Abstract
The aim of the present study was to examine the association between prenatal psychosocial stress exposure and subsequent prefrontal cortex-dependent working memory performance in human adults. Working memory performance was assessed using an item-recognition task under 10 mg hydrocortisone (cortisol) and placebo conditions in a sample of 32 healthy young women (mean age = 25 ± 4.34 years) whose mothers experienced a major negative life event during their pregnancy (Prenatal Stress, PS group), and in a comparison group of 27 healthy young women (mean age = 24 ± 3.4 years). The two groups did not differ in the placebo condition, however, subjects in the PS group showed longer reaction times after hydrocortisone administration compared with subjects in the comparison group (p = .02). These findings provide support for an association between prenatal stress exposure and the potential modulatory effect of cortisol on working memory performance in young adults, which may reflect compromised development of the prefrontal cortex in prenatal life.
Keywords: prenatal stress, psychosocial, working memory, hydrocortisone
The notion of developmental programming of adult health and disease is based on a large number of epidemiological studies across the world that have reported associations between markers of an individual’s birth phenotype, such as low birth weight or small body size, and subsequent risk of disease in adult life (Barker, 1998; Cannon, Jones, & Murray, 2002; Gluckman & Hanson, 2004a; Thompson, Syddall, Rodin, Osmond, & Barker, 2001). There is emerging evidence that in humans, cognitive and intellectual performance also is affected by prenatal factors. For example, very low birth weight children perform poorer in academic achievement tests (Finnstrom, Gaddlin, Leijon, Samuelsson, & Wadsby, 2003), and low birth weight is associated with lower scores on tests measuring language, spatial, fine motor, tactile, and attention abilities (Breslau, Chilcoat, DelDotto, Andreski, & Brown, 1996). In line with these results, higher birth weight was associated with better intelligence test scores at age 17 years (Seidman et al., 1992), and intrauterine growth restriction had an impact on spatial navigation at 6 years of age (Leitner, Heldman, Harel, & Pick, 2005).
It is unlikely that birth phenotypes, per se, play a causal role in increasing the risk for impairments in cognitive performance later in life. Instead, birth phenotypes more likely constitute a crude marker of developmental processes in intrauterine life that also may influence the structure and function of physiological systems that underlie health and disease risk in later life (Gluckman & Hanson, 2004b; Morley, Owens, Blair, & Dwyer, 2002). The link between prenatal environment and adult health and disease may not necessarily be mediated via adverse birth outcomes, and measures of prenatal conditions may be more sensitive predictors of subsequent outcomes than birth size and weight at birth.
The association between prenatal factors and learning and memory has been reported very early in development. Elevated levels of placental corticotrophin-releasing hormone (CRH) concentrations during the last trimester of gestation, potentially reflecting high levels of physiological stress, are associated with impaired fetal learning (Sandman, Wadhwa, Chicz-DeMet, Porto, & Garite, 1999). A small but growing literature indicates that the consequences of prenatal maternal stress persist into the postpartum period (e.g., Entringer et al., 2008; Luoma et al., 2001; O’Connor, Heron, Golding, Beveridge, & Glover, 2002; Van den Bergh, Van Calster, Smits, Van Huffel, & Lagae, 2007). Several recent prospective studies in humans have reported that prenatal adversity is associated with cognitive development (Buitelaar, Huizink, Mulder, de Medina, & Visser, 2003; Laplante, Brunet, Schmitz, Ciampi, & King, 2008). Moreover, studies in rodents and nonhuman primates also report that prenatal stress exposure influences postnatal cognitive performance (e.g., Kofman, 2002; Lemaire, Lamarque, Moal, Piazza, & Abrous, 2006; Szuran, Zimmermann, & Welzl, 1994; Vallee et al., 1999) and suggest underlying functional mechanisms may explain this association, including impairment of neurogenesis (Coe et al., 2003; Lemaire, Koehl, Le Moal, & Abrous, 2000), and long-term potentiation (Yaka, Salomon, Matzner, & Weinstock, 2007; Yang et al., 2007).
Many of these studies have examined hippocampal-dependent declarative memory because the hippocampus has been shown to be a prominent target for early adverse effects (e.g., Bremner, 2003; Driessen et al., 2000), and hippocampal dysfunction is believed to be involved in hypothalamic–pituitary–adrenal (HPA) axis dysregulation associated with adverse neonatal experience (Meaney et al., 1996). Less work has been done with regard to the effects of early adversity (i.e., prenatal as well as early neonatal influences) on prefrontal-dependent working memory performance, which constitutes another important aspect of cognitive function. Working memory is the cognitive mechanism that underlies temporary storage and manipulation of limited amounts of information (Baddeley, 1986), allowing the brain to perform higher cognitive functions such as language, comprehension, and reasoning. The prefrontal cortex is known to develop later in terms of myelination and synaptic density (Yakovlev & Lecours, 1967). Because of its protracted development and the expression of glucocorticoid (GC) receptors (GRs; Teicher et al., 2003), the prefrontal cortex also may be prone to early insults. This is supported by a recent study in rats reporting an association between maternal stress during pregnancy and reductions of dendritic spine densities in the prefrontal cortex in the offspring (Murmu et al., 2006), and by several studies that found associations between early postnatal adverse experience and changes in the development of synaptic circuits in the prefrontal cortex of rodents (Helmeke, Ovtscharoff, Poeggel, & Braun, 2001; Helmeke, Poeggel, & Braun, 2001; Ovtscharoff & Braun, 2001; Poeggel et al., 2003). In addition, studies that examined the effects of a different kind of prenatal adversity—prenatal alcohol exposure—found associations with frontal cortical volume reductions (Wass, Persutte, & Hobbins, 2001) and a decreased number of neurons in the medial prefrontal cortex (Mihalick, Crandall, Langlois, Krienke, & Dube, 2001). Effects of exogenous GC administration (Lupien, Gillin, & Hauger, 1999; Mizoguchi, Ishige, Takeda, Aburada, & Tabira, 2004), as well as acute psychosocial stress (Schoofs, Preuss, & Wolf, 2008) on working memory performance have been observed, and they are believed to be modulated by GRs. In rodents, prenatal stress was associated with reduced hippocampal GR density (Barbazanges, Piazza, Le Moal, & Maccari, 1996; Maccari et al., 1995; Weinstock, Matlina, Maor, Rosen, & McEwen, 1992), and early neonatal adversity was associated with reduced hippocampal and frontal GR density (Ladd, Huot, Thrivikraman, Nemeroff, & Plotsky, 2004; Meaney et al., 1985).
Taken together, theses findings suggest that working memory function may be impaired by acute and early developmental stress exposure. In some studies, differential effects of GC on memory performance in patient populations compared to healthy controls have been noted (Bremner, Vythilingam, Vermetten, Afzal, et al., 2004; Bremner, Vythilingam, Vermetten, Anderson, et al., 2004). Thus, these studies suggest that if there is a subtle vulnerability, small differences between groups may not emerge under basal conditions but may emerge only when there is a challenge imposed on the system. Thus, the objective of the present study was to investigate in a double-blind, placebo-controlled, within-subject design in young adults (a) whether there is a general effect of prenatal psychosocial stress exposure on working memory performance (i.e., basal or placebo condition), and (b) whether the effects of prenatal stress on working memory are modulated by exogenous hydrocortisone administration.
Method
Subjects
The study sample included a total of 59 subjects. Thirty-two young women (mean age = 25 years, SD = ± 4.34 years), whose mothers experienced a high level of psychosocial stress (negative life events during pregnancy), constituted the prenatal stress group (PS). A sample of 27 women of comparable age (24 ± 3.40 years) constituted the comparison group (CG). Subjects were recruited through an announcement in local newspapers and via e-mails that were sent to students and staff of the University of Trier, Germany. Before entering the study, the absence of acute or chronic physical and mental health problems was ascertained by self-report and confirmed by a medical examination, and subjects were asked about their medical history. All subjects were nonsmokers, reported to be medication free and to have no history of psychiatric disorders. A copy of the maternal prenatal medical record (which is handed to the mother by the obstetrician during her first prenatal visit) was obtained from each participant. From this record, information about maternal parity, maternal age at birth, length of gestation and subjects’ weight, height and head circumference at birth were extracted. Written informed consent was obtained from all subjects. The investigation described in this manuscript was conducted in accordance with the guidelines described in the declaration of Helsinki, and the study protocol was approved by the ethics committee of the German Psychological Society (DGPs).
Conceptualization and Assessment of Prenatal Psychosocial Stress Exposure
We adopted a conservative strategy for the conceptualization of prenatal stress in the present study. We defined a high level of prenatal psychosocial stress exposure as the presence of major negative life events that occurred to the mother while she was pregnant (see Table 1 for list and frequency of events). Psychosocial stress is a multicomponent construct that includes the occurrence of negative life events, appraisal of the stress (e.g., degree of predictability and control), and psychological symptoms such as anxiety and negative affect. Because retrospective assessment of stress appraisals and symptoms is known to be unreliable, we focused on only the presence or absence of negative life events during the index pregnancy. Moreover, we selected those events that are considered as highly stressful across individuals (Table 1).
Table 1.
List of Events During Pregnancy Included in the Study (N = 32)
| Event | n | % |
|---|---|---|
| Relationship conflicts (divorce, break up, paternity denial, marital infidelity) |
13 | 41 |
| Death of someone close (partner, parent, other child) | 7 | 22 |
| Severe illness of someone close (cancer, heart attack, stroke) | 6 | 19 |
| Severe financial problems (loss of house because of flooding, sudden unemployment of husband, foreclosure) |
3 | 9 |
| Car accident | 2 | 6 |
| Unmarried, father not accepted by family | 1 | 3 |
| Becoming political refugee | 2 | 5 |
Note. Each woman is listed with one life event. One woman that reported marital infidelity of her husband followed by a divorce during her pregnancy is only listed once under the category “Relationship Conflicts.” Three other women that lost a family member suffering of a disease were each listed once under “Death of Someone Close” and not under “Severe Illness of Someone Close.”
In all subjects we conducted semistructured interviews based on a questionnaire about exposure to major negative life events during the prenatal period that subjects were instructed to review with their mothers prior to the interview. In most of the cases (70%) we were able to verify this information by communicating directly with the mothers by phone, e-mail, or letters. The subjects that were recruited to constitute the comparison group were asked to review the same questionnaire with their mothers to ascertain that their mothers had not experienced any negative life events during pregnancy.
Working Memory Task
Working memory performance was tested twice in each individual with 1-week intervals between test sessions. At the beginning of the first session, participants’ height and body weight were measured and body mass index (BMI) was computed. Subjects were asked to ingest 10 mg of hydrocortisone or placebo at 1400 h (1 h prior to testing) to allow uptake of hydrocortisone. This dose of orally administered hydrocortisone has been found to have an effect on memory performance in previous studies (e.g., Buss, Wolf, Witt, & Hellhammer, 2004). The order of administration was randomized in a double-blind fashion. Unfortunately, because the present sample was a subgroup of a larger study, complete counterbalancing in regard to the order of hydrocortisone/placebo administration could not be achieved. Among the 32 PS subjects, 23 received hydrocortisone on Day 1, and 9 subjects received placebo on Day 1, whereas 9 CG subjects received hydrocortisone and 18 received placebo on Day 1. Subjects remained at the research facility until testing at 1500 h, and no further tests were conducted during this time period.
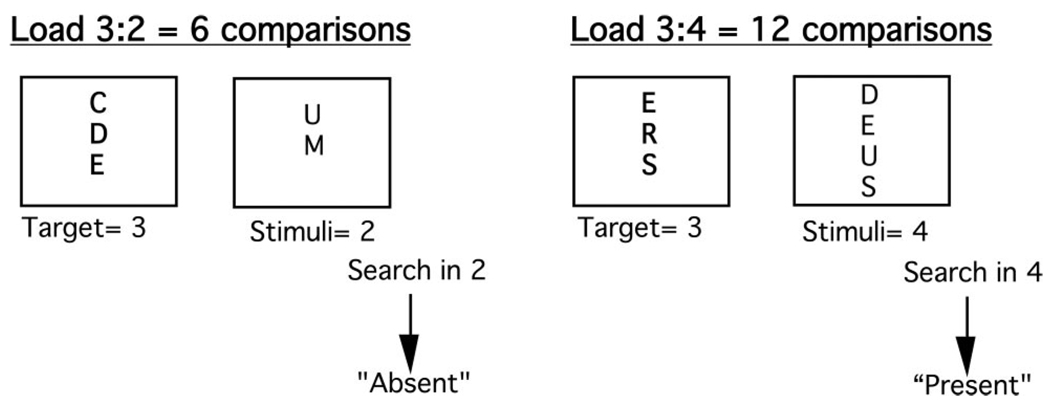
Working memory performance was assessed by using a computerized item-recognition task adapted from Sternberg (1966) using EPrime (Version 1.0) and experimental procedures were identical across test days. The task included a series of discrete trials. Each condition consisted of the presentation of three to four uppercase letters, followed by a recognition display of two to four uppercase letters, to which participants responded yes (present-target trials) or no (absent-target trials), by pressing one of two buttons on a computer keyboard, indicating whether or not one of the targets was identical to one of the stimuli in the recognition display. There was only one possible target present in the display on present-target trials. Each condition comprised 20 trials, and the number of comparisons determined processing load. The processing capacity load was manipulated by varying the number of targets to be held in memory for later item recognition or by varying the number of stimuli presented in the recognition display, or by varying both. Three to four targets to be held in memory with two to four stimuli in the recognition display led to a range in processing loads of 6 to 16 comparisons. For example, a comparison load of 6 (3:2, target:display) means that 3 targets have to be held in working memory while there are 2 stimuli on the item-recognition display, leading to six possible comparisons to perform before answering (see Figure 1). For all conditions, the stimuli were uppercase letters and there were 20 trials per each of the five conditions (i.e., five comparison loads: 6 vs. 8 vs. 9 vs. 12 vs. 16), yielding a total of 100 trials. Errors and reaction times were recorded by the software and the order of processing load was randomized across participants.
Figure 1.
Schematic presentation of trials in the item recognition task. After presentation of the target, the stimuli were presented on the item-recognition display. Comparison load was defined by the number of targets (3 to 4) to hold in working memory multiplied by the number of stimuli (2 to 4) in the item-recognition display (adapted from Lupien et al., 1999).
Cortisol Measures
Salivary cortisol concentrations were assessed using a Salivette sampling device (Sarstedt, Nümbrecht, Germany). Saliva samples were obtained before the administration of hydrocortisone or placebo at 1400 h and immediately before testing at 1500 h. Saliva samples were frozen at −20 °C until analysis. Salivary cortisol was analyzed with a time-resolved immunoassay with fluorescence detection as described elsewhere (Dressendorfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). Interassay and intraassay coefficients of variance were below 10% and 12%, respectively.
Questionnaires
Because it is possible that prenatal stress exposure is associated with adverse postnatal experiences such as poor maternal care and presence of other stressors during childhood, we administered several measures to assess and control for these potential confounding factors. To measure an important aspect of the family environment during the postnatal period, we administered the maternal care scale of the Parental Bonding Inventory (PBI; German version by Lutz, Heyn, and Kommer [1995], originally developed by Parker, Tupling, and Brown [1979]). The PBI measures the self-reported perception of being parented to the age of 16 years. Studies assessing retest reliability of the PBI suggest that the parental evaluation is a rather stable measure, which is not affected by confounding variables like dysthymia, neuroticism, depressive episode, or gender (Lizardi & Klein, 2005; Parker, 1990; Plantes, Prusoff, Brennan, & Parker, 1988; Wilhelm, Niven, Parker, & Hadzi-Pavlovic, 2005). Good validity of the PBI can be concluded for example, from high agreement between sibling ratings (Parker, 1990). Furthermore, subjects’ and subjects’ mothers’ socioeconomic status (SES) was assessed by educational level.
A translated version of the Childhood Traumatic Events Survey (Pennebaker & Susman, 1988) was administered. The instrument screens adverse experience during childhood in six questions. In addition, subjects completed a German version of the Centre for Epidemiological Studies Depression Scale (CES-D, (Hautzinger & Bailer, 1993), and the Neuroticism Extraversion Openness Five Factor Inventory (NEO FFI; Borkenau & Ostendorf, 1993).
Statistical Analysis
T tests and General Linear Models (GLMs) were computed for the analyses of cortisol concentrations, with repeated measures time (1400 h vs.1500 h) and treatment (hydrocortisone vs. placebo) and between-subjects factors group (PS vs. CG). The interrelationship between BMI and cortisol levels after hydrocortisone ingestion was assessed applying Pearson correlations.
To analyze the association between prenatal stress exposure and working memory performance, GLMs for reaction time were computed with group as the between subjects factors and treatment (hydrocortisone vs. placebo), target type (absent vs. present) and comparison load (6 vs. 8 vs. 9 vs. 12 vs. 16) as the within-subjects factors. Further GLMs were performed to analyze a potential sequence effect, as subjects may profit on the second testing day from learning experience on the first testing day. First, test day (Day 1 vs. Day 2) and comparison load were entered as the within-subjects factors, while reaction times were averaged across groups, target type and treatment. Second, further models were computed for Day 1 only, separately for hydrocortisone and placebo treatment, with group as the between subjects factor and comparison load as the within-subjects factors. Third, an additional model was conducted for Day 1 within PS subjects only, with comparison load as the within-subject and treatment as the between-subjects factor.
Additional GLM analyses were performed to assess differences between the two groups in birth weight, length of gestation, maternal age and parity, SES, depression, degree of neuroticism, and maternal care. χ2 analyses were conducted to assess differences between the two groups in the frequency of the events assessed by the Childhood Traumatic Events Survey.
All results shown are the mean ± SEM. Greenhouse-Geisser corrections were applied where appropriate and only adjusted results are reported.
Results
Birth Weight, Length of Gestation, and Mode of Delivery
There was no difference between the two groups in birth weight, PS = 3280 g ± 83, CG = 3258 g ± 74, F(1, 55) = .03, p = .86; length of gestation, PS = 39.33 weeks ± .30, CG = 39.39 weeks ± .37, F(1, 53) = .01, p = .91, or birth weight adjusted for length of gestation as expressed by growth percentiles at birth, PS = 37.8 ± 1.9, CG = 40.8 ± 2.6, F(1, 50) = .36, p = .55. Birth weight and length of gestation of all subjects were within the normal range. There were no cesarean section deliveries in the PS group, whereas two subjects in the CG were delivered by cesarean section. Birth weight and length of gestation of these two subjects were within the normal range. The two groups did not differ in maternal parity, PS = 1.1 ± .21, CG = 1.0 ± .18, F(1, 49) = .14, p = .71, and maternal age at birth, PS = 28.23 ± .90, CG = 30.13 ± 1.1, F(1, 49) = 1.8, p = .18.
Cortisol Levels
Cortisol levels increased significantly 1 hour after hydrocortisone administration, 6.31 ± 1.17 vs. 54.06 ± 5.11, t(60) = −9.77, p < .001, affirming that subjects ingested the hydrocortisone tablet. After placebo administration, cortisol concentrations were not different one hour after ingestion, 5.28 ± .37 vs. 5.63 ± 1.21, t(60) = −.31, p = .76. In addition, no differences between the two groups in cortisol concentrations before or after hydrocortisone or placebo administration were observed (ps > .36 for main effect group, interaction group by time, interaction group by treatment). There was no association between cortisol concentrations after hydrocortisone administration and BMI (r = −.11, p = .38).
Working Memory Performance
Detection errors with regard to each comparison load, presence of target, and treatment were all below 5% and were not subjected to further analyses.
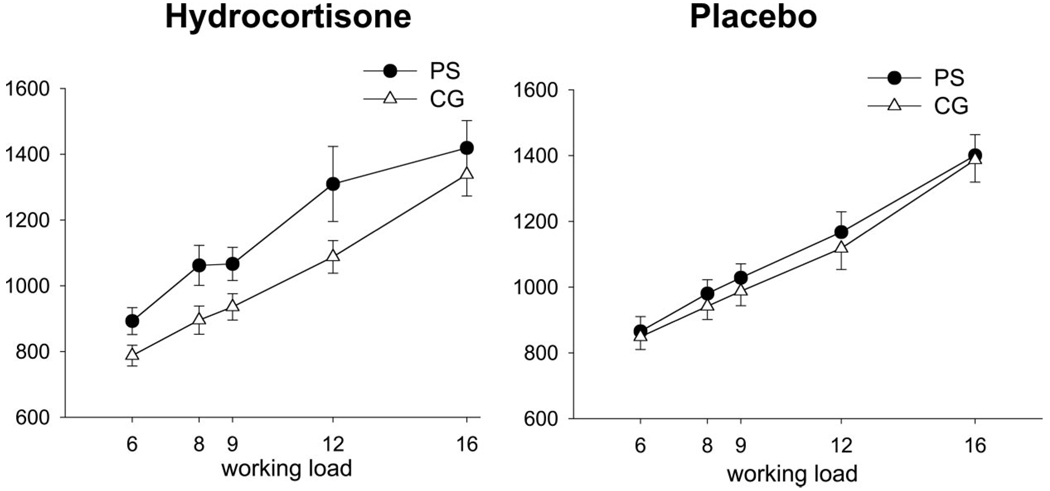
As expected, participants performed faster when the target was present, main effect target type: F(1, 52) = 119.14, p < .001, and reaction time increased with higher working loads, F(2.67,138.96) = 100.58, p < .001. There were no main effects of treatment, F(1, 52) = .07, p = .79 or group, F(1, 52) = 1.18, p = .28. However, a significant group × treatment interaction emerged, F(1, 53) = 5.74, p = .02, with PS subjects performing slower compared CG subjects under hydrocortisone, whereas the two groups did not differ under placebo treatment (see Figure 2). Because there were no interactions between group and target type, reaction times for all further analyses were averaged across target present and absent conditions.
Figure 2.
Reaction times in a working memory task in prenatally stressed (PS) and comparison group (CG) subjects after hydrocortisone (left) and placebo (right) administration. The two groups did not differ in the placebo condition; however, PS subjects showed longer reaction times after hydrocortisone administration compared with CG subjects (p = .02).
Averaged across target type, group and treatment, there was a significant effect of test day, F(1, 54) = 21.2, p < .001: participants performed faster on Day 2 than on Day 1, suggesting a practice effect. Because the order of treatment was not completely counterbalanced (relatively more PS than CG subjects received hydrocortisone on Test Day 1), further analyses were performed for Test Day 1 only to investigate whether the above reported Group × Treatment interaction was driven by practice effects. On Day 1, PS subjects under hydrocortisone performed slower CG subjects under hydrocortisone (marginally significant main effect group, F(1, 30) = 3.67, p = .06, whereas there was no difference between PS and CG subjects under placebo, F(1, 24) = .33, p = .57.
Examining reaction times on Day 1 within PS subjects only, descriptively PS subjects under hydrocortisone performed slower than PS subjects under placebo, but the main effect treatment was not significant, F(1, 29) = 1.28, p = .27.
Questionnaires
Subjects and subjects’ mothers did not differ in SES, assessed by educational level, and there were no differences in maternal care scores between the two groups, F(1, 49) = 1.74, p = .19. The two groups neither differed in the frequency of the events assessed by the Childhood Traumatic Events Survey (p > .25 for all events), nor in their depression score, F(1, 54) = .12, p = .73 or their degree of neuroticism, F(1, 58) = 1.29, p = .24.
Discussion
Our results demonstrate an association in humans between maternal psychosocial stress exposure during pregnancy and subsequent working memory performance in the adult (female) offspring, potentially modulated by cortisol.
Young women who were exposed to prenatal stress exhibited an impaired performance under hydrocortisone treatment in a cognitive task that has been related to prefrontal cortex functioning, while they did not differ from a comparison group under basal (i.e., after placebo) condition. The observed changes are independent of birth weight and length of gestation, as well as postnatal factors including maternal care, exposure to traumatic events during childhood, and subjects’ present depression and neuroticism scores.
Consistent with our findings, Mennes, Stiers, Lagae, and Van den Bergh (2006) did not find an effect of antenatal maternal anxiety on working memory performance in adolescents tested under basal conditions. In young adults, changes in working memory performances after prenatal exposure to maternal adversity only seem to emerge when taking the modulatory effects of GC into account. It is possible that differences in working memory performance under basal condition between the two groups may emerge later in life, because animal models suggest that lower cognitive performance in association with early adversity are observed over the course of aging and are not distinct at younger age (Brunson et al., 2005).
Wadhwa and others (e.g., Huizink, Mulder, & Buitelaar, 2004; Wadhwa, 2005) have discussed possible mechanisms of how stress is transduced from the pregnant mother to the fetus, such as (a) transplacental transport of maternal stress hormones to the fetus, (b) maternal stress-induced release of placental hormones that enter the fetal circulation, and (c) maternal stress-induced effects on placental physiology including blood flow and changes in fetal metabolism impacting on oxygen and glucose usage. These changes may produce deleterious effects on brain development during critical periods, impairing its maturation with effects on morphology, physiology, and neurochemistry (Coe et al., 2003).
The question arises why the PS subjects show impaired working memory function only after exogenous hydrocortisone administration. Although speculative, we propose the following model: Cortisol has been shown to modulate working memory performance (Lupien et al., 1999; Mizoguchi et al., 2004). The cortisol-modulating effect on working memory is believed to be mediated by GC receptors (GRs), which are expressed in the prefrontal cortex (McEwen, de Kloet, & Rostene, 1986; McEwen, Weiss, & Schwartz, 1968; Meaney et al., 1985; Reul & de Kloet, 1985; Seidman, Laor, Gale, Stevenson, & Danon, 1991). Changes in the expression rates of mineralocorticoid receptors (MRs) and GRs are associated with cognitive function in animal models (see, for example, Ferguson & Sapolsky, 2008). In rodents, early maternal separation has been found to be associated with reduced hippocampal and frontal GR density (Ladd et al., 2004; Meaney et al., 1985). Circulating GCs bind to two receptor subtypes: the mineralocorticoid (MR or Type I) and GC (GR or Type II) receptors (Reul & de Kloet, 1985). Given their differential affinity for GCs, the MRs will be saturated at lower concentrations of GCs than the GRs. In both animals and humans, many studies reveal the presence of an inverted U-shaped function between circulating stress hormone levels and memory performance (for a complete review, see Lupien & McEwen, 1997). de Kloet, Oitzl, and Joels (1999) explain this inverted U-shaped function with a MR/GR ratio hypothesis. In this view, cognitive function can be enhanced when most of the MRs and only part of the GRs are activated (top of the inverted U-shape function; increased MR/GR ratio). However, when circulating levels of GCs are significantly decreased or increased (extremes of the inverted U-shaped function; low MR/GR ratio), cognitive impairments will result. This model has been developed to explain effects of GC on hippocampus dependent memory; however, we speculate that it can also be applied to prefrontal cortex dependent memory function, given high expression of MR and GR in the prefrontal cortex (McEwen et al., 1986; Meaney et al., 1985; Patel et al., 2000; Sarrieau et al., 1988; Watzka et al., 2000; Xing, Russell, Webster, & Post, 2004). The proposed model would suggest that in the comparison group, the relatively low amount of orally administered hydrocortisone in our study compared with other studies (e.g., Lupien et al., 1999) was not sufficient to result in a low ratio of MR/GR occupation by saturating MRs and GRs. Thus, working memory performance was not impaired by hydrocortisone administration in the CG. In the PS subjects, however, we speculate that exposure to prenatal stress may have resulted in lower frontal GR expression. Thus, the relatively low amount of hydrocortisone that we administered may have led to a lower MR/GR ratio because of higher occupancy of GRs. Consequently, PS subjects showed a decreased memory performance under hydrocortisone treatment.
There are some limitations to our study. First and foremost, prenatal stress exposure was assessed retrospectively. Although retrospective assessments of psychosocial factors such as stress are prone to biases such as “after-the-fact” reporting, biases produced by personality/mood, and memory-related biases, we believe it is unlikely that any of these biases operated in our study sample. Subjects with adverse health outcomes are more prone to retrospectively reporting higher levels of prior adverse exposures (i.e., after-the-fact retrospective reporting bias); however, it is unlikely that this bias was present in the current study because all subjects were healthy and subjects (as well as the experimenters) were blind to and had no a priori knowledge about the results of the study outcome. In terms of reporting bias produced by personality/mood state, the subjects in the two groups did not differ in either neuroticism or depression scores (i.e., the major personality/mood constructs that underlie self-report bias). Last, retrospective assessments also are prone to memory biases, but it also is unlikely that this potential bias affected one group of subjects more than the other. If at all, memory bias and underreporting stress in the comparison group could only bias the results in the direction of possibly diluting the observed effects.
Retrospective life event assessments certainly are less biased than retrospective assessments of other components of stress such as perceived severity of stress appraisals and symptoms. Thus, instead of relying on a subjective distress measure of mothers’ stress appraisal, we focused on only the presence of negative life events during the index pregnancy, and we selected those events that are considered as highly stressful across individuals. Although it is known that stressful life events are more likely to occur in women of lower social class, there were no differences in SES in our two study groups. It is possible that prenatal stress exposure is associated with adverse postnatal experiences such as poor maternal care and presence of other stressors during childhood. We, however, assessed these constructs in our sample and found no differences across the two study groups in perceived maternal care after birth, in the frequency of early traumatic events assessed by the Childhood Traumatic Events Survey, in depression and neuroticism levels.
Because the present sample was a subgroup of a larger study, complete counterbalance in regard to the order of hydrocortisone/placebo administration could not be achieved in this within-subject design. However, the direction of the observed effect was the same when—in a cross sectional model—comparing the two groups under hydrocortisone and placebo treatment at Day 1 only, and when comparing working memory performance of PS subjects under hydrocortisone versus under placebo on Day 1 only. This suggests that the observed interaction between hydrocortisone treatment and prenatal stress exposure was not because of practice effects.
Unfortunately, very few men responded to our study announcements and thus were not included in the present analyses because we did not have the power to examine potential gender effects. As a result, our findings cannot be assumed to generalize to men.
In conclusion, the present findings provide support for an impact of prenatal stress exposure on working memory performance in humans, which might reflect compromised development of the prefrontal cortex in prenatal life.
Acknowledgments
This work was supported, in part, by the German Research Foundation Grant WU 324/ 3-(1-3) to SW and U.S. PHS (NIH) Grants HD-047609, HD-041696, and HD-33506 to PDW.
Contributor Information
Sonja Entringer, Department of Theoretical and Clinical Psychobiology, University of Trier, Germany, and Department of Psychiatry and Human Behavior, University of California, Irvine, Irvine, California.
Claudia Buss, Department of Theoretical and Clinical Psychobiology, University of Trier, Germany, and Department of Psychiatry and Human Behavior, University of California, Irvine, Irvine, California.
Robert Kumsta, Department of Theoretical and Clinical Psychobiology, University of Trier, Germany, and MRC Social, Genetic, and Developmental Psychiatry Centre, Institute of Psychiatry, King’s College London, United Kingdom.
Dirk H. Hellhammer, Department of Theoretical and Clinical Psychobiology, University of Trier, Germany
Pathik D. Wadhwa, Department of Psychiatry and Human Behavior, University of California, Irvine, Irvine, California
Stefan Wüst, Department of Theoretical and Clinical Psychology, University of Trier, Germany, and Department of Genetic Epidemiology in Psychiatry, Central Institute of Mental Health, Mannheim, Germany.
References
- Baddeley A. Working memory. New York: Oxford University Press; 1986. [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. Journal of Neuroscience. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Mothers, babies and health in later life. Livingstone: Churchill; 1998. [Google Scholar]
- Borkenau P, Ostendorf F. NEO-Fünf-Faktoren Inventar (NEO-FFI) nach Costa und McCrae. Handanweisung. Göttingen: Hogrefe; 1993. [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child and Adolescent Psychiatric Clinics of North America. 2003;12:271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Afzal N, Nazeer A, Newcomer JW, et al. Effects of dexamethasone on declarative memory function in posttraumatic stress disorder. Psychiatry Research. 2004;129:1–10. doi: 10.1016/j.psychres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Anderson G, Newcomer JW, Charney DS. Effects of glucocorticoids on declarative memory function in major depression. Biological Psychiatry. 2004;55:811–815. doi: 10.1016/j.biopsych.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat H, DelDotto J, Andreski P, Brown G. Low birth weight and neurocognitive status at six years of age. Biological Psychiatry. 1996;40:389–397. doi: 10.1016/0006-3223(95)00399-1. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, et al. Mechanisms of late-onset cognitive decline after early-life stress. Journal of Neuroscience. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buitelaar JK, Huizink AC, Mulder EJ, de Medina PG, Visser GH. Prenatal stress and cognitive development and temperament in infants. Neurobiology of Aging. 2003;24 Suppl 1:S53–S60. doi: 10.1016/s0197-4580(03)00050-2. discussion S67-58. [DOI] [PubMed] [Google Scholar]
- Buss C, Wolf OT, Witt J, Hellhammer DH. Autobiographic memory impairment following acute cortisol administration. Psychoneuroendocrinology. 2004;29:1093–1096. doi: 10.1016/j.psyneuen.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: Historical and meta-analytic review. American Journal of Psychiatry. 2002;159:1080–1092. doi: 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biological Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: Are corticosteroids good or bad guys? Trends in Neurosciences. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- Dressendorfer RA, Kirschbaum C, Rohde W, Stahl F, Strasburger CJ. Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology. 1992;43:683–692. doi: 10.1016/0960-0760(92)90294-s. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, et al. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Entringer S, Wust S, Kumsta R, Layes IM, Nelson EL, Hellhammer DH, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. American Journal of Obstetrics and Gynecology. 2008;199(498):e491–e497. doi: 10.1016/j.ajog.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson D, Sapolsky R. Overexpression of mineralocorticoid and transdominant glucocorticoid receptor blocks the impairing effects of glucocorticoids on memory. Hippocampus. 2008;18:1103–1111. doi: 10.1002/hipo.20467. [DOI] [PubMed] [Google Scholar]
- Finnstrom O, Gaddlin PO, Leijon I, Samuelsson S, Wadsby M. Very-low-birth-weight children at school age: Academic achievement, behavior and self-esteem and relation to risk factors. Journal of Maternal Fetal Neonatal Medicine. 2003;14:75–84. doi: 10.1080/jmf.14.2.75.84. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004a;15:183–187. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004b;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M. Allgemeine Depressions Skala: Manual. Göttingen: Beltz Test GmbH; 1993. [Google Scholar]
- Helmeke C, Ovtscharoff W, Jr, Poeggel G, Braun K. Juvenile emotional experience alters synaptic inputs on pyramidal neurons in the anterior cingulate cortex. Cerebral Cortex. 2001;11:717–727. doi: 10.1093/cercor/11.8.717. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Poeggel G, Braun K. Differential emotional experience induces elevated spine densities on basal dendrites of pyramidal neurons in the anterior cingulate cortex of Octodon degus. Neuroscience. 2001;104:927–931. doi: 10.1016/s0306-4522(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Mulder EJ, Buitelaar JK. Prenatal stress and risk for psychopathology: Specific effects or induction of general susceptibility? Psychological Bulletin. 2004;130:115–142. doi: 10.1037/0033-2909.130.1.115. [DOI] [PubMed] [Google Scholar]
- Kofman O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neuroscience and Biobehavioral Reviews. 2002;26:457–470. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mRNA and negative feedback on the hypothalamo-pituitary-adrenal axis following neonatal maternal separation. Biological Psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Laplante DP, Brunet A, Schmitz N, Ciampi A, King S. Project Ice Storm: Prenatal maternal stress affects cognitive and linguistic functioning in 5(1/2)-year-old children. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1063–1072. doi: 10.1097/CHI.0b013e31817eec80. [DOI] [PubMed] [Google Scholar]
- Leitner Y, Heldman D, Harel S, Pick CG. Deficits in spatial orientation of children with intrauterine growth retardation. Brain Research Bulletin. 2005;67:13–18. doi: 10.1016/j.brainresbull.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Lamarque S, Moal ML, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biological Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Lizardi H, Klein DN. Long-term stability of parental representations in depressed outpatients utilizing the Parental Bonding Instrument. Journal of Nervous and Mental Disease. 2005;193:183–188. doi: 10.1097/01.nmd.0000154838.16100.36. [DOI] [PubMed] [Google Scholar]
- Luoma I, Tamminen T, Kaukonen P, Laippala P, Puura K, Salmelin R, et al. Longitudinal study of maternal depressive symptoms and child well-being. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:1367–1374. doi: 10.1097/00004583-200112000-00006. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Gillin CJ, Hauger RL. Working memory is more sensitive than declarative memory to the acute effects of corticosteroids: A dose-response study in humans. Behavioral Neuroscience. 1999;113:420–430. doi: 10.1037//0735-7044.113.3.420. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Research. Brain Research Reviews. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Lutz R, Heyn C, Kommer D. Questionnaire to assess parental bonding. In: Lutz R, Mark N, editors. How healthy are the sick? Mental health of the sick. Göttingen: Verlag für angewandte Psychologie; 1995. pp. 183–199. [Google Scholar]
- Maccari S, Piazza PV, Kabbaj M, Barbazanges A, Simon H, Le Moal M. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. Journal of Neuroscience. 1995;15(1 Pt 1):110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, de Kloet ER, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiological Reviews. 1986;66:1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature. 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Aitken DH, Bodnoff SR, Iny LJ, Tatarewicz JE, Sapolsky RM. Early postnatal handling alters glucocorticoid receptor concentrations in selected brain regions. Behavioral Neuroscience. 1985;99:765–770. doi: 10.1037//0735-7044.99.4.765. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, et al. Early environmental regulation of forebrain glucocorticoid receptor gene expression: Implications for adrenocortical responses to stress. Developmental Neuroscience. 1996;18:49–72. doi: 10.1159/000111395. [DOI] [PubMed] [Google Scholar]
- Mennes M, Stiers P, Lagae L, Van den Bergh B. Long-term cognitive sequelae of antenatal maternal anxiety: Involvement of the orbitofrontal cortex. Neuroscience and Biobehavioral Reviews. 2006;30:1078–1086. doi: 10.1016/j.neubiorev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Mihalick SM, Crandall JE, Langlois JC, Krienke JD, Dube WV. Prenatal ethanol exposure, generalized learning impairment, and medial prefrontal cortical deficits in rats. Neurotoxicology and Teratology. 2001;23:453–462. doi: 10.1016/s0892-0362(01)00168-4. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. Journal of Neuroscience. 2004;24:5492–5499. doi: 10.1523/JNEUROSCI.0086-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley R, Owens J, Blair E, Dwyer T. Is birthweight a good marker for gestational exposures that increase the risk of adult disease? Paediatric and Perinatal Epidemiology. 2002;16:194–199. doi: 10.1046/j.1365-3016.2002.00428.x. [DOI] [PubMed] [Google Scholar]
- Murmu MS, Salomon S, Biala Y, Weinstock M, Braun K, Bock J. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. European Journal of Neuroscience. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years: Report from the Avon Longitudinal Study of Parents and Children. British Journal of Psychiatry. 2002;180:502–508. doi: 10.1192/bjp.180.6.502. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Jr, Braun K. Maternal separation and social isolation modulate the postnatal development of synaptic composition in the infralimbic cortex of Octodon degus. Neuroscience. 2001;104:33–40. doi: 10.1016/s0306-4522(01)00059-8. [DOI] [PubMed] [Google Scholar]
- Parker G. The Parental Bonding Instrument: A decade of research. Social Psychiatry and Psychiatric Epidemiology. 1990;25:281–282. doi: 10.1007/BF00782881. [DOI] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. A parental bonding instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- Patel PD, Lopez JF, Lyons DM, Burke S, Wallace M, Schatzberg AF. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. Journal of Psychiatric Research. 2000;34:383–392. doi: 10.1016/s0022-3956(00)00035-2. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Susman JR. Disclosure of traumas and psychosomatic processes. Social Science and Medicine. 1988;26:327–332. doi: 10.1016/0277-9536(88)90397-8. [DOI] [PubMed] [Google Scholar]
- Plantes MM, Prusoff BA, Brennan J, Parker G. Parental representations of depressed outpatients from a U.S.A. sample. Journal of Affective Disorders. 1988;15:149–155. doi: 10.1016/0165-0327(88)90083-3. [DOI] [PubMed] [Google Scholar]
- Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P, Braun K. Juvenile emotional experience alters synaptic composition in the rodent cortex, hippocampus, and lateral amygdala. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:16137–16142. doi: 10.1073/pnas.2434663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Wadhwa PD, Chicz-DeMet A, Porto M, Garite TJ. Maternal corticotropin-releasing hormone and habituation in the human fetus. Developmental Psychobiology. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Sarrieau A, Dussaillant M, Sapolsky RM, Aitken DH, Olivier A, Lal S, et al. Glucocorticoid binding sites in human temporal cortex. Brain Research. 1988;442:157–160. doi: 10.1016/0006-8993(88)91444-8. [DOI] [PubMed] [Google Scholar]
- Schoofs D, Preuss D, Wolf OT. Psychosocial stress induces working memory impairments in an n-back paradigm. Psychoneuroendocrinology. 2008;33:643–653. doi: 10.1016/j.psyneuen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Seidman DS, Laor A, Gale R, Stevenson DK, Danon YL. Is low birth weight a risk factor for asthma during adolescence? Archives of Disease in Childhood. 1991;66:584–587. doi: 10.1136/adc.66.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Birth weight and intellectual performance in late adolescence. Obstetrics and Gynecology. 1992;79:543–546. [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Szuran T, Zimmermann E, Welzl H. Water maze performance and hippocampal weight of prenatally stressed rats. Behavioural Brain Research. 1994;65:153–155. doi: 10.1016/0166-4328(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Thompson C, Syddall H, Rodin I, Osmond C, Barker DJ. Birth weight and the risk of depressive disorder in late life. British Journal of Psychiatry. 2001;179:450–455. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- Vallee M, MacCari S, Dellu F, Simon H, Le Moal M, Mayo W. Long-term effects of prenatal stress and postnatal handling on age- related glucocorticoid secretion and cognitive performance: A longitudinal study in the rat. European Journal of Neuroscience. 1999;11:2906–2916. doi: 10.1046/j.1460-9568.1999.00705.x. [DOI] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: A prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2007;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wass TS, Persutte WH, Hobbins JC. The impact of prenatal alcohol exposure on frontal cortex development in utero. American Journal of Obstetrics and Gynecology. 2001;185:737–742. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]
- Watzka M, Bidlingmaier F, Beyenburg S, Henke RT, Clusmann H, Elger CE, et al. Corticosteroid receptor mRNA expression in the brains of patients with epilepsy. Steroids. 2000;65:895–901. doi: 10.1016/s0039-128x(00)00205-1. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Research. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Niven H, Parker G, Hadzi-Pavlovic D. The stability of the Parental Bonding Instrument over a 20-year period. Psychological Medicine. 2005;35:387–393. doi: 10.1017/s0033291704003538. [DOI] [PubMed] [Google Scholar]
- Xing GQ, Russell S, Webster MJ, Post RM. Decreased expression of mineralocorticoid receptor mRNA in the prefrontal cortex in schizophrenia and bipolar disorder. International Journal Neuropsychopharmacology. 2004;7:143–153. doi: 10.1017/S1461145703004000. [DOI] [PubMed] [Google Scholar]
- Yaka R, Salomon S, Matzner H, Weinstock M. Effect of varied gestational stress on acquisition of spatial memory, hippocampal LTP and synaptic proteins in juvenile male rats. Behavioural Brain Research. 2007;179:126–132. doi: 10.1016/j.bbr.2007.01.018. [DOI] [PubMed] [Google Scholar]
- Yakovlev PL, Lecours AR. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1967. pp. 3–70. [Google Scholar]
- Yang J, Hou C, Ma N, Liu J, Zhang Y, Zhou J, et al. Enriched environment treatment restores impaired hippocampal synaptic plasticity and cognitive deficits induced by prenatal chronic stress. Neurobiology of Learning and Memory. 2007;87:257–263. doi: 10.1016/j.nlm.2006.09.001. [DOI] [PubMed] [Google Scholar]