Abstract
Brain-computer interfaces (BCIs) allow their users to communicate or control external devices using brain signals rather than the brain's normal output pathways of peripheral nerves and muscles. Motivated by the hope of restoring independence to severely disabled individuals and by interest in further extending human control of external systems, researchers from many fields are engaged in this challenging new work. BCI research and development have grown explosively over the past two decades. Efforts have recently begun to provide laboratory-validated BCI systems to severely disabled individuals for real-world applications. In this review, we discuss the current status and future prospects of BCI technology and its clinical applications. We will define BCI, review the BCI-relevant signals from the human brain, and describe the functional components of BCIs. We will also review current clinical applications of BCI technology, and identify potential users and potential applications. Finally, we will discuss current limitations of BCI technology, impediments to its widespread clinical use, and expectations for the future.
1. Introduction
The possibility of establishing a direct communication and control channel between the human brain and computers or robots has been a topic of scientific speculation and even science fiction for many years. Over the past twenty years, this idea has been brought to fruition by numerous research and development programs, and has evolved into one of the fastest-growing areas of scientific research. This technology, called brain-computer interface (BCI) technology, provides a new output channel for brain signals to communicate or control external devices without using the normal output pathways of peripheral nerves and muscles. A BCI recognizes the intent of the user through the electrophysiological or other signals of the brain. Electrophysiological signals may be recorded over the scalp, underneath the scalp, or within the brain; other types of physiological signals may be recorded by magnetic sensors or other means. In real time, a brain signal is translated into output commands that accomplish the desire of the user. The most common example of use of such technology is the direct control of a computer cursor by a person or animal using a BCI based on electrophysiological signals.
A BCI allows a person to communicate with or control the external world without using conventional neuromuscular pathways. That is, messages and control commands are delivered not by muscular contractions but rather by brain signals themselves. This BCI feature brings hope to individuals who are suffering from the most severe motor disabilities, including people with amyotrophic lateral sclerosis (ALS), spinal cord injury, stroke, and other serious neuromuscular diseases or injuries. BCI technology holds promise to be particularly helpful to people who are “locked-in,” cognitively intact but without useful muscle function. Restoration of basic communication capabilities for these people would significantly improve their quality of life as well as that of their caregivers, increase independence, reduce social isolation, and potentially reduce cost of care [1].
BCI research has undergone an explosive growth in recent years. At present, there are over 400 groups worldwide engaging in a wide spectrum of research and development programs, using a variety of brain signals, signal features, and analysis and translational algorithms [2]. In this review, we discuss the current status and future prospects of BCI technology and its clinical applications. We will define BCI, review the BCI-relevant source signals from the human brain, and describe the functional components of BCIs. We will also review current clinical applications of BCI technology, and identify potential users and potential applications. Finally we will discuss current limitations of BCI technology, impediments to its widespread clinical use, and expectations for the future.
2. BCI Definition, Signal Types, and Operation
Definition of a BCI
A brain-computer interface (BCI), also sometimes referred to as a brain-machine interface (BMI), is a communication and/or control system that allows real-time interaction between the human brain and external devices. A BCI user's intent, as reflected by brain signals, is translated by the BCI system into a desired output: computer-based communication or control of an external device.
The term “Brain-computer Interface” was first introduced by the pioneering works of Dr. J. Vidal [3, 4] in the early 1970's. Motivated by the hope of creating an alternative output pathway for severely disabled individuals and an interest in further extending human direct control of external systems, BCI researchers have engaged in this new challenging work. Researchers hail from a large variety of fields, including clinical neurology and neurosurgery, rehabilitation engineering, neurobiology, engineering, psychology, computer science, and mathematics, and have led to an explosive growth in BCI research and development over the past two decades [3, 5-22].
A BCI is defined as a system that measures and analyzes brain signals and converts them in real-time into outputs that do not depend on the normal output pathways of peripheral nerves and muscles [23]. Systems that measure electrical activity generated by muscles do not satisfy the above definition, and therefore are not BCIs. Systems that measure brain activity that depends on muscle control are not pure, or independent BCIs, but might rather be called dependent BCIs. Thus, for example, a system that uses visual evoked potentials (VEPs) to detect gaze direction [24, 25] is a dependent BCI because it requires neuromuscular control of eye (or head) movements. (It should be noted that several recent studies [26-28] indicate that some VEP-based BCI systems are not totally dependent on gaze direction, and thus are to a limited degree independent.)
BCIs do not read minds. Rather, a BCI changes electrophysiological signals from mere reflections of central nervous system (CNS) activity into messages and commands that act on the world and that, like output in conventional neuromuscular channels, accomplish the person's intent. Thus, a BCI replaces nerves and muscles and the movements they produce with hardware and software that measure brain signals and translate those signals into actions [14].
Successful BCI operation depends on the interaction of two adaptive controllers: the user, who produces specific brain signals that encode intent and the BCI, which translates these signals into output that accomplishes the user's intent. Aiming to replace the conventional neuromuscular output channels, a BCI must function as an adaptive close-loop control system. It must provide real-time feedback to the user, by which the user can fine-tune the brain signals in order to optimize the desired output. It should be noted that a system that simply records and analyses brain signals and does not provide the results of the analysis to the user in a real-time interactive way is not a BCI [29].
Types of Brain Signals
In principle, a variety of neurophysiologic signals reflecting in-vivo brain activities might be recorded and used to drive a BCI. Depending on the biophysical nature of the signal source, these signals can be broadly grouped into three categories: electrophysiological, magnetic, and metabolic.
Electrophysiological Signals
Electrophysiological signals resulting from brain activity can be broadly classified according to the degree of invasiveness of the recording device [30]. Figure 1 illustrates BCI systems based on electrophysiological signals measured by noninvasive (EEG), cortical surface (ECoG), and intracortical recording devices. Each of the recording methods has its own advantages and disadvantages. Electroencephalographic (EEG) signals are recorded from the scalp. Since EEG-based BCIs are non-invasive, they provide the simplest and safest BCI recording methods. However, EEG has limited frequency range and spatial resolution, and it is more susceptible to powerline interference and other artifacts like electromyographic (EMG) signals from cranial muscles or electrooculographic (EOG) activity. Figure 1a shows two noninvasive BCI systems based on different EEG signal features. Electrocorticographic (ECoG) signals are recorded from electrodes surgically placed on the surface of the cortex. These electrodes measure the same signals as in EEG, but their closer proximity to the brain and the elimination of the insulating features of the skull and dura result in greater signal amplitude, wider detectable frequency range, and better topographical resolution. Figure 1b shows an example of human ECoG signals and topographies during two-dimensional movement control. Finally, intracortical methods can be used for a BCI by recording local field potentials (LFPs) and neuronal action potentials (spikes). These intracortical methods represent the most invasive BCI methods since they record electrical activity from electrodes implanted in the brain. Figure 1c shows a microelectrode array for intracortical recording, its placement location in human motor cortex, and results from intracortical BCI studies in monkeys. Both ECoG and intracortical recordings provide wider frequency range, higher topographical resolution, and better signal quality and dimensionality than EEG. However, both methods require surgery, and issues such as risk of tissue damage and infection, and long-term recording stability arise [18, 30]. It remains possible that intracortical recording might not produce speed and accuracy of performance substantially greater than what can be achieved with EEG, a non-invasive method not requiring surgery or any of its attendant risks [31]. The comparative merits of noninvasive (EEG) methods and invasive (ECoG and intracortical) methods remain unresolved at present. With each method having its strengths and weaknesses, the decision of a potential BCI user for one method over the other may ultimately depend on the overall goal of the individual's BCI use [32].
1.
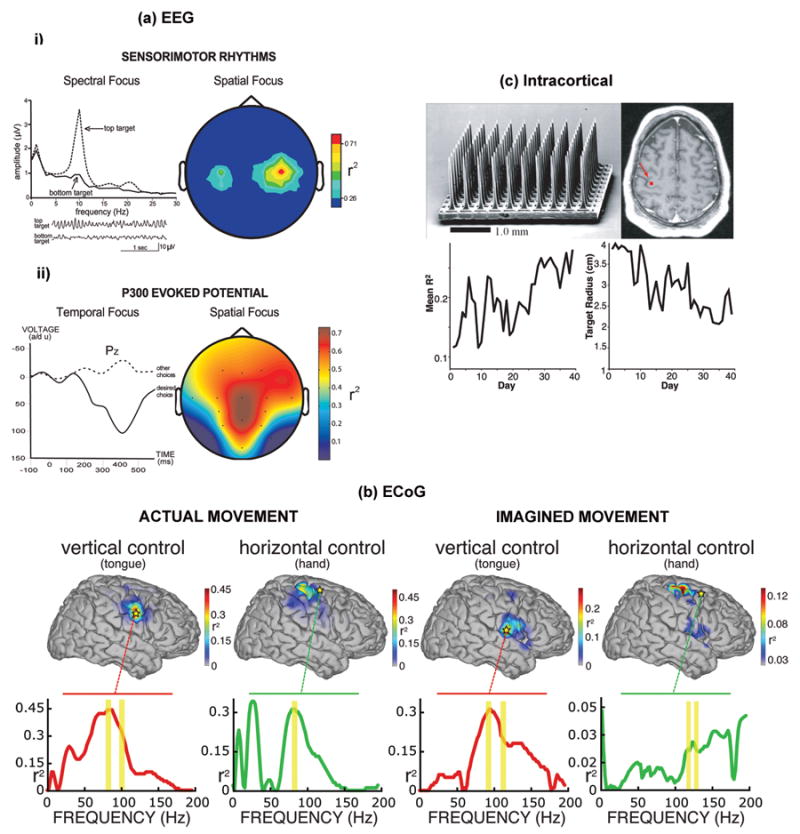
(a) EEG-based BCI systems: i) Sensorimotor rhythm (SMR) BCI [83, 109]. EEG activity is recorded over the sensorimotor cortex. Users are trained to control the amplitude of the μ rhythm (8-12 Hz) or the β rhythm (18-26 Hz) in order to move a computer cursor to a top target or a bottom target on a computer screen. Frequency spectra for vertical cursor movement (top or bottom target) indicate that the user's control focuses in the μ-rhythm frequency band. Sample EEG traces (bottom) show that the μ rhythm is prominent with top targets and minimal with bottom targets. An SMR BCI can provide two or even three-dimensional movement control. (Adapted from Wolpaw JR et al [136], with permission from the Institute of Electrical and Electronics Engineers); ii) P300 event-related potential BCI [6, 19]. A matrix of possible selections is shown on a computer screen. EEG activity is recorded over the centroparietal cortex while these selections flash in succession. Only the selection desired by the user elicits a P300 potential (e.g., a positive voltage deflection about 300ms after the flash). (Adapted from Donchin E et al [11], with permission from the Institute of Electrical and Electronics Engineers);
(b) ECoG-based BCI systems: Sample topographies of vertical and horizontal control using ECoG signals. These topographies show the color-coded correlation (i.e., r2 values) of the cortical activity with vertical or horizontal movement. The level of task-related control of different cortical areas is indicated. Actual and imagined tongue movements were used for vertical control, while actual and imagined hand movements were used for horizontal control. The traces below each topography show r2 values for the locations (stars) used online. The frequency bands used online are indicated by yellow bars. Actual and imagined tasks presented similar activity patterns over locations active with motor and motor imagery tasks. (Adapted from Schalk G et al [20], with permission from the Institute of Physics Publishing);
(c) Intracortical-based BCI systems:
Top left panel - An example of a 100-microelectrode array for chronic implantation in human motor cortex to record neuronal action potentials and/or local field potentials. Top right panel - Placement of an electrode array in the human motor cortex (arrow). (Adapted from Hochberg LR et al [18], with permission from Macmillan Publisher Ltd.) Bottom panel - three-dimensional cursor movements by groups of individual neurons in the motor cortex of a monkey; (left) average correlation of the firing rate of a single cortical neuron with target direction over daily training sessions; (right) resulting improvement in BCI performance, measured as the mean target radius required to maintain a 70% target hit rate. The size of the target needed decreased as the correlations of the firing rates of the neurons controlling cursor movement with the target direction increased. (Reproduced from Taylor DM et al [13], with permission from the American Association for the Advancement of Science.)
Most studies of BCI clinical applications in humans have been performed with EEG-based BCIs. These have shown that EEG-based BCIs can allow a person to control a computer cursor in at least three dimensions, to select letters to perform word-processing, to run computer-based Windows™ programs, and to perform environmental control. Several people severely disabled by ALS have begun using the Wadsworth Center's EEG-based BCI at home for everyday, independent use [33]. Human ECoG studies have been performed with volunteers who are under evaluation for epilepsy surgery. Prior to surgery, the neurosurgeons evaluate the patient's brain activity using an array of ECoG electrodes to localize critical cortical functions [16, 20, 34, 35]. If a patient volunteers, a BCI researcher can perform BCI studies during the patient's evaluation period, which usually lasts only one or two weeks. Thus, the availability of ECoG research subjects is somewhat limited and is always secondary to the clinical needs of the patients [36]. Moreover, the location of the ECoG electrode array is based solely on the patient's clinical needs, and thus might not include cortical regions associated with brain patterns useful for BCI [37]. Despite these obstacles, impressive results have emerged from short-term ECoG studies, focusing mainly on the capacities of ECoG signals for use in a human BCI [16, 20, 34, 35, 38-48]. Most intracortical BCI data to date have been obtained from animals, primarily from monkeys [45-52]. While monkeys have been shown to be able to use intracortical signals to control a robotic arm, comparable human data are very limited [8, 18, 49]. Due to the present practical limitations on invasive BCI studies in humans, BCI clinical applications to date have used primarily scalp-recorded EEG signals.
Magnetic and Metabolic Signals
Magnetoencephalography (MEG) was recently proposed as a potential new source of brain-derived signals to operate a BCI [50-53]. MEG is attractive because it is non-invasive, able to detect frequency ranges above those available in EEG recordings [54], and has slightly higher spatial resolution than EEG [55]. MEG measures very small magnetic fields produced by the electrical activity of the brain [56]. Researchers have explored the potential of MEG BCI in the rehabilitation of stroke patients with encouraging initial results [17, 50]. Recent BCI research using MEG signals demonstrated reliable self-control of sensorimotor rhythm amplitude [51] and satisfactory two-dimensional BCI control [53]. Despite its wider frequency range and excellent spatiotemporal resolution, MEG currently requires bulky and expensive equipment and a protected environment, and thus is at present impractical for widespread clinical use [30].
In recent years, there has been growing interest in BCIs based on metabolic signals derived from the brain [57-69]. Metabolic activity is assessed by measuring blood oxygenation (the blood oxygen level dependent (BOLD) response) through functional magnetic resonance imaging (fMRI) or near-infrared spectroscopy (NIRS). These measures are known to be correlated with neural activity in the brain [17, 70-72]. They take advantage of the fact that during mental activation there is an increase in oxyhemoglobin and a decrease in deoxyhemoglobin.
Weiskopf et al [63] showed that a person can control the fMRI BOLD response from circumscribed cortical and subcortical regions. Studies have repeatedly demonstrated that there is a tight correlation between voluntary changes in brain metabolism and behavior (for review see [73]). Benefiting from fMRI's high spatial resolution and recent advances in simultaneous acquisition, analysis, and visualization of whole brain images, researchers have successfully trained human subjects to volitionally control localized brain regions using feedback from a real-time fMRI BCI (for complete review see [74, 75]). Furthermore, recent work by Lee et al. [59] has demonstrated the possibility of two-dimensional real-time control of a robotic arm through a motor imagery task by human subjects, using an fMRI-based BCI.
Despite the above mentioned outstanding features of MEG and fMRI in acquiring valuable neural information from the brain, their clinical application in BCI systems for real-life daily use is currently not realistic, due to the high cost and size of the equipment, and the technical difficulties involved [76]. Furthermore, the BOLD response is a relatively low-frequency signal (<1 Hz) compared to EEG or MEG, and this limits its BCI information-carrying capacity. At present, MEG and fMRI's use remains reserved for identification of function-specific foci for subsequent placement of cortical electrodes [60], for rehabilitation training of patients, and for other non-BCI research purposes.
Near-infrared (NIRS) spectroscopy, which also measures blood oxygenation, is another potential BCI method. In NIRS, light in the near infrared range (700-100nm) [73] tracks neural metabolism by monitoring the relative amounts of oxyhemoglobin and deoxyhemoglobin. An inrush of oxygen-rich blood occurs in an active area and in surrounding tissue [70]. Thus far, NIRS-based BCIs have not matched the performance of EEG-based BCIs [67]. Nevertheless, they are promising because, unlike fMRI and MEG, NIRS is inexpensive and portable [65, 67-69]. Thus, NIRS BCI methods might be of practical value for clinical applications in the near future.
BCI Operation
Any BCI, regardless of its recording methods or applications, consists of four essential elements, as described by Wolpaw [14] : 1) signal acquisition, 2) feature extraction; 3) feature translation; and 4) device output. Figure 2 illustrates the essential elements and operation of a BCI system, as well as its clinical applications. These four elements are managed through the system's operating protocol. Since BCIs based on electrophysiological signals are in the most advanced state of development and have resulted in some clinical applications, the remainder of this article focuses on BCIs of this type.
2.
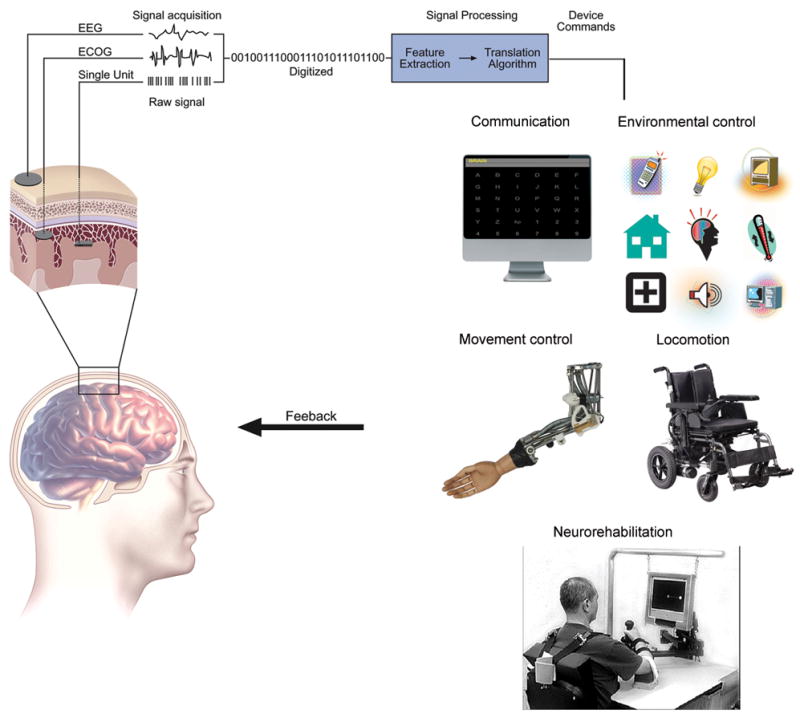
Essential elements and operation of a BCI system (modified from Wolpaw JR et al [14], and Leuthardt EC et al [36], with permission from Elsevier and Wolters Kluwer respectively). Brain signals that carry the intent of the user are first acquired by electrodes placed on the scalp (EEG), beneath the skull and over the cortical surface (ECoG), or within brain tissue (intracortical). These brain signals are digitized, and specific signal features are extracted. The extracted signal features are translated into device commands that activate and control assistive technology used for: communication (e.g., spelling on a computer screen); movement control (e.g., robotic arm) (Credit: Copyright Fraunhofer IPA)); environmental control (e.g., TV, light, temperature, etc); locomotion (e.g., electric wheelchair); or neurorehabilitation (adapted from Daly JJ et al [129], with permission from the Journal of Rehabilitation Research and Development).
Signal Acquisition
Signal acquisition is the measurement of the neurophysiologic state of the brain. In BCI operation, the recording interface (i.e., electrodes, for electrophysiological BCI systems) tracks neural information reflecting a person's intent embedded in the ongoing brain activity. As discussed in the last section, the most common electrophysiological signals employed for BCI systems include: EEG recorded by electrodes on the scalp; ECoG recorded by electrodes placed beneath the skull and over the cortical surface; and local field potentials (LFPs) and neuronal action potentials (spikes) recorded by microelectrodes within brain tissue. The brain electrical signals used for BCI operation are acquired by the electrodes, amplified, and digitized.
Feature Extraction
The signal-processing stage of BCI operation occurs in two steps. The first step, feature extraction, extracts signal features that encode the intent of user. In order to have effective BCI operation, the electrophysiological features extracted should have strong correlations with the user's intent. The signal features extracted can be in the time-domain or the frequency-domain [6, 11, 49, 77-80], or both [81]. The most common signal features used in current BCI systems include: amplitudes or latencies of event-evoked potentials (e.g., P300), frequency power spectra (e.g., sensorimotor rhythms), or firing rates of individual cortical neurons. An algorithm filters the digitized data and extracts the features that will be used to control the BCI. In this step, confounding artifacts (such as 60-Hz noise or EMG activity) are removed to ensure accurate measurement of the brain signal features.
Feature Translation
The second step of signal processing is accomplished by the translation algorithm, which converts the extracted signal features into device commands. Brain electrophysiological features or parameters are translated into commands that will produce output such as letter selection, cursor movement, control of a robot arm, or operation of another assistive device. A translation algorithm must be dynamic to accommodate and adapt to the continuing changes of the signal features and to ensure that the possible range of the specific signal features from the user covers the full range of device control [14, 82, 83].
Device Output
The signal features thus extracted and translated provide the output to operate an external device. The output might be used to operate a spelling program on a computer screen through letter selection [6, 11], to move a cursor on a computer screen [12, 84, 85], to drive a wheelchair [86, 87] or other assistive devices [88], to manipulate a robotic arm [89, 90], or even to control movement of a paralyzed arm through a neuroprosthesis [15, 91]. At present, the most commonly used output device is the computer screen, and it is used for communication.
Operating Protocol
The operating protocol determines the interactive functioning of the BCI system. It defines the onset/offset control, the details of and sequence of steps in the operation of the BCI, and the timing of BCI operation. It defines the feedback parameters and settings, and possibly also any switching between different device outputs. An effective operating protocol allows a BCI system to be flexible, serving the specific needs of an individual user. At present, since most BCI studies occur in laboratories under controlled conditions [1], investigators typically control most of the parameters in the protocol, providing simple and limited functionality to the BCI user. More flexible and complete operating protocols will be important for BCI use in real life, outside of the laboratory.
3. BCI Clinical Applications
Potential BCI Users
Individuals who are severely disabled by disorders such as ALS, cerebral palsy, brainstem stroke, spinal cord injuries, muscular dystrophies, or chronic peripheral neuropathies might benefit from BCIs. To help determine the value of BCIs for different individuals, Wolpaw et al [30] suggested that potential BCI users be categorized by the extent, rather than the etiology, of their disability. Evaluated in this way, potential BCI users fall into three reasonably distinct groups: (1) people who have no detectable remaining useful neuromuscular control and are thus totally locked-in; (2) people who retain only a very limited capacity for neuromuscular control such as weak eye-movements or a slight muscle twitch; and (3) people who still retain substantial neuromuscular control and can readily use conventional muscle-based assistive communication technology.
It is not yet clear to what extent BCIs can serve people in the first group, those who are totally locked-in (e.g., by late-stage ALS or severe cerebral palsy). Resolution of this issue requires extensive and prolonged evaluation of each individual in order to resolve basic issues of alertness, attention, visual or auditory capacities, and higher cortical function. While it has been hypothesized that the totally locked-in state constitutes a unique BCI-resistant condition [17], the issue remains unresolved at present. It is worth mentioning that researchers have speculated that individuals in this group might be able to retain the capacity for BCI use if they begin it before becoming totally locked-in [17, 30].
At present, people in the second group constitute the primary prospective user population for current BCI systems. This group, which outnumbers the first group, includes people with late-stage ALS patients who rely on artificial ventilation as their disease progresses, people with brainstem strokes, and people with severe cerebral palsy. Typically, they retain only very limited, easily fatigued, and/or unreliable eye movements or other minimal muscle function and thus cannot be adequately served by conventional muscle-based assistive communication technology. For people in this group, BCI systems may be able to provide basic communication and control that is more convenient and reliable than that provided by conventional technology [30].
The third and largest group of potential BCI users consists of people who retain substantial neuromuscular control. For most in this group, present-day BCI systems, with their limited capacities, have little to offer. These individuals are usually much better served by conventional technology. Nevertheless, some in this group, such as those with high-cervical spinal cord injuries, may prefer a BCI over conventional assistive devices that coopt their remaining voluntary muscle control (e.g., systems that depend on gaze direction or EMG from facial muscles). In the future, as the capacities, reliability, and convenience of BCI systems continue to improve, more people in this group could find them of value, and the number of people using BCIs could substantially increase.
The different conditions mentioned above impair the CNS in different ways, and different BCIs depend on different aspects of brain activity. Thus, some people may be better served by one BCI than by another. For example, people who have sensorimotor cortex impairment due to severe cerebral palsy may not be able to use BCIs based on EEG or single-neuron activity from these cortical areas. In such people, BCI systems that use other EEG components (e.g., P300 [6, 11, 19, 92]) or neuronal activity from other brain regions might be good alternatives.
Possible BCI Uses
It is important to distinguish between a BCI and its applications [23]. The term BCI refers to the system that records, analyzes, and translates the input (i.e., the user's brain signals) into device commands. In contrast, the term application refers to the specific purposes or devices to which the output commands are applied. Recent focus on the real-world applications of BCI technology [93, 94] is speeding the transition of BCI research from the laboratory to clinical products useful in everyday life. Although BCI applications could conceivably be clinical or non-clinical (e.g., computer games), this review discusses clinical applications only.
The potential clinical uses of BCIs can be classified as: (1) direct control of assistive technologies; and (2) neurorehabilitation. Since the BCI serves as a replacement of normal neuromuscular pathways, the most obvious BCI applications are those that activate and control assistive technologies that are already in place to enable communication and control of the environment. These applications of BCIs to assistive technology encompass the areas of communication, movement control, environmental control, and locomotion. The possible uses of BCIs in neurorehabilitation have just begun to be explored [29, 73].
Communication
Communication for people who are “locked in” probably represents the most pressing area in need of intervention with BCI technology [93, 95]. Although other applications are under development, restoring communication has been the main focus of the BCI research community to date [83, 96, 97].
Distinguished from one another by the specific electrophysiological features measured, three types of EEG-based BCI systems have been tested in human subjects for the purpose of communication, specifically those based on: 1) slow cortical potentials (SCPs); 2) P300 event-related potentials; and 3) sensorimotor rhythms (SMRs). Both the SCP BCI and the SMR BCI require significant training of the users to gain sufficient control of their brain activity to produce signals that can be effectively applied to BCI use. In contrast, a P300 BCI measures the brain's response to stimuli (visual or auditory) of special significance and requires minimal user training.
SCPs are slow voltage changes in cortex. They occur over 0.5–10.0 sec and are among the lowest-frequency features of EEG. Negative shifts of SCPs represent cortical activation associated with movement or other functions, while positive SCP shifts accompany reduced cortical activation [98, 99]. Early studies [100] confirmed the importance of the anterior brain regions in physiological regulation of SCP signals, and suggested these areas are important for successful use of this type of BCI [73]. With extensive training, sometimes months [17], the user learns to control SCP positive or negative voltage shifts. The BCI translates these voltage shifts into vertical movement of a cursor or an object on a computer screen. By this means, binary selection or control can be achieved. Based on this principle, Birbaumer et al. developed an early BCI-controlled spelling device [10, 101]. A series of studies in people with ALS and other severe neurological diseases in different stages of physical impairment confirmed the ability of the SCP BCI to provide basic communication capability [102-104]. Despite these achievements, SCPs provide only very slow communication (e.g., one minute per letter [17]). Moreover, a comparative study [17] suggested that, for people severely disabled by ALS, an SMR-based or P300-based BCI system is a better option than an SCP-based BCI.
Sensorimotor rhythms (SMRs), also recorded by EEG, have also proved to provide features suitable for BCI-enabled communication and have been used successfully by several research groups [37, 83, 85]. Typically, the SMRs are recorded over sensorimotor cortex and the features useful for BCI are the μ rhythm (8-12 Hz) and the β rhythm (18-26 Hz). Figure 1a (i) illustrates a BCI based on SMRs. Changes in μ and β rhythm amplitudes are referred to as event-related desynchronization (ERD) (i.e., decrease) and event-related synchronization (ERS) (i.e., increase). Typically, changes in μ and β rhythms are associated with movement, sensation, and motor imagery. The rhythms decrease or desynchronize with movement or its preparation, and increase or synchronize after movement and with relaxation [105]. However, people can learn to use motor imagery, rather than actual movement, to change SMR amplitudes, and can use that control to operate a BCI. Work in several laboratories has shown that an SMR-based BCI can enable basic word processing and icon selection [7, 12, 37, 83, 85, 106-111]. These studies amply demonstrate that, with training, most people with or without motor disabilities can use SMR amplitudes to select targets by controlling the one, two, or three dimensional movements of a cursor [22, 83].
The third major type of EEG-based BCI communication uses the well-studied P300 even-related brain potential [6, 11] to indicate the response to a salient or infrequent stimulus within a stream of frequent standard stimuli. Figure 1a (ii) illustrates a BCI based on P300. Detected in EEG recordings over the central and parietal regions, the P300 signal is a positive deflection of brain wave at a latency of about 300 msec [112-114]. The stimuli used in most P300 BCI systems reported to date are visual, and are based on the P300 speller first developed by Donchin et al. [11, 112]. In this BCI, the user faces a 6 × 6 matrix of letters, numbers, symbols, and/or function keys. The rows and columns in the matrix flash in a random order, and the user attends to the matrix item s/he wishes to select. By detecting the row and column that elicit the largest P300, the BCI recognizes the user's target letter/symbol. Because the P300 response occurs normally, use of a P300 BCI does not require substantial training. This quality, combined with the relative ease of acquisition of brain signals by EEG, makes a P300-based BCI potentially very practical for clinical use. Sellers et al. [19] and Nijboer et al. [92] have reported that ALS patients are able to communicate using a P300 speller. Successful use of a P300 BCI has also been reported for people with disabilities resulting from stroke, spinal cord injury, cerebral palsy, multiple sclerosis, and other disorders [115, 116]. In these systems, communication can be greatly enhanced by appropriate software such as a text-to-speech synthesizer and a word-prediction program. A P300 BCI system based on auditory stimuli would be useful for patients with limited or restricted eye movement or eyesight; and such BCIs are currently under development [19, 21, 117, 118].
Movement control
Restoration of motor control in paralyzed patients is another key application of BCI and is the main goal of many researchers in the field. The research in this clinical application is sparse and has used mainly SMR-based systems. Wolpaw and his colleagues have demonstrated one-dimensional, two-dimensional [83], and even three-dimensional cursor control using an SMR system [22] and have done preliminary experiments with SMR control of a robotic arm [119]. These experiments indicate that SMR BCI systems might be able to support multidimensional control of the movement of motor neuroprosthesis or an orthotic device such as a robotic arm. Pfurtscheller and colleagues tested an SMR-based BCI for restoration of motor control in paralyzed patients (for review see [37]). A tetraplegic patient was trained to control an electrically driven hand orthosis with EEG signals recorded over sensorimotor cortex [120]. By learning to generate separable motor imagery tasks, this patient was able to open and close his paralyzed hand with the hand orthosis.
Functional electrical stimulation (FES) can also be used for restoration of motor function in paralyzed patients with intact lower motor neuron and peripheral nerve function. With the goal of further enhancing motor restoration in paralyzed patients, Pfurtscheller and his colleagues combined the SMR BCI with FES systems and tested the combined system in two patients with high spinal cord injury [67,68].
Environmental Control
BCI-based environmental control could greatly improve the quality of life of severely disabled people. People with severe motor disabilities are often home-bound. Effective means for controlling their environments (e.g., controlling room temperature, light, power beds, TV, etc.) would increase their well-being and sense of independence [88, 93, 121]. A recent pilot study by Cincotti et al [88] attempted to integrate BCI technology into a domestic environmental control system. With unified control through EEG-based BCI technology, the user is able to operate remotely domestic devices such as neon lights and bulbs, TV and stereo sets, a motorized bed, an acoustic alarm, a front door opener, and a telephone, as well as to monitor the surrounding environment through wireless cameras. The clinical validation of the system prototype took place in a simulated home environment in an occupational therapy department. Fourteen healthy normal subjects and four subjects suffering from spinal muscular atrophy type II (SMA II) or Duchenne Muscular Dystrophy (DMD) were tested. The patients were able to control the system with an average accuracy of 60-75% over the last three testing session (8-12 sessions in total). Preliminary findings from this study suggested that the self-control of the domestic environment realized with BCI technology increased the patient's sense of independence. Also, caregivers could be relieved to some extent from the need to be continually present.
Locomotion
Restoration of independent locomotion is another important issue for paralyzed people. In light of this, several BCI research groups have attempted to develop BCI-driven wheelchairs in order to restore some form of mobility. Tanaka et al. developed an electric wheelchair controlled by EEG [122]. Directional commands were detected by EEG and were then applied to direct control of the wheelchair. Such precise control may be quite demanding on the user. Rebsamen et al. reported a wheelchair controlled by a P300-BCI system [123] in which the user simply selects a destination from a menu of destinations. While this approach is less demanding for the user, the capacity for real-time directional control of the wheelchair is limited by the selections, and prior definition of the possible paths is needed. Millán and his group studied a BCI-controlled wheelchair that is based on the EEG activity associated with various mental tasks and a shared control system [86, 124]. It employed intelligent algorithms to assist the user in obtaining continuous command of the system during wheelchair navigation. Further work is required to confirm the usability of a BCI-driven wheelchair in the real-world environment. For this application, due to considerations of safety, there must be stricter requirements for accuracy than for many other BCI applications.
Neurorehabilitation
In addition to their uses for communication and control, BCI systems also have potential to serve as therapeutic tools to help people whose neuromuscular function has been impaired by trauma or disease to relearn useful motor function. Neurorehabilitation using BCI systems promotes functional recovery and may improve quality-of-life (QoL) [95]. This specific application of BCI systems seeks to augment current rehabilitation therapies by reinforcing and thereby increasing effective use of impaired brain areas and connections [125, 126]. This approach to rehabilitation was first evaluated with MEG signals in people with strokes, and found cortical reorganization after BCI-based training [50].
In a recent review, Daly and Wolpaw [29] classified the possible BCI-based motor learning strategies into two categories. In the first, patients are trained to produce more normal brain activity to control motor function. This strategy is based on the idea that more normal activity will result in more normal CNS function, and will thereby improve motor control. Since preliminary results in stroke patients demonstrate that they can gain control of specific brain activity patterns [50, 73], a BCI might be used to enhance this control by measuring and extracting EEG features that can be translated into feedback to the user. Daly and coworkers [127] measured EEG activity from stroke patients before and after this EEG-based neurorehabilition. After the motor re-learning intervention, EEG features were found to change in parallel with improvement in motor function. Moreover, a recent study by Enzinger and colleagues [128] reported that sensorimotor rehabilitation using BCI training and motor imagery improved motor function after CNS injury.
The second strategy for producing improved motor control is to use the output from a BCI to activate a device that assists movement. This approach is based on the hypothesis that the CNS plasticity induced by the sensory input produced during the improved motor function provided by the device will lead to improved motor control. In past studies, neurorehabilitation training with robotic devices that assisted movement has been effective in stroke patients [129]. Daly and coworkers have done promising preliminary work combining BCI with FES or assistive robotics for motor re-learning in stroke patients [130]. BCI-based therapy might provide a useful complement to standard neurorehabilitation methods, and might lower cost by reducing the need for the constant presence of a rehabilitation therapist.
4. Limitations of Current BCIs
All of the BCIs currently under development have limitations. Issues of safety and the long-term stability of the recording electrodes used in invasive BCI systems remain to be resolved satisfactorily. Some, but not all of these concerns may be resolved when it becomes possible to fully implant a telemetric device to transmit the recorded brain signals. Nevertheless, the electrodes can be implanted in only a relatively small number of areas and can record from relatively limited populations of cells. In contrast, EEG-based BCI systems, which are noninvasive and do not require surgery or the long-term maintenance of implanted electrodes, do not have the risks of surgery or the questions of long-term stability of the electrodes since the electrodes are external and easily replaced. On the other hand, the brain signals detected by EEG-based systems are relatively weak and of limited frequency range. Nevertheless, EEG-based BCIs are currently adaptable for practical independent use by disabled people outside of the laboratory [131] and are in fact currently in use by a small number of paralyzed people at home in their daily lives [33]. Despite this achievement, continuing development of practical EEG-based BCI systems is needed to address existing issues. The extent of available independent control channels in such recordings remains to be determined. Continued development of noninvasive BCI systems with multiple independent control channels could substantially expand the capacity of BCI applications (e.g., multidimensional control of neuroprosthesis).
Another possible obstacle to moving BCI technology into practical use is their demand on the user's attention. Rapid fatigue of users has been previously reported in certain studies of BCI control [49, 123] and inconsistent performance by individual users is characteristic of most methods. Ongoing changes in the user's performance (due to fatigue, distraction, disease progression, etc.) require continuing adaptation of the BCI system. Some BCI applications currently require an exhaustive series of user commands (e.g., different mental activation tasks); these might be reduced by development of intelligent adaptation and learning algorithms. Further advances in other areas, such as speed, accuracy, consistency, convenience, and cosmesis, are also important for successful implementation of practical BCI systems.
5. Problems of Dissemination and Support
All current BCIs require significant efforts to set up, calibrate, and operate [1]. The kind and degree of effort vary substantially across BCIs. LFP-, spike- or ECoG-driven BCIs require surgery and constant monitoring of the scalp-port through which the wires run to the electrodes. EEG electrodes, which can be applied in a few minutes, require periodic reapplications. Daily recalibration may be needed (particularly for intracortical BCIs). A number of key issues have to be addressed in order to transfer BCI technology from the laboratory to clinical setting [30]. These include the ease and convenience of daily use, cosmesis, safety, reliability, usefulness of the BCI applications in the user's daily life, and the need for ongoing expert technical oversight. The cost of ongoing technical support may be high, and such support may only be available from a few research groups. Therefore, the development of standardized BCI systems with reduced complexity and minimal need for ongoing technical support is essential for the widespread dissemination of BCI technology.
The physical and social circumstances of potential BCI users, including their home situation, families, friends, and caregivers are also important. Unlike laboratory BCI systems, home BCI systems must be compact and able to fit into the user's environment with little or no inconvenience or disruption. In addition, home BCI systems must perform reliably in complex and unstable environments that often contain sources of electronic noise such as ventilators. Family or employed caregivers play an important role in the daily operation and maintenance of BCI systems. For EEG-based systems to be used at home, it is necessary to train caregivers in electrode application, recognition and correction of poor EEG signal quality, and initiation of software operation. The development of more user-friendly electrodes, such as dry, capacitance-based electrodes [132-134], could reduce the demands on caregivers. Customization of BCI systems to suit the needs of the individual user is important. In addition, to avoid unrealistic expectations and disappointment, users and their families should be made aware of the modest capacities of present-day BCI technology [1]. Thus, thorough preliminary discussions with prospective BCI users and their families and caregivers are important.
6. Expectations for the future
BCI research and development is a multidisciplinary effort involving neuroscientists, engineers, applied mathematicians, computer scientists, psychologists, neurologists, and clinical rehabilitation specialists. Although most of the published BCI literature to date concerns development of improved signal processing or other engineering facets of BCI technology, incorporation of professionals from all the above mentioned disciplines is critical for success [135].
As a field of practice and a subject of study, BCI technology is still in its infancy. Further research on different components of BCI development is ongoing and challenging. These include explorations of: useful brain signals; signal recording techniques; feature extraction and translation methods; methods for engaging short- and long-term adaptations between user and system so as to optimize performance; appropriate BCI applications; and clinical validation, dissemination, and support.
Efforts have recently begun to translate laboratory-validated BCI technologies into home systems for severely disabled individuals [33]. These home systems are currently limited to applications for simple communication (e.g., word processing, speech synthesizing, and email, etc) and simple environmental control (e.g., TV, room temperature, etc.) Widespread dissemination of these BCI systems may be difficult, since the fact that the limited capacities of current BCIs make them useful to only relatively small populations of users means that they are unlikely to attract significant commercial interest. In response to this problem, a new noncommercial option for BCI dissemination has recently been initiated (www.braincommunication.org). Other BCI applications, such as restoration of motor function, have been confined mainly to laboratory settings or limited lab-based demonstrations, and are not yet being used in everyday life [17]. Further work in all these areas is needed for BCIs to be validated and shown to be practical for the real-life environments of home-bound users. The use of BCI applications in neurorehabilitation is another promising area that is as yet still in its infancy.
Acknowledgments
Work in the authors' laboratory has been supported by the National Institutes of Health (NIH) (Grants HD30146 (NCMRR/NICHD) and EB00856 (NIBIB & NINDS)), the James S. McDonnell Foundation, the NEC Foundation, the Altran Foundation, the ALS Hope Foundation, and the Brain Communication Foundation.
Contributor Information
Joseph N. Mak, Laboratory of Neural Injury and Repair, Wadsworth Center, New York State Department of Health, Albany, NY 12201-0509 USA, (jmak@wadsworth.org).
Jonathan R. Wolpaw, Laboratory of Neural Injury and Repair, Wadsworth Center, New York State Department of Health, Albany, NY 12201-0509 USA, and State University of New York, Albany, NY 12222 USA, (wolpaw@wadsworth.org)
References
- 1.Kubler A, Mushahwar VK, Hochberg LR, Donoghue JP. BCI Meeting 2005--workshop on clinical issues and applications. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):131–4. doi: 10.1109/tnsre.2006.875585. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan TM, Wolpaw JR. The Third International Meeting on Brain-Computer Interface Technology: making a difference. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):126–7. [PubMed] [Google Scholar]
- 3.Vidal JJ. Toward direct brain-computer communication. Annu Rev Biophys Bioeng. 1973;2:157–80. doi: 10.1146/annurev.bb.02.060173.001105. [DOI] [PubMed] [Google Scholar]
- 4.Vidal JJ. Real-time detection of brain events in EEG. Proceedings of the IEEE. 1977;65(5):633–641. [Google Scholar]
- 5.Konger C, Principe JC. Neural network classification of event related potentials for the development of a new computer interface. Proc Proc IJCNN′90; 1990; Pages. [Google Scholar]
- 6.Farwell LA, Donchin E. Talking off the top of your head: toward a mental prosthesis utilizing event-related brain potentials. Electroencephalogr Clin Neurophysiol. 1988 Dec;70(6):510–23. doi: 10.1016/0013-4694(88)90149-6. [DOI] [PubMed] [Google Scholar]
- 7.Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. Electroencephalogr Clin Neurophysiol. 1991 Mar;78(3):252–9. doi: 10.1016/0013-4694(91)90040-b. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy PR, Bakay RA. Restoration of neural output from a paralyzed patient by a direct brain connection. Neuroreport. 1998 Jun 1;9(8):1707–11. doi: 10.1097/00001756-199806010-00007. [DOI] [PubMed] [Google Scholar]
- 9.Chapin JK, Moxon KA, Markowitz RS, Nicolelis MA. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci. 1999 Jul;2(7):664–70. doi: 10.1038/10223. [DOI] [PubMed] [Google Scholar]
- 10.Birbaumer N, Kubler A, Ghanayim N, Hinterberger T, Perelmouter J, Kaiser J, Iversen I, Kotchoubey B, Neumann N, Flor H. The thought translation device (TTD) for completely paralyzed patients. IEEE Trans Rehabil Eng. 2000 Jun;8(2):190–3. doi: 10.1109/86.847812. [DOI] [PubMed] [Google Scholar]
- 11.Donchin E, Spencer KM, Wijesinghe R. The mental prosthesis: assessing the speed of a P300-based brain-computer interface. IEEE Trans Rehabil Eng. 2000 Jun;8(2):174–9. doi: 10.1109/86.847808. [DOI] [PubMed] [Google Scholar]
- 12.Pfurtscheller G, Neuper C, Guger C, Harkam W, Ramoser H, Schlogl A, Obermaier B, Pregenzer M. Current trends in Graz Brain-Computer Interface (BCI) research. IEEE Trans Rehabil Eng. 2000 Jun;8(2):216–9. doi: 10.1109/86.847821. [DOI] [PubMed] [Google Scholar]
- 13.Taylor DM, Tillery SI, Schwartz AB. Direct cortical control of 3D neuroprosthetic devices. Science. 2002 Jun 7;296(5574):1829–32. doi: 10.1126/science.1070291. [DOI] [PubMed] [Google Scholar]
- 14.Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clin Neurophysiol. 2002 Jun;113(6):767–91. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- 15.Pfurtscheller G, Muller GR, Pfurtscheller J, Gerner HJ, Rupp R. ‘Thought’--control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett. 2003 Nov 6;351(1):33–6. doi: 10.1016/s0304-3940(03)00947-9. [DOI] [PubMed] [Google Scholar]
- 16.Leuthardt EC, Schalk G, Wolpaw JR, Ojemann JG, Moran DW. A brain-computer interface using electrocorticographic signals in humans. J Neural Eng. 2004 Jun;1(2):63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 17.Birbaumer N. Breaking the silence: brain-computer interfaces (BCI) for communication and motor control. Psychophysiology. 2006 Nov;43(6):517–32. doi: 10.1111/j.1469-8986.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 18.Hochberg LR, Serruya MD, Friehs GM, Mukand JA, Saleh M, Caplan AH, Branner A, Chen D, Penn RD, Donoghue JP. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature. 2006 Jul 13;442(7099):164–71. doi: 10.1038/nature04970. [DOI] [PubMed] [Google Scholar]
- 19.Sellers EW, Donchin E. A P300-based brain-computer interface: initial tests by ALS patients. Clin Neurophysiol. 2006 Mar;117(3):538–48. doi: 10.1016/j.clinph.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Schalk G, Miller KJ, Anderson NR, Wilson JA, Smyth MD, Ojemann JG, Moran DW, Wolpaw JR, Leuthardt EC. Two-dimensional movement control using electrocorticographic signals in humans. J Neural Eng. 2008 Mar;5(1):75–84. doi: 10.1088/1741-2560/5/1/008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubler A, Furdea A, Halder S, Hammer EM, Nijboer F, Kotchoubey B. A brain-computer interface controlled auditory event-related potential (p300) spelling system for locked-in patients. Ann N Y Acad Sci. 2009 Mar;1157:90–100. doi: 10.1111/j.1749-6632.2008.04122.x. [DOI] [PubMed] [Google Scholar]
- 22.McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic (EEG) control of three-dimensional movement. Proc Society for Neuroscience; November 2008; Pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolpaw JR, Birbaumer N, Heetderks WJ, McFarland DJ, Peckham PH, Schalk G, Donchin E, Quatrano LA, Robinson CJ, Vaughan TM. Brain-computer interface technology: a review of the first international meeting. IEEE Trans Rehabil Eng. 2000 Jun;8(2):164–73. doi: 10.1109/tre.2000.847807. [DOI] [PubMed] [Google Scholar]
- 24.Middendorf M, McMillan G, Calhoun G, Jones KS. Brain-computer interfaces based on the steady-state visual-evoked response. IEEE Trans Rehabil Eng. 2000 Jun;8(2):211–4. doi: 10.1109/86.847819. [DOI] [PubMed] [Google Scholar]
- 25.Sutter EE. The brain response interface: communication through visually-induced electrical brain responses. Journal of Microcomputer Applications. 1992;15(1):31–45. [Google Scholar]
- 26.Allison BZ, McFarland DJ, Schalk G, Zheng SD, Jackson MM, Wolpaw JR. Towards an independent brain-computer interface using steady state visual evoked potentials. Clin Neurophysiol. 2008 Feb;119(2):399–408. doi: 10.1016/j.clinph.2007.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly SP, Lalor EC, Finucane C, McDarby G, Reilly RB. Visual spatial attention control in an independent brain-computer interface. IEEE Trans Biomed Eng. 2005 Sep;52(9):1588–96. doi: 10.1109/TBME.2005.851510. [DOI] [PubMed] [Google Scholar]
- 28.Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Visual spatial attention tracking using high-density SSVEP data for independent brain-computer communication. IEEE Trans Neural Syst Rehabil Eng. 2005 Jun;13(2):172–8. doi: 10.1109/TNSRE.2005.847369. [DOI] [PubMed] [Google Scholar]
- 29.Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurol. 2008 Nov;7(11):1032–43. doi: 10.1016/S1474-4422(08)70223-0. [DOI] [PubMed] [Google Scholar]
- 30.Wolpaw JR, Loeb GE, Allison BZ, Donchin E, do Nascimento OF, Heetderks WJ, Nijboer F, Shain WG, Turner JN. BCI Meeting 2005--workshop on signals and recording methods. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):138–41. doi: 10.1109/TNSRE.2006.875583. [DOI] [PubMed] [Google Scholar]
- 31.Rokni U, Richardson AG, Bizzi E, Seung HS. Motor learning with unstable neural representations. Neuron. 2007 May 24;54(4):653–66. doi: 10.1016/j.neuron.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Kübler A, Müller KR. An Introduction to Brain-Computer Interfacing. In: Guido Dornhege JdRM, Hinterberger Thilo, McFarland Dennis, Müller Klaus-Robert., editors. Toward Brain-Computer Interfacing. Canbridge, MA: MIT press; 2007. pp. 1–25. [Google Scholar]
- 33.Vaughan TM, McFarland DJ, Schalk G, Sarnacki WA, Krusienski DJ, Sellers EW, Wolpaw JR. The Wadsworth BCI Research and Development Program: at home with BCI. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):229–33. doi: 10.1109/TNSRE.2006.875577. [DOI] [PubMed] [Google Scholar]
- 34.Schalk G, Kubanek J, Miller KJ, Anderson NR, Leuthardt EC, Ojemann JG, Limbrick D, Moran D, Gerhardt LA, Wolpaw JR. Decoding two-dimensional movement trajectories using electrocorticographic signals in humans. J Neural Eng. 2007 Sep;4(3):264–75. doi: 10.1088/1741-2560/4/3/012. [DOI] [PubMed] [Google Scholar]
- 35.Leuthardt EC, Miller KJ, Schalk G, Rao RP, Ojemann JG. Electrocorticography-based brain computer interface--the Seattle experience. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):194–8. doi: 10.1109/TNSRE.2006.875536. [DOI] [PubMed] [Google Scholar]
- 36.Leuthardt EC, Schalk G, Moran D, Ojemann JG. The emerging world of motor neuroprosthetics: a neurosurgical perspective. Neurosurgery. 2006 Jul;59(1):1–14. doi: 10.1227/01.NEU.0000221506.06947.AC. discussion 1-14. [DOI] [PubMed] [Google Scholar]
- 37.Pfurtscheller G, Muller-Putz GR, Schlogl A, Graimann B, Scherer R, Leeb R, Brunner C, Keinrath C, Lee F, Townsend G, Vidaurre C, Neuper C. 15 years of BCI research at Graz University of Technology: current projects. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):205–10. doi: 10.1109/TNSRE.2006.875528. [DOI] [PubMed] [Google Scholar]
- 38.Shenoy P, Miller KJ, Ojemann JG, Rao RP. Generalized features for electrocorticographic BCIs. IEEE Trans Biomed Eng. 2008 Jan;55(1):273–80. doi: 10.1109/TBME.2007.903528. [DOI] [PubMed] [Google Scholar]
- 39.Demirer RM, Ozerdem MS, Bayrak C. Classification of imaginary movements in ECoG with a hybrid approach based on multi-dimensional Hilbert-SVM solution. J Neurosci Methods. 2009 Mar 30;178(1):214–8. doi: 10.1016/j.jneumeth.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 40.Felton EA, Wilson JA, Williams JC, Garell PC. Electrocorticographically controlled brain-computer interfaces using motor and sensory imagery in patients with temporary subdural electrode implants. Report of four cases. J Neurosurg. 2007 Mar;106(3):495–500. doi: 10.3171/jns.2007.106.3.495. [DOI] [PubMed] [Google Scholar]
- 41.Blakely T, Miller KJ, Zanos SP, Rao RP, Ojemann JG. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurg Focus. 2009 Jul;27(1):E13. doi: 10.3171/2009.4.FOCUS0977. [DOI] [PubMed] [Google Scholar]
- 42.Darvas F, Miller KJ, Rao RP, Ojemann JG. Nonlinear phase-phase cross-frequency coupling mediates communication between distant sites in human neocortex. J Neurosci. 2009 Jan 14;29(2):426–35. doi: 10.1523/JNEUROSCI.3688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gunduz A, Sanchez JC, Carney PR, Principe JC. Mapping broadband electrocorticographic recordings to two-dimensional hand trajectories in humans Motor control features. Neural Netw. 2009 Jul 2; doi: 10.1016/j.neunet.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 44.Kubanek J, Miller KJ, Ojemann JG, Wolpaw JR, Schalk G. Decoding flexion of individual fingers using electrocorticographic signals in humans. J Neural Eng. 2009 Oct 1;6(6):66001. doi: 10.1088/1741-2560/6/6/066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pistohl T, Ball T, Schulze-Bonhage A, Aertsen A, Mehring C. Prediction of arm movement trajectories from ECoG-recordings in humans. J Neurosci Methods. 2008 Jan 15;167(1):105–14. doi: 10.1016/j.jneumeth.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Scherer R, Zanos SP, Miller KJ, Rao RP, Ojemann JG. Classification of contralateral and ipsilateral finger movements for electrocorticographic brain-computer interfaces. Neurosurg Focus. 2009 Jul;27(1):E12. doi: 10.3171/2009.4.FOCUS0981. [DOI] [PubMed] [Google Scholar]
- 47.Wilson JA, Felton EA, Garell PC, Schalk G, Williams JC. ECoG factors underlying multimodal control of a brain-computer interface. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):246–50. doi: 10.1109/TNSRE.2006.875570. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez JC, Gunduz A, Carney PR, Principe JC. Extraction and localization of mesoscopic motor control signals for human ECoG neuroprosthetics. J Neurosci Methods. 2008 Jan 15;167(1):63–81. doi: 10.1016/j.jneumeth.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 49.Kennedy PR, Bakay RA, Moore MM, Adams K, Goldwaithe J. Direct control of a computer from the human central nervous system. IEEE Trans Rehabil Eng. 2000 Jun;8(2):198–202. doi: 10.1109/86.847815. [DOI] [PubMed] [Google Scholar]
- 50.Buch E, Weber C, Cohen LG, Braun C, Dimyan MA, Ard T, Mellinger J, Caria A, Soekadar S, Fourkas A, Birbaumer N. Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008 Mar;39(3):910–7. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mellinger J, Schalk G, Braun C, Preissl H, Rosenstiel W, Birbaumer N, Kubler A. An MEG-based brain-computer interface (BCI) Neuroimage. 2007 Jul 1;36(3):581–93. doi: 10.1016/j.neuroimage.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tecchio F, Porcaro C, Barbati G, Zappasodi F. Functional source separation and hand cortical representation for a brain-computer interface feature extraction. J Physiol. 2007 May 1;580(Pt.3):703–21. doi: 10.1113/jphysiol.2007.129163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Gerven M, Jensen O. Attention modulations of posterior alpha as a control signal for two-dimensional brain-computer interfaces. J Neurosci Methods. 2009 Apr 30;179(1):78–84. doi: 10.1016/j.jneumeth.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser J, Walker F, Leiberg S, Lutzenberger W. Cortical oscillatory activity during spatial echoic memory. Eur J Neurosci. 2005 Jan;21(2):587–90. doi: 10.1111/j.1460-9568.2005.03867.x. [DOI] [PubMed] [Google Scholar]
- 55.Bradshaw LA, Wijesinghe RS, Wikswo JP., Jr Spatial filter approach for comparison of the forward and inverse problems of electroencephalography and magnetoencephalography. Ann Biomed Eng. 2001 Mar;29(3):214–26. doi: 10.1114/1.1352641. [DOI] [PubMed] [Google Scholar]
- 56.Cohen D. Magnetoencephalography: detection of the brain's electrical activity with a superconducting magnetometer. Science. 1972 Feb 11;175(22):664–6. doi: 10.1126/science.175.4022.664. [DOI] [PubMed] [Google Scholar]
- 57.Hinterberger T, Veit R, Wilhelm B, Weiskopf N, Vatine JJ, Birbaumer N. Neuronal mechanisms underlying control of a brain-computer interface. Eur J Neurosci. 2005 Jun;21(11):3169–81. doi: 10.1111/j.1460-9568.2005.04092.x. [DOI] [PubMed] [Google Scholar]
- 58.Hinterberger T, Weiskopf N, Veit R, Wilhelm B, Betta E, Birbaumer N. An EEG-driven brain-computer interface combined with functional magnetic resonance imaging (fMRI) IEEE Trans Biomed Eng. 2004 Jun;51(6):971–4. doi: 10.1109/TBME.2004.827069. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Ryu J, Jolesz FA, Cho ZH, Yoo SS. Brain-machine interface via real-time fMRI: preliminary study on thought-controlled robotic arm. Neurosci Lett. 2009 Jan 23;450(1):1–6. doi: 10.1016/j.neulet.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramsey NF, van de Heuvel MP, Kho KH, Leijten FS. Towards human BCI applications based on cognitive brain systems: an investigation of neural signals recorded from the dorsolateral prefrontal cortex. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):214–7. doi: 10.1109/TNSRE.2006.875582. [DOI] [PubMed] [Google Scholar]
- 61.Sitaram R, Caria A, Veit R, Gaber T, Rota G, Kuebler A, Birbaumer N. FMRI brain-computer interface: a tool for neuroscientific research and treatment. Comput Intell Neurosci. 2007:25487. doi: 10.1155/2007/25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weiskopf N, Mathiak K, Bock SW, Scharnowski F, Veit R, Grodd W, Goebel R, Birbaumer N. Principles of a brain-computer interface (BCI) based on real-time functional magnetic resonance imaging (fMRI) IEEE Trans Biomed Eng. 2004 Jun;51(6):966–70. doi: 10.1109/TBME.2004.827063. [DOI] [PubMed] [Google Scholar]
- 63.Weiskopf N, Veit R, Erb M, Mathiak K, Grodd W, Goebel R, Birbaumer N. Physiological self-regulation of regional brain activity using real-time functional magnetic resonance imaging (fMRI): methodology and exemplary data. Neuroimage. 2003 Jul;19(3):577–86. doi: 10.1016/s1053-8119(03)00145-9. [DOI] [PubMed] [Google Scholar]
- 64.Yoo SS, Fairneny T, Chen NK, Choo SE, Panych LP, Park H, Lee SY, Jolesz FA. Brain-computer interface using fMRI: spatial navigation by thoughts. Neuroreport. 2004 Jul 19;15(10):1591–5. doi: 10.1097/01.wnr.0000133296.39160.fe. [DOI] [PubMed] [Google Scholar]
- 65.Bauernfeind G, Leeb R, Wriessnegger SC, Pfurtscheller G. Development, set-up and first results for a one-channel near-infrared spectroscopy system. Biomed Tech (Berl) 2008;53(1):36–43. doi: 10.1515/BMT.2008.005. [DOI] [PubMed] [Google Scholar]
- 66.Coyle S, Ward T, Markham C, McDarby G. On the suitability of near-infrared (NIR) systems for next-generation brain-computer interfaces. Physiol Meas. 2004 Aug;25(4):815–22. doi: 10.1088/0967-3334/25/4/003. [DOI] [PubMed] [Google Scholar]
- 67.Coyle SM, Ward TE, Markham CM. Brain-computer interface using a simplified functional near-infrared spectroscopy system. J Neural Eng. 2007 Sep;4(3):219–26. doi: 10.1088/1741-2560/4/3/007. [DOI] [PubMed] [Google Scholar]
- 68.Luu S, Chau T. Decoding subjective preference from single-trial near-infrared spectroscopy signals. J Neural Eng. 2009 Feb;6(1):016003. doi: 10.1088/1741-2560/6/1/016003. [DOI] [PubMed] [Google Scholar]
- 69.Sitaram R, Zhang H, Guan C, Thulasidas M, Hoshi Y, Ishikawa A, Shimizu K, Birbaumer N. Temporal classification of multichannel near-infrared spectroscopy signals of motor imagery for developing a brain-computer interface. Neuroimage. 2007 Feb 15;34(4):1416–27. doi: 10.1016/j.neuroimage.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Matthews F, Pearlmutter BA, Ward TE, Soraghan C, Markham C. Hemodynamics for Brain-Computer Interfaces. Signal Processing Magazine, IEEE. 2008;25(1):87–94. [Google Scholar]
- 71.Sitaram R, Weiskopf N, Caria A, Veit R, Erb M, Birbaumer N. fMRI Brain-Computer Interfaces. Signal Processing Magazine, IEEE. 2008;25(1):95–106. [Google Scholar]
- 72.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003 May 15;23(10):3963–71. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birbaumer N, Cohen LG. Brain-computer interfaces: communication and restoration of movement in paralysis. J Physiol. 2007 Mar 15;579(Pt 3):621–36. doi: 10.1113/jphysiol.2006.125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weiskopf N, Scharnowski F, Veit R, Goebel R, Birbaumer N, Mathiak K. Self-regulation of local brain activity using real-time functional magnetic resonance imaging (fMRI) J Physiol Paris. 2004 Jul-Nov;98(4-6):357–73. doi: 10.1016/j.jphysparis.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 75.Weiskopf N, Sitaram R, Josephs O, Veit R, Scharnowski F, Goebel R, Birbaumer N, Deichmann R, Mathiak K. Real-time functional magnetic resonance imaging: methods and applications. Magn Reson Imaging. 2007 Jul;25(6):989–1003. doi: 10.1016/j.mri.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 76.Birbaumer N, Weber C, Neuper C, Buch E, Haapen K, Cohen L. Physiological regulation of thinking: brain-computer interface (BCI) research. Prog Brain Res. 2006;159:369–91. doi: 10.1016/S0079-6123(06)59024-7. [DOI] [PubMed] [Google Scholar]
- 77.Krusienski DJ, Sellers EW, McFarland DJ, Vaughan TM, Wolpaw JR. Toward enhanced P300 speller performance. J Neurosci Methods. 2008 Jan 15;167(1):15–21. doi: 10.1016/j.jneumeth.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McFarland DJ, Krusienski DJ, Wolpaw JR. Brain-computer interface signal processing at the Wadsworth Center: mu and sensorimotor beta rhythms. Prog Brain Res. 2006;159:411–9. doi: 10.1016/S0079-6123(06)59026-0. [DOI] [PubMed] [Google Scholar]
- 79.McFarland DJ, Wolpaw JR. Sensorimotor rhythm-based brain-computer interface (BCI): model order selection for autoregressive spectral analysis. J Neural Eng. 2008 Jun;5(2):155–62. doi: 10.1088/1741-2560/5/2/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pardey J, Roberts S, Tarassenko L. A review of parametric modelling techniques for EEG analysis. Med Eng Phys. 1996 Jan;18(1):2–11. doi: 10.1016/1350-4533(95)00024-0. [DOI] [PubMed] [Google Scholar]
- 81.Schalk G, Wolpaw JR, McFarland DJ, Pfurtscheller G. EEG-based communication: presence of an error potential. Clin Neurophysiol. 2000 Dec;111(12):2138–44. doi: 10.1016/s1388-2457(00)00457-0. [DOI] [PubMed] [Google Scholar]
- 82.Ramoser H, Wolpaw JR, Pfurtscheller G. EEG-based communication: evaluation of alternative signal prediction methods. Biomed Tech (Berl) 1997 Sep;42(9):226–33. doi: 10.1515/bmte.1997.42.9.226. [DOI] [PubMed] [Google Scholar]
- 83.Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a noninvasive brain-computer interface in humans. Proc Natl Acad Sci U S A. 2004 Dec 21;101(51):17849–54. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kayagil TA, Bai O, Henriquez CS, Lin P, Furlani SJ, Vorbach S, Hallett M. A binary method for simple and accurate two-dimensional cursor control from EEG with minimal subject training. J Neuroeng Rehabil. 2009;6:14. doi: 10.1186/1743-0003-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McFarland DJ, Krusienski DJ, Sarnacki WA, Wolpaw JR. Emulation of computer mouse control with a noninvasive brain-computer interface. J Neural Eng. 2008 Jun;5(2):101–10. doi: 10.1088/1741-2560/5/2/001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Galan F, Nuttin M, Lew E, Ferrez PW, Vanacker G, Philips J, Millan Jdel R. A brain-actuated wheelchair: asynchronous and non-invasive Brain-computer interfaces for continuous control of robots. Clin Neurophysiol. 2008 Sep;119(9):2159–69. doi: 10.1016/j.clinph.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 87.Pires G, Castelo-Branco M, Nunes U. Visual P300-based BCI to steer a wheelchair: a Bayesian approach. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:658–61. doi: 10.1109/IEMBS.2008.4649238. [DOI] [PubMed] [Google Scholar]
- 88.Cincotti F, Mattia D, Aloise F, Bufalari S, Schalk G, Oriolo G, Cherubini A, Marciani MG, Babiloni F. Non-invasive brain-computer interface system: towards its application as assistive technology. Brain Res Bull. 2008 Apr 15;75(6):796–803. doi: 10.1016/j.brainresbull.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Taylor DM, Tillery SI, Schwartz AB. Information conveyed through brain-control: cursor versus robot. IEEE Trans Neural Syst Rehabil Eng. 2003 Jun;11(2):195–9. doi: 10.1109/TNSRE.2003.814451. [DOI] [PubMed] [Google Scholar]
- 90.Lebedev MA, Nicolelis MA. Brain-machine interfaces: past, present and future. Trends Neurosci. 2006 Sep;29(9):536–46. doi: 10.1016/j.tins.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Muller-Putz GR, Scherer R, Pfurtscheller G, Rupp R. EEG-based neuroprosthesis control: a step towards clinical practice. Neurosci Lett. 2005 Jul 1-8;382(1-2):169–74. doi: 10.1016/j.neulet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 92.Nijboer F, Sellers EW, Mellinger J, Jordan MA, Matuz T, Furdea A, Halder S, Mochty U, Krusienski DJ, Vaughan TM, Wolpaw JR, Birbaumer N, Kubler A. A P300-based brain-computer interface for people with amyotrophic lateral sclerosis. Clin Neurophysiol. 2008 Aug;119(8):1909–16. doi: 10.1016/j.clinph.2008.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moore MM. Real-world applications for brain-computer interface technology. IEEE Trans Neural Syst Rehabil Eng. 2003 Jun;11(2):162–5. doi: 10.1109/TNSRE.2003.814433. [DOI] [PubMed] [Google Scholar]
- 94.Karim AA, Hinterberger T, Richter J, Mellinger J, Neumann N, Flor H, Kubler A, Birbaumer N. Neural internet: Web surfing with brain potentials for the completely paralyzed. Neurorehabil Neural Repair. 2006 Dec;20(4):508–15. doi: 10.1177/1545968306290661. [DOI] [PubMed] [Google Scholar]
- 95.Dobkin BH. Brain-computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. J Physiol. 2007 Mar 15;579(Pt 3):637–42. doi: 10.1113/jphysiol.2006.123067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kubler A, Neumann N. Brain-computer interfaces--the key for the conscious brain locked into a paralyzed body. Prog Brain Res. 2005;150:513–25. doi: 10.1016/S0079-6123(05)50035-9. [DOI] [PubMed] [Google Scholar]
- 97.Neuper C, Muller GR, Kubler A, Birbaumer N, Pfurtscheller G. Clinical application of an EEG-based brain-computer interface: a case study in a patient with severe motor impairment. Clin Neurophysiol. 2003 Mar;114(3):399–409. doi: 10.1016/s1388-2457(02)00387-5. [DOI] [PubMed] [Google Scholar]
- 98.Rockstroh B, Elbert T, Canavan A, Lutzenberger W, Birbaumer N. Slow cortical potentials and behavior. Baltimore, MD: Urban and Schwarzenberg; 1989. [Google Scholar]
- 99.Birbaumer N. Slow cortical potentials: their origin, meaning, and clinical use. In: van Boxtel GJM, Böcker KBE, editors. Brain and behavior: past, present and future. Tilburg: Tilburg University Press; 1997. pp. 25–39. [Google Scholar]
- 100.Lutzenberger W, Birbaumer N, Elbert T, Rockstroh B, Bippus W, Breidt R. Self-regulation of slow cortical potentials in normal subjects and patients with frontal lobe lesions. Prog Brain Res. 1980;54:427–30. doi: 10.1016/S0079-6123(08)61655-6. [DOI] [PubMed] [Google Scholar]
- 101.Birbaumer N, Ghanayim N, Hinterberger T, Iversen I, Kotchoubey B, Kubler A, Perelmouter J, Taub E, Flor H. A spelling device for the paralysed. Nature. 1999 Mar 25;398(6725):297–8. doi: 10.1038/18581. [DOI] [PubMed] [Google Scholar]
- 102.Kaiser J, Kubler A, Hinterberger T, Neumann N, Birbaumer N. A non-invasive communication device for the paralyzed. Minim Invasive Neurosurg. 2002 Mar;45(1):19–23. doi: 10.1055/s-2002-23578. [DOI] [PubMed] [Google Scholar]
- 103.Kubler A, Birbaumer N. Brain-computer interfaces and communication in paralysis: extinction of goal directed thinking in completely paralysed patients? Clin Neurophysiol. 2008 Nov;119(11):2658–66. doi: 10.1016/j.clinph.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kubler A, Neumann N, Kaiser J, Kotchoubey B, Hinterberger T, Birbaumer NP. Brain-computer communication: self-regulation of slow cortical potentials for verbal communication. Arch Phys Med Rehabil. 2001 Nov;82(11):1533–9. doi: 10.1053/apmr.2001.26621. [DOI] [PubMed] [Google Scholar]
- 105.Pfurtscheller G. EEG event-related desynchronization (ERD) and event-related synchronization (ERS) In: Niedermeyer E, Lopes da Silva FH, editors. Electroencephalography: basic principles, clinical applications and related fields. 4th. Baltimore, MD: williams and Wilkin; 1999. pp. 958–967. [Google Scholar]
- 106.Wolpaw JR, McFarland DJ, Vaughan TM, Schalk G. The Wadsworth Center brain-computer interface (BCI) research and development program. IEEE Trans Neural Syst Rehabil Eng. 2003 Jun;11(2):204–7. doi: 10.1109/TNSRE.2003.814442. [DOI] [PubMed] [Google Scholar]
- 107.Wolpaw JR, McFarland DJ. Multichannel EEG-based brain-computer communication. Electroencephalogr Clin Neurophysiol. 1994 Jun;90(6):444–9. doi: 10.1016/0013-4694(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 108.Pfurtscheller G, Neuper C, Muller GR, Obermaier B, Krausz G, Schlogl A, Scherer R, Graimann B, Keinrath C, Skliris D, Wortz M, Supp G, Schrank C. Graz-BCI: state of the art and clinical applications. IEEE Trans Neural Syst Rehabil Eng. 2003 Jun;11(2):177–80. doi: 10.1109/TNSRE.2003.814454. [DOI] [PubMed] [Google Scholar]
- 109.Neuper C, Muller-Putz GR, Scherer R, Pfurtscheller G. Motor imagery and EEG-based control of spelling devices and neuroprostheses. Prog Brain Res. 2006;159:393–409. doi: 10.1016/S0079-6123(06)59025-9. [DOI] [PubMed] [Google Scholar]
- 110.Kostov A, Polak M. Parallel man-machine training in development of EEG-based cursor control. IEEE Trans Rehabil Eng. 2000 Jun;8(2):203–5. doi: 10.1109/86.847816. [DOI] [PubMed] [Google Scholar]
- 111.Roberts SJ, Penny WD. Real-time brain-computer interfacing: a preliminary study using Bayesian learning. Med Biol Eng Comput. 2000 Jan;38(1):56–61. doi: 10.1007/BF02344689. [DOI] [PubMed] [Google Scholar]
- 112.Donchin E, Smith DB. The contingent negative variation and the late positive wave of the average evoked potential. Electroencephalogr Clin Neurophysiol. 1970 Aug;29(2):201–3. doi: 10.1016/0013-4694(70)90124-0. [DOI] [PubMed] [Google Scholar]
- 113.Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965 Nov 26;150(700):1187–8. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- 114.Walter WG, Cooper R, Aldridge VJ, McCallum WC, Winter AL. Contingent Negative Variation: An Electric Sign of Sensorimotor Association and Expectancy in the Human Brain. Nature. 1964 Jul 25;203:380–4. doi: 10.1038/203380a0. [DOI] [PubMed] [Google Scholar]
- 115.Piccione F, Giorgi F, Tonin P, Priftis K, Giove S, Silvoni S, Palmas G, Beverina F. P300-based brain computer interface: reliability and performance in healthy and paralysed participants. Clin Neurophysiol. 2006 Mar;117(3):531–7. doi: 10.1016/j.clinph.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 116.Hoffmann U, Vesin JM, Ebrahimi T, Diserens K. An efficient P300-based brain-computer interface for disabled subjects. J Neurosci Methods. 2008 Jan 15;167(1):115–25. doi: 10.1016/j.jneumeth.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 117.Sellers EW, Kubler A, Donchin E. Brain-computer interface research at the University of South Florida Cognitive Psychophysiology Laboratory: the P300 Speller. IEEE Trans Neural Syst Rehabil Eng. 2006 Jun;14(2):221–4. doi: 10.1109/TNSRE.2006.875580. [DOI] [PubMed] [Google Scholar]
- 118.Furdea A, Halder S, Krusienski DJ, Bross D, Nijboer F, Birbaumer N, Kubler A. An auditory oddball (P300) spelling system for brain-computer interfaces. Psychophysiology. 2009 May;46(3):617–25. doi: 10.1111/j.1469-8986.2008.00783.x. [DOI] [PubMed] [Google Scholar]
- 119.McFarland DJ, Wolpaw JR. Brain-Computer Interface Operation of Robotic and Prosthetic Devices. Computer. 2008;41(10):52–56. [Google Scholar]
- 120.Pfurtscheller G, Guger C, Muller G, Krausz G, Neuper C. Brain oscillations control hand orthosis in a tetraplegic. Neurosci Lett. 2000 Oct 13;292(3):211–4. doi: 10.1016/s0304-3940(00)01471-3. [DOI] [PubMed] [Google Scholar]
- 121.Gao X, Xu D, Cheng M, Gao S. A BCI-based environmental controller for the motion-disabled. IEEE Trans Neural Syst Rehabil Eng. 2003 Jun;11(2):137–40. doi: 10.1109/TNSRE.2003.814449. [DOI] [PubMed] [Google Scholar]
- 122.Tanaka K, Matsunaga K, Wang HO. Electroencephalogram-Based Control of an Electric Wheelchair. Robotics, IEEE Transactions on. 2005;21(4):762–766. [Google Scholar]
- 123.Rebsamen B, Burdet E, Guan C, Zhang H, Teo CL, Zeng Q, Laugier C, Ang MH., Jr Controlling a Wheelchair Indoors Using Thought. Intelligent Systems, IEEE. 2007;22(2):18–24. [Google Scholar]
- 124.Vanacker G, Del RMJ, Lew E, Ferrez PW, Moles FG, Philips J, Van Brussel H, Nuttin M. Context-based filtering for assisted brain-actuated wheelchair driving. Comput Intell Neurosci. 2007:25130. doi: 10.1155/2007/25130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Leuthardt EC, Schalk G, Roland J, Rouse A, Moran DW. Evolution of brain-computer interfaces: going beyond classic motor physiology. Neurosurg Focus. 2009 Jul;27(1):E4. doi: 10.3171/2009.4.FOCUS0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004 Mar;55(3):400–9. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- 127.Daly JJ, Yin F, Perepezko EM, Siemionow V, Yue GH. Prolonged cognitive planning time, elevated cognitive effort, and relationship to coordination and motor control following stroke. Neural Systems and Rehabilitation Engineering, IEEE Transactions on. 2006;14(2):168–171. doi: 10.1109/TNSRE.2006.875554. [DOI] [PubMed] [Google Scholar]
- 128.Enzinger C, Ropele S, Fazekas F, Loitfelder M, Gorani F, Seifert T, Reiter G, Neuper C, Pfurtscheller G, Muller-Putz G. Brain motor system function in a patient with complete spinal cord injury following extensive brain-computer interface training. Exp Brain Res. 2008 Sep;190(2):215–23. doi: 10.1007/s00221-008-1465-y. [DOI] [PubMed] [Google Scholar]
- 129.Daly JJ, Hogan N, Perepezko EM, Krebs HI, Rogers JM, Goyal KS, Dohring ME, Fredrickson E, Nethery J, Ruff RL. Response to upper-limb robotics and functional neuromuscular stimulation following stroke. J Rehabil Res Dev. 2005 Nov-Dec;42(6):723–36. doi: 10.1682/jrrd.2005.02.0048. [DOI] [PubMed] [Google Scholar]
- 130.Daly JJ, Cheng RC, Hrovat K, Litinas KH, Rogers JM, Dohring ME. Development and testing of non-invasive BCI + FES/robot system for use in motor re-learning after stroke. Proc International Functional Electrical Stimulation Society; 2008; Pages. [Google Scholar]
- 131.McFarland DJ, Müller KR. Invasive BCI approaches: Preface. In: Guido Dornhege JdRM, Hinterberger Thilo, McFarland Dennis, Müller Klaus-Robert., editors. Toward Brain-Computer Interfacing. Canbridge, MA: MIT press; 2007. pp. 123–127. [Google Scholar]
- 132.Alizadeh-Taheri B, Smith RL, Knight RT. An active, microfabricated, scalp electrode array for EEG recording. Sensors and Actuators A: Physical. 1996;54(1-3):606–611. [Google Scholar]
- 133.Popescu F, Fazli S, Badower Y, Blankertz B, Muller KR. Single trial classification of motor imagination using 6 dry EEG electrodes. PLoS One. 2007;2(7):e637. doi: 10.1371/journal.pone.0000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harland CJ, Clark TD, Prance RJ. Remote detection of human electroencephalograms using ultrahigh input impedance electric potential sensors. Applied Physics Letters. 2002;81(17):3284–3286. [Google Scholar]
- 135.Wolpaw JR, Birbaumer N. Brain-computer interfaces for communication and control. In: Selzer ME, Clarke S, Cohen LG, Duncan P, Gage FH, editors. Textbook of Neural Repair and Rehabilitation; NeuralRepair and Plasticity. Cambridge: Cambridge University Press; 2006. pp. 602–614. [Google Scholar]
- 136.Wolpaw JR, McFarland DJ, Vaughan TM. Brain-computer interface research at the Wadsworth Center. IEEE Trans Rehabil Eng. 2000 Jun;8(2):222–6. doi: 10.1109/86.847823. [DOI] [PubMed] [Google Scholar]


