Abstract
“Barker’s hypothesis” emerged almost 25 years ago from epidemiological studies of birth and death records that revealed a high geographic correlation between rates of infant mortality and certain classes of later adult deaths as well as an association between birthweight and rates of adult death from ischemic heart disease. These observations led to a theory that undernutrition during gestation was an important early origin of adult cardiac and metabolic disorders due to fetal programming that permanently shaped the body’s structure, function, and metabolism and contributed to adult disease. This theory stimulated interest in the fetal origins of adult disorders, which expanded and coalesced ~5 years ago with the formation of an international society for developmental origins of health and disease (DOHaD). Here we review a few examples of the many emergent themes of the DOHaD approach, including theoretical advances related to predictive adaptive responses of the fetus to a broad range of environmental cues, empirical observations of effects of overnutrition and stress during pregnancy on outcomes in childhood and adulthood, and potential epigenetic mechanisms that may underlie these observations and theory. Next, we discuss the relevance of the DOHaD approach to reproductive medicine. Finally, we consider the next steps that might be taken to apply, evaluate, and extend the DOHaD approach.
Keywords: Barker’s hypothesis, DOHaD, predictive adaptive response, IUGR, premature birth, obesity, stress, epigenetics
FROM EPIDEMIOLOGICAL OBSERVATIONS TO THE FETAL ORIGINS HYPOTHESIS
The Developmental Origins of Health and Disease (DOHaD) approach evolved from epidemiological studies of infant and adult mortality. A trio of articles in The Lancet by Barker and colleagues1–3 represent perhaps the most influential early publications in this area that led to the fetal origins hypothesis (often called “Barker’s hypothesis”). There are many reviews of Barker’s hypothesis, and one of the best is provided by Barker himself, who summarized the genesis of the developmental origins hypothesis.4 The main points are best described by quotes from these original sources, which we provide later.
Barker4 gives a personal account of a program of epidemiological research of the geographic distributions of diseases across local authorities of England and Wales, which provided the countrywide data used by Barker et al1 to show a large positive geographic correlation (~0.7) for standardized rates for infant mortality from 1921 to 1925 and ischemic heart disease from 1968 to 1978. An interpretation of this relationship was based on several factors: the association of neonatal deaths in the 1920 with low birthweight, the dependence on adverse intrauterine rather than postnatal factors, the paradoxical rise in heart disease with rising prosperity but lower rates in the most prosperous locations, a review of the literature on maternal and infant nutrition, and other factors. This led to the insight and hypothesis that the geographic relationship of infant and adults death rates “reflects variations in nutrition in early life, which are expressed pathologically on exposure to later dietary influences” (p. 1081).
According to Barker’s4 account, the next step of investigation “required studies of a kind that had not hitherto been carried out” (p. 415). This was initiated within a sample of adults (men born from 1911 to 1930 in Hertfordshire) with good records of size at birth, weight in infancy, and death from ischemic health disease, which Barker et al2 used to confirm (in individuals) the deductions from the geographic study: Men with the lowest birthweights had the highest death rates, those with the highest birthweights had the lowest death rates, and standardized death rates fell steeply with increasing weight at 1 year of age. This led to the hypothesis that “an environment which produces poor fetal and infant growth is followed by an adult environment that determines high risk for ischemic heart disease” (p. 579).
To develop the hypothesis further, Baker et al3 reviewed how fetal undernutrition at different stages of gestation can be linked to different birth phenotypes, each linked to adaptations associated with changes in concentrations of placental and fetal hormone and later with different metabolic abnormalities in adulthood. This integration proposed that “undernutrition during gestation reprograms the relationship between glucose and insulin and between growth hormone and IGF [insulin-like growth factor]” (p. 940), which permanently changes the body’s structure, function and metabolism that increases risk for coronary heart disease in later life.
FROM FETAL ORIGINS OF ADULT DISEASE TO DEVELOPMENTAL ORIGINS OF HEALTH AND DISEASE
Barker’s hypothesis stimulated a great deal of worldwide interest and activity in the area of developmental plasticity, Gillman et al5 summarized in a report of the meetings of the World Congress on Fetal Origins of Adult Disease that were convened in 2001 (Mumbai, India) and 2003 (Brighton, United Kingdom) and the transition to DOHaD that was formed subsequently “to recognize the broader scope of developmental cues, extending from the oocyte to the infant and beyond, and the concept that the early life environment has widespread consequences for later health” (p. 625). The DOHaD society has sponsored meetings in 2005 (Toronto, Ontario, Canada), 2006 (Utrecht, The Netherlands), 2007 (Perth, Western Australia), and 2009 (Santiago, Chile), and these international congresses provided an important forum for exchange of ideas and progress in this rapidly expanding field (www.dohadsoc.org).
Here we review some of the expanded themes, including: (1) the development of theory based on the concept of predictive adaptive responses of the fetus to a variety of environmental cues and consequences of mismatch between prenatal and postnatal environments, (2) the emphasis on the fetal origins of obesity and overnutrition as well as undernutrition during gestation and in infancy as pathways into obesity in childhood and adulthood, (3) the evaluation of psychobiological effects of stress during pregnancy on fetal development and later outcomes, and (4) the consideration of epigenetic mechanisms to account for some of the observations based on the DOHaD approach.
The field is now so large that a selective review is necessary. For relevance to the topic of reproductive medicine, we chose to focus our review on important details from three specialized research programs (the Southampton Women’s Survey (SWS), Project VIVA, and the Behavioral Perinatology Research Program) that emphasize prospective evaluations of fetal development during pregnancy.
The Southampton Women’s Survey: Background and Current Status
Barker and collaborators developed the first generation of theories to account for the observations of correlations of fetal and adult death rates across geographic locations1 and birthweight and ischemic heart disease death rates across individuals,2 which included the “thrifty phenotype” theory of Hales and Barker6 and the “developmental plasticity” theory of Bateson et al.7 With this scientific background (see www.mrc.soton.ac.uk), the next stage of a program of research at the University of Southampton focused on data from cohorts of individuals born in the first half of the 20th century. Using data from one of these cohorts, Roseboom et al8 investigated effects of timing of fetal undernutrition based on the Dutch cohort exposed to the 1944–1945 famine at the end of World War II and showed that fetal undernutrition may affect different organs of the body depending on different critical phases of development (i.e., in the Dutch famine, those individuals conceived before the famine and exposed to an energy-poor fetal environment late in gestation as adults had increased risk for insulin resistance and impaired glucose tolerance, but those conceived during the famine as adults had increased risk for high serum cholesterol and coronary heart disease). Using data from another cohort, Barker et al9 investigated the trajectory of growth during infancy and childhood in the Helsinki 1934 to 1944 Birth Cohort (see Eriksson et al10) and showed that adult outcomes were moderated by the tempo of growth in infancy and childhood as well as by fetal growth and birthweight (i.e., the risk of coronary events in adulthood was more strongly related to the tempo of childhood body mass index [BMI] gain from ages 2 to 11 years than to BMI itself at any other age).
This program of research also initiated a new a cohort study of contemporary births. Initial goals were to directly test some of the assumptions of the DOHaD approach (i.e., documentation of adaptations occurring in the fetus when undernourished, including changes in metabolism, alterations in hormone production and tissue sensitivity to these alterations, and changes in the relative growth rates of organs and structures of the body). The SWS started with interviews of 12,500 young female residents of Southampton to obtained measures of prepregnancy characteristics, followed by detailed, prospective evaluation of this cohort that was described by Inskip et al.11 According to the SWS protocol, detailed measures were obtained of fetal development during conceptions and gestations that produced 3,000 live births, of birth phenotypes, and of outcomes in infancy and childhood. The ambitious goals of the SWS include evaluation of (1) influences of a mother’s diet, body composition, and endocrine profile on fetal growth, placental, and fetal adaptive responses, and (2) interactions of maternal and intrauterine factors with genes and postnatal environments of offspring that influence growth in infancy, pathways that lead to poor adult health, and risk factors for diseases in childhood (obesity, cardiorespiratory function, and asthma) and adulthood (coronary heart disease, type 2 diabetes, and osteoporosis).
An impressive list of publications from 2004 to 2009 is available from the SWS section of the MRC Epidemiology Resource Centre Web site (http://www.mrc.soton.ac.uk/index.asp?page=4). Two examples from this research program are described here to address methodological issues that are critical for the evaluation of some basic assumptions of the DOHaD approach (e.g., the tracking of nutrients and oxygen supply by the measurement of fetal blood flow12 and the characterization of infant size by measurement of body composition13). These are crucial measures in the theory of developmental plasticity or predictive adaptive response, which predicts that maternal diet may regulate blood flow to developing organs (i.e., to the brain versus the liver) and may elicit fetal programming that affects body composition at birth (fat mass versus lean mass). Haugen et al12 evaluated the effects of maternal adiposity and diet in 381 low-risk pregnancies in the SWS at 36 weeks of gestation using Doppler ultrasound to estimate blood flow in the umbilical cord and ductus venosus, which shunts well-oxygenated placental blood from the liver to the brain and heart. The low-risk group was selected to gain a better understanding of these factors in normal conditions rather than in extreme conditions in a high-risk group. Two independent effects were documented: the fetuses of women with low versus high central adiposity and operationally defined imprudent versus healthy diet had reduced ductus venosus shunting and increased liver blood flow. The observed fetal adaptations of cardiovascular responses to nutrient availability in this low-risk group suggested that these maternal characteristics were associated with liver-sparing response that “contrast with the brain-sparing response to fetal hypoxemia, which reduces hepatic flow and increases ductus venosus shunting” (p. 14). Harvey et al13 reported on parental determinants of neonatal body composition in 448 births in the SWS, with dual-energy x-ray absorptiometry scan assessment of fat and muscle mass components of body composition in the offspring within 2 weeks of birth. With this rigorous measurement of neonatal body composition, this study documented that total fat mass was related to maternal lifestyle factors (smoking and physical activity) as well as maternal height, parity, and triceps skinfold thickness. One conclusion was that if these influences on fat mass have persisting effects, this information could point the way to early life interventions that may prevent later obesity. These examples of rigorous and prospective early evaluations in the SWS protocol show how modern methods can be used to assess fetal adaptations and their effects on structures and functions that may alter body composition (rather than just weight) at birth. This may provide improved estimates of the underlying DOHaD-related factors that contribute to risk for common adult disorders (e.g., obesity) later in life.
Collaboration between centers (see www.liggins.auckland.ac.nz/uoa/affiliations) directed by the first two chairs of the DOHaD Society (Peter Gluckman from the Liggins Institute in Auckland and Mark Hanson from the MRC Epidemiology Resource Centre at the University of Southampton) led to the next generation of theory based on the concept of predictive adaptive response (see Gluckman and Hanson14). Applications of the predictive adaptive response concept, presented and discussed in additional detail in a book by Gluckman and Hanson,15 suggest that the fetus forecasts the future by sensing the current environment in utero and develops adaptively to match capabilities with expected demands. Gluckman et al16 emphasized one of the premises of this hypothesis that the association of outcomes with birthweight is an epiphenomenon of the relationship between nutrient availability to the fetus and the predictive adaptive responses that this elicits. Gluckman et al17 extended the concept of developmental plasticity by emphasizing that fetal programming may operate across the range from undernutrition to over-nutrition, with a U-shaped curve relating prenatal nutrition to adult metabolic disease.
Project VIVA: Background and Current Status
Gillman18 noted limitations of undernutrition and low birthweight as markers of prenatal etiological pathways related to postnatal health outcomes. This led to consideration of fetal, infant, and child body composition as a phenotype and abnormalities anywhere in the maternal-fetal supply line of nutrients as a common final pathway that may alter body composition. Oken and Gillman19 addressed the fetal origins of obesity and noted two different relationships with birthweight: a direct relationship held for birthweight with BMI in childhood and adulthood, but an inverse relationship held for low birth-weight with central adiposity, insulin resistance, and the metabolic syndrome. Gillman et al20,21 used the life course approach to focus investigations on general in utero conditions and placental function and physiological processes regulating fetal development across a broad range of birth sizes, rather than on abnormal or pathological processes at one extreme.
Project VIVA was initiated in 1999 in eight offices of Harvard Vanguard Medical Associates, a large multi-specialty group practice in Massachusetts. Its initial goals were to identify women early in pregnancy and to enter them into a protocol for prospective assessments twice during pregnancy, within 3 days of birth, and at 6 months, and at 1, 2 and 3 years of age.20 In 2006 it was extended to conduct follow-up through 7 years of age. The Web site for Project VIVA lists an impressive set of publications (see www.dacp.org/viva/publications.htm), describing the initial evaluations of factors associated with blood pressure of the newborn, including maternal age20 and maternal prenatal smoking,22 and factors related to obesity at 3 years of age, including gestational weight gain23 and weight in the first 6 months of life.24 One example will be described in detail here that is particularly relevant to recent extensions of the DOHaD approach to consider more than birthweight as a predictor of early adaptations that are permanent and affect later outcomes by programming. Taveras et al24 evaluated 559 children in Project VIVA to determine association of weight early in life (weight-for-length at birth and 6 months of age) with obesity (BMI >95th percentile) at 3 years of age. Weight early in life was directly associated with higher BMI at 3 years of age, but the association was larger for standardized weight at 3 years of age than for birth-weight. This suggest that increases in weight in the first 6 months of life may produce additional programming effects that increased risk for obesity in early childhood and thus may influence risk for later obesity more than birthweight alone that is assumed to reflect fetal programming.
Behavioral Perinatology Research Program: Background and Current Status
An early extension of the DOHaD approach was to go beyond nutrition hypotheses and to relate fetal development to exposure to other factors such as prenatal maternal stress and maternal-placental-fetal biological mediators of stress. Wadhwa25 reviewed how psychoneuroendocrine processes in human pregnancy influence fetal development and health. Drake et al26 reviewed the fetal glucocorticoid overexposure hypothesis as an alternative to the fetal undernutrition hypothesis to account for the relationship of the prenatal environment to adult disorders related to cardiovascular, metabolic, neuroendocrine, and behavioral phenotypes.
A multi-investigator research program at the University of California, Irvine, was initiated in 1993, and Wadhwa25 reviewed the contributions of this program over the initial 12 years, which generated extensive information on the short-term effects of exposure to maternal psychosocial stress during pregnancy. This focused research documented that length of gestation and fetal growth are mediated, in part, by maternal-placental-fetal stress physiology, particularly placental corticotrophin-releasing hormone. Because simple birth phenotypes such as birthweight may represent a crude marker of intrauterine conditions that are likely to exert a causal role, this research program was recently extended based on the DOHaD approach to evaluate healthy young adults born with a normal birth-size phenotype (i.e., no low birthweight or preterm birth) but exposed during intrauterine life to maternal psychosocial stress, defined by a major stressful life event during pregnancy. Entringer et al27–29 showed that compared with healthy young individuals without this history, these prenatal stress-exposed individuals exhibited primary insulin resistance and a lipid profile consistent with the metabolic syndrome,27 altered immune function,28 altered endocrine function,29 and compromised cognitive function.30 These findings suggest that in utero exposure to maternal stress may have long-term negative physiological consequences and may directly influence adult health even in the absence of adverse birth phenotypes such as low birthweight. Also directed by the DOHaD approach, Buss et al31 evaluated brain morphology in young adults and revealed an association between birthweight and postnatal environment: Lower birth-weight was associated with smaller hippocampal volume (a well-established risk factor for depression and psychopathology) only in individuals exposed to postnatal adversity (low levels of parental bonding). Swanson and Wadhwa32 have suggested that the DO-HaD approach also may be relevant to the origins of some child mental health disorders.
EPIGENETIC PROCESSES AND THE DOHaD APPOACH
In a review of the emerging science of epigenomics, Callinan and Feinberg33 defined epigenetics as “the study of heritable changes other than those in the DNA sequence that encompass two major modifications of DNA or chromatin: DNA methylation, the covalent modification of cytosine, and post-translational modification of histones including methylation, acetylation, phosphorylation and sumoylation” (p. R95). They provide an excellent description of the potentially unique contributions of epigenetics, a glossary of terms, and a thorough description of methods used to detect genome-wide variation in DNA methylation and chromatin modification.
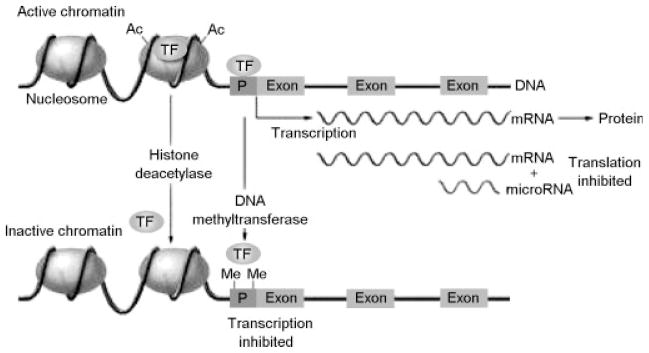
Outstanding reviews were published in 2007 that provide background on how the principles and concepts of epigenetics have been applied to the DOHaD approach.34–36 If some of the mechanisms for developmental plasticity described in the DOHaD approach are epigenetic, and disease-related outcomes are related to disruptions of epigenetic processes elicited by the fetal environment, then this emerging field may provide explanatory mechanisms that underlie some of the enduring effects of adverse fetal, infant, and childhood environments. In their review of the DOHaD approach, Gluckman et al17 provided an excellent summary of epigenetic modification of histones or of DNA itself in a figure (p. 66) that we reproduce here (Fig. 1). This figure summarizes the important epigenetic processes of DNA methylation and histone acetylation and methylation that have been discussed and reviewed elsewhere31–34 in detail and thus are not repeated here.
Figure 1.
Regulation of gene expression through epigenetic processes. Epigenetic modification of histones or of DNA itself controls access of transcription factors (TFs) to the DNA sequence, thereby modulating the rate of transcription to messenger RNA (mRNA). Transcriptionally active chromatin (top) characterized by the presence of acetyl groups (Ac) on specific lysine residues of core histones in the nucleosome, which decreases their binding to DNA and results in a more open chromatin structure that permits access of transcription factors. In addition, cytidine-guanosine (CpG) sequences in the promoter regions (P) of actively transcribed genes are generally unmethylated, allowing for the binding of transcription factors. Transcriptionally inactive chromatin (bottom) is characterized by histone deacetylation, promoter CpG methylation (as indicated by methyl groups [Me]), and decreased binding of transcription factors. (For simplicity, other histone modifications [such as methylation] and additional regulatory factors [such as methyl-CpG binding proteins] are not shown.) A further level of epigenetic control is provided by microRNA molecules (19 to 22 nucleotides in length), which bind to complementary sequences in the 3′ end of mRNA and reduce the rate of protein synthesis. From Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359(1):61–73.
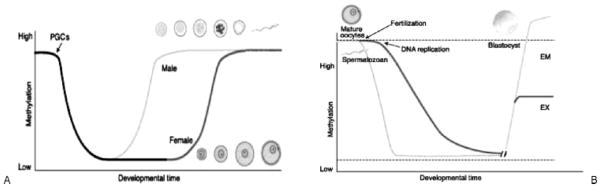
Two types of genes are modified epigenetically (i.e., are epigenetically liable): imprinted genes and genes with metastable epialleles. Imprinted genes are those in which specifically either the maternally derived or the paternally derived allele is suppressed, thereby rendering them functionally haploid (i.e., with parent-of-origin monoallelic expression). In other nonimprinted genes, one or both alleles are regulated epigenetically, and these metastable epialleles result in varying levels of gene expression. The modification of DNA from conception forward occurs to establish the epigenetic influence of gene expression, which is clearly presented in an article by Reik et al37 that is often cited in reviews of imprinting. This article provided a figure outlining the processes of methylation reprogramming in the germ line and in preimplantation embryos that is reproduced here (Fig. 2). As Leudi et al38 discuss, many undiscovered genes are predicted to be imprinted, and this set of genes is likely to have special importance for reproductive medicine and fetal growth. They speculate about the evolutionary benefits of imprinted genes, which likely are the product of positive Darwinian selection despite potential drawbacks associated with a haploid gene compared with a diploid gene that has a backup copy that may protect from “single-hit” effects of DNA damage. Waterland and Michels35 pointed out that in the DOHaD field “direct evidence of an involvement of epigenetic dysregulation in human cardiovascular disease, type 2 diabetes, and obesity is scant” compared with the field of cancer. They noted that this may be due to temporal and tissue specificity of the relevant tissue that may have epigenetic dysregulation associated diseases that have been the focus of the DOHaD approach, and they concluded that any disease with a genetic basis is also likely to have an epigenetic basis, but the “tissue-specificity of epigenetic regulation (and dysregulation) will be the major obstacle to epigenetic epidemiology of DOHaD” (p. 379). However, as pointed out by Gluckman and Hanson,15 there is a strong epigenetic basis for the DOHaD model of disease pathogenesis based on animal studies, which (for example) show that minor alterations in the maternal diet during pregnancy can produce lasting changes in the physiology and metabolism of offspring. We summarize here classic findings from two research programs that have provided evidence about possible epigenetic mechanisms involved in this type of phenotypic plasticity in the DOHaD approach. More detailed description and review was provided as background for a 2006 meeting on Genes, Environments and Human Development, Health and Disease.39
Figure 2.
(A) Methylation reprogramming in the germ line. Primordial germ cells (PGCs) in the mouse become demethylated early in development. Remethylation begins in prospermatogonia on E16 in male germ cells and after birth in growing oocytes. Some stages of germ cell development are shown (modified from 73). (B) Methylation reprogramming in preimplantation embryos. The paternal genome (blue) is demethylated by an active mechanism immediately after fertilization. The maternal genome (red) is demethylated by a passive mechanism that depends on DNA replication. Both are remethylated around the time of implantation to different extents in embryonic (EM) and extraembryonic (EX) lineages. Methylated-imprinted genes and some repeat sequences (dashed line) do not become demethylated. Unmethylated imprinted genes (dashed line) do not become methylated. From Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science 2001;293(5532):1089–1093.
The Agouti Mouse Model
A group at Duke University has used a mouse model to investigate the effects of maternal diet during pregnancy on the phenotype manifested in the offspring (www.geneimprint.com). The viable yellow agouti (Avy) mouse has a mutation that causes yellow hair pigmentation. Avy/a animals manifest a broad range of coat-color phenotypes from brown to mottled to yellow. Waterland and Jirtle40 investigated methyl supplementation of the diet of mothers during pregnancy and showed when a standard diet is supplemented by methyl donors, methylation of the Avy gene increases and the coat-color distribution shifts toward the brown phenotype. Dolinoy et al41 extended this work by investigation of effects of soy-rich diets before and during pregnancy in a/a females. The distribution of coat color in Avy offspring was shifted toward brown, and adult weight of the yellow phenotype was ~50% higher than in the brown phenotype. This demonstrated that for the Avy/a genotype of the viable yellow agouti mouse model, a high-soy diet results in epigenetic changes (increased methylation of CpG during fetal development), affects coat color, and also reduces obesity.
The Rat Model of Nurturing
A group at McGill University has used a rodent model to investigate epigenetic effects of maternal care. In rats, an important component of maternal care consists of licking and grooming, which varies widely across individuals. Meaney and Szyf42 showed that increased licking and grooming increased hippocampal expression of the glucocorticoid receptor (GR) mRNA and protein, decreased hypothalamic corticotrophin-release factor, and reduced hypothalamic-pituitary-adrenal response to stress. This work showed a direct relationship between maternal behavior and DNA methylation in the rat hippocampal GR gene (specifically in the exon 17 promoter). Weaver et al43 demonstrated further that the stress responses in adult rats that are programmed early in life by maternal care can be reversed by central infusion of methionine (a methyl donor), suggesting that the inherently stable epigenomic marks established by behavioral programming at a critical period early in life are potentially reversible later in life. This provides a biological basis for speculations about the effects of poverty on early experience, and how exposure to abuse, family strife, emotional neglect, and harsh discipline may have epigenetic effects that produce individual differences in neural and endocrine response to stress and may increase the susceptibility to common adult disorders such as depression and anxiety, drug abuse, and diabetes, heart disease, and obesity.
Recent Epigenetic Studies in Primates
Aaggard-Tillery et al44 used a nonhuman primate model to investigate the effects of maternal diet on alternations to the epigenome that may be related to obesity. A high-fat diet (35% fat) was established that produced obesity in pregnant monkeys. In comparison with control animals with a control diet (13% fat), the offspring of the obese monkeys were obese. In the first stage of investigation, Aagaard-Tillery et al44 identified an epigenetic change in the liver of these offspring (hyperacetylation of fetal hepatic tissue) that was associated with the high-fat diet and the resulting obesity. In the second stage, they altered the fat content of the monkeys’ diets during pregnancy. Even though obesity was maintained, the epigenetic changes in offspring were no longer present. This finding suggests that obesity may, in part, be due to the effects of maternal diet rather than maternal obesity on the fetal environment. This study in primates is important because it showed “in utero exposure (caloric-dense high-fat maternal diet) induces site-specific alterations in fetal hepatic H3 acetylation” and that this leads “to epigenetically altered fetal chromatin structure in primates via covalent modifications of histones and hence lends a molecular basis to the fetal origins of adult disease hypothesis” (p. 91).
Tyckol45 addressed the special nature of imprinted genes in placental growth. The placenta is the principal metabolic, respiratory, excretory, and endocrine organ of the fetus, which has substantial molecular variation across the fetal and maternal compartment (see Sood et al46) and affects birthweight even after adjustment for placental weight (see Salafia et al47). There are ~30 known imprinted genes, and a large percentage are expressed in trophoblasts and regulate placental growth.48 In this review, placental phenotypes controlled by imprinted genes were discussed, as well as the role of oppositely imprinted genes (e.g., Igf2 and Igf2r) related to the evolutionary theory of genetic conflict described by Haig49 that proposes different maternal and paternal self-interest in fetal growth. Some genes expressed in the placenta normally are maternally silenced/paternally expressed genes that promote growth (e.g., MEST) and others normally are maternally expressed/paternally silenced that limit growth (e.g., PHLDA2). In a study of intrauterine growth restriction (IUGR) and non-IUGR placenta, an altered expression of imprinted genes in placental response to maternal vascular underperfusion was investigated by McMinn et al.50 In this study, IUGR was characterized by increased expression of PHLDA2 and decreased expression of MEST in placenta tissue. This suggested unbalanced expression of these two oppositely imprinted genes was one component of the adaptive response of placental tissue to chronic maternal vascular underperfusion associated with IUGR, which provides some support for the conflict hypothesis. New methods are emerging for assessing epigenetic marks, such as the MSNP approach for determining allele-specific methylation and allele-specific expression patterns that may be dependent or independent of genome sequence (see Kerkel et al51). In the study of IUGR, there was no evidence of altered DNA methylation in imprinting centers of the PHLDA2 and MEST genes, which led McNinn et al50 to conclude that “the high PHLDA2/MEST mRNA ratios in this subset of IUGR may reflect altered DNA methylation in as yet uncharacterized cis-acting regulatory sequences, but more likely reflects conventional transcriptional dysregulation by transacting factors in placental cytotrophoblasts” (p. 543).
RELEVANCE OF EPIGENETIC AND THE DOHaD HYPOTHESIS TO REPRODUCTIVE MEDICINE
Niemtz and Feinberg52 provide an example of early epigenetic research in reproductive medicine.52 They investigated a possible a link between assisted reproductive therapy (ART) and Beckwith-Wiedemann syndrome (BWS).53 This syndrome is characterized by genetic heterogeneity, but about half the cases are associated with loss of imprinting in genes related to growth (e.g., the LIT1 gene located on the tip of chromosome 11). Niemitz and Feinberg52 suggested that “epigenetic alterations could arise from some aspect of ART” (e.g., in the in vitro culture itself or the media used) or that “epigenetic alteration could be a significant cause for infertility, rather than a consequence of the procedures used to treat it” (p. 605). Chang et al54 tested that hypothesis that culture media would be implicated as a common factor among children with BWS conceived after ART, but in a small sample of 19 they reported that in vitro fertilization (IVF) was a common factor but otherwise “no common factor was identified among reproductive endocrine records,” and they concluded “larger prospective studies are needed to systematically assess the potential risk factors associated with BWS and ART” (p. 353).
A summary of recent studies relevant to reproductive medicine is facilitated by two recent reviews in the series of Seminars in Reproductive Medicine,55,56 which provide detailed background for the application of the DOHaD approach to reproductive medicine and epigenetic processes (Fig. 1) that may operate at the time of conception (Fig. 2).
Rinaudo and Lamb55 summarized the literature on in utero stress related to deficient maternal-placental nutrient supply and some adverse childhood and adult outcomes (i.e., cardiovascular disease, hypertension, diabetes, and dysregulation of the hypothalamic-pituitary-adrenal axis) associated with stressful fetal environments during the postimplantation period of fetal growth. They also provided a brief review of perinatal morbidity associated with stress during the preimplantation period related to in vitro culture in assisted reproduction. They concluded that the evidence linking stress in utero to risk for adult disorders is convincing, and that the preimplantation embryo development is particularly sensitive to epigenetic regulation and dysregulation.
Kalra and Molinaro56 summarized the literature on association of in vitro fertilization with perinatal morbidity and the risk for congenital abnormalities, preterm birth, low birthweight, and other pregnancy-related complications. They concluded that children “conceived after IVF do seem to be at an increased risk for congenital and chromosomal anomalies compared with that of natural conceptions, particularly if ICSI [intracytoplasmic sperm injection] is used” (p. 432), but that effects have not yet been determined for long-term outcomes related to the DOHaD approach and physical, emotional, or cognitive development.
Other reviews of reproductive outcomes after IVF57 and ICSI58 also are available and add to the growing recognition that alteration of biochemical and biophysical conditions at conception and during early embryonic life associated with ARTs may result in changes in epigenetic processes and produce short- and long-term effects on development and health.
NEXT STEPS: LARGE HUMAN COHORT STUDIES
There are many recent developments in the field of epigenetics. The NIH Roadmap program on the Epigenomics of Human Health and Disease (www.nih.gov) was initiated to accelerate new developments, including the characterization of reference epigenomes in non-disease states and the perturbation of these epigenomes in disease states that may be temporally and tissue specific. Recent workshops (Epigenomics of Addiction) and conferences (Epigenomics of Human Health and Disease) have summarized these new developments in the understanding of mechanisms and methods that are being applied in the current investigations. The basic processes that describe how cells with the same molecular instructions (the DNA code) become differentiated during development through differential expression of genes have been reviewed in detail, with information available on the NIH Web site (see www.nida.nih.gov).
The next phase of research to address epigenetic mechanisms in humans would benefit substantially from large birth cohort studies with prospective measures of broad domains of exposures and outcomes.35,59 There are good examples in the literature. Recently, the Avon Longitudinal Study of Parents and Children (ALSPAC) study,60 the Danish National Birth Cohort,61 and the SWS11 have taken initial steps to identify critical developmental processes that underlie fetal growth and development13,62,63 and later outcomes in childhood related to the overnutrition hypothesis,64 preterm birth,61 and body composition.13
A prospective birth cohort, the National Children’s Study (NCS), is now underway in the United States. The NCS will recruit a nationally representative birth cohort at 105 sites with ~100,000 children. The recruitment will start with randomly designated neighborhoods and a survey of households within them to identify ~750,000 women between the 18 and 40 years of age who are not pregnant or within the first trimester of pregnancy. These women will be evaluated and followed until ~1000 births occur at each of the 105 locations (for an expected total of ~105,000). Broad exposures and outcome domains will be assessed at multiple times across stages of development (before conception, during pregnancy, and at birth; in infancy, childhood, adolescence; and into adulthood). A recent summary of the background, controversies, and status of the NCS was provided by Landrigan et al.65 The NCS will provide a prospective evaluation of a large birth cohort with early and frequent direct observation of the same individuals over time, which will provide rich information about health and disease that will be stored in clinical databases as well as biological and environmental samples that will be stored in biospecimen repositories. This information will be used initially to evaluate 29 “priority” hypotheses that were developed during the early consensus-development phase of the project in 2002 and then updated by the Vanguard Centers of the NCS in 2007 (see www.nationalchildrensstudy.gov). However, with such a large and representative birth cohort characterized by broad exposure and outcome domains, many more hypotheses will be tested and will emerge as the NCS progresses.
Although the NCS does not provide specific funding for genetic or epigenetic analyses, it does have a goal to provide the NCS clinical databases and biospecimen repositories for use in approved and separately funded adjunct studies designed to take advantage of the extensive infrastructure of the NCS. Swanson and Wadhwa32 presented and discussed three critical issues that highlight the extraordinary value of the birth cohort design used by the NCS, and here we note some significant issues and problems associated with each one:
If the nature of the combined effect of multiple genetic and environmental risk factors on complex common disorders is nonadditive, as assumed by the DOHaD approach, then the best way to elucidate these interactive effects is to assess the various risk factors simultaneously in the same cohort rather than in separate cohorts as proposed by Willet et al.66 However, the cost and subject burden required to conduct a birth cohort study are limiting factors that are now being considered in the NCS.67
If vulnerability to a particular risk factor or disease phenotype is determined not only by the genome acquired at conception but by the nature of its interplay with the environments during successive critical periods of development, a key feature of the DOHaD approach, then a longitudinal assessment of the environment from before conception through pregnancy, fetal life, birth, and infancy may be necessary, instead of relying on assessment of the environment in adult life as proposed by Collins and Manolio,68 to better understand disease susceptibilities that may have fetal or developmental origins. However, the investment in time, the retention of the sample, and the continuity of funding are issues that are facing the NCS.69
If the interplay between genes and environment is characterized by dynamic modifications to the genome, as assumed by the DOHaD approach, then not only the stable DNA sequence but also the epigenetic modifications to nuclear DNA and chromatin structure must be investigated serially during critical periods in early development over time and under various environmental conditions to assess gene–environment interactions adequately. However, the issues of temporal and tissue specificity must be addressed.35 The need for multiple collections of environmental exposures and biological specimens and the limitation inherent in human studies of the availability of many specific tissue types create truly daunting tasks that must be addressed before the full study can be initiated.70 The Vanguard Centers (7 of the 105 locations) are now conducting a field test of the NCS draft protocol that is intended to provide an empirical basis for decisions about feasibility and costs, which will be used to establish the final protocol for implementation in all 105 locations.71
The NCS provides an extraordinary opportunity to develop genetic and epigenetic projects and make use of the DOHaD approach to address specific mechanisms related to common disorders (e.g., obesity72) that are increasing by epidemic proportions around the world and define critical issues for consideration in the area of reproductive medicine.
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 5.Gillman MW, Barker D, Bier D, et al. Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD) Pediatr Res. 2007;61(5 Pt 1):625–629. doi: 10.1203/pdr.0b013e3180459fcd. [DOI] [PubMed] [Google Scholar]
- 6.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 7.Bateson P, Barker D, Clutton-Brock T, et al. Developmental plasticity and human health. Nature. 2004;430(6998):419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 8.Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185(1–2):93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ, Forsén T, Uutela A, Osmond C, Eriksson JG. Size at birth and resilience to effects of poor living conditions in adult life: longitudinal study. BMJ. 2001;323(7324):1273–1276. doi: 10.1136/bmj.323.7324.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D. Size at birth, childhood growth and obesity in adult life. Int J Obes Relat Metab Disord. 2001;25(5):735–740. doi: 10.1038/sj.ijo.0801602. [DOI] [PubMed] [Google Scholar]
- 11.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C SWS Study Group. Cohort profile: the Southampton Women’s Survey. Int J Epidemiol. 2006;35(1):42–48. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haugen G, Hanson M, Kiserud T, Crozier S, Inskip H, Godfrey KM. Fetal liver-sparing cardiovascular adaptations linked to mother’s slimness and diet. Circ Res. 2005;96(1):12–14. doi: 10.1161/01.RES.0000152391.45273.A2. [DOI] [PubMed] [Google Scholar]
- 13.Harvey NC, Poole JR, Javaid MK, et al. SWS Study Group. Parental determinants of neonatal body composition. J Clin Endocrinol Metab. 2007;92(2):523–526. doi: 10.1210/jc.2006-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 15.Gluckman P, Hanson M. Developmental Origins of Health and Disease. Cambridge, United Kingdom: Cambridge University Press; 2006. [Google Scholar]
- 16.Gluckman PD, Lillycrop KA, Vickers MH, et al. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci U S A. 2007;104(31):12796–12800. doi: 10.1073/pnas.0705667104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillman MW. Epidemiological challenges in studying the fetal origins of adult chronic disease. Int J Epidemiol. 2002;31(2):294–299. [PubMed] [Google Scholar]
- 19.Oken E, Gillman MW. Fetal origins of obesity. Obes Res. 2003;11(4):496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- 20.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144(2):240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 21.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353(17):1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oken E, Huh SY, Taveras EM, Rich-Edwards JW, Gillman MW. Associations of maternal prenatal smoking with child adiposity and blood pressure. Obes Res. 2005;13(11):2021–2028. doi: 10.1038/oby.2005.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken E, Taveras EM, Kleinman KP, Rich-Edwards JW, Gillman MW. Gestational weight gain and child adiposity at age 3 years. Am J Obstet Gynecol. 2007;196(4):322.e1–8. doi: 10.1016/j.ajog.2006.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taveras EM, Rifas-Shiman SL, Belfort MB, Kleinman KP, Oken E, Gillman MW. Weight status in the first 6 months of life and obesity at 3 years of age. Pediatrics. 2009;123(4):1177–1183. doi: 10.1542/peds.2008-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30(8):724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci (Lond) 2007;113(5):219–232. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- 27.Entringer S, Wust S, Kumsta R, et al. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008;498(5):498.e1–7. doi: 10.1016/j.ajog.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Entringer S, Kumsta R, Nelson EL, Hellhammer DH, Wadhwa PD, Wüst S. Influence of prenatal psychosocial stress on cytokine production in adult women. Dev Psychobiol. 2008;50(6):579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Entringer S, Kumsta R, Hellhammer DH, Wadhwa PD, Wüst S. Prenatal exposure to maternal psychosocial stress and HPA axis regulation in young adults. Horm Behav. 2009;55(2):292–298. doi: 10.1016/j.yhbeh.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Entringer S, Buss C, Kumsta R, et al. Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behav Neurosci. 2009;123(4):886–893. doi: 10.1037/a0016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buss C, Lord C, Wadiwalla M, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27(10):2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanson JD, Wadhwa PM. Developmental origins of child mental health disorders. J Child Psychol Psychiatry. 2008;49(10):1009–1019. doi: 10.1111/j.1469-7610.2008.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callinan PA, Feinberg AP. The emerging science of epigenomics. Hum Mol Genet. 2006;15(Spec No 1):R95–101. doi: 10.1093/hmg/ddl095. [DOI] [PubMed] [Google Scholar]
- 34.Gluckman PD, Hanson MA, Beedle AS. Non-genomic transgenerational inheritance of disease risk. Bioessays. 2007;29(2):145–154. doi: 10.1002/bies.20522. [DOI] [PubMed] [Google Scholar]
- 35.Waterland RA, Michels KB. Epigenetic epidemiology of the developmental origins hypothesis. Annu Rev Nutr. 2007;27:363–388. doi: 10.1146/annurev.nutr.27.061406.093705. [DOI] [PubMed] [Google Scholar]
- 36.Junien C, Nathanielsz P. Report on the IASO Stock Conference 2006: early and lifelong environmental epigenomic programming of metabolic syndrome, obesity and type II diabetes. Obes Rev. 2007;8(6):487–502. doi: 10.1111/j.1467-789X.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- 37.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293(5532):1089–1093. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 38.Luedi PP, Dietrich FS, Weidman JR, Bosko JM, Jirtle RL, Hartemink AJ. Computational and experimental identification of novel human imprinted genes. Genome Res. 2007;17(12):1723–1730. doi: 10.1101/gr.6584707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swanson JM, Wadhwa PD. Genes, Environments and Human Development, Health and Disease (GEHDHD) meeting, Arnold and Mabel Beckman Center of the National Academy of Sciences; September 7–8, 2006; Irvine, CA. [Google Scholar]
- 40.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20(1):63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114(4):567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meaney MJ, Szyf M. Maternal care as a model for experience-dependent chromatin plasticity? Trends Neurosci. 2005;28(9):456–463. doi: 10.1016/j.tins.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Weaver IC, Champagne FA, Brown SE, et al. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25(47):11045–11054. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aagaard-Tillery KM, Grove K, Bishop J, et al. Developmental origins of disease and determinants of chromatin structure: maternal diet modifies the primate fetal epigenome. J Mol Endocrinol. 2008;41(2):91–102. doi: 10.1677/JME-08-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tycko B. Imprinted genes in placental growth and obstetric disorders. Cytogenet Genome Res. 2006;113(1–4):271–278. doi: 10.1159/000090842. [DOI] [PubMed] [Google Scholar]
- 46.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci U S A. 2006;103(14):5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salafia CM, Zhang J, Miller RK, et al. Placental growth patterns affect birth weight for given placental weight. Birth Defects Res A Clin Mol Teratol. 2007;79(4):281–288. doi: 10.1002/bdra.20345. [DOI] [PubMed] [Google Scholar]
- 48.Tycko B, Morison IM. Physiological functions of imprinted genes. J Cell Physiol. 2002;192(3):245–258. doi: 10.1002/jcp.10129. [DOI] [PubMed] [Google Scholar]
- 49.Haig D. Altercation of generations: genetic conflicts of pregnancy. Am J Reprod Immunol. 1996;35(3):226–232. doi: 10.1111/j.1600-0897.1996.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 50.McMinn J, Wei M, Schupf N, et al. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta. 2006;27(6–7):540–549. doi: 10.1016/j.placenta.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Kerkel K, Spadola A, Yuan E, et al. Genomic surveys by methylation-sensitive SNP analysis identify sequence-dependent allele-specific DNA methylation. Nat Genet. 2008;40(7):904–908. doi: 10.1038/ng.174. [DOI] [PubMed] [Google Scholar]
- 52.Niemitz EL, Feinberg AP. Epigenetics and assisted reproductive technology: a call for investigation. Am J Hum Genet. 2004;74(4):599–609. doi: 10.1086/382897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeBaun MR, Niemitz EL, Feinberg AP. Association of in vitro fertilization with Beckwith-Wiedemann syndrome and epigenetic alterations of LIT1 and H19. Am J Hum Genet. 2003;72(1):156–160. doi: 10.1086/346031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang AS, Moley KH, Wangler M, Feinberg AP, Debaun MR. Association between Beckwith-Wiedemann syndrome and assisted reproductive technology: a case series of 19 patients. Fertil Steril. 2005;83(2):349–354. doi: 10.1016/j.fertnstert.2004.07.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rinaudo PF, Lamb J. Fetal origins of perinatal morbidity and/or adult disease. Semin Reprod Med. 2008;26(5):436–445. doi: 10.1055/s-0028-1087109. [DOI] [PubMed] [Google Scholar]
- 56.Kalra SK, Molinaro TA. The association of in vitro fertilization and perinatal morbidity. Semin Reprod Med. 2008;26(5):423–435. doi: 10.1055/s-0028-1087108. [DOI] [PubMed] [Google Scholar]
- 57.Mukhopadhaya N, Arulkumaran S. Reproductive outcomes after in-vitro fertilization. Curr Opin Obstet Gynecol. 2007;19(2):113–119. doi: 10.1097/GCO.0b013e32807fb199. [DOI] [PubMed] [Google Scholar]
- 58.Palermo GD, Neri QV, Takeuchi T, Rosenwaks Z. ICSI: where we have been and where we are going. Semin Reprod Med. 2009;27(2):191–201. doi: 10.1055/s-0029-1202309. [DOI] [PubMed] [Google Scholar]
- 59.Jirtle RL, Randy L. Randy L. Jirtle, PhD: epigenetics a window on gene dysregulation, disease. Interview by Bridget M Kuehn. JAMA. 2008;299(11):1249–1250. doi: 10.1001/jama.299.11.1249. [DOI] [PubMed] [Google Scholar]
- 60.Golding J, Pembrey M, Jones R ALSPAC Study Team. ALSPAC—the Avon Longitudinal Study of Parents and Children. I. Study methodology. Paediatr Perinat Epidemiol. 2001;15(1):74–87. doi: 10.1046/j.1365-3016.2001.00325.x. [DOI] [PubMed] [Google Scholar]
- 61.Olsen J. The Danish National Birth Cohort—a data source for studying preterm birth. Acta Obstet Gynecol Scand. 2005;84(6):539–540. doi: 10.1111/j.0001-6349.2005.00784.x. [DOI] [PubMed] [Google Scholar]
- 62.Ong KK, Dunger DB. Thrifty genotypes and phenotypes in the pathogenesis of type 2 diabetes mellitus. J Pediatr Endocrinol Metab. 2000;13(suppl 6):1419–1424. doi: 10.1515/jpem-2000-s616. [DOI] [PubMed] [Google Scholar]
- 63.Olsen EM, Petersen J, Skovgaard AM, Thomsen BL, Jørgensen T, Weile B. The growth pattern of 0–1-year-old Danish children, when screened by public health nurses—the Copenhagen County Child Cohort 2000. Ann Hum Biol. 2005;32(3):297–315. doi: 10.1080/03014460500068360. [DOI] [PubMed] [Google Scholar]
- 64.Lawlor DA, Timpson NJ, Harbord RM, et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 2008;5(3):e33. doi: 10.1371/journal.pmed.0050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Landrigan PJ, Trasande L, Thorpe LE, et al. The National Children’s Study: a 21-year prospective study of 100,000 American children. Pediatrics. 2006;118(5):2173–2186. doi: 10.1542/peds.2006-0360. [DOI] [PubMed] [Google Scholar]
- 66.Willett WC, Blot WJ, Colditz GA, Folsom AR, Henderson BE, Stampfer MJ. Merging and emerging cohorts: not worth the wait. Nature. 2007;445(7125):257–258. doi: 10.1038/445257a. [DOI] [PubMed] [Google Scholar]
- 67.Wadman M. Congress probes NIH stimulus funds. Nature. 2009;458(7238):556. doi: 10.1038/458556a. [DOI] [PubMed] [Google Scholar]
- 68.Collins FS, Manolio TA. Merging and emerging cohorts: necessary but not sufficient. Nature. 2007;445(7125):259. doi: 10.1038/445259a. [DOI] [PubMed] [Google Scholar]
- 69.Alexander D. Memo to the National Children’s Study Steering Committee (March 23, 2009) [Accessed July 2, 2009];National Children’s Study—update. 2009 Available at: http://appropriations.house.gov/Witness_testimony/LHHS/Raynard_Kington_03_26_09.pdf.
- 70.National Academy of Sciences. The National Children’s Study Research Plan. Washington, DC: The National Academies Press; 2008. [Google Scholar]
- 71.Scheidt P, Dellarco M, Dearry A. [Accessed July 2, 2009];A major milestone for the National Children’s Study. 2009 doi: 10.1289/ehp.12416. Available at: http://www.nationalchildrensstudy.gov/newsevents/updatesevents/announcements/Pages/NCS-Editorial-Environmental-Health-Perspectives-Jan2009.pdf. [DOI] [PMC free article] [PubMed]
- 72.Trasande L, Cronk C, Durkin M, et al. Environment and obesity in the National Children’s Study. Environ Health Perspect. 2009;117(2):159–166. doi: 10.1289/ehp.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]