEM and incubated again overnight in growth medium to elute any absorbed ethanol from the chamber. A new device was used for each experiment. Salt bridges of 2% agarose in DMEM were cast in 15 cm long, 1.6 cm outer diameter, 1.3 cm inner diameter, U-shaped glass tubing. A circuit was created to pulse a DC electric field across the cell culture area using a power supply (Agilent 6614C) switched with an NPN transistor (Fairchild BU406) driven by a function generator (Agilent 33250A) creating a square wave of 50 Hz and 6 V/cm peak-to-peak amplitude (Fig. 1B). The waveform was monitored by an oscilloscope to verify the applied voltage and frequency. The whole assembly was placed in a humidified incubator at 37°C and 5% CO2 (Fig. 1C).
FIG. 1.
Design of stimulation chamber. (A) Schematic of chamber design. (B) Circuit diagram of the function generator using a transistor to switch the power supply at the appropriate frequency. (C) Assembled chambers and agarose salt bridges connecting the cell culture chamber to the electrodes and saline reservoirs. The design allows for the easy exchange of new salt bridges. Color images available online at www.liebertonline.com/ten.
Actin imaging and immunofluorescence
Before permeabilization, mASCs were treated for 6 h in a 50 Hz, 6 V/cm peak-to-peak, DC electric field. mASCs were permeabilized to label areas of active actin polymerization.25 The cells were then washed with PBS and permeabilized for 3 min in cytoskeleton buffer containing sucrose (CBS) (10 mM 2-(N-morpholino)ethane-sulfonic acid (MES), pH 6.1, 138 mM KCl, 3 mM MgCl2, 2 mM ethylene glycol tetraacetic acid (EGTA), 0.2 mg/mL saponin, 1 mM ATP, and 15 μg/mL Alexa Fluor 488–conjugated actin) in the presence of the electric field. The cells were washed with CBS and then fixed in CBS with 0.5% Triton X-100 and 0.25% glutaraldehyde and postfixed in CBS with 1% glutaraldehyde. The cells were washed again with CBS and incubated for 15 min with rhodamine phalloidin. The cells were then washed with buffer and mounted on a Zeiss LSM confocal microscope for imaging.
For indirect fluorescent imaging of osteogenic markers, cells were stimulated for 7 or 21 days with electric fields. The cells were fixed for 15 min with 4% paraformaldehyde in PBS. The cells were washed in 1% Triton X-100 in PBS for 5 min and then blocked in 10% normal serum in PBS containing 1% bovine serum albumin, 0.1% cold fish skin gelatin, 0.1% Triton X-100, and 0.05% Tween 20 for 15 min. The cells were incubated at 4°C overnight in primary antibody at 1:100 dilution in PBS containing 1% bovine serum albumin, 0.5% Triton X-100, and 0.1% cold fish skin gelatin. The cells were washed extensively to remove primary antibody against OPN, Col I, runt-related transcription factor 2 (Runx2), or OCN and detected with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies diluted at 1:200 in PBS containing 0.5% Tween 20. The plates were imaged on a Zeiss LSM confocal microscopy with the appropriate filters for a FITC fluorophore with the pinhole set to maximum.
Cell proliferation
Cell proliferation was performed by cell counting. mASCs were seeded in stimulation chambers at a density of 104 cells/cm2. To assess the effects of applied electric fields on cell proliferation, cells were grown in standard growth medium and exposed to 50 Hz, 6 V/cm electric fields for 6 h per day. The growth medium was changed immediately after each field application. On days 1, 3, 5, 7, and 10, cells were trypsinized and counted. The means of total chamber counts and standard deviations were calculated. The data were displayed as mean ± standard error of the mean (SEM).
Osteogenic differentiation, alkaline phosphatase, and alizarin red staining
Initially, cells were cultured in growth medium containing DMEM with 10% FBS and 1% penicillin–streptomycin. After subculturing and expansion, mASCs were replated in electric stimulation chambers at a density of 104 cells/cm2. After overnight attachment, mASCs were treated with basic osteogenic differentiation media (ODM) (DMEM, 10% FBS, 100 mg/mL of ascorbic acid, 10 mM β-glycerophosphate, 100 IU/mL of penicillin, and 100 IU/mL of streptomycin), and the electric field treatments were begun on the experimental group for up to 21 days. The medium was changed every day. Staining and quantification of alkaline phosphatase (ALP) activity were determined after 7 days. Alizarin red S staining was performed after 21 days of osteogenic differentiation in the presence of intermittent electric fields. RNA was harvested on day 21 to assess gene expression of OPN, Col I, Runx2, and OCN.
The medium was removed, and the cells were washed twice with PBS. The cells were fixed with 60% acetone and 40% citrate working solution for 30 s. Subsequently, the cells were washed with deionized water for 45 s and stained for 30 min with a diazonium salt solution comprised of Fast Blue RR salt (0.024%) and 4% naphthol AS-MX phosphate alkaline solution (Sigma-Aldrich). ALP-positive cells were stained blue.
Calcium deposition was assessed with alizarin red S (Cat. No. A5533; Sigma-Aldrich). Cultured cells were washed with PBS, fixed for 15 min in 100% ethanol, and stained with 0.2% alizarin red solution (pH 4.2) for 30 min. Stained cells were extensively washed with deionized water to remove the nonspecific precipitation. The positive red staining represents calcium deposits on the differentiated cells. Photographs were obtained and presented for the analysis of late-stage osteogenic differentiation.
Quantitative alkaline phosphatase activity
ALP activity was also determined using a biochemical colorimetric assay with the Sigma kit (Document 104) as described by the manufacturer. Briefly stated, the cells were washed with cold PBS. The cells were scraped into a radio immuno precipitation assay (RIPA) buffer (containing 50 mM Tris–HCl of pH 7.5, 150 mM NaCl, 5% glycerol, 1 mM ethylenediaminetetraacetic acid, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, and 0.25% Na-deoxycholate) and centrifuged. The enzymatic ALP activity in the supernatant of the cell lysate was assayed by measuring the p-nitrophenol formed from the enzymatic hydrolysis of p-nitrophenylphosphate, used as the substrate, at 405 nm. This quantity was normalized to total protein quantity measured using the bicinchoninic acid assay (BCA) protein assay reagent kit (Pierce, Rockford, IL) according to the manufacturer's instructions.
Quantitative reverse-transcription–polymerase chain reaction
To study gene regulation by periodic exposure to electric fields, mASCs were plated in culture chambers and treated with either standard growth medium alone or standard growth medium supplemented with a 6 V/cm peak-to-peak, 50 Hz field for 6 h each day for 7 and 21 days. At each time point, cells were harvested, and the RNA was isolated by RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocol. The RNA was quantified by spectrophotometry. The RNA was treated with DNase I (Applied Biosystems/Ambion, Austin, TX) according to the manufacturer's instructions. Reverse transcription was performed on 1 μg of the total RNA isolated from each sample using oligo(dT)-priming in separate 50 μL reactions according to the manufacturer's recommendations (Taqman Reverse Transcription Reagent Kit; Applied Biosystems, Foster City, CA). Quantitative real-time polymerase chain reaction analysis was performed in 20 μL reactions using Sybr Green Mastermix (Applied Biosystems) for 15 min at 95°C for initial denaturing, followed by 40 cycles of 95°C for 30 s and 60°C for 1 min in an ABI Prism 7900HT sequence detection system. The target genes and primer sequences are listed in Table 1. Each primer set was first tested to determine the optimal concentrations, and the products were run out on a 2% agarose gel to confirm the appropriate size. Complimentary DNA prepared from the pooled samples was used to construct a calibration curve for each gene. Gene expression of each sample was calculated by normalizing the expression levels of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Applied Biosystems). Each reaction was performed in triplicate, and the mean and standard deviation were calculated. Multiple independent experiments were conducted with similar trends.
Table 1.
Gene Primer Sequences Used for Quantitative Real-Time Polymerase Chain Reaction
| Gene name | Accession number | Primer sequence (5′ to 3′) |
|---|---|---|
| Alkaline phosphatase | NM_007431 | Forward: GTTGCCAAGCTGGGAAGAACAC Reverse: CCCACCCCGCTATTCCAAAC |
| Collagen I | S67530 | Forward: AACCCGAGGTATGCTTGATCT Reverse: CCAGTTCTTCATTGCATTGC |
| Osteopontin | AF_515708 | Forward: GATTTGCTTTTGCCTGTTTGG Reverse: TGAGCTGCCAGAATCAGTCACT |
| Runx2 | NM_009820 | Forward: CGGTCTCCTTCCAGGATGGT Reverse: GCTTCCGTCAGCGTCAACA |
| Osteocalcin | 12096 | Forward: GGGAGACAACAGGGAGGAAAC Reverse: CAGGCTTCCTGCCAGTACCT |
| GAPDH | NM_008084 | Forward: AGGTCGGTGTGAACGGATTTG Forward: TGTAGACCATGTAGTTGAGGTCA |
Runx2, runt-related transcription factor 2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
Reactive oxygen species detection
Reactive oxygen species (ROS) generation was assessed by loading cells with 5-(and-6)-carboxy-2′,7′–dichlorodihydrofluorescein diacetate and measuring its intracellular conversion to highly fluorescent dichlorofluorescein.26 Cells were exposed to a 6 V/cm peak-to-peak, 50 Hz field for 6 h. Control cells were maintained in the same conditions with salt bridges, but without an applied field. Positive controls were incubated with 100 μM H2O2 for 90 min before harvesting. After 6 h, the medium was discarded and replaced with 25 μM 5-(and-6)-carboxy-2′,7′–dichlorodihydrofluorescein diacetate in growth medium and incubated for 30 min at 37°C. For imaging, the cells were washed in PBS multiple times and resuspended in PBS and imaged with an LSM 5 Pascal confocal laser scanning on a Zeiss 200M axiovert (Carl Zeiss Microimaging, Thornwood, NY). The cells were excited with a 488 nm argon laser and detected with a 515 nm long pass emission filter with the pinhole set to maximum. For quantitation, the medium was aspirated, and the cells were washed in PBS and harvested with trypsin. The cells were centrifuged, washed, and resuspended in PBS and pipetted into black Corning Costar 96-well plates. The wells were excited at 495 nm and detected at 529 nm with the well scan function on a Molecular Devices Spectramax M2 fluorescent plate reader. The means and SEMs were calculated, and the data were expressed as the mean ± SEM.
Calcium imaging
Real-time intracellular levels of calcium were quantified using ratiometric imaging in chambers similar in construction to the culture chambers. Quartz chambers were constructed of acrylic laminated to quartz slides with Melinex, creating a channel for cell culture and medium reservoirs. The chambers were incubated with 10 μg/mL fibronectin to promote cell adhesion. Cells were loaded into the channel area through the machined reservoirs and allowed to adhere for 24 h. Before electric field exposure, cells were incubated with 10 μM Fura-2 AM (Molecular Probes, Eugene, OR) for 30 min at 31°C in phenol red–free DMEM and then washed with fresh DMEM and 1% FBS. Following Fura-2-AM loading, the cell-seeded chambers were mounted on the stage of the fluorescent microscope, and electrodes and 6 cm agarose salt bridges were connected. The cells were left undisturbed for 30 min to equilibriate. Fluorescent images were collected at 10-s intervals for 5 min before applying a voltage across the cells, for 5 min during which voltage was applied, and 5 min after the electric field was terminated. All experiments were performed at room temperature to reduce Fura-2 AM dye compartmentalization.
Cyclic adenosine monophosphate quantification
Cells were plated and allowed to adhere for 24 h in stimulation chambers. The chambers were then stimulated with pulsed electric fields for 6 h. Control cells were maintained in the same conditions but not exposed to the electric fields. The cells were then enzymatically lifted from the chambers with trypsin, centrifuged, washed with PBS, and lysed. The activity of cyclic adenosine monophosphate (cAMP) in the supernatant of the cell lysate was assayed with a competitive immunoassay for quantitation of total cAMP (Sigma CA200) according to the manufacturer's protocol. This quantity was normalized to total protein amount, measured using the bicinchoninic acid protein assay reagent kit (Pierce) according to the manufacturer's instructions.
Cell mechanical property investigation
Cell mechanical properties before and after electric field stimulation were characterized by force spectroscopy with an atomic force microscope (AFM). An Agilent 5500 AFM (Agilent Technologies, Santa Clara, CA) mounted on a Zeiss 200M Axiovert inverted microscope (Carl Zeiss Microimaging) was used to precisely position the AFM tip on the cell. A silicon cantilever (Novascan Technologies, Ames, IA) with a nominal spring constant of 30 mN/m with a 5 μm diameter borosilicate glass sphere attached to the tip was mounted on the AFM. The spring constant of each cantilever was calibrated with Sader's method by measuring the thermal noise spectrum of the cantilever deflection signal with a Labview DAQ card (NI 9215; National Instruments Corporation, Austin, TX). mASCs were enzymatically lifted from preconfluent 10 cm culture dishes. Cells were plated on 22 × 22 mm No. 2 glass coverslips (VWR International, West Chester, PA) and allowed to adhere overnight. Coverslips with cells for electrical stimulation were placed carefully into a chamber similar to the previous chambers fabricated from an acrylic top plate affixed to a tissue culture dish with a 300 μm space created by two layers of Melinex. The coverslips were positioned in the space such that they formed the bottom of the channel with the cells plated on the top surface. This created a 100 μm conductive medium channel over the top of the cells. U-shaped glass tubes filled with 2% agarose salt bridges were used to complete the circuit between the culture chamber and saline electrolysis reservoirs. The cells were stimulated at 10 V/cm for 6 h. The coverslips were washed in standard growth medium and fitted into the AFM stage within a liquid cell. The liquid cell was subsequently filled with 0.6 mL CO2 independent medium (Cat. No. 18045-088; Invitrogen) containing 10% serum and 1% penicillin/streptomycin. The AFM stage and liquid cell were mounted on the AFM, and the cantilever with a closed-loop piezo scanner was lowered into the liquid cell. Three to five locations within each cell were chosen for force deflection curves to reflect distinct regions within the cell: the nucleus, perinuclear cell body, and extended lamellipodium. The AFM probe approached the cell from a free deflection point and indented at a speed of 0.4 μm/s. The maximum indentation depth was controlled by a preset force limit to avoid damaging the cell. Force distance curves were recorded during the indentation and retraction of the probe from these curves; the cell apparent elastic modulus could be extracted by fitting experiment data with Hertz model for elastic indentation.27
 |
Where F is the indentation force, E the Young's modulus, ν the Poisson ratio (typically between 0.3 and 0.5 for soft samples such as cells, 0.5 was used for this study), R the radius of the indenting sphere, and δ the indentation depth.28
Cells (n = 25) were chosen randomly to be probed in three to five locations within the cell. These points were averaged, and the overall population modulus was expressed as the mean ± SEM. Statistically significant differences in cell stiffness between population densities were assessed with a paired Student's t-test.
Data analysis
Data are expressed as mean ± SEM. Runx2, Col I, OPN, ALP, and OCN gene expression levels were normalized against GAPDH assayed in the same sample tube. Paired Student's t-tests were performed to compare the control cells and cells exposed to electric fields. A value of p ≤ 0.05 was considered significant.
Results
Cell proliferation and morphology
We quantified the effects of pulsed DC electric fields on mASC proliferation. mASCs were plated in stimulation chambers at 104 cells/cm2. Experimental chambers were exposed to a square wave with a fixed polarity, a 50 Hz, 6 V peak-to-peak, pulsed DC electric field, for 6 h per day. Control cells were maintained in the stimulation chambers with no exposure to electric fields. All cells were cultured in standard growth medium. Twenty-four hours after plating, mASCs were spread with distinct cell edges and lamellipodia extending in random directions (Fig. 2A). Control cultures showed typical confluent morphology with randomized cell orientation and increasing cell density over time in culture (Fig. 2B–D). One of the most remarkable effects of the electric field was its influence on cell morphology and orientation. The pulsed DC fields caused the cells to align perpendicularly to the field vector after the first treatment duration of 6 h on the first day (Fig. 2E). The alignment was more clearly discernable over successive treatments as shown on days 3, 5, and 10 (Fig. 2F–H) compared with controls on days 3, 5, and 10 (Fig. 2B–D). A preponderance of cell orientation persisted even 24 h after the field exposure (data not shown). Over the course of electric field exposure, the cells increased their elongation in the direction perpendicular to the field. The cells continued to proliferate and became densely packed in arrays perpendicular to the electric field vector. The difference between the control and the electric field-exposed cells was profound even after only 3 days of stimulation (Fig. 2F). Other researchers have reported mitogenic effects of electric fields on cell populations; we did not observe the same.29 Over time both the control and the field-exposed cells grew and increased in density equally. Despite the dramatic changes in cell shape and 16 orientations, it appeared that the applied electric fields had little influence on the growth of mASCs (Fig. 2I).
FIG. 2.
The electric field caused dramatic changes in cell morphology and alignment perpendicular to the field direction but did not cause changes in proliferation images of (A–D) the control cells and (E–H) the field-exposed cells after 6 V/cm, 50 Hz electric field stimulation for 6 h per day after (A) and (E) 1 day; (B) and (F) 3 days; (C) and (G) 5 days; and (D) and (H) 10 days. Strong alignment perpendicular to the electric field can be seen even after only (E) 1 day, and the alignment persists with daily stimulation. The arrow shows the electric field vector with the anode on the left and cathode on the right. Scale bar represents 500 μm. (I) Growth curve comparing cells stimulated electrically and control populations. The electric field did not show a significant effect on cell proliferation over a 10-day period. Color images available online at www.liebertonline.com/ten.
To examine the nature of the cytoskeletal reorganization, we permeabilized cells and used fluorescently conjugated actin to label sites of actively polymerizing actin in response to the electric field treatment. The preexisting actin filaments within the cell were indicated by rhodamine phalloidin staining in red. Control cells did not show significant green staining, suggesting that they are not polymerizing actin and are fairly immobile in the control cultures (Fig. 3A). After 6 h of exposure to pulsed DC electric fields, there was evidence of actin accumulation on the cathode-facing side of the cells (Fig. 3B) and often on the trailing edges (Fig. 3C) as indicated by the incorporation of green fluorescent actin. The detail of Figure 3C (Fig. 3D) shows almost exclusively new actin incorporation at the trailing edges of a cell in response to the electric fields. The incidence of newly incorporated actin along stress fibers and at what appear to be the focal adhesion sites suggested an increase in cytoskeletal stresses in cells exposed to electric fields.
FIG. 3.
mASCs exposed to pulsed electric fields showed changes in actin cytoskeletal architecture. (A) A single control mASC, not exposed to an electric field, does not show extensive incorporation of newly polymerized actin (green) at its focal adhesion sites along the cell periphery; the green fluorescent actin is mostly incorporated in the perinuclear region of the cell body, while rhodamine phalloidin (red) strongly partitions to the preexisting mature stress fibers in the cell. (B) An mASC treated for 6 h in an electric field shows alignment of the stress fibers (red) perpendicular to the electric field direction (large white arrow) with accumulation of newly incorporated actin (green, small white arrows) on the cathode-facing side of the cell. (C) A mASC treated for 6 h with 50 Hz, 6 V/cm peak-to-peak, DC electric field shows evidence of increasing cytoskeletal stress by accumulation of new actin (green) at focal adhesions on the trailing edge as the cell migrates toward the cathode. The white arrow indicates the direction of the field with the cathode on the right. (D) A magnified view of the white box in (C) shows the adhesion sites at the trailing edge (small white arrows) are clearly new actin incorporation by the exclusive green clolor and the lack of rhodamine phalloidin (red) staining at the edge. mASC, mouse adipose-derived stromal cell; DC, direct current. Color images available online at www.liebertonline.com/ten.
Osteogenic gene expression
The marked changes in cell morphology induced by the electric field inspired us to investigate the electric field's ability to influence lineage commitment in mASCs. Previous studies have confirmed that mechanical cues driven by changes in cell shape can be linked to differentiation.30 We examined the expression of a number of critical osteogenic markers including the transcription factor, runt-related transcription factor 2 (Runx2/Cbfa), which is essential for osteoblastic differentiation and the formation and maintenance of bone.31 Many osteogenic genes including the early osteogenic marker, Col I, contain consensus Runx2-binding sites in their promoter regions. OPN, another protein expressed early in the osteogenic differentiation cascade, is one of the most abundant noncollagenous proteins in the extracellular matrix of the bone.32,33 The role of OPN in extracellular matrix has been shown to be central to the formation of bone, regulating both bone cell attachment and mineralization.34,35 Additionally, OPN and ALP expression has been found to be temporally concurrent.36 Because of the integral role of these four osteogenic factors in bone formation, we quantified changes in the mRNA levels in response to the electric field stimulation for Runx2, Col I, OPN, and ALP. For evidence of terminal differentiation, we assessed OCN expression and stained with alizarin red to detect bone nodule formation.
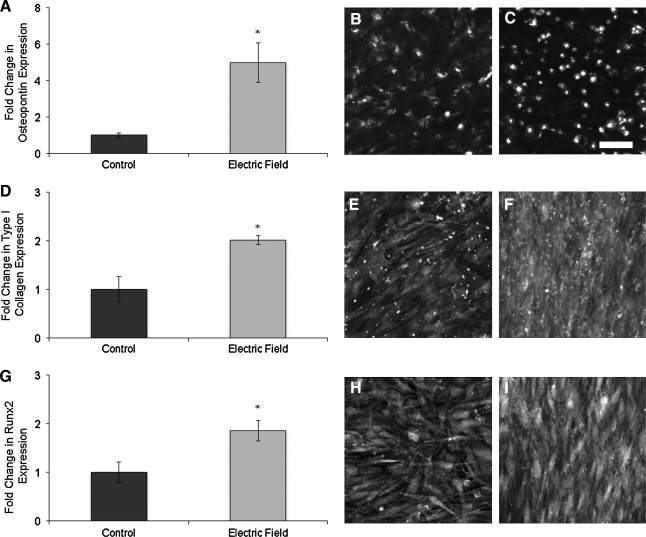
mASCs were plated in stimulation chambers and cultured in ODM. Controls were maintained only in ODM, while the experimental group was exposed to 50 Hz, 6 V/cm peak-to-peak, pulsed DC electric fields for 6 h per day for 7 and 21 days. The expression levels of ALP, OPN, Col I, Runx2, and OCN were normalized to GAPDH expression in the same sample. After 7 days of electrical stimulation in the presence of osteogenic factors, ALP showed an increase in both gene expression and enzymatic activity over controls (Fig. 4A, B). The ALP staining was congruent with the gene expression results, and the alignment of the cells in response the electric field was even evident in the micrograph (Fig. 4C, D). Of the early osteogenic markers, OPN showed the greatest increase in gene expression in response to the electric field stimulation, a fivefold increase (p < 0.05). This was also reflected in the immunofluorescence imaging (Fig. 5B, C). The expression of Col I and Runx2 also increased to a lesser extent, but the increases were both significant (p < 0.05) (Fig. 5D, G).
FIG. 4.
mASCs exposed to a pulsed electric fields for 7 days showed an increase in alkaline phosphatase (ALP) expression. After 7 days, both (A) enzymatic activity and (B) ALP gene expression were increased over unstimulated control cells. ALP (C) control and (D) experimental staining showed a similar trend. (D) The perpendicular alignment and organization can be seen in the electric field–treated cells. *p < 0.05 relative to controls. Color images available online at www.liebertonline.com/ten.
FIG. 5.
Gene expression of early osteogenic markers. (A) Osteopontin shows the greatest increase in expression after 21 days of electric field treatment over controls. Immunofluorescence detection of (B) control and (C) electric field–treated groups show a similar trend. (D) Type I Collagen (Col I) and (G) runt-related transcription factor 2 (Runx2) show similar levels of increased gene expression which is also reflected in their immunostains. (E) Control Col I versus (F) electric field–treated Col I, and (H) control Runx2 versus (I) electric field–treated Runx2 expression. *p < 0.05.
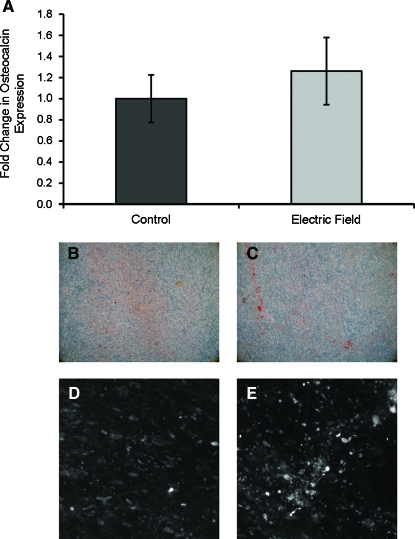
Terminal osteogenic differentiation was assessed with alizarin red staining and OCN gene expression by quantitative real-time polymerase chain reaction after 21 days of differentiation under the influence of electrical fields. No significant differences in OCN expression between electric field stimulated and control cultures were evident (Fig. 6A). This was also seen in the alizarin staining and immunofluorescence labeling (Fig. 6B–E). Additionally, the overall matrix accumulation was very low in both the control and experimental groups as evidenced by the apparent lack of alizarin red staining and low signal-to-noise ratio in the immunofluorescence images.
FIG. 6.
Terminal osteogenic differentiation. (A) Osteocalcin was not expressed at increased levels over controls after 21 days of electric field treatment. (B) Control and (C) electric field–treated cultures for 21 days did not show significant differences in alizarin red staining, indicating an overall lack of calcium accumulation in both control and experimental cultures. This lack in terminal osteogenic response was mirrored by (D) control and (E) electric field immunofluorescence staining of osteocalcin which did not show significant differences. Color images available online at www.liebertonline.com/ten.
Reactive oxygen species
We investigated the possible transduction pathways for the observed influence of electric fields on early osteogenic gene expression by examining the potential biochemical signaling pathways. While the fields constitute nonionizing radiation, other reports have traced changes in gene expression to oxidative stress caused by similarly low intensity electric fields.23,37 Thus, we sought to investigate the possible accumulation of ROS in mASCs in response to the pulsed DC fields. We showed by quantifying dichlorofluorescein fluorescence that there was no significant accumulation of ROS generated by the application of pulsed DC electric fields (p > 0.05) (Fig. 7A–D).
FIG. 7.
Biochemical mediators of electric fields in mASCs. (A) Control and (B) electric field–treated cells did not show an obvious difference in dichlorofluorescein fluorescence, indicating these electric fields did not cause reactive oxygen species accumulation. (C) The positive control did show a significant increase in dichlorofluorescein fluorescence, confirming the presence of reactive oxygen species in mASCs in response to H2O2 exposure. Quantification of fluorescence in a similar experiment (D) also shows the same trend. *p < 0.05 relative to controls. (E) Traces of individual cells' fluorescence intensity ratios demonstrating an increase in cytosolic calcium after the initiation of a 6 V/cm peak-to-peak 50 Hz DC electric field across a population of mASCs. Corresponding pseudocolored ratiometric images; (F) before the field, (G) 1 min after field initiation (4 min after start of experiment), and (H) 2 min after cessation of the field (8 min after beginning of experiment). These images demonstrated cells respond to the field with transient increases in cytosolic calcium as indicated by the change in color from blue to green to red. (I) Cyclic adenosine monophosphate is significantly reduced after exposure of mASCs to pulsed DC electric fields for 6 h at 6 V/cm peak-to-peak and 50 Hz. *p ≤ 0.01. Color images available online at www.liebertonline.com/ten.
Cyclic AMP and calcium transients
We also quantified key intracellular second messengers such as calcium and cAMP to determine other signaling pathways that could be responsible for the observed gene expression changes. mASCs were plated in electric stimulation chambers and loaded with Fura 2. The cells were allowed to equilibriate in phenol red–free medium and then an electric field was initiated. Our data demonstrate a small, but measurable increase in cytosolic-free calcium in response to the exposure of these cells to the pulsed DC electric field (Fig. 7E). Pseudocolored images showed that the peak calcium response occurred 30 s after initiation of the field (Fig. 7G) and declined to baseline values (Fig. 7F) within a minute of termination of the pulsed electric field (Fig. 7H). To observe changes in cAMP synthesis, mASCs were exposed to a pulsed DC, 50 Hz, 6 V/cm peak-to-peak electric field for 6 h. Control cells were cultured in stimulation chambers but not exposed to electric fields. Consistent with other studies, we showed a significant decrease in cAMP in response to an electric field treatment (p < 0.05) (Fig. 7I).38
Cytoskeletal tension in response to electric field stimulation
The accumulation of newly polymerized actin at focal adhesion sites (Fig. 3B) suggested an increase in stress fiber formation in response to cytoskeletal stresses. We, therefore, used an AFM to analyze the elastic modulus of the cells before and after exposure of the cells to pulsed electric fields. The cells increased in modulus signifying an increase in cytoskeletal tension in response to the electric fields (Fig. 8A). We postulated that this increased tension may be related to the rho kinase activity. Therefore, we used the specific inhibitor of rho-associated protein kinase (ROCK), Y27632, 10 μM, to abolish electric field–mediated increases in cytoskeletal tension. The effect of Y27632 on static cell cultures caused a twofold (p < 0.05) decrease in cell modulus, most likely a result of disrupting actin stress fibers (Fig. 8A). The pharmacologic inhibition of rho-associated kinases on electrically stimulated cells caused nearly a 10-fold decrease (p < 0.05) in cell modulus relative to the electric field–treated cells alone (Fig. 8A). The cytoskeletal effects can be viewed with the same actin permeabilization treatment used previously. In control cells exposed only to electric fields, 20 newly polymerizing actins accumulate on the cathode-facing side of the cell (green), while preexisting stress fiber formation perpendicular to the field vector was visible in red (Fig. 8E). In ROCK-inhibited cells, after electric field exposure, the same newly polymerizing actin (green) can still be seen on the cathode-facing side of the cell; however, there is a clear lack of organized actin stress fibers indicated by diffuse rhodamine staining (Fig. 8F).
FIG. 8.
Cytoskeletal stresses and their effects on gene expression. (A) The cell modulus was nearly doubled relative to controls by the electric field treatment. While treatment with a selective ROCK inhibitor, Y27632, 10 μM, caused a decrease in cell modulus, surprisingly, the combination of ROCK inhibitor and electric field treatment caused the greatest overall decrease in cell elastic modulus. The increase in elastic modulus was accompanied by an increase in gene expression of (B) OPN, (C) Col I, and (D) Runx2. *p < 0.05 relative to controls, **p < 0.05 relative to the electric field treatment alone. The combination of the ROCK inhibition with electric fields resulted in a decreased cell modulus but an increase in osteogenic marker expression. (E) A control cell indicating well-organized stress fibers and cathodal accumulation of the newly formed actin filaments. (F) Disruption of the cytoskeleton with ROCK inhibitor caused an apparent loss of organized stress fibers but does not interfere with the cell's ability to polymerize new actin on the cathode-facing side (green). Color images available online at www.liebertonline.com/ten.
To determine if the observed increase in cytoskeletal tension caused the increases in early osteogenic markers, we examined these markers after 7 days in response to the electric field–driven increases in cytoskeletal stresses. We also used the specific ROCK inhibitor, Y27632, to abolish the electric field effects on cytoskeletal stress during the field exposure. Expectedly, in response to the treatment of static cultures with ROCK inhibitor, expression of OPN, Col I, and Runx2 decreased. Also, as demonstrated earlier, electric field exposure alone caused increases in the expression in these same genes. Interestingly, the combination of ROCK inhibitor and electric field treatment, which previously caused a dramatic decrease in cytoskeletal tension, caused an unexpected increase in OPN and Col I expression over both controls and electric field–treated cells (Fig. 8B, C).
Discussion
We investigated the effects of pulsed DC electric fields on the differentiation of mASCs. We showed that the cells dramatically reorganized their cytoskeleton in response to the electrical stimulation. It has been shown repeatedly that cell shape is inextricably linked to phenotype and thus function.39,40 We explored the extent to which the changes in morphology led to changes in phenotype. The change in cell shape in the mASCs was indeed accompanied by a change in gene expression. Early osteogenic markers were increased in mASCs in response to the pulsed electric field exposure. However, inhibiting cell shape changes with ROCK inhibitors did not abolish the osteogenic response.
Because previous research has demonstrated that ROS are produced by exposure of embryoid bodies to pulsed electric fields, we first characterized the extent of oxygen radical production by the pulsed electric fields. Oxidative stress occurs when oxygen-free radical production exceeds endogenous antioxidant scavenging capabilities. Oxidative stress has been shown to have direct effects on bone metabolism including osteoblast differentiation.41 We showed here that ROS do not accumulate appreciably, and thus the changes in gene expression were not simply a byproduct of oxidative stress induced by the electric fields.
The mechanisms for the transduction of pulsed electric fields into changes in osteogenic gene expression remain unclear. A possible explanation lies in the similarity between osteoblasts in response to the oscillatory fluid flow and mASCs in response to the electric fields, both show features including an elicited calcium response and upregulation in Runx2 and OPN.42 Osteoblasts are well-characterized mechanosensing cells; ASCs also appear to be mechanosensitive, responding to shear stresses resulting from pulsatile fluid flow in a bone-cell-like manner.43 We hypothesized that the increase in early osteogenic gene markers in mASCs in response to electric fields may be because of the cytoskeletal reorganization. Rho GTPases are the best characterized regulators of actin, controlling not only cytoskeletal organization but also transcription, cell cycle, and vesicle trafficking.44–46 Cytoskeletal tension induced by ROCK is implicated as a key adipogenic to osteogenic commitment switch.30 The new actin incorporation at the focal adhesion sites at the trailing edge of the cell in response to the electric field suggested increases in actin polymerization to augment stress fibers in response to the increasing isometric tension within the cell. Newly incorporated actin was not witnessed at focal adhesion sites on the trailing edge of the ROCK-inhibited cells, suggesting that the increases in cytoskeletal tension could be abrogated with rho kinase inhibitors. AFM studies of the elastic modulus of mASCs in response to the electric field yielded evidence of the expected increase in cytoskeletal tension but surprisingly showed a decrease in cell modulus in the combined scenario of ROCK inhibitor treatment with the addition of electric fields. It appears that the changes cytoskeletal tension may give rise to changes in osteogenic expression in mASCs. However, the transduction of electric fields into gene expression is not explicitly dependent on the cytoskeletal pathways dependent on ROCK.
We were not able to establish a strong influence on terminal differentiation in mASCs in response to the electric field stimulation. However, there was similarly low OCN expression and calcium accumulation in the control populations as well. The stimulation chambers were created to confine the electric field within the area directly surrounding the cultured cells. However, a byproduct of this requisite design is that perhaps the confined culture negatively impacts the terminal osteogenic marker expression.
In addition to the cytoskeletal changes, we demonstrated a number of biochemical mediators of the electric fields in mASCs, including a transient increase in the cytosolic-free calcium accompanied by a decrease in cAMP. These represent potential alternate pathways to impact gene expression. The decrease in cAMP is to be expected because calcium entry inhibits adenylyl cyclases that in turn suppress cAMP synthesis.47 The increased calcium concentration can also activate phosphodiesterases that catalyze the hydrolysis of cyclic nucleotides such as cAMP. The decrease in cAMP reduces downstream signaling by the transcription factor cAMP response element-binding protein. Recently, Siddappa et al. have shown a decrease in osteogenic differentiation in rodent models as a result of cAMP signaling.48 If the opposite is also true, then the increases in osteogenic gene expression could be in part a result of decreased cAMP signaling. Further study is required to elucidate the mechanisms behind the osteogenic effects of electric fields on mASCs.
After the piezoelectric properties of bone were first described by Fukada and Yasuda, many researchers were intensely interested in the effects of electromagnetic fields on bone.49 Currently, orthopedic surgeons continue to apply gross electromagnetic stimulus to promote healing in challenging fracture nonunions. There are a number of cell types that may be responding to this field in vivo, including osteoblasts and mesenchymal stem cells from bone marrow. This study investigates a clinically attractive method of biasing lineage commitment in a tissue-specific multipotent cell population. There is a great interest in the response of mesenchymal stem cells to electromagnetic stimulation. Only through a systematic examination of the influence of electromagnetic fields on these types of cells can we learn to use the electromagnetic fields in concert with multipotent cells for skeletal regenerative medicine.
Acknowledgments
The authors thank Dr. Yue Xu for experimental assistance with this research. And also thank Dr. Chris Jacobs for the generous use of his Nikon microscope for the calcium imaging experiments.
Disclosure Statement
No competing financial interests exist.
References
- 1.Panetta N.J. Gupta D.M. Slater B.J. Kwan M.D. Liu K.J. Longaker M.T. Tissue engineering in cleft palate and other congenital malformations. Pediatr Res. 2008;63:545. doi: 10.1203/PDR.0b013e31816a743e. [DOI] [PubMed] [Google Scholar]
- 2.Warren S.M. Fong K.D. Chen C.M. Loboa E.G. Cowan C.M. Lorenz H.P. Longaker M.T. Tools and techniques for craniofacial tissue engineering. Tissue Eng. 2003;9:187. doi: 10.1089/107632703764664666. [DOI] [PubMed] [Google Scholar]
- 3.Cowan C.M. Aalami O.O. Shi Y.Y. Chou Y.F. Mari C. Thomas R. Quarto N. Nacamuli R.P. Contag C.H. Wu B. Longaker M.T. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 4.Song H.Y. Jeon E.S. Kim J.I. Jung J.S. Kim J.H. Oncostatin M promotes osteogenesis and suppresses adipogenic differentiation of human adipose tissue-derived mesenchymal stem cells. J Cell Biochem. 2007;101:1238. doi: 10.1002/jcb.21245. [DOI] [PubMed] [Google Scholar]
- 5.Dragoo J.L. Choi J.Y. Lieberman J.R. Huang J. Zuk P.A. Zhang J. Hedrick M.H. Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 6.McFarland R. Draft Guidance for Industry: cell selection devices for point of care production of minimally manipulated autologous peripheral blood stem cells (PBSC). U.S. Department of Health and Human Services, Food and Drug Administration Center for Biologics Evaluation and Research, July 2007. www.fda.gov/CbER/gdlns/pbsc.htm www.fda.gov/CbER/gdlns/pbsc.htm
- 7.Pittenger M.F. Mackay A.M. Beck S.C. Jaiswal R.K. Douglas R. Mosca J.D. Moorman M.A. Simonetti D.W. Craig S. Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 8.Zuk P.A. Zhu M. Ashjian P. De Ugarte D.A. Huang J.I. Mizuno H. Alfonso Z.C. Fraser J.K. Benhaim P. Hedrick M.H. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Y. Malladi P. Wagner D.R. Longaker M.T. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Curr Opin Mol Ther. 2005;7:300. [PubMed] [Google Scholar]
- 10.Malladi P. Xu Y. Yang G.P. Longaker M.T. Functions of vitamin D, retinoic acid, and dexamethasone in mouse adipose-derived mesenchymal cells. Tissue Eng. 2006;12:2031. doi: 10.1089/ten.2006.12.2031. [DOI] [PubMed] [Google Scholar]
- 11.Cowan C.M. Shi Y.Y. Aalami O.O. Chou Y.F. Mari C. Thomas R. Quarto N. Contag C.H. Wu B. Longaker M.T. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 12.Lendeckel S. Jodicke A. Christophis P. Heidinger K. Wolff J. Fraser J.K. Hedrick M.H. Berthold L. Howaldt H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Mehlhorn A.T. Niemeyer P. Kaschte K. Muller L. Finkenzeller G. Harti D. Sudkamp N.P. Schmal H. Differential effects of BMP-2 and TGF-beta1 on chondrogenic differentiation of adipose derived stem cells. Cell Prolif. 2007;40:809. doi: 10.1111/j.1365-2184.2007.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee W.C. Maul T.M. Vorp D.A. Rubin J.P. Marra K.G. Effects of uniaxial cyclic strain on adipose-derived stem cell morphology, proliferation, and differentiation. Biomech Model Mechanobiol. 2007;6:265. doi: 10.1007/s10237-006-0053-y. [DOI] [PubMed] [Google Scholar]
- 15.Nuccitelli R. Endogenous ionic currents and DC electric fields in multicellular animal tissues. Bioelectromagnetics. 1992;1(Suppl):147. doi: 10.1002/bem.2250130714. [DOI] [PubMed] [Google Scholar]
- 16.Hotary K.B. Robinson K.R. Endogenous electrical currents and voltage gradients in Xenopus embryos and the consequences of their disruption. Dev Biol. 1994;166:789. doi: 10.1006/dbio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 17.Bassett C.A. Pawluk R.J. Pilla A.A. Augmentation of bone repair by inductively coupled electromagnetic fields. Science. 1974;184:575. doi: 10.1126/science.184.4136.575. [DOI] [PubMed] [Google Scholar]
- 18.Fitzsimmons R.J. Farley J. Adey W.R. Baylink D.J. Embryonic bone matrix formation is increased after exposure to a low-amplitude capacitively coupled electric field, in vitro. Biochim Biophys Acta. 1986;882:51. doi: 10.1016/0304-4165(86)90054-1. [DOI] [PubMed] [Google Scholar]
- 19.Aaron R.K. Ciombor D.M. Simon B.J. Treatment of nonunions with electric and electromagnetic fields. Clin Orthop Relat Res. 2004;419:21. doi: 10.1097/00003086-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Sauer H. Bekhite M.M. Hescheler J. Wartenberg M. Redox control of angiogenic factors and CD31-positive vessel-like structures in mouse embryonic stem cells after direct current electrical field stimulation. Exp Cell Res. 2005;304:380. doi: 10.1016/j.yexcr.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 21.Serena E. Flaibani M. Carnio S. Boldrin L. Vitiello L. De Coppi P. Elvassore N. Electrophysiologic stimulation improves myogenic potential of muscle precursor cells grown in a 3D collagen scaffold. Neurol Res. 2008;30:207. doi: 10.1179/174313208X281109. [DOI] [PubMed] [Google Scholar]
- 22.Lohmann C.H. Schwartz Z. Liu Y. Guerkov H. Dean D.D. Simon B. Boyan B.D. Pulsed electromagnetic field stimulation of MG63 osteoblast-like cells affects differentiation and local factor production. J Bone Joint Surg. 2000;18:637. doi: 10.1002/jor.1100180417. [DOI] [PubMed] [Google Scholar]
- 23.Sauer H. Rahimi G. Hescheler J. Wartenberg M. Effects of electrical fields on cardiomyocyte differentiation of embryonic stem cells. J Cell Biochem. 1999;75:710. doi: 10.1002/(sici)1097-4644(19991215)75:4<710::aid-jcb16>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 24.Gupta T.D. Jain V.K. Tandon P.N. Comparative study of bone growth by pulsed electromagnetic fields. Med Biol Eng Comput. 1991;29:113. doi: 10.1007/BF02447095. [DOI] [PubMed] [Google Scholar]
- 25.Symons M.H. Mitchison T.J. Control of actin polymerization in live and permeabilized fibroblasts. J Cell Biol. 1991;114:503. doi: 10.1083/jcb.114.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royall J.A. Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 27.Hertz H. Über die Berührung fester elastischer Körper. J Reine Angew Mathematik. 1882;92:156. [Google Scholar]
- 28.Briscoe B.J. Sebastian K.S. Adams M.J. The effect of indenter geometry on the elastic response to indentation. J Phys D: Appl Phys. 1994;27:1156. [Google Scholar]
- 29.Tsai M.T. Chang W.H. Chang K. Hou R.J. Wu T.W. Pulsed electromagnetic fields affect osteoblast proliferation and differentiation in bone tissue engineering. Bioelectromagnetics. 2007;28:519. doi: 10.1002/bem.20336. [DOI] [PubMed] [Google Scholar]
- 30.McBeath R. Pirone D.M. Nelson C.M. Bhadriraju K. Chen C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev Cell. 2004;6:483. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 31.Ducy P. Zhang R. Geoffroy V. Ridall A.L. Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 32.Denhardt D.T. Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7:1475. [PubMed] [Google Scholar]
- 33.Gerstenfeld L.C. Uporova T. Ashkar S. Salih E. Gotoh Y. McKee M.D. Nanci A. Glimcher M.J. Regulation of avian osteopontin pre- and posttranscriptional expression in skeletal tissues. Ann NY Acad Sci. 1995;760:67. doi: 10.1111/j.1749-6632.1995.tb44621.x. [DOI] [PubMed] [Google Scholar]
- 34.Reinholt F.P. Hultenby K. Oldberg A. Heinegard D. Osteopontin—a possible anchor of osteoclasts to bone. Proc Natl Acad Sci USA. 1990;87:4473. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giachelli C.M. Steitz S. Osteopontin: a versatile regulator of inflammation and biomineralization. Matrix Biol. 2000;19:615. doi: 10.1016/s0945-053x(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 36.Gerstenfeld L.C. Gotoh Y. McKee M.D. Nanci A. Landis W.J. Glimcher M.J. Expression and ultrastructural localization of a major 66 kDa phosphoprotein synthesized by chicken osteoblasts during in vitro miner-alization. Anat Rec. 1990;228:93. doi: 10.1002/ar.1092280113. [DOI] [PubMed] [Google Scholar]
- 37.Wartenberg M. Wirtz N. Grob A. Niedermeier W. Hescheler J. Peters S.C. Sauer H. Direct current electrical fields induce apoptosis in oral mucosa cancer cells by NADPH oxidase-derived reactive oxygen species. Bioelectromagnetics. 2008;29:47. doi: 10.1002/bem.20361. [DOI] [PubMed] [Google Scholar]
- 38.Norton L.A. Rodan G.A. Bourret L.A. Epiphyseal cartilage cAMP changes produced by electrical and mechanical perturbations. Clin Orthop Rel Res. 1977;124:59. [PubMed] [Google Scholar]
- 39.Folkman J. Moscona A. Role of cell shape in growth control. Nature. 1978;273:345. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- 40.Chen C.S. Mrksich M. Huang S. Whitesides G.M. Ingber D.E. Geometric control of cell life and death. Science. 1997;276:1425. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 41.Mody N. Parhami F. Sarafian T.A. Demer L.L. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med. 2001;31:509. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 42.You J. Reilly G.C. Zhen X. Yellowley C.E. Chen Q. Donahue H.J. Jacobs C.R. Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem. 2001;276:13365. doi: 10.1074/jbc.M009846200. [DOI] [PubMed] [Google Scholar]
- 43.Knippenberg M. Helder M.N. Zandieh Doulabi B. Semeins C.M. Wuisman P.I. Klein-Nulend J. Adipose tissue-derived mesenchymal stem cells acquire bone cell-like responsiveness to fluid shear stress on osteogenic stimulation. Tissue Eng. 2005;11:1780. doi: 10.1089/ten.2005.11.1780. [DOI] [PubMed] [Google Scholar]
- 44.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 45.Bishop A.L. Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241. [PMC free article] [PubMed] [Google Scholar]
- 46.Etienne-Manneville S. Hall A. Rho GTPases in cell biology. Nature. 2002;420:629. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 47.Cooper D.M. Mons N. Karpen J.W. Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature. 1995;374:421. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- 48.Siddappa R. Mulder W. Steeghs I. van de Klundert C. Fernandes H. Liu J. Arends R. van Blitterswijk C. de Boer J. cAMP/PKA signaling inhibits osteogenic differentiation and bone formation in rodent models. J Tissue Eng Part A. 2009;15:2135. doi: 10.1089/ten.tea.2008.0512. [DOI] [PubMed] [Google Scholar]
- 49.Fukada E. Yasuda I. On the piezoelectric effect of bone. J Phys Soc Jpn. 1957;12:1158. [Google Scholar]