Abstract
Background and Aims:
Patients with chronic hepatitis C virus (HCV) infection display great variability in disease activity and progression. While virus-specific adaptive immune responses have been extensively characterized and found to be impaired in chronic hepatitis C, the role of innate immune responses in disease activity and progression of chronic hepatitis C is not well understood.
Methods and Results:
Studying 42 HCV-infected patients and 12 healthy uninfected controls, we found an increased frequency of NK cells expressing TRAIL, NKp44, NKG2C and CD122 in chronic hepatitis C as compared to healthy controls (p<0.05 for all markers) and stronger activation of NK cells in the liver than in the blood (p<0.05). This NK cell phenotype was associated with polarization of NK cell function towards CD107a expression as a marker of degranulation, but with not increased interferon (IFN)-γ-production of CD56dim NK cells. The polarized NK cell phenotype correlated with alanine aminotransferase levels (r2=0.312, p=0.03). To investigate whether in vivo exposure of NK cells to HCV-induced type I IFN was causing this NK cell phenotype, peripheral blood mononuclear cells from 10 healthy controls and 8 HCV-infected patients were stimulated in the presence of IFN-α, which resulted in increased NK cell expression of TRAIL and CD107a (p<0.001), but not IFN-γ.
Conclusions:
Collectively, these results describe a polarized NK cell phenotype induced by chronic exposure to HCV-induced IFN-α. This phenotype may contribute to liver injury through TRAIL expression and cytotoxicity whereas the lacking increase in IFN-γ production may facilitate the inability to clear HCV.
Introduction
Infection with hepatitis C virus (HCV) results in viral persistence in about 70-80% of cases, and is associated with chronic liver inflammation and an increased risk for cirrhosis and hepatocellular carcinoma. Liver injury and disease progression are thought to be driven by host immune responses 1. However, the relative contributions of the adaptive and innate host immune responses and their respective effector functions have not been well defined.
Most studies on the immunology of hepatitis C have focused on the adaptive immune response. In acute self-limited hepatitis C, the HCV-specific CD4 and CD8 T cell response can be vigorous with more than 10% of all peripheral blood lymphocytes recognizing HCV antigens 2, 3. The recruitment of HCV-specific CD4 and CD8 T cells to the liver coincides with the onset of liver injury (as determined by increased alanine aminotransferase (ALT) levels, the decrease in HCV titer and ultimately, with HCV clearance. In chronic hepatitis C, HCV-specific T cells are described as ineffective and functionally impaired: First, HCV-specific T cells are present at very low frequency in both blood and liver of chronically HCV-infected patients, typically comprising less than 0.05% of all peripheral blood lymphocytes 4-6. Second, they are terminally differentiated and do not proliferate well 6. Third, they are impaired in their effector functions due to upregulation of inhibitory molecules, such as programmed death-1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4) 7. This impaired T cell phenotype is even more pronounced in the liver, the site of HCV replication, than in the blood. Fourth, HCV actively escapes from recognition of the few remaining, functional HCV-specific T cells via mutations and quasispecies shifts 8. These findings suggest that the adaptive immune response is not the sole or even the primary contributor to disease progression.
In contrast to T cells, the role of innate immune cells has not been extensively studied. This is a significant omission because the liver is selectively enriched in key components of the innate immune system. Natural killer (NK) cells for example, represent only 5-10% of peripheral blood lymphocytes, but about 30% of lymphocytes in the healthy human liver 9, and an increase in the NK cell frequency in the liver has been observed in mouse models of viral hepatitis 10.
As opposed to T cells, NK cells do not require antigen-specific priming to recognize virus-infected cells. Once activated, NK cells exert cytotoxicicty and produce antiviral cytokines, such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α, and chemokines, such as MIP-1α and MIP-1β. Thus, NK cells are not only in the right location but also able to mount immediate effector responses to hepatotropic viruses. Indeed, this has been shown for inflammatory flares in patients with chronic hepatitis B virus infection, where NK cells contribute to liver injury via a TRAIL-dependent mechanism 11.
The overall role of NK cells in HCV infection, however, is not well understood. Immunogenetic studies suggest that distinct haplotypes of killer cell immunoglobulin-like receptors (KIRs) and their HLA ligands influence the outcome of acute and chronic HCV infection. Homozygosity for KIR2DL3 and HLA-C group 1 alleles, for example, is associated with an increased likelihood of HCV clearance 12, whereas KIR2DS3 is associated with an increased risk of liver injury in chronic HCV infection 13. These associations are probably the result of differential NK cell activation and function due to KIR/HLA interaction. As shown in vitro, NK cells from subjects with KIR2DL3 and HLA-C group 1 compound genotype respond faster and more efficiently to influenza A virus infection than NK cells from subjects with less favorable genotypes 14. Importantly, activated NK cells have also been shown to recognize and lyse HCV replicon-containing hepatoma cells in vitro 15 and should therefore be able of recognizing and killing HCV-infected hepatocytes in vivo. However, published data is controversial in regards to up- or downregulation of specific NK cell markers and NK cell effector functions in HCV infection 16-20. Furthermore, the mechanisms underlying these phenotypic and/or functional changes remain unknown. While it has been proposed that individual HCV proteins alter NK cell functions based on in vitro assays 21, 22, it should be noted that HCV-infected hepatocytes do not secrete soluble viral proteins and that HCV particles with either genotype 1a or 2a envelope proteins do not directly modulate NK cell function 23.
The current study describes the phenotype and function of NK cells in liver and blood of patients with chronic HCV infection. NK cells were activated in chronic hepatitis, but displayed a polarized phenotype with increased degranulation and expression of TRAIL, but not IFN-γ. This polarized phenotype correlated with markers of liver injury and was induced by exposure to IFN-α. Based on these results, we propose a model in which polarization of NK cell function by HCV-induced IFN-α contributes to both the progression of liver disease and the failure to clear HCV.
Materials and Methods
Study cohort
All subjects gave written informed consent for research testing under protocols approved by the NIDDK Institutional Review Board. Blood samples were drawn in citrate dextrose (CPDA) tubes from 42 patients with chronic HCV infection and from 12 healthy blood donors. PBMCs were separated on Ficoll-Histopaque (Mediatech, Manassas, VA) density gradients, washed three times with phosphate buffered saline (PBS; Mediatech), and cryopreserved. Paired analysis of liver and blood NK cells was performed for 10 patients. After sufficient tissue was submitted for pathological evaluation, a 5 mm segment was homogenized, washed in PBS, and resuspended in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; US Bio-Technologies, Pottstown, PA), 10 mM HEPES, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 2 mM L-glutamine (Mediatech) (complete medium). Liver-infiltrating lymphocytes (LIL) were directly stained with the antibodies indicated below.
NK cell analysis
(i) Frequency and phenotype
PBMCs and LIL were stained with ethidium monoazide (EMA), anti-CD19-PeCy5 (BD Biosciences, San Jose, CA), anti-CD14-PeCy5 (Serotec, Raleigh, NC) and anti-CD3-AlexaFluor700 (BD Biosciences) to exclude dead cells, B cells, monocytes and T cells, respectively, and with anti-CD56-PeCy7 (BD Biosciences) and anti-CD16-PacificBlue (BD Biosciences) to identify NK cells. Cells were additionally stained with either (i) anti-TRAIL-PE (BD Biosciences), (ii) anti-CD122-FITC and anti-NKG2C-PE (R&D Systems, Minneapolis, MN), (iii) anti-NKp44-PE, (iv) anti-NKp46-PE, (v) anti-NKG2A-PE or (vi) anti-NKG2D-PE (all from Beckman Coulter).
(ii) Degranulation
NK cell degranulation, i.e. an increase in cell surface CD107a expression 24, was assessed in response to MHC class I-negative K562 cells (ATCC, Manassas, VA). Specifically, cryopreserved PBMCs were thawed and cultured at 4 × 106 cells/ml at 37°C in complete medium without any exogenously added cytokines. After 14h, PBMCs were washed, resuspended at 106 cells/ml and stimulated in the presence of anti-CD107a (15 μl/ml) with either (i) K562 cells at an effector to target ratio of 1:1, (ii) IL-12 (0.5 ng/ml; R&D Systems) and IL-15 (20 ng/ml; R&D Systems), (iii) K562 cells at an effector to target ratio of 1:1 in the presence of IL-12 (0.5 ng/ml; R&D Systems) and IL-15 (20 ng/ml; R&D Systems) 25, or in (iv) complete medium without K562 and cytokines. After 6h at 37°C, PBMCs were washed, stained with the same EMA/antibody combination used for phenotyping and analyzed by flow cytometry. The frequency of CD107a-positive NK cells that were incubated without K562 cells and cytokines was subtracted from the frequency of CD107a-positive NK cells stimulated with K562 and/or cytokines.
(iii) Cytokine release
Cryopreserved PBMCs were thawed and cultured with either (i) IL-12 (0.5 ng/ml; R&D Systems) and IL-15 (20 ng/ml R&D Systems) 25, (ii) IL-12 (0.5 ng/ml; R&D Systems) and IL-18 (100 ng/ml R&D Systems) 25, or (iii) complete medium without cytokines at 37°C. After 14h, Brefeldin A (BD Biosciences) was added for another 4h. Finally, cells were washed, stained with the same EMA/antibody combination used for phenotyping and analyzed by flow cytometry. The frequency of IFN-γ-positive NK cells incubated in complete medium without cytokines was subtracted from the frequency of IFN-γ-positive NK cells stimulated with cytokines.
(iv) IFN-α stimulation
Cryopreserved PBMCs were thawed and cultured at 4 × 10 6 cells/ml at 37°C in complete medium with or without 3 ng/ml consensus sequence IFN-α (IFN-α–con1; InterMune Inc., Brisbane, CA) for 14h. PBMCs were then either directly stained with antibodies against cell surface markers, or used for a degranulation assay (see above) with or without IFN-α–con1 or subjected to 4h cytokine release assays in the presence or absence of IFN-α–con1, followed by the assays described above.
Flow cytometry
Stained cells were analyzed on an LSRII using FacsDiva Version 4.1 (BD Biosciences) and FlowJo Version 6.7 (Tree Star, Ashland, OR) software.
Statistical Analysis
Statistical analyses were performed with GraphPad Prism Version 5.0a (GraphPad). Mann-Whitney U tests were employed to compare NK cell frequency, phenotype and response, as well as cytokine levels between samples. Paired Student t-tests were used to assess differences in NK cell phenotype between paired liver and blood samples, and to assess changes in NK cell cytotoxicity or cytokine release upon in vitro stimulation with IFN-α. Linear regression analysis was used to examine correlations. A two-sided p-value of less than 0.05 was considered significant.
Results
NK cell phenotype in chronic HCV infection
The studied patients with chronic hepatitis C were on average 52 + 9 years old and treatment-naive at the time of this study. Seventeen patients (52%) were male, and most (33/42, 79%) were infected with HCV genotype 1. Serum ALT levels ranged from 15 to 373 U/mL (median 50 U/mL; IQR 36 – 108 U/mL) and HCV RNA levels ranged from 1.08 × 104 IU/mL to 2.5 × 107 IU/mL (median 1.85 × 106 IU/mL; IQR 4.7 × 105 −5.34 × 106 IU/mL).
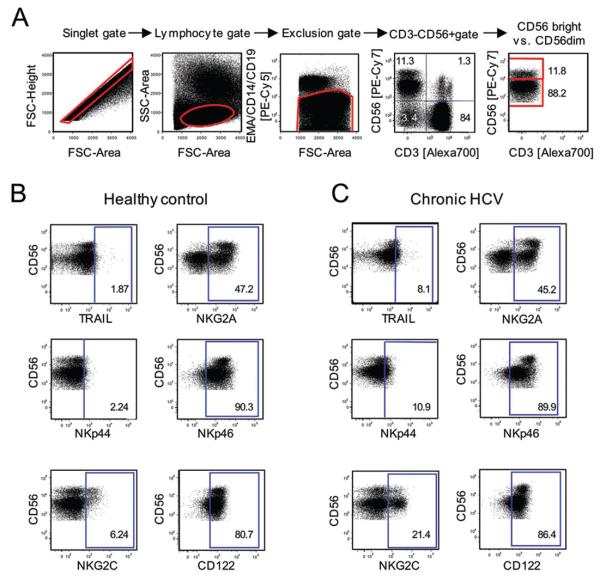
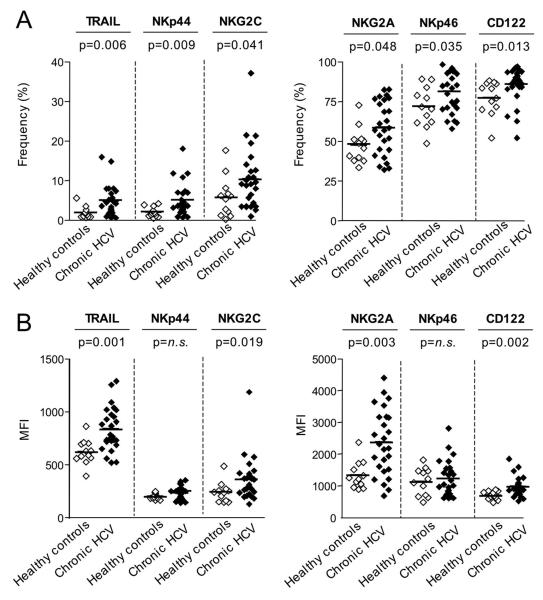
CD3-CD56+ NK cells and their CD56bright and CD56dim subsets were identified in PBMC by multicolor flow cytometry by gating on single cells (forward scatter–height versus forward scatter–area), lymphocytes (forward scatter versus side scatter), and exclusion of CD14+ monocytes, CD19+ B cells, and ethidium monoazide–positive (EMA+) dead cells, followed by staining for CD3 and CD56 (Fig. 1A) and cell surface markers of interest (Fig. 1B, C). As shown in figure 2A, the percentage of CD3-CD56+ NK cells expressing TRAIL and the natural cytotoxicity receptors NKp44 and NKp46 were significantly higher in patients with chronic HCV infection than in healthy controls (Fig. 2A). In addition, the activating receptor NKG2C was expressed on a higher percentage of NK cells and at a higher mean fluorescence intensity (MFI) per cell in patients with chronic HCV infection, suggesting that NK cells may be activated in vivo in chronic HCV infection (Fig. 2A, B). Consistent with these findings, the percentage of NK cells that expressed CD122 (Fig. 2A) and the MFI of CD122-expression (Fig. 2B) were significantly higher in patients with chronic HCV infection than in healthy controls. CD122, a subunit of the IL-2 receptor, is essential for signaling in response to IL-2 and IL-15, which results in NK cell activation. Furthermore, both the percentage of NK cells that expressed NKG2A (Fig. 2A) and the MFI of NKG2A-expression (Fig. 2B) were also increased in patients with chronic HCV infection as compared to healthy controls. NKG2A ligation is known to mediate an inhibitory signal, but has previously been shown to be increased on NK cells in HCV infection 18, possibly mediating a negative feedback mechanism in a chronic inflammatory situation that supports the expression of the NKG2A ligand, HLA-E 18, 26. The expression of these markers was also analyzed for separately for CD56bright NK cells, which produce IFN-γ and contribute to T helper cell type 1 priming 27, and for CD56dim NK cells, which represent a fully mature highly cytotoxic NK cell subset. With the exceptions of NKp44 with enhanced expression only on CD56bright NK cells and NKG2A with enhanced expression only on CD56dim NK cells, none of the other cell surface markers differed between CD56bright (Suppl. Fig. 1) and CD56dim NK cells (Suppl. Fig. 2). The overall percentage of CD56bright and CD56dim NK cells did not differ between chronic HCV patients and healthy controls (not shown).
Fig. 1. Multicolor flow cytometry to analyze NK cells in PBMC.
(A) Gating strategy. (B-C) Representative dot plots showing the expression of cell surface markers on NK cells from healthy controls (B) and chronic HCV patients (C).
Fig. 2. Phenotype of peripheral blood NK cells.
Frequency (A) and MFI (B) of NK cells displaying the indicated surface markers in the blood of patients with chronic HCV infection and healthy, uninfected controls. Horizontal lines indicate the mean. n.s., not significant.
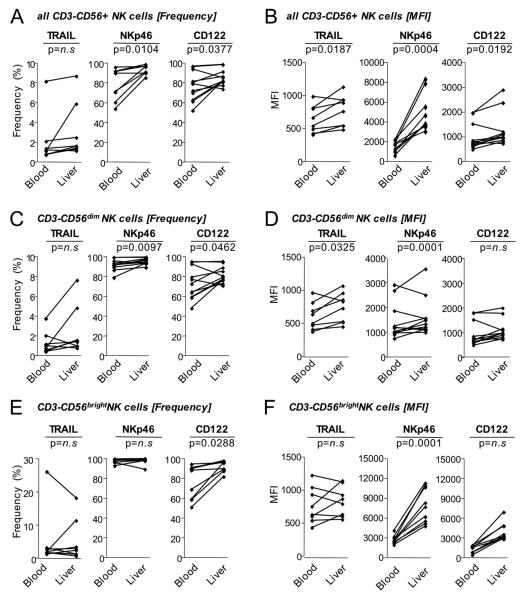
To study NK cells in the liver, the site of HCV replication, lymphocytes were isolated on the same day from paired liver biopsies and blood samples of 10 HCV-infected patients. The median age of the patients was 57 years [IQR 48-62 years], median ALT 66 U/mL [IQR 43-156 U/mL] and median HCV RNA 890,000 IU/ml [IQR 214,000-3,960,000 IU/ml]. The median Ishak inflammatory score was 6 [IQR 5-8] and the median Ishak fibrosis score 1 [IQR 1-3] (Suppl. Table 1). The percentage of NK cells expressing TRAIL, NKp46 and CD122 (Fig. 3A) and the MFI were significantly higher in the liver than in the blood (Fig. 3B). This was more pronounced for the CD56dim (Fig. 3C, D) than the CD56bright NK cell subset (Fig. 3E, F).
Fig. 3. Comparison of NK cell phenotypes in blood and liver in chronic hepatitis C.
Frequency and MFI of CD3-CD56+ NK cells (A-B), CD3-CD56dim NK cells (C-D) and CD3-CD56bright NK cells (E-F) with the indicated surface markers in blood and liver of patients with chronic HCV infection.
NK cell function in chronic HCV infection
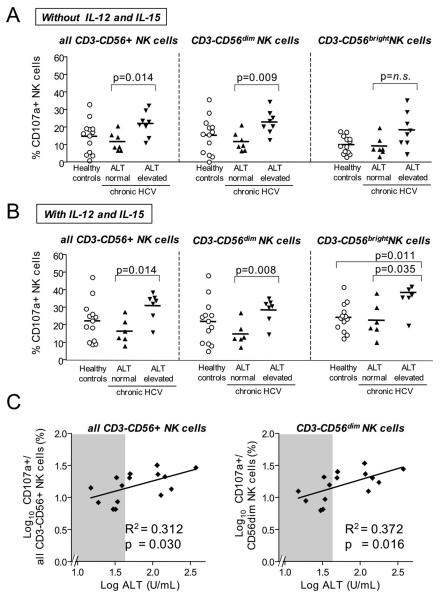
NK cell activation typically results in cytotoxicity/degranulation and release of antiviral cytokines such as IFN-γ. When stimulated with MHC-class I negative K562 cells, significantly more NK cells from HCV-infected patients with elevated ALT levels degranulated than NK cells from HCV-infected patients with normal ALT levels (p=0.014, Fig. 4A). This was particularly significant for the CD56dim NK cell subset (p=0.0093) and became even more significant in the presence of IL-12 and IL-15 (p=0.0082, Fig. 4B). Overall, the percentage of degranulating NK cells correlated with serum ALT levels (R2 = 0.3122, p=0.030 for all CD3-CD56+ NK cells; R2=0.372, p=0.016 for CD56dim NK cell subset, Fig. 4C).
Fig. 4. NK cell degranulation, a marker for cytotoxicity, correlates to liver injury in chronic hepatitis C.
(A, B) The frequency of degranulating (CD107a+) NK cells within all CD3-CD56+ NK cells (left panel), the CD3-CD56dim NK cell subset (middle panel) and the CD3-CD56brightNK cell subset (right panel) is higher in HCV-infected patients with elevated ALT levels than in those with normal ALT levels. NK cells were exposed to MHC-negative K562 cells (A) or to MHC-negative K562 cells in the presence of IL-12 and IL-15 (B) to induce degranulation.
(C) The frequency of degranulating (CD107a+) NK cells within all CD3-CD56+ NK cells (left panel) and within the CD3-CD56dim NK cell subset (right panel) correlates to ALT levels. Shaded areas indicate the range of normal (< 41 U/l) ALT levels. Horizontal lines indicate the mean.
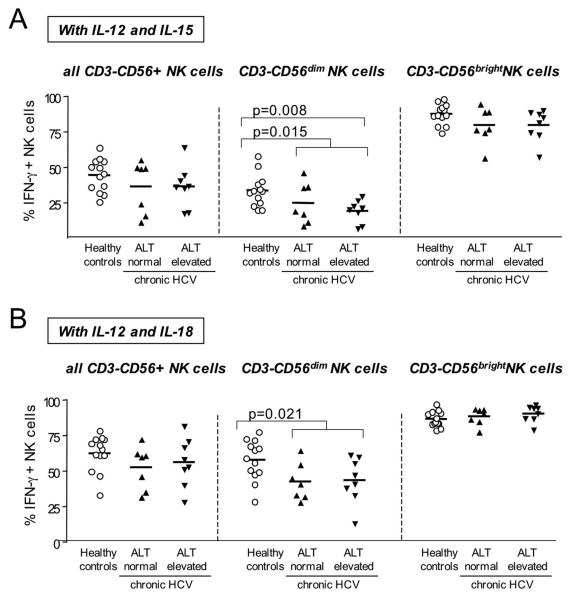
However, in contrast to the observed increased degranulation, we did not detect increased IFN-γ production by activated NK cells from HCV-infected patients. In fact, CD56dim NK cells of HCV-infected patients produced significantly less IFN-γ than CD56dim NK cells of healthy controls (Fig. 5). This finding was independent of serum ALT levels and was seen when NK cells were stimulated with either a combination of IL-12 and IL-15 (Fig. 5A) or with a combination of IL-12 and IL-18 (Fig. 5B). Moreover, of the NK cells producing IFN-γ in response to IL-12/IL-15 an average of 59+18% were CD56dim in healthy controls, 57+21% were CD56dim in HCV patients with normal ALT values and 37+11% were CD56dim in HCV patients with elevated ALT values. Thus, CD56dim NK cells contributed to a significantly lesser degree to IFN-γ production in patients with chronic hepatitis C, particularly in those with high ALT levels, than in healthy controls (p=0.0074), supporting the notion of a polarization of NK cell function in chronic hepatitis C.
Fig. 5. NK cell IFN-γ secretion does not increase in patients with chronic hepatitis C.
The frequency of IFN-γ-producing NK cells within all CD3-CD56+ NK cells (left panel), the CD3-CD56dim NK cell subset (middle panel) and the CD3-CD56bright NK cell subset (right panel) is not higher in HCV-infected patients with elevated ALT levels than in those with normal ALT levels. IFN-γ secretion was induced by stimulation with IL-12 and IL-15 (A) or with IL-12 and IL-18 (B). Only significant p-values (<0.05) are indicated. Horizontal lines indicate the mean.
Collectively, these results suggest polarization of CD56dim NK cell function towards increased release of cytotoxic granules without a concomitant increase in the production of antiviral IFN-γ in HCV patients with elevated ALT levels.
Mechanism underlying polarization of NK cell function in chronic HCV infection
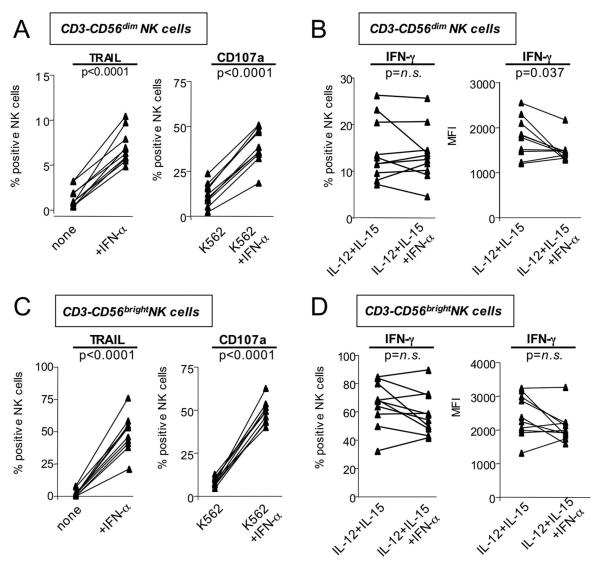
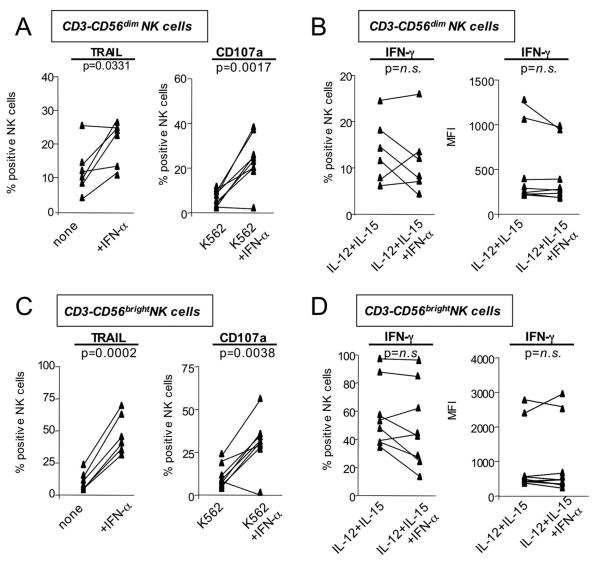
Degranulation, TRAIL expression and IFN-γ production of NK cells can all be induced via activation of the IFN-α/β receptor 28. To investigate whether the driving force behind the polarized NK cell phenotype was in vivo exposure of NK cells to HCV-induced type I IFN 29, PBMCs from healthy, uninfected blood donors were stimulated in the presence of IFN-α in vitro. Activation of NK cells in the presence of IFN-α significantly increased TRAIL expression and degranulation of CD56dim NK cells (p<0.0001, Fig. 6A). In contrast, the frequency of IFN-γ producing NK cells did not increase and the corresponding MFI showed a significant decrease (p=0.0369, Fig. 6B). Even CD56bright NK cells, the main producers of IFN-γ in the body 27, responded to IFN-α with a significant increase in TRAIL expression and degranulation (p<0.0001 Fig. 6C), but no increase in IFN-γ production (Fig. 5D). Similar results were obtained for NK cells from HCV-infected patients (Fig. 7). To exclude the possibility that the differential TRAIL/CD107a and IFN-γ responses were the result of the different assay stimulation conditions IFN-γ EIAs were performed on the supernatants from the TRAIL and CD107a assays. The EIAs confirmed that TRAIL and CD107a expression, but not IFN-γ expression increased in response to IFN-α exposure (not shown). Thus, in vitro exposure of NK cells to IFN-α reproduced the polarized NK cell phenotype that we had observed ex vivo in patients with chronic HCV infection.
Fig. 6. NK cells of healthy, uninfected control subjects upregulate markers of cytotoxicity (CD107a, TRAIL) but not IFN-γ-production after in vitro exposure to IFN-α.
CD3-CD56dim (A, B) and CD3-CD56bright (C, D) NK cells increase TRAIL and CD107a expression (A, C) but not IFN-γ-production (B, D) after in vitro exposure to IFN-α. n.s., not significant.
Fig. 7. NK cells of chronic HCV patients upregulate markers of cytotoxicity (CD107a, TRAIL) but not IFN-γ-production after in vitro exposure to IFN-α.
CD3-CD56dim (A, B) and CD3-CD56bright (C, D) NK cells increase TRAIL and CD107a expression (A, C) but not IFN-γ-production (B, D) after in vitro exposure to IFN-α. n.s., not significant.
Discussion
This study provides insights into the role of innate immune cells in chronic hepatitis C. While it has consistently been reported that the NK cell phenotype is altered in chronically HCV-infected patients as compared to healthy, uninfected controls, published findings differ regarding up- or downregulation of activation markers, natural cytotoxicity receptors, and effector functions 16-20, 30. Based on in vitro studies, it has also been proposed that plate-bound recombinant HCV envelope proteins impair NK cell activation and function 21, 22 and that HCV may utilize this mechanism to establish persistence. However, consistent with our recent finding that HCV envelope proteins do not inhibit NK cell activation and function when they are part of viral particles 23, we now report that NK cells are not inhibited, but activated in the blood of HCV-infected patients. TRAIL, NKp44 and NKG2C, markers only expressed by activated NK cells, were expressed by a higher percentage of NK cells of patients with chronic hepatitis C than on NK cells from healthy, uninfected controls (Fig. 2). Moreover, the expression of CD122 and NKp46, which can also be observed to some extent on NK cells of healthy controls, was further increased on NK cells of patients with chronic hepatitis.
Surprisingly, NK cell activation did not result in equal induction of all effector functions. Markers of NK cell cytotoxicity such as TRAIL expression and degranulation were clearly enhanced, consistent with a report by Morishima et al. 17, and and correlated to ALT levels (r2=0.3122, p=0.0304, Fig. 4C). In contrast, and consistent with a recent report 20 IFN-γ production was not enhanced and even lower in HCV-infected patients than in healthy controls (Fig. 4). This polarized NK cell phenotype was reproduced in vitro when NK cells were stimulated in the presence of IFN-α (Fig. 6, 7), suggesting that exposure to HCV-induced IFN-α causes this phenomenon in vivo in HCV-infected patients. This represents an indirect, IFN-α-mediated rather than a direct virus-mediated effect because HCV itself does not activate NK cells 23.
In HCV infection, hepatocytes and plasmacytoid dendritic cells are the most likely sources of IFN-α. HCV induces type I IFN within days after infection 31, 32 but is relatively resistant to its antiviral activity. Thus, mRNA levels of IFN-β, IFN-α subtypes, and IFNinducible genes, such as 2′5′OAS and chemokines remain upregulated in the liver for the duration of infection 31-34, but are not sufficient to achieve viral clearance. As a result, immune cells are continuously exposed to HCV-induced IFN-α, which may contribute to the observed polarization of the NK cell phenotype towards cytotoxicity. Specifically, IFN-α is known to initiate Signal Transducers and Activators of Transcription 1 (STAT1) signaling, which improves gene expression of interferon regulatory factor-1, a key transcription factor necessary for cytotoxicity of NK cells and expression of the effector molecules Fas-L and perforin 35. Exposure to IFN-α also increases expression of STAT1, which upon stimulation of the IFN-α/β receptor results in downregulation of IFN-γ production and promotion of STAT1-mediated cytotoxicity in a mouse model of lymphocytic choriomeningitis virus infection 29.
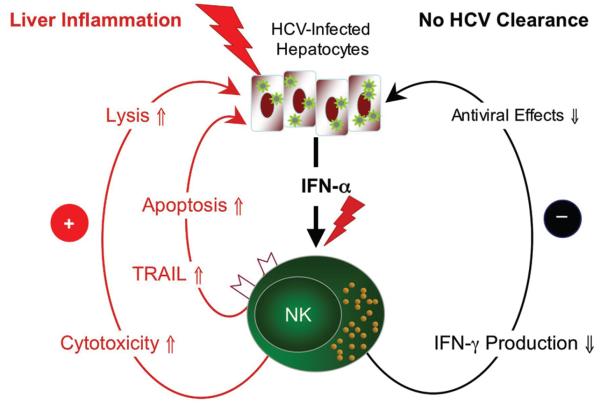
What are the consequences of this polarized NK cell phenotype? We propose a model in which polarization of NK cell function contributes to liver injury and persistence of HCV (Fig. 8). IFN-β is secreted by hepatocytes in response to double-stranded RNA, an obligatory product of HCV replication. IFN-β binds to the IFN-α/β receptor and induces interferon-stimulated genes and IFN-α via the Jak-Stat pathway. NK cell stimulation in the presence of IFN-α polarizes their phenotype towards cytotoxicity as evidenced by upregulation of TRAIL and increased expression of the degranulation marker CD107a on the cell membrane. This results apoptosis of HCV-infected hepatocytes with ALT release as suggested by the correlation between CD107a expression and ALT elevation (Fig. 4C). Consistent with this scenario, TRAIL expression and apoptosis are increased in the HCV-infected liver 36, and activated NK cells have been shown to lyse human hepatoma cells transfected with HCV replicons 15. HCV infection of primary human hepatocytes and hepatoma cells increases their sensitivity to TRAIL-induced apoptosis via inhibition of Bcl-2 and Bcl-xL, release of cytochrome C from the mitochondria into the cytosol 37, 38. Combined, these findings may explain the correlation of NK cell activation/degranulation and serum ALT levels observed in our study and the published correlation between NK cell cytotoxicity and fibrosis 17.
Fig. 8. Model.
Chronic exposure to HCV-induced IFN-α induces a polarized NK cell phenotype that contributes to liver inflammation due to increased cytotoxicity but not to viral clearance due to lack of IFN-γ production (see discussion for details).
At the same time we propose that polarization of NK cells towards cytotoxic functions, but without increased IFN-γ production contributes to HCV persistence. Evidence for the antiviral potential of IFN-γ is provided by several studies: First, IFN-γ suppresses HCV replication in human hepatocytes in vitro which can be abolished by IFN-γ neutralizing antibodies 39. Second, the few patients who are able to recover spontaneously from acute HCV infection display a strong temporal association between the appearance of IFN-γ producing T cells and HCV clearance 1. A similar correlation has also been observed between the increase of IFN-γ mRNA levels in the liver and HCV clearance in the chimpanzee model of HCV infection 33, 40. IFN-γ-mediated viral clearance is considered more effective than elimination of virus-infected hepatocytes by cytotoxic mechanisms 41, because elimination of infected hepatocytes via cytotoxicity requires a 1:1 interaction between NK cell and hepatocyte, whereas IFN-γ secreted by a single NK cell may reach 100-fold more hepatocytes. Once infection is established, however, both the frequency of HCV-specific T cells and their ability produce IFN-γ are significantly reduced 1, 6, and the same phenomenon of reduced IFN-γ production in chronic infection is also observed for NK cells as we now describe. Both observations may be linked because NK cell-produced IFN-γ is necessary for the development of a vigorous T helper 1 response 42.
In summary, the results of this study suggest that NK cells contribute to liver inflammation due to a polarized phenotype induced by chronic exposure to HCV-induced IFN-α. At the same time, the NK cells' ability to produce IFN-γ does not increase, explaining the paradox that NK cells are activated and cytotoxic, but unable to clear HCV, once chronic infection is established. These data are also of interest in the context of HCV therapy. Patients with higher pre-treatment levels of IFN-induced genes are less likely to respond to IFN-α-based therapy 43. Studies to monitor NK cell responses prospectively prospectively during IFN-α-based therapy are currently in progress in our laboratory.
Supplementary Material
Acknowledgments:
This study was supported by the intramural research program of NIDDK, NIH G Ahlenstiel is the recipient of grant AH173/1-1 from the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany.
Abbreviations
- ALT
alanine aminotransferase
- EIA
enzyme immunoassay
- EMA
ethidium monoazide
- HCV
hepatitis C virus
- HLA
human leukocyte antigen
- IFN
interferon
- NK
natural killer
- MFI
mean fluorescence intensity
- PBMCs
peripheral blood mononuclear cells
- TRAIL
TNF-related apoptosis-inducing ligand
Footnotes
Financial Disclosures and Conflict of Interest Statement: None of the authors has any financial arrangements to disclose No conflict of interest exist for any of the authors
References
- 1.Rehermann B, Nascimbeni M. Immunology of hepatitis B virus and hepatitis C virus infection. Nat Rev Immunol. 2005;5:215–29. doi: 10.1038/nri1573. [DOI] [PubMed] [Google Scholar]
- 2.Urbani S, Amadei B, Fisicaro P, Pilli M, Missale G, Bertoletti A, Ferrari C. Heterologous T cell immunity in severe hepatitis C virus infection. J Exp Med. 2005;201:675–80. doi: 10.1084/jem.20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, Robbins G, Phillips R, Klenerman P, Walker BD. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang KM, Thimme R, Melpolder JJ, Oldach D, Pemberton J, Moorhead-Loudis J, McHutchison JG, Alter HJ, Chisari FV. Differential CD4 and CD8 T-cell responsiveness in hepatitis C virus infection. Hepatology. 2001;33:267–276. doi: 10.1053/jhep.2001.21162. [DOI] [PubMed] [Google Scholar]
- 5.Lauer GM, Barnes E, Lucas M, Timm J, Ouchi K, Kim AY, Day CL, Robbins GK, Casson DR, Reiser M, Dusheiko G, Allen TM, Chung RT, Walker BD, Klenerman P. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127:924–36. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Wedemeyer H, He X-S, Nascimbeni M, Davis AR, Greenberg HB, Hoofnagle JH, Liang TJ, Alter H, Rehermann B. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 7.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, Altman JD, Rouse BT, Freeman GJ, Ahmed R, Grakoui A. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–53. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutebemberwa A, Ray SC, Astemborski J, Levine J, Liu L, Dowd KA, Clute S, Wang C, Korman A, Sette A, Sidney J, Pardoll DM, Cox AL. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215–25. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norris S, Collins C, Doherty DG, Smith F, McEntee G, Traynor O, Nolan N, Hegarty J, O'Farrelly C. Resident human hepatic lymphocytes are phenotypically different from circulating lymphocytes. J Hepatol. 1998;28:84–90. doi: 10.1016/s0168-8278(98)80206-7. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre KW, Welsh RM. Accumulation of natural killer and cytotoxic T large granular lymphocytes in the liver during virus infection. J Exp Med. 1986;164:1667–81. doi: 10.1084/jem.164.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn C, Brunetto M, Reynolds G, Christophides T, Kennedy PT, Lampertico P, Das A, Lopes AR, Borrow P, Williams K, Humphreys E, Afford S, Adams DH, Bertoletti A, Maini MK. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J Exp Med. 2007;204:667–80. doi: 10.1084/jem.20061287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 13.Paladino N, Flores AC, Marcos CY, Fainboim H, Theiler G, Arruvito L, Williams F, Middleton D, Fainboim L. Increased frequencies of activating natural killer receptors are associated with liver injury in individuals who do not eliminate hepatitis C virus. Tissue Antigens. 2007;69(Suppl 1):109–11. doi: 10.1111/j.1399-0039.2006.762_7.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahlenstiel G, Martin MP, Gao X, Carrington M, Rehermann B. Distinct KIR/HLA compound genotypes affect the kinetics of human antiviral natural killer cell responses. J Clin Invest. 2008;118:1017–26. doi: 10.1172/JCI32400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin J, Bost A, Glass J, Tan S. Cytokine-activated NK cells exert direct killing ofhepatoma cellsharboringhepatitis C virus replicons. J Interferon Cytokine Res. 2006;26:854–65. doi: 10.1089/jir.2006.26.854. [DOI] [PubMed] [Google Scholar]
- 16.Golden-Mason L, Madrigal-Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE, O'Farrelly C, Doherty DG. Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut. 2008;57:1121–8. doi: 10.1136/gut.2007.130963. [DOI] [PubMed] [Google Scholar]
- 17.Morishima C, Paschal DM, Wang CC, Yoshihara CS, Wood BL, Yeo AE, Emerson SS, Shuhart MC, Gretch DR. Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology. 2006;43:573–80. doi: 10.1002/hep.21073. [DOI] [PubMed] [Google Scholar]
- 18.Nattermann J, Feldmann G, Ahlenstiel G, Langhans B, Sauerbruch T, Spengler U. Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut. 2006;55:869–77. doi: 10.1136/gut.2005.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Maria A, Fogli M, Mazza S, Basso M, Picciotto A, Costa P, Congia S, Mingari MC, Moretta L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur J Immunol. 2007;37:445–55. doi: 10.1002/eji.200635989. [DOI] [PubMed] [Google Scholar]
- 20.Oliviero B, Varchetta S, Paudice E, Michelone G, Zaramella M, Mavilio D, De Filippi F, Bruno S, Mondelli MU. Natural Killer Cell Functional Dichotomy in Chronic Hepatitis B and Chronic Hepatitis C Virus Infections. Gastroenterology. 2009 May 24; doi: 10.1053/j.gastro.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 21.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–9. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crotta S, Stilla A, Wack A, D'Andrea A, Nuti S, D'Oro U, Mosca M, Filliponi F, Brunetto RM, Bonino F, Abrignani S, Valiante NM. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C Virus envelope protein. J Exp Med. 2002;195:35–42. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon JC, Shiina M, Ahlenstiel G, Rehermann B. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–20. [PubMed] [Google Scholar]
- 26.Saez-Borderias A, Romo N, Magri G, Guma M, Angulo A, Lopez-Botet M. IL-12-dependent inducible expression of the CD94/NKG2A inhibitory receptor regulates CD94/NKG2C+ NK cell function. J Immunol. 2009;182:829–36. doi: 10.4049/jimmunol.182.2.829. [DOI] [PubMed] [Google Scholar]
- 27.Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32:1205–11. doi: 10.1002/1521-4141(200205)32:5<1205::AID-IMMU1205>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.Biron CA, Dalod M. Innate immunity and viral infections. American Society for Microbiology. 2001 [Google Scholar]
- 29.Miyagi T, Gil MP, Wang X, Louten J, Chu WM, Biron CA. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med. 2007;204:2383–96. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meier UC, Owen RE, Taylor E, Worth A, Naoumov N, Willberg C, Tang K, Newton P, Pellegrino P, Williams I, Klenerman P, Borrow P. Shared alterations in NK cell frequency, phenotype, and function in chronic human immunodeficiency virus and hepatitis C virus infections. J Virol. 2005;79:12365–74. doi: 10.1128/JVI.79.19.12365-12374.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG, Chisari FV. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99:15669–74. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75:7059–66. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin EC, Seifert U, Kato T, Rice CM, Feinstone SM, Kloetzel PM, Rehermann B. Virus-induced type I IFN stimulates generation of immunoproteasomes at the site of infection. J Clin Invest. 2006;116:3006–14. doi: 10.1172/JCI29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patzwahl R, Meier V, Ramadori G, Mihm S. Enhanced expression of interferon-regulated genes in the liver of patients with chronic hepatitis C virus infection: detection by suppression-subtractive hybridization. J Virol. 2001;75:1332–8. doi: 10.1128/JVI.75.3.1332-1338.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang S, Wei H, Sun R, Tian Z. IFNalpha regulates NK cell cytotoxicity through STAT1 pathway. Cytokine. 2003;23:190–9. doi: 10.1016/s1043-4666(03)00226-6. [DOI] [PubMed] [Google Scholar]
- 36.Mundt B, Wirth T, Zender L, Waltemathe M, Trautwein C, Manns MP, Kuhnel F, Kubicka S. Tumour necrosis factor related apoptosis inducing ligand (TRAIL) induces hepatic steatosis in viral hepatitis and after alcohol intake. Gut. 2005;54:1590–6. doi: 10.1136/gut.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lan L, Gorke S, Rau SJ, Zeisel MB, Hildt E, Himmelsbach K, Carvajal-Yepes M, Huber R, Wakita T, Schmitt-Graeff A, Royer C, Blum HE, Fischer R, Baumert TF. Hepatitis C virus infection sensitizes human hepatocytes to TRAIL-induced apoptosis in a caspase 9-dependent manner. J Immunol. 2008;181:4926–35. doi: 10.4049/jimmunol.181.7.4926. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Dong H, Eksioglu E, Hemming A, Cao M, Crawford JM, Nelson DR, Liu C. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology. 2007;133:1649–59. doi: 10.1053/j.gastro.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 39.Wang SH, Huang CX, Ye L, Wang X, Song L, Wang YJ, Liang H, Huang XY, Ho WZ. Natural killer cells suppress full cycle HCV infection of human hepatocytes. J Viral Hepat. 2008;15:855–864. doi: 10.1111/j.1365-2893.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thimme R, Bukh J, Spangenberg HC, Wieland S, Pemberton J, Steiger C, Govindarajan S, Purcell RH, Chisari FV. Viral and immunological determinants of hepatitis C virus clearance, persistence, and disease. Proc Natl Acad Sci U S A. 2002;99:15661–8. doi: 10.1073/pnas.202608299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guidotti LG, Chisari FV. To kill or to cure: options in host defense against viral infection. Curr Opin Immunol. 1996;8:478–483. doi: 10.1016/s0952-7915(96)80034-3. [DOI] [PubMed] [Google Scholar]
- 42.Morandi B, Bougras G, Muller WA, Ferlazzo G, Munz C. NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion. Eur J Immunol. 2006;36:2394–400. doi: 10.1002/eji.200636290. [DOI] [PubMed] [Google Scholar]
- 43.Feld JJ, Nanda S, Huang Y, Chen W, Cam M, Pusek SN, Schweigler LM, Theodore D, Zacks SL, Liang TJ, Fried MW. Hepatic gene expression during treatment with peginterferon and ribavirin: Identifying molecular pathways for treatment response. Hepatology. 2007;46:1548–63. doi: 10.1002/hep.21853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.