Abstract
To examine the role of transient receptor potential vanilloid type 4 (TRPV4) channels in development of salt sensitive hypertension, male Dahl salt-sensitive (DS) and -resistant (DR) rats were fed a low (LS) or high (HS) salt diet for 3 weeks. DS-HS but not DR-HS rats developed hypertension. 4α-phorbol 12, 13-didecanoate (4α-PDD, a selective TRPV4 activator, 2.5 mg/kg iv) decreased mean arterial pressure (MAP) in all groups with the greatest effects in DR-HS and the least in DS-HS rats (p<0.05). Depressor effects of 4α-PDD but not dihydrocapsaicin (DHC, a selective TRPV1 agonist, 30 μg/kg iv) were abolished by ruthenium red (RuR, a TRPV4 antagonist, 3mg/kg iv) in all groups. Blockade of TRPV4 with RuR increased MAP in DR-HS rats only (p<0.05). TRPV4 protein contents were decreased in the renal cortex, medulla, and dorsal root ganglia (DRG) in DS-HS compared to DS-LS rats, but increased in DRG and mesenteric arteries (MA) in DR-HS compared to DR-LS rats (p<0.05). MAP responses to blockade of small- and large-/intermediate-conductance Ca2+-activated K+ channels (Maxiκ channels) with apamin and charybdotoxin, respectively, were examined. Apamin (100 μg/kg) plus charybdotoxin (100 μg/kg) abolished 4α-PDD-induced hypotension in DR-LS, DR-HS, and DS-LS rats only. Thus, HS-induced enhancement of TRPV4 function and expression in sensory neurons and resistant vessels in DR rats may prevent salt-induced hypertension possibly via activation of Maxiκ channels given that blockade of TRPV4 elevates MAP. In contrast, HS-induced suppression of TRPV4 function and expression in sensory neurons and kidneys in DS rats may contribute to increased salt sensitivity.
Keywords: Dahl salt sensitive hypertension, transient receptor potential (TRP) channels, TRPV4, TRPV1, Ca2+-activated K+ channels
Introduction
As a major risk factor for cardiovascular disease, sodium plays an important role in the pathogenesis and therapy of hypertension that affects 25% to 35% of the world population older than 18 years. 1, 2 The increment in blood pressure driven by a salt load is characteristic of salt-sensitive hypertension, a condition affecting more than two thirds of individuals with essential hypertension who are older than 60 years.3 Various lines of evidence suggest black patients are more salt sensitive than whites, which may be due to a tendency to retain sodium in the kidney though complete explanation for the difference has yet to be developed.4 It has been proposed that the kidney and the central nervous system are the two major sites for salt sensing, but the underlying molecular mechanisms are largely unclear.5 As a genetic model of salt-sensitive hypertension mimicking that of humans, Dahl-salt sensitive (DS) hypertensive rats have been extensively used for the study of molecular mechanisms mediating increased salt sensitivity.
The transient receptor potential (TRP) vanilloid subtype 4 (V4), a member of the TRP family, is a non-selective cationic channel. TRPV4 can be activated by a wide variety of stimuli including thermal, physical, and chemical stimuli such as the synthetic agonists 4α-phorbol-12,13-didecanoate (4α-PDD) and GSK1016790A, the endocannabinoid anandamine, or the arachidonic acid metabolite epoxyeicosatrienoic acid (EET).6∼9 Indeed, broad expression of TRPV4 in various tissues including the heart, liver, lung, spleen, kidney, sympathetic ganglia, and dorsal root ganglia (DRG) and trigeminal ganglia suggests a polymodal role of TRPV4 in diverse cell functions.10,11 Specifically, TRPV4 expresses in neurons of the circumventricular organs including the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO) that sense and modulate osmotic pressure by feedback regulation.12 Furthermore, expression of TRPV4 in the rat kidney is restricted to water-impermeant nephon segment.13 All of the evidence support osmo-sensitive nature of TRPV4, indicating that TRPV4 may play a key role in the regulation of sodium and water homeostasis and that dysfunction of TRPV4 may contribute to the development of salt-sensitive hypertension.
We have shown that function and expression of TRPV1, a highly homologous channel of TRPV4, are impaired in DS rats, rendering these rats disadvantageous in terms of blood pressure regulation due to the weakening of the protective effect of TRPV1 in the face of salt load.14 Similarly as TRPV1, TRPV4 plays a compensatory role in preventing salt-induced increases in blood pressure in Wistar rats.15 However, it is unknown whether altered expression and function of TRPV4 constitute a potential molecular mechanism contributing to increased salt sensitivity in a genetically predisposed hypertensive model. Accordingly, the present study was designed to examine this possibility. Blood pressure responses to a selective TRPV4 agonist, 4α-PDD, or a TRPV4 antagonist, ruthenium red (RuR), were assessed with or without blockade of the small- and large-/intermediate-conductance Ca2+-activated K+ channels (Maxiκ channels) with apamin and charybdotoxin, respectively, in DS and Dahl-salt resistant (DR) rats fed a low (LS) or high (HS) salt diet. Previous reports indicate that RuR is an effective blocker of TRPV4 but not TRPV1 in rats,15 and Maxiκ channels are likely involved in TRPV4 action. 10,11 Differential expression and regulation of TRPV4 in the kidney, mesenteric resistance arteries (MA), and sensory neurons in response to low or high salt intake were also determined in DS and DR rats.
Methods
Preparation of animals and samples
All experiments were approved by the Institutional Animals Care and Use Committee. Experiments were performed using male DR and DS (Charles River laboratory, Wilmington, MA). All rats (5 weeks old) housed in the animal facility 1 week before the experiments were randomly assigned to a LS (0.15% of Na+ by weight, Harlan Teklad) or HS (4% of Na+ by weight; Harlan Teklad) diet for 3 weeks and grouped as DRLS, DRHS, DSLS, and DSHS. All rats drank water ad libitum throughout the experiment. Systolic blood pressure was measured by the use of the Blood Pressure Analysis System (Hatteras instruments, Cary, North Carolina) before dietary treatment and at the end of each week after dietary treatment.
At the end of the third week, subgroups from each of the 4 groups were euthanized by decapitation without subjecting to acute experiments. The cervical, thoracic, and lumbar DRG, MA), and renal cortex and medulla were dissected and collected for Western blot analysis and immunohistochemistry staining.
To serve as a hypertensive control group, Sprague-Dawley (SD, Charles River laboratories) rats were treated with deoxycorticosterone acetate (DOCA) and salt or vehicle for 3 weeks as described previously.14,16 Briefly, rats underwent uninephrectomy via a flank incision on the left hand side and a silicone rubber DOCA implant (200 mg/kg) was placed subcutaneously between the shoulder blades. DOCA-salt rats received 1.0% NaCl and 0.2% KCl in water to drink whereas control SD rats received tap water. Systolic blood pressure was measured, and DRG, MA, and the renal cortex and medulla dissected and collected for Western blot analysis as depicted above.
Surgical preparation
The rats were anesthetized with ketamine and xylazine (80 and 4 mg/kg, intraperitoneally, respectively) for implantation of vascular catheters or with pentobarbital (50 mg/kg, intraperitoneally) when the animals subjected to injection of dihydrocapsaicin (DHC, 30 μg/kg, iv). The left jugular vein and carotid artery were cannulated under anesthesia for administration of drugs or monitoring of mean arterial pressure (MAP) with a Statham 231D pressure transducer coupled to a Gould 2400s recorder (Gould Instruments), respectively. For the rats anesthetized with ketamine and xylazine, baseline MAP and its responses to various chemicals were obtained three hours after surgery with the rats fully awake and unrestrained. Each animal recovered in a separate cage.
MAP responses to TRPV4 activation with or without blockade of TRPV4 or Maxiκ channels
This protocol examined the effect of TRPV4 activation when TRPV4 was intact or blocked or when Maxiκ channels were blocked. Rats fed a NS or HS diet in DR or DS groups were subjected to intravenous administration of 2.5 mg/kg 4α- PDD, a selective TRPV4 agonist, alone or in combination with 3 mg/kg RuR, a TRPV4 antagonist, or with 100 μg/kg apamin (a blocker of small-conductance Ca2+-activated K+ channels) plus 100 μg/kg charybdotoxin (a blocker of large- and intermediate-conductance Ca2+-activated K+ channels). The doses of 4α- PDD, RuR, apamin, and charybdotoxin were chosen based on previous studies showing specific and effective activation or blockade of the corresponding targets.15, 17∼19 Baseline MAP and its responses to the aforementioned drugs were recorded. In the cases of administration of combined drugs, 4α- PDD was administered 20 and 30 minutes after intravenous injection of RuR or apamin plus charybdotoxin, respectively.
MAP responses to TRPV1 activation with or without blockade of TRPV4
To examine the effectiveness of RuR in blockade of TRPV4 but not TRPV1, rats were intravenously injected with 30 μg/kg DHC, a selective TRPV1 agonist, alone or in combination with 3 mg/kg RuR. The dose of DHC was chosen based on previous studies showing specific and effective activation of TRPV1.14,20 DHC was administered 20 minutes after intravenous injection of RuR. In light of the fact that DHC is an irritant and causes pain in conscious rats, this protocol was performed under anesthesia as described above.
MAP responses to TRPV4 blockade alone
To determine whether blockade of TRPV4 conveyed significant changes in MAP, rats were subjected to intravenous injection of RuR at 3 mg/kg. Baseline MAP and its response to RuR were obtained three hours after surgery with the rats fully awake and unrestrained.
Western blot analysis
Membrane proteins were extracted as described previously 14 and 20 μg proteins were used for Western blot analysis. The mesenteric arteries from 2 rats were pooled together and used as one sample. Western blot analysis was performed with the use of primary antibody targeted to TRPV4 (1:500, Alomone labs, Jerusalem, Israel) and secondary antibody conjugated with horseradish peroxidase (1:800, Santa Cruz Biotechnology). The membranes were developed using an ECL kit (Amersham Pharmacia Biotech) and exposed to films (Hyperfilm-ECL, Amersham Pharmacia Biotech). The films were scanned and analyzed with the use of the Image Quantity Program (Scion) to obtain integrated densitometric values. β-Actin was used to normalize protein loading on membranes.
Immunohistochemistry
Mesenteric resistance arteries were placed in liquid nitrogen and embedded in OTC compound. Freshly frozen samples were sectioned at 20 μm on a Leica CM1850 Cryostat (Leica Microsystems Inc, Deutschland), placed on 3-aminoalkyethoxysilane coated slides, fixed in acetone for 15 minutes, washed in saline, incubated in 0.1% Triton X-100 in PBS for 20 minutes, and then incubated in 5% sheep serum (Chemicon International, Temecula, CA) in PBS for 30 minutes. The sections were subsequently incubated in rabbit anti-rat TRPV4 (1:200, Alomone labs, Jersalem, Israel) for 1 h at room temperature, washing in PBS, and incubated in goat anti-rabbit Cy3 (1:50, Jackson ImmunoResearch) for 1 h at room temperature. The slides were viewed under Zeiss Pascal Confocal Laser Scanning Microscope using 543-nm laser. Negative controls from DRHS rats were performed by omission of primary antibodies, which showed no specific immunoreactivity.
Drugs
Both 4α- PDD (LC Laboratories) and DHC (Sigma) were dissolved using the same vehicle, i.e., ethanol (5% v/v), Tween-80 (5% v/v), and saline. RuR (Sigma), apamin (Sigma), charybdotoxin (Sigma) were dissolved in saline.
Statistical analysis
All values were expressed as means ± SE. Differences among groups were analyzed using one-way ANOVA followed by a Bonferroni's adjustment for multiple comparisons. Differences between 2 groups were analyzed by the use of the unpaired Student t test. Differences were considered statistically significant at P<0.05.
Results
Baseline MAP in DS and DR rats
Before dietary treatment, there was no significant difference in systolic blood pressure between DS and DR rats, and HS treatment significantly increased systolic blood pressure in DS rats compared to DR rats (Figure 1). The increase in systolic blood pressure was confirmed by elevated baseline MAP in conscious DSHS (164 ± 5 mmHg, P<0.05) compared to DSLS (107 ± 4 mmHg), DRLS (106 ± 3 mmHg), and DRHS (110 ± 4 mmHg) rats, consistent with the fact that blood pressure is sensitive to HS intake in DS rats.
Figure 1.
Time course of systolic blood pressure in conscious DR or DS rats measured by the tail-cuff method. Values are mean ± SE (n=5∼7). *P<0.05 compared with the corresponding DSLS group.
Effects of TRPV4 activation in the presence or absence of TRPV4 blockade
As a selective TRPV4 activator, 2.5 mg/kg 4α-PDD opened TRPV4 channels and decreased MAP in all four groups (Figure 2A∼D). The depressor effect of MAP began at 2∼3 min, reached the lowest points at 4∼7 min, and lasted for 15∼25 min after 4 α –PDD administration. The magnitude of the decreases in MAP induced by 4α-PDD was the biggest in DRHS (-39 ± 1 mmHg, P<0.05) rats and the shallowest in DSHS (-14 ± 2 mmHg, P<0.05) rats compared to DRLS (-30 ± 2 mmHg) and DSLS (-31 ± 2 mmHg) rats (Figure 2E), indicating that enhanced TRPV4 function occurs in DR rats fed a HS diet and that diminished function of TRPV4 takes place in DS rats fed a HS diet. Intravenous bolus pre-administration of 3 mg/kg RuR abolished 4α-PDD-induced hypotensive effects in all groups (DRLS -5 ± 3 mmHg; DRHS -6 ± 2 mmHg; DSLS -5 ± 2 mmHg; DSHS -4 ± 2 mmHg) (Figures 2A∼E), indicating that RuR is an effective antagonist of TRPV4 in all settings.
Figure 2.
Responses of MAP to bolus administration of 4α-PDD (2.5 mg/kg, iv) with or without TRPV4 blockade (RuR, 3 mg/kg, iv) in conscious DR or DS rats. A∼D, time course responses of MAP to bolus administration of 4α-PDD with or without RuR in conscious DR or DS rats. E, peak responses of MAP to bolus administration of 4α-PDD with or without RuR in conscious DR or DS rats. Values are mean ± SE (n=4∼7). *P<0.05 compared with the corresponding 4α-PDD-treated group. †P<0.05 compared with the corresponding DRLS group. ‡P<0.05 compared with the corresponding DSLS group.
Effects of TRPV1 activation in the presence or absence of TRPV4 blockade
DHC (30 μg/kg, i.v.), a selective TRPV1 agonist, evoked a triphasic MAP response and reached the lowest points 1∼3 min after its administration, consistent with the previous reports. 14,15 The degree of the decreases in MAP induced by DHC was greater in DRHS rats compared to DRLS, DSLS, and DSHS rats (Table 1). RuR (3 mg/kg, iv), weakly but significantly, attenuated the depressor effect of DHC in DRHS rats but not in DRLS, DSLS, or DSHS rats, indicating somewhat attenuated function of TRPV1 by RuR under certain conditions (Table 1).
Table 1.
Effects of Dihydrocapsaicin (DHC) on Mean Arterial Pressure (MAP) in the Presence or Absence of TRPV Blockade in Pentobarbital-Anesthetized DR or DS Rats Fed a LS or HS Diet for 3 Weeks.
| Treatment Groups | Change of MAP (mmHg) | |||
|---|---|---|---|---|
| DRLS | DRHS | DSLS | DSHS | |
| DHC | -28±3 | -39±3* | -28±4 | -27±1 |
| RuR+DHC | -21±2 | -31±1† | -22±1 | -23±3 |
Values are mean ± SE (n=4∼5).
P<0.05 compared with the corresponding DRLS group.
P<0.05 compared with the corresponding DHC-treated group.
Effects of blockade of TRPV4
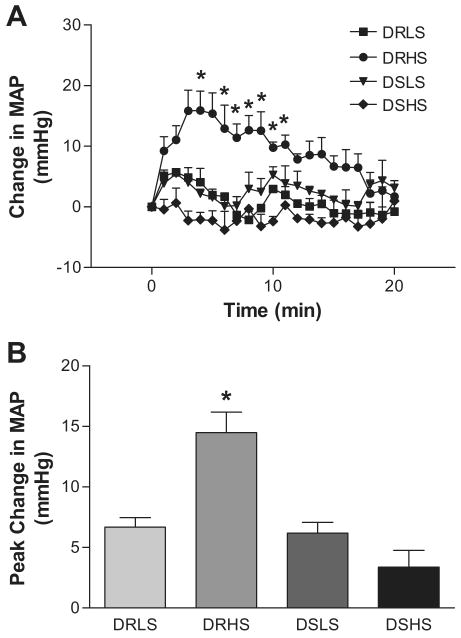
In response to intravenous bolus administration of 3 mg/kg RuR, MAP elevated immediately and reached the peak 2∼5 min after administration in all groups (Figure 3). The pressor effect of RuR lasted for 15∼20 min. Changes of MAP were bigger in DRHS (15 ± 2 mmHg, P<0.05) rats compared to other three groups (DRLS 7 ± 1 mmHg; DSLS 7 ± 1 mmHg; DSHS 4 ± 2 mmHg), indicating that TRPV4 plays a compensatory role in preventing salt-induced elevation of blood pressure in DR rats and that this counterbalancing effect of TRPV4 is impaired in DS rats.
Figure 3.
Time course responses (Panel A) and peak responses (Panel B) of MAP to bolus administration of RuR (3 mg/kg, iv) in conscious DR or DS rats. Values are mean ± SE (n=4∼8). *P<0.05 compared with the three other groups.
Expression and regulation of TRPV4 in the kidney, DRG, and MA
A clear single band representing TRPV4 protein was observed by western blot analysis. The data showed that HS treatment decreased TRPV4 expression in the kidneys of DS rats (renal cortex: DSLS 0.509 ± 0.035 versus DSHS 0.355 ± 0.020% of β-actin arbitrary, P<0.05; renal medulla: DSLS 0.341 ± 0.047 versus DSHS 0.114 ± 0.020% of β-actin arbitrary, P<0.05), but it had no effect in the kidneys of DR rats (renal cortex: DRLS 0.563 ± 0.034 versus DRHS 0.581 ± 0.024% of β-actin arbitrary, P>0.05; renal medulla: DRLS 0.441 ± 0.017 versus DRHS 0.451 ± 0.040% of β-actin arbitrary, P>0.05) (Figure 4). In DRG (Figure 5), HS treatment enhanced TRPV4 expression in DR rats (DRLS 0.284 ± 0.041 versus DRHS 0.673 ± 0.058% of β-actin arbitrary, P<0.05) but decreased TRPV4 expression in DS rats (DSLS 0.518 ± 0.012 versus DSHS 0.220 ± 0.046% of β-actin arbitrary, P<0.05). In MA (Figure 5), HS treatment enhanced TRPV4 expression in both DR (DRLS 0.108 ± 0.011 versus DRHS 0.173 ± 0.013% of β-actin arbitrary, P<0.05) and DS (DSLS 0.164 ± 0.022 versus DSHS 0.318 ± 0.043% of β-actin arbitrary, P<0.05) rats, a result confirmed by immunohistochemistry studies that revealed intense TRPV4 expression in the endothelial and adventitial layers of MA in DR and DS rats fed a HS diet (Figure 6). Thus, TRPV4 was differentially regulated in DR and DS rats by salt in such that salt-induced expression of TRPV4 in the kidney and DRG but not MA was impaired in DS rats. In contrast to DS rats, DOCA-salt treatment had no effect on TRPV4 expression in the kidney, DRG, or MA (Table 2) despite the fact that DOCA-salt-hypertensive rats had comparable systolic blood pressure as that of DS on a HS diet (DOCA-salt rats: 182 ± 9 mmHg versus vehicle control SD rats: 126 ± 6 mmHg, P<0.05). Theses results support the notions that salt-induced changes in TRPV4 expression in DS rats is specific and that these changes in DS rats are not the consequence but potentially the cause of elevated blood pressure.
Figure 4.

Western blot analysis of protein expression of TRPV4 in the renal cortex and medulla of DR or DS rats. Values are mean ± SE (n=4∼5). *P<0.05 compared with the corresponding LS-treated group.
Figure 5.

Western blot analysis of protein expression of TRPV4 in DRG and MA of DR or DS rats. Values are mean ± SE (n=4∼5). *P<0.05 compared with the corresponding LS-treated group.
Figure 6.

Confocal microscopic images of Cy3-labeled TRPV4 staining of mesenteric arteries in DR or DS rats fed a LS or HS diet. Negative controls were performed by omission of primary antibodies using DR rats fed a HS diet. Scale bars, 20μm
Table 2.
Western blot analysis of TRPV4 expression in the kidney, dorsal root ganglia (DRG) and mesenteric resistance arteries (MA) in Sprague-Dawley (SD) and DOCA-salt-hypertensive rats.
| Tissues Examined | %β-Actin Arbitrary Units | |
|---|---|---|
| SD | DOCA | |
| Renal Cortex | 0.741±0.035 | 0.894±0.075 |
| Renal Medulla | 0.401±0.040 | 0.417±0.046 |
| DRG | 0.433±0.056 | 0.385±0.023 |
| MA | 0.860±0.055 | 1.009±0.054 |
Values are mean ± SE (n=4∼5).
Effects of TRPV4 activation in the presence or absence of blockade of Maxiκ channels
Blockade of Maxiκ channels with combinational administration of apamin (100 μg/kg, iv) and charybdotoxin (100 μg/kg, iv) blocked 4α-PDD-induced hypotensive effects in all groups (DRLS -10± 1 mmHg; DRHS -13 ± 2 mmHg; DSLS -10 ± 2 mmHg, DSHS -7 ± 2 mmHg, and the 4α-PDD alone groups were the same as in Figures 2). These results indicate a key role of Maxiκ channels in TRPV4-mediated hypotensive effects (Figure 7).
Figure 7.
Responses of MAP to bolus administration of 4α-PDD (2.5 mg/kg, iv) with or without Apamin (100 μg/kg, iv) plus Charybdotoxin (100 μg/kg, iv) in conscious DR or DS rats. A∼D, time course responses of MAP to bolus administration of 4α-PDD with or without Apamin plus Charybdotoxin in conscious DR or DS rats. E, peak responses of MAP to bolus administration of 4α-PDD with or without apamin plus charybdotoxin in conscious DR or DS rats. Values are mean ± SE (n=4∼7). *P<0.05 compared with the corresponding 4α-PDD-treated group. †P<0.05 compared with the corresponding DRLS group. ‡P<0.05 compared with the corresponding DSLS group.
Discussion
This study was designed to test the hypothesis that impaired function and expression of TRPV4 channels occur in DS rats in the face of salt load, which contributes to the development of hypertensive in this genetically predisposed strain that mimics human salt-sensitive hypertension. Our data show (1) that activation of TRPV4 conveys a similar degree of depressor effects in DR and DS rats on a LS diet, and that the depressor effect of TRPV4 is enhanced in DR but diminished in DS rats in response to HS intake; (2) that baseline blood pressure is markedly elevated in DR but not DS rats fed a HS diet when TRPV4 is blocked; (3) that TRPV4 is differentially regulated in DR and DS rats by salt, i.e., HS intake upregulates or maintains a steady state of TRPV4 expression in DRG or the renal cortex/medulla, respectively, in DR rats, but downregulates TRPV4 expression in DRG and the renal cortex/medulla in DS rats; and (4) that blockade of the Maxiκ channels impedes the hypotensive effects induced by 4α-PDD in DR and DS rats fed a LS or HS diet. Taken together, these data show for the first time that TRPV4 function and expression are enhanced in response to HS intake in DR rats in such that blockade of TRPV4 leads to an increase in blood pressure, indicating that TRPV4 activation and up-regulation may constitute a counter-regulatory mechanism to prevent salt-induced increases in blood pressure in DR rats possibly via activation of the Maxiκ channels. In contrast, TRPV4 function and expression are suppressed in response to salt load in DS rats, which may serve as a potential mechanism underlying increased salt sensitivity of arterial pressure in DS rats.
While 4α-PDD is a potent hypotensive agent via its known effects on opening TRPV4 channels, it may also weakly activate TRPV1.21 However, the depressor effects of 4α-PDD are predominantly mediated by activation of TRPV4 but not TRPV1 at the dose used in the present study given that blockade of TRPV4 but not TRPV1 abolishes 4α-PDD-induced hypotensive effects. 15 TRPV4 has been showed to be extensively expressed in smooth muscle cells (SMC) and endothelial cells of blood vessels, and can be activated by various endogenous vasoactive agents including endocannabinoids, arachidonic acid (AA) and its metabolites EETs leading to vasodilation or vasoconstriction depending on specific vascular beds. 22∼24 Interestingly, activation of TRPV4 conveys a similar degree of depressor effects in DR and DS rats fed a LS diet, indicating that TRPV4 function is intact in DS rats without salt challenge. In contrast, TRPV4 function is altered in an opposite direction in DR and DS rats in response to salt load, i.e., the depressor effect of TRPV4 is enhanced in DR but diminished in DS rats in response to HS intake. These results may have at least the following implications. First, enhanced TRPV4 function in DR rats may be a compensatory response to HS intake to counteract salt-induced increases in blood pressure. On the other hand, diminished TRPV4 function occurs in DS rats in response to salt load, indicating that there may be a genetic predisposition of salt-induced impairment of TRPV4 contributing to increased salt sensitivity of arterial pressure in this strain.
Given the lack of highly specific TRPV4 antagonists, RuR has been used as a pharmacological tool to block TRPV4. The specific issue relevant to the present study is the fact that TRPV4 co-expresses with TRPV1, a known cardiovascular regulator.14,25 Thus, specificity of RuR was examined. Our results show that RuR abolishes the hypotensive effects induced by 4α-PDD in both DR and DS rats fed a NS or HS diet, but only weakly attenuates DHC-induced depressor effects in DR rats fed a HS diet. These results indicate that blockade of 4α-PDD-induced hypotension by RuR is mainly mediated by antagonizing TRPV4 instead of TRPV1, a result consistent with previous reports. 15
Direct examination of protein expression of TRPV4 reveals differential expression in DR and DS rats, which may underlie distinct functional responses of TRPV4 in DR and DS rats fed a HS diet. HS intake upregulates or maintains a steady state of TRPV4 expression in sensory neurons or the renal cortex/medulla, respectively, in DR rats, whereas HS intake downregulates TRPV4 expression in sensory neurons and the renal cortex/medulla in DS rats. Thus, it is conceivable that upregulated TRPV4 expression leads to robust depressor effects in response to 4α-PDD-induced activation of TRPV4 whereas blockade of TRPV4 with RuR elevates baseline MAP in DR rats fed a HS diet. In contrast, suppressed TRPV4 expression results in dim or lack of responses of blood pressure when TRPV4 is activated or blocked in DS rats on a HS diet. Interestingly, HS intake upregulates TRPV4 expression in mesenteric arteries especially in endothelial cells of these vessels in both DR and DS rats, indicating that HS-induced impairment in TRPV4 expression in DS rats is tissue-specific. Furthermore, while DOCA-salt-hypertensive rats have similar blood pressure as that of DS on a HS diet, TRPV4 expression in sensory neurons, kidneys, or mesenteric arteries is not altered by DOCA-salt treatment. Theses results indicate that salt-induced impairment in TRPV4 expression in DS rats is model specific and that abnormalities in TRPV4 expression may not be the consequence but potentially the cause of elevated blood pressure in DS rats. Moreover, the lack of elevated TRPV4 expression in DOCA salt rats may contribute, at least in part, to increased blood pressure given that compensatory upregulation of TRPV4 appears to prevent salt-induced increases in blood pressure in DR rats or Wistar rats. 15 Indeed, our previous studies in Wistar rats indicated that TRPV4 expression and activity could be altered as early as 7 days after high salt treatment (unpublished). Given that changes in TRPV4 expression and activity in DR rats fed a HS diet mimic that observed in Wistar rats fed a HS diet, 15 it is conceivable that the failure of such changes early on in DS rats fed a HS diet likely contributes to, rather than results from, increased blood pressure and associated end-organ damage.
To determine the potential downstream pathway(s) mediating TRPV4 action in DR and DS rats, Maxiκ channels function was examined. Vascular responses to TRPV4 activation are mainly mediated by endothelium-derived hyperpolarizing factors (EDHF).26∼28 EDHF opens Maxiκ channels, leading to hyperpolarization of endothelial cells and the subsequent vasodilation. We have recently studied the relation between TRPV4 and EDHF in Wistar rats by using inhibitors specific for three major endothelium-dependent pathways, including indomethacin, L-NA, and combinational administration of apamin and charybdotoxin, to block 4α-PDD-induced depressor effect.29 The results showed that administration of apamin plus charybdotoxin, but not indomethacin or L-NA, markedly attenuated TRPV4-mediated depressor effects. 29 Consistently, our current data show that blockade of Maxiκ channels with apamin plus charybdotoxin inhibits the hypotensive effect induced by TRPV4 activation in DR and DS rats, indicating that TRPV4-mediated depressor effects are largely endothelium- and Maxiκ channel-dependent. Endothelial dysfunction, known to occur in DS rats as well as other salt-dependent hypertensive models including DOCA-salt hypertensive rats, 30∼33 has been linked to impaired EDHF-induced vasodilatation.34∼36 EDHF-induced vasodilatation is particularly critical in resistance arteries, making EDHF a key determinant in controlling vascular resistance.37,38 Decreased generation of EDHFs has also been shown to contribute to impairment of endothelium-dependent vasodilation in hypertension.30 Thus, impaired endothelial function with disturbed EDHF release and/or action would lead to diminished function of Maxiκ and TRPV4 channels, which may contribute to the development of hypertension in DS rats in the face of salt intake.36,39
Taken together of previous results on TRPV1 14 with the data presented in the current study, there are similarities and differences in function and expression between TRPV1 and TRPV4 in DR and DS rats. HS intake upregulates TRPV1 in kidneys and mesenteric arteries and TRPV4 in sensory neurons and mesenteric arteries in DR rats, leading to augmented depressor effects when these channels are activated or elevated blood pressure when these channels are blocked. 14 On the other hand, HS intake suppresses TRPV1 in kidneys and mesenteric arteries and TRPV4 in kidneys and sensory neurons in DS rats, resulting in attenuated depressor effects when these channels are activated which may constitute an impaired compensatory mechanism in the face of salt load. 14 While TRPV1-mediated vasodilation appears to be largely CGRP-dependent, 14 TRPV4-induced hypotension is likely mediated by activation of Maxiκ channels.
Perspectives
With over half of hypertensives being salt sensitive,40 increased attention to strategies that target specifically in reducing salt sensitivity particularly in high risk individuals is urgently needed. It has been reported that salt intake restriction decreases systolic and diastolic blood pressure in both hypertensive and normotensive individuals with a bigger magnitude in the hypertensive group.41 However, the molecular mechanisms underlying salt-dependent regulation of blood pressure remain to be defined. Several powerful endocrine/paracrine/autocrine systems or factors have been implicated to be involved, which include but not limited to the renin-angiotension system, the endothelin system, transforming growth factor β, nuclear factor-κB, and TRPV1,41,42 The data from the present study provide further evidence that salt intake would enhance TRPV4 expression and function to counterbalance salt-induced elevation in blood pressure in a salt resistant strain of rats. On the other hand, loss of function of TRPV4, at least in part, occurs in a salt sensitive strain in response to salt load, which may be a potential molecular mechanism for increased salt sensitivity in this strain. It follows that protecting or enhancing TRPV4 expression and function may be therapeutic in treating salt-dependent hypertension.
Acknowledgments
Sources of Funding: This work was supported in part by National Institutes of Health (grants HL-57853, HL-73287, and DK67620) and a grant from Michigan Economic Development Corporation.
Footnotes
Disclosures: None.
References
- 1.Staessen JA, Wang J, Bianchi G, Birkenhäger WH. Essential hypertension. Lancet. 2003;361:1629–1641. doi: 10.1016/S0140-6736(03)13302-8. [DOI] [PubMed] [Google Scholar]
- 2.He FJ, MacGregor GA. Salt, blood pressure and cardiovascular disease. Curr Opin Cardiol. 2007;22:298–305. doi: 10.1097/HCO.0b013e32814f1d8c. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiological mechanisms of salt-dependent hypertension. Am J Kidney Dis. 2007;50:655–672. doi: 10.1053/j.ajkd.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 4.Lindhorst J, Alexander N, Blignaut J, Rayner B. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. 2007;18:241–247. [PMC free article] [PubMed] [Google Scholar]
- 5.Orlov SN, Mongin AA. Salt-sensing mechanisms in blood pressure regulation and hypertension. Am J Physiol Heart Circ Physiol. 2007;293:H2039–2053. doi: 10.1152/ajpheart.00325.2007. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 7.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 8.Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ES, Gordon E, Evans L, Misajet BA, Demarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, Sheardown SA, Xu X, Brooks DP, Laping NJ, Westfall TD. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: Part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- 9.Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther. 2008;326:443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- 10.Plant TD, Strotmann R. TRPV4. Handb Exp Pharmacol. 2007;179:189–205. doi: 10.1007/978-3-540-34891-7_11. [DOI] [PubMed] [Google Scholar]
- 11.Nilius B, Watanabe H, Vriens J. The TRPV4 channel: structure-function relationship and promiscuous gating behaviour. Pflugers Arch. 2003;446:298–303. doi: 10.1007/s00424-003-1028-9. [DOI] [PubMed] [Google Scholar]
- 12.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tian W, Salanova M, Xu H, Lindsley JN, Oyama TT, Anderson S, Bachmann S, Cohen DM. Renal expression of osmotically responsive cation channel TRPV4 is restricted to water-impermeant nephron segments. Am J Physiol Renal Physiol. 2004;287:F17–24. doi: 10.1152/ajprenal.00397.2003. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Wang DH. A novel mechanism contributing to development of Dahl salt-sensitive hypertension: role of the transient receptor potential vanilloid type 1. Hypertension. 2006;47:609–614. doi: 10.1161/01.HYP.0000197390.10412.c4. [DOI] [PubMed] [Google Scholar]
- 15.Gao F, Sui D, Garavito RM, Worden RM, Wang DH. Salt intake augments hypotensive effects of transient receptor potential vanilloid 4: functional significance and implication. Hypertension. 2009;53:228–235. doi: 10.1161/HYPERTENSIONAHA.108.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gavras H, Brunner HR, Laragh JH, Vaughan ED, Jr, Koss M, Cote LJ, Gavras I. Malignant hypertension resulting from deoxycorticosterone acetate and salt excess: role of renin and sodium in vascular changes. Circ Res. 1975;36:300–309. doi: 10.1161/01.res.36.2.300. [DOI] [PubMed] [Google Scholar]
- 17.Naida AM, Ghosh TK, Mathew OP. Airway protective reflexes elicited by laryngeal ammonia: role of C-fiber afferents. Respir Physiol. 1996;103:11–17. doi: 10.1016/0034-5687(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 18.Lin YS, Ho CY, Chang SY, Kou YR. Laryngeal C-fiber afferents are not involved in the apneic response to laryngeal wood smoke in anesthetized rats. Life Sci. 2000;66:1695–1704. doi: 10.1016/s0024-3205(00)00492-6. [DOI] [PubMed] [Google Scholar]
- 19.Kawabata A, Nakaya Y, Kuroda R, Wakisaka M, Masuko T, Nishikawa H, Kawai K. Involvement of EDHF in the hypotension and increased gastric mucosal blood flow caused by PAR-2 activation in rats. Br J Pharmacol. 2003;140:247–254. doi: 10.1038/sj.bjp.0705433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller MS, Brendel K, Buck SH, Burks TF. Dihydrocapsaicin-induced hypothermia and substance P depletion. Eur J Pharmacol. 1982;83:289–292. doi: 10.1016/0014-2999(82)90263-1. [DOI] [PubMed] [Google Scholar]
- 21.Bhave G, Hu HJ, Glauner KS, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW., 4th Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1) Proc Natl Acad Sci U S A. 2003;100:12480–12485. doi: 10.1073/pnas.2032100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich A, Chubanov V, Kalwa H, Rost BR, Gudermann T. Cation channels of the transient receptor potential superfamily: their role in physiological and pathophysiological processes of smooth muscle cells. Pharmacol Ther. 2006;112:744–760. doi: 10.1016/j.pharmthera.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Wang DH. Function and regulation of the vanilloid receptor in rats fed a high salt diet. J Hypertens. 2003;21:1525–1530. doi: 10.1097/00004872-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Köhler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- 27.Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation. 2008;117:1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- 28.Kotlikoff MI. EDHF redux: EETs, TRPV4, and Ca2+ sparks. Circ Res. 2005;97:1209–1210. doi: 10.1161/01.RES.0000196741.99904.e4. [DOI] [PubMed] [Google Scholar]
- 29.Gao F, Wang DH. Hypotension induced by TRPV4 activation: role of Ca2+-activated K+ channels and sensory nerves. J Hypertension. 2010;28:102–110. doi: 10.1097/HJH.0b013e328332b865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lind L, Granstam SO, Millgård J. Endothelium-dependent vasodilation in hypertension: a review. Blood Press. 2000;9:4–15. [PubMed] [Google Scholar]
- 31.Schiffrin EL. Vascular endothelin in hypertension. Vascul Pharmacol. 2005;43:19–29. doi: 10.1016/j.vph.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Li JS, Larivière R, Schiffrin EL. Effect of a nonselective endothelin antagonist on vascular remodeling in deoxycorticosterone acetate-salt hypertensive rats. Evidence for a role of endothelin in vascular hypertrophy. Hypertension. 1994;24:183–188. doi: 10.1161/01.hyp.24.2.183. [DOI] [PubMed] [Google Scholar]
- 33.Schiffrin EL. Endothelin: potential role in hypertension and vascular hypertrophy. Hypertension. 1995;25:1135–1143. doi: 10.1161/01.hyp.25.6.1135. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Zhou Z, Jiang DJ, Li D, Tan B, Liu H, Li YJ. Reduction of NO- and EDHF-mediated vasodilatation in hypertension: role of asymmetric dimethylarginine. Am J Physiol Heart Circ Physiol. 2007;293:H1673–1681. doi: 10.1080/10641960701616194. [DOI] [PubMed] [Google Scholar]
- 35.Büssemaker E, Popp R, Fisslthaler B, Larson CM, Fleming I, Busse R, Brandes RP. Aged spontaneously hypertensive rats exhibit a selective loss of EDHF-mediated relaxation in the renal artery. Hypertension. 2003;42:562–568. doi: 10.1161/01.HYP.0000088852.28814.E2. [DOI] [PubMed] [Google Scholar]
- 36.Si H, Heyken WT, Wölfle SE, Tysiac M, Schubert R, Grgic I, Vilianovich L, Giebing G, Maier T, Gross V, Bader M, de Wit C, Hoyer J, Köhler R. Impaired endothelium-derived hyperpolarizing factor-mediated dilations and increased blood pressure in mice deficient of the intermediate-conductance Ca2+-activated K+ channel. Circ Res. 2006;99:537–544. doi: 10.1161/01.RES.0000238377.08219.0c. [DOI] [PubMed] [Google Scholar]
- 37.Berman RS, Martin PE, Evans WH, Griffith TM. Relative contributions of NO and gap junctional communication to endothelium-dependent relaxations of rabbit resistance arteries vary with vessel size. Microvasc Res. 2002;63:115–128. doi: 10.1006/mvre.2001.2352. [DOI] [PubMed] [Google Scholar]
- 38.McGuire JJ, Ding H, Triggle CR. Endothelium-derived relaxing factors: a focus on endothelium-derived hyperpolarizing factor(s) Can J Physiol Pharmacol. 2001;79:443–470. [PubMed] [Google Scholar]
- 39.Plüger S, Faulhaber J, Fürstenau M, Löhn M, Waldschütz R, Gollasch M, Haller H, Luft FC, Ehmke H, Pongs O. Mice with disrupted BK channel beta1 subunit gene feature abnormal Ca(2+) spark/STOC coupling and elevated blood pressure. Circ Res. 2000;87:E53–60. doi: 10.1161/01.res.87.11.e53. [DOI] [PubMed] [Google Scholar]
- 40.Haddy FJ. Role of dietary salt in hypertension. Life Sci. 2006;79(17):1585–92. doi: 10.1016/j.lfs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 41.He FJ, MacGregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev. 2004;3 doi: 10.1002/14651858.CD004937. CD004937. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Iturbe B, Romero F, Johnson RJ. Pathophysiological mechanisms of salt-dependent hypertension. Am J Kidney Dis. 2007;50:655–672. doi: 10.1053/j.ajkd.2007.05.025. [DOI] [PubMed] [Google Scholar]