Abstract
The water soluble poly(ethylene glycol) (PEG) functionalized and hydroxylated endohedral trimetallic nitride metallofullerene derivatives, Gd3N@C80[DiPEG(OH)x], have been synthesized and characterized. The 1H MRI relaxivities in aqueous solution were measured for the derivatives with four different molecular weights of PEG (350–5000 Da) at 0.35 T, 2.4 T and 9.4 T. The 350/750 Da PEGs derivatives were found to have the highest relaxivities values among the derivatives, 237/232 mM−1s−1 for r1 and 460/398 mM−1s−1 for r2 (79/77 mM−1s−1 and 153/133 mM−1s−1 based on Gd3+ ion) respectively, at a clinical-range magnetic field of 2.4 T. These represent some of the highest relaxivities reported in the literatures for any commercial or investigational MRI contrast agent. Dynamic light scattering results confirm a larger size distribution for 350/750 Da PEGs derivatives (95/96 nm) relative to longer chain length derivatives, 5000 Da PEG derivatives (37nm). Direct infusion of the optimized 350 Da PEG derivatives into live tumor-bearing rat brains demonstrated an initial uniform distribution and hence, the potential for effective brachytherapy applications when the encapsulated Gd3+ ions are replaced with radioactive 177Lu.
Keywords: Gadofullerenes, Relaxivity, Contrast Agents
Introduction
Magnetic resonance imaging (MRI) is widely used in the clinical diagnosis of cancer and other diseases. Improved contrast can be obtained by the use of intravenously-injected paramagnetic contrast agents due to their extravasation across leaking vasculature and subsequent accumulation in lesions. Since contrast agents are widely employed in clinical MRI studies, these agents must be biologically safe and have a high relaxivity in order to minimize the amount delivered. Currently, the most widely used MRI contrast agents are gadolinium chelates: the large number of unpaired electrons (seven) per Gd3+ ion and favorably long electron spin-lattice relaxation time significantly increase the relaxation rate of water hydrogens.(1, 2) However, it has been recently reported that nephrogenic systemic fibrosis (NSF), a painful and debilitating disorder of the skin and systemic tissues, has been associated with exposure to Gd3+ ions based contrast agents in patients with renal insufficiency.(3, 4) New contrast agents with higher relaxivity and lower toxicity are an important target of current investigations.
Because of their shape, capacity for multiple endo encapsulants and isolation of the Gd3+ ions from the bio-environment, endohedral metallofullerenes (EMFs) represent ideal next generation nanoparticles as diagnostic biomedical nanoprobes. Several new classes of MRI contrast agents, water-soluble endohedral metallofullerenes, have been reported.(5–17) EMFs have been functionalized with hydrophilic groups, e.g., -OH or –COOH.(8–12) These water soluble EMF derivatives show significantly greater relaxivities than the chelated Gd3+ compounds in current clinical use.
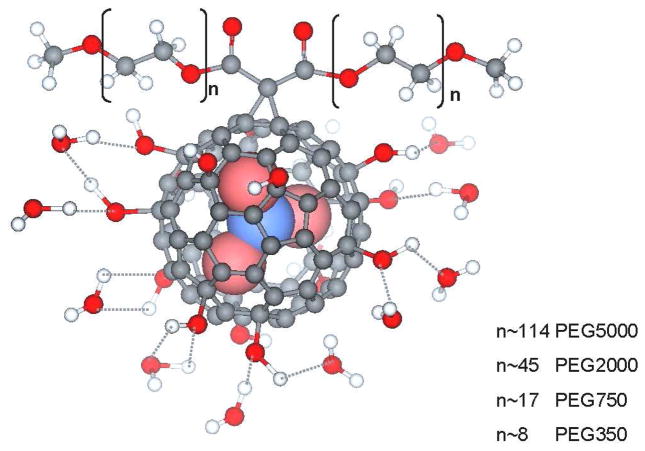
We have previously reported the synthesis of trimetallic nitride templated (TNT) EMFs, AxB3-xN@C80 (A,B=metal, x=0–3).(18) The TNT EMFs have an additional advantage: they can encapsulate up to three Gd3+ ions inside the cage, resulting in higher relaxivity. If different metal atoms/ions are encapsulated, e.g., Lu, Tb, Ho, etc., TNT EMFs can also perform as multimodal (MRI and radiotherapy) platforms with the same biodistribution. We previously reported the synthesis of Gd3N@C80[DiPEG5000(OH)x] (PEG=poly(ethylene glycol)),(7) which is water soluble and exhibits high relaxivity. In vitro and in vivo studies demonstrated the potential applicability of this MRI contrast agent. More recent studies have also demonstrated high relaxivity for related TNT EMFs with different functionality.(13, 15, 16) The high relaxivity of the TNT EMFs arises from the following factors: (1) the large number of exchangeable water molecules coordinated to the hydroxyl groups which are attached to the carbon cage (Fig. 1) and are simultaneously relaxed by the (Gd3N)6+ endohedral cluster, (2) the slow rotational correlation time caused by the long PEG chain and aggregation effects.
Figure 1.
The Gd3N@C80[DiPEG(OH)x] nanoparticle with several hydrogen-bonded water molecules shown.
In the current study, we have synthesized pegylated and hydroxylated Gd3N@C80 with different PEG chain lengths (350–5000MW) and the relaxivities of these derivatives were measured at three different magnetic field strengths. The optimum PEG chain of these pegylated and hydroxylated Gd3N@C80 derivativesis is an important structure property for potential MRI studies at different magnetic field strengths. To demonstrate the in vivo applicability of these high-relaxivity contrast agents, a convection-enhanced delivery (CED) model was used to infuse Gd3N@C80[DiPEG350(OH)x] directly into a brain-tumor-bearing rat.
Materials and Methods
Synthesis of Gd3N@C80[DiPEG(OH)x]
Gd3N@C80 was produced by the standard Krätschmer-Huffman (K–H) method with the introduction of nitrogen gas into the K–H generator.(18) The product was purified by HPLC as previously reported.(19) 1, 8-Diazabicyclo(5.4.0)undec-7-ene (DBU, FW 152.24), 98%, was purchased from Sigma-Aldrich; 99% CBr4, 99% 18-crown-6, 50% sodium hydroxide solution and 30 wt.% hydrogen peroxide were used as obtained from Aldrich Chem. Co.. Di{ω-methyl-poly(ethylene glycol)} malonate (DiPEG) 350, 750, 2000 and 5000 (MW. 768, 1568, 4068, 10068, respectively) were prepared from ω-methyl-poly(ethylene glycol)s (PEGs) as following: To a solution of PEG and triethylamine (TEA) in CH2Cl2, a solution of malonyl dichloride in CH2Cl2 was added dropwise. The molar ratio of PEG:TEA:malonyl dichloride was kept as 2:2:1. The solution was stirred at room temperature under N2 protection for 3 h. The final mixture was concentrated and dropped into large amount of ethyl ether, and then fractionated by a silica gel column.
Gd3N@C80, DiPEG, DBU, and CBr4 were dissolved in chlorobenzene in molar ratio 1:20:20:67. After deaeration with bubbling argon for 30 minutes, the solution was stirred overnight at room temperature. The resultant mixture was evaporated and the residue was dissolved in toluene. To this solution the 18-crown-6 and 50% sodium hydroxide solution was added and the resultant solution was stirred for 4 hours. 1.5mL hydrogen peroxide was added drop by drop and more water was added. The mixture was stirred overnight at room temperature. The resultant solution was concentrated and separated by Sephadex G25 (Pharmacia) size-exclusion gel column with distilled water as eluent to afford a narrow golden band (first fraction, pH≤7). (See Supporting Information Figure 1.) The yield was up to 95%.
Relaxivity Measurements
The concentrations of Gd3+ ions were determined by inductively coupled plasma optical emission spectroscopy (ICP-OES, Perkin-Elmer Optima 5300DV) at 342.247 nm. The instrument was calibrated by a 10 ppm ICP standard solution. Each molecule was assumed to encapsulate three Gd3+ ions, so the concentration of Gd3N@C80[DiPEG(OH)x] was assumed to be one third of the Gd3+ ion concentration.
The proton relaxation times were measured at three different magnetic field strengths: 0.35 T (TEACH SPIN PS1-B), 2.4 T (Bruker/Biospec) and 9.4 T (Varian Inova 400). The inversion-recovery method was used to measure T1 and Carr-Pucell-Meiboom-Gill method was used for measurement of T2. The relaxivities were extracted from linear fits of plots of relaxation rates (1/T1 and 1/T2) versus the concentrations of the paramagnetic species.
Dynamic light scattering
Dynamic light scattering (DLS) measurements were carried out with an ALV/CGS-3 compact goniometer system and ALV/LSE-5003 multi-τ digital correlator at 25 °C. He-Ne laser producing vertically polarized light of λ0 = 632.8 nm was used as a light source. Each sample was filtered with a 0.5 μm cellulose acetate membrane filter.
Animal Studies
We used a convection-enhanced delivery method (7) to infuse 0.0235 mM Gd3N@C80[DiPEG350(OH)x] into a live rat brain tumor and followed the functionalized EMF redistribution over the course of several days. Infusion was applied for 180 min at a rate of 0.2 μL/min with a total infusion volume of 36 μL.
Results and Discussion
1H NMR
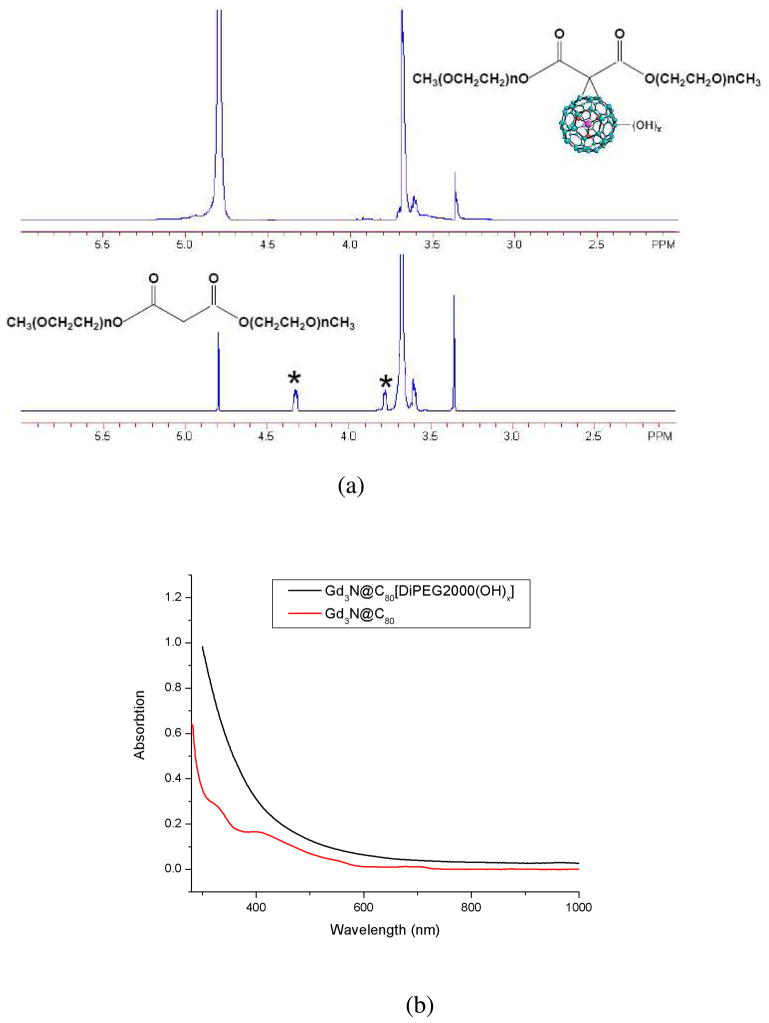
1H NMR of Gd3N@C80[DiPEG(OH)x] was performed on an Inova 400. Figure 2a displays the NMR spectra of Gd3N@C80[DiPEG350(OH)x] and malonyl DiPEG350 in D2O. In Gd3N@C80[DiPEG350(OH)x] the -OCH3 and –OCH2CH2- signals appeared at δ3.35 ppm (s, 6H) and 3.67 ppm (m, 52H), respectively; the signals for –COOCH2- and COOCH2CH2- centered at 4.32 ppm (t, 4H) and 3.78 ppm (t, 4H) in the malonate disappeared in the EMF apparently due to the strong paramagnetic effect of the Gd3+ ions. The NMR spectra of other PEG derivatives give similar results.
Figure 2.
(a) 400MHz 1H NMR spectra of Gd3N@C80[DiPEG350(OH)x] (top) in comparison with DiPEGM350 malonate (bottom) in D2O; (b) UV-Vis spectra of Gd3N@C80 and Gd3N@C80[DiPEG2000(OH)x]. Note the disappearance of the characteristic peaks in the derivative at 330 and 410 nm.
UV-Vis
The UV-Vis spectra of Gd3N@C80 and Gd3N@C80[DiPEG2000(OH)x] are shown in Figure 2b. For Gd3N@C80, there is an absorption peak at about 710nm and a second absorption occurs at 410 nm.(20) These absorptions are attributed to the Π→Π* transitions.(21) For the functionalized metallofullerenes, however, these characteristic features are significantly reduced. This indicates that the conjugated π structure of the carbon cage has been significantly altered. Similar results were obtained from other samples with different molecular weight (Gd3N@C80[DiPEG5000, 750, 350(OH)x]).
Mass Spectra
To further confirm the successful functionalization, MALDI-TOF mass spectra were obtained for DiPEG750, Gd3N@C80[DiPEG750] and Gd3N@C80[DiPEG750(OH)x]. (See supporting information Figure 2) The spectrum for DiPEG 750 was a series of peaks centered at about m/z 1388. The peaks were separated by m/z 44, which is the mass of (CH2CH2O) unit. The spectrum for Gd3N@C80[DiPEG750] was a series peaks centered at about m/z 2907. The separation of m/z 1 is attributed to the isotope effect of Gd3N@C80. We can clearly see that Gd3N@C80 was successfully functionalized with DiPEG750. However, in the mass spectrum of Gd3N@C80[DiPEG750(OH)x] we could only observe the peaks for the metallofullerene (m/z 1447) and PEG fragments (centered at m/z 759 and separated by 44). Even though the molecular ion peakwas not observed, the metallofullerene and PEG fragments indicate the successful functionalization.
MRI Relaxivity
The ability of paramagnetic compounds to enhance the relaxation rate can be described by the following equation:(1, 2)
| (1) |
1/T1 and 1/T2 are the longitudinal and transverse relaxation rates. 1/Ti,obs is the observed value of the solution and 1/Ti,para is the contribution of the paramagnetic compound. [M] is the concentration of the paramagnetic species. r1 and r2 are defined as longitudinal and transverse relaxivities respectively, which is expressed in (mM·s)−1.
A major contribution to 1/Ti,para arises from the simultaneous relaxation of multiple water molecules. This is the result of the dipolar electron-nuclear spin interaction between the large fluctuating Gd3+ electron magnetic moment and the water hydrogen nucleus. These relaxed water molecules undergo subsequently rapid exchange with bulk water. Dipole-dipole interactions are described by the Solomon-Bloembergen-Morgan (SBM) equations. In the high-field region (above 10 MHz) these equations reduce to: (1, 2)
| (2) |
| (3) |
| (4) |
If water exchange process exists, then the relaxation rates are:(1, 2)
| (5) |
| (6) |
Where [M] is in unit of mM, τR is the rotational correlation time, τM is the residence time of the coordinated water molecules, ωH is the Larmor Frequency of proton, τle is the longitudinal relaxation time of electron and q is the number of water molecules that coordinate to the paramagnetic molecules. τC is dominated by the shortest correlation time among τR, τM and τle. If τR is the dominant time, the relaxivity as a function of frequency shows a broad maximum peak that increases with increasing τR.(1)
In the present investigation, the relaxivity data of all the Gd3N@C80[DiPEG(OH)x] samples were measured. The results are summarized in Table 1. Our previously reported relaxivity data of Gd3N@C80[DiPEG5000(OH)x](7) were also listed and they agree with the current data. All the Gd3N@C80[DiPEG(OH)x] samples have high r1 and r2 values at all three magnetic field strengths. The r1 values range from 139 mM−1 s−1 (Gd3+ based relaxivity 46.3 mM−1 s−1) to 237 mM−1 s−1 (Gd3+ based relaxivity 79.0 mM−1 s−1) for the different PEG chains at 2.4 T to be compared to the value of about 4 mM−1s−1 for commercially available Gd3+ ion chelated contrast agents. Several other groups have also reported r1 values of mono gadofullerene derivatives, that is, one Gd3+ ion per fullerene cage. These r1 values are typically higher than those of extracellular commercial MRI contrast agents. In our work, the relaxivities of all the Gd3N@C80[DiPEG(OH)x] samples are significantly higher on a per molecule basis. A summary of these other studies and the relaxivitiy of Gd3N@C80[DiPEG350(OH)x] in the current study are presented in Table 2. (6, 8, 9, 11, 12, 22)
TABLE 1.
Relaxivity data of Gd3N@C80[DiPEG(OH)x] series. The relaxivities were measured at room temperature in water.
| Mol. Weight of PEG | Conc. Range (μM) | r1 (0.35T) (mM−1s−1) | r2 (0.35T) (mM−1s−1) | r1 (2.4T) (mM−1s−1) | r2 (2.4T) (mM−1s−1) | r1 (9.4T) (mM−1s−1) | r2 (9.4T) (mM−1s−1) |
|---|---|---|---|---|---|---|---|
| 5000 | 0.4–6.5 | 107±8 | 127±36 | 139±6 | 221±11 | 52.5±2.4 | 186±12 |
| 5000(7) | 1.6–12.6 | 102 | 144 | 143 | 222 | 32 | 137 |
| 2000 | 1.0–15.2 | 130±4 | 148±8 | 158±6 | 249±12 | 41.9±3.0 | 218±11 |
| 750 | 1.1–17.4 | 152±5 | 169±20 | 232±10 | 398±22 | 63.3±1.8 | 274±9 |
| 350 | 1.5–23.5 | 227±31 | 268±19 | 237±9 | 460±23 | 68.2±3.3 | 438±5 |
TABLE 2.
Relaxivity data of Gd3N@C80[DiPEG(OH)x] series. The relaxivities were measured at room temperature in water.
| Metallofullerene Derivatives | r1 (mM−1s−1) | Magnetic Field Strength (T) |
|---|---|---|
| Gd@C82O6(OH)16(NH2CH2CH2COOH)8 (6) | 9.1 | 1.5 |
| Gadofullerenol(22) | 47 | 9.4 |
| Gd@C82(OH)40 (12) | 67 | 0.47 |
| 81 | 1.0 | |
| 31 | 4.7 | |
| Gd@C60(OH)x (26) | 83.2 | 1.4 |
| Gd@C60[C(COOH)2]10 (11, 26) | 24.0 | 1.4 |
| 4.6 | 0.47 | |
| Gd3N@C80[DiPEG750(OH)x] | 150 (50.0 Gd3+ based) | 0.35 |
| 246 (82.0 Gd3+ based) | 2.4 | |
| 58.1 (19.7 Gd3+ based) | 9.4 |
As previously indicated, a primary reason for the high molar relaxivity of the Gd3N@C80[DiPEG(OH)x] samples in comparison with other endohedral metallofullerenes derivatives originate from the presence of three Gd3+ ions within the cage: the charge transfer between the (Gd3N)6+ cluster and the (C80)6− cage leads to a very stable nanoparticle. Previous studies have confirmed ferromagnetic coupling in this cluster gives rise to a large magnetic moment, 21 μB.(23) The dipolar electron-nuclear spin interaction between the fluctuating Gd3+ electron magnetic moment and the large number of exchangeable water hydrogen atoms induces efficient proton relaxation. This is in contrast with the conventional Gd-DTPA complex, which has a single water molecule bound in the first coordination sphere of the metal ion, exchanges rather slowly with the bulk water and the entire complex tumbles very rapidly.
Further factors contributing to the relaxation process relate to the unique property of the fullerenes to aggregate and form large nanoclusters. Aggregation occurs frequently for water soluble metallofullerenes and affects the relaxivity significantly as previous studies have shown.(5, 9) For the low molar mass Gd3N@C80[DiPEG(OH)x] samples, the aggregates are formed by hydrogen bonding between –OH groups; however, the hydrophobic fullerene-fullerene interactions are also strong. Hence, fullerene aggregates can be formed. According to the Debye-Stokes equation for rigid spherical particles:
the rotational correlation time τR increases as the particle radius increases which is an important parameter influencing relaxivity as a function of field strength. It has also been postulated that the presence of these aggregates might lead to a ‘confined’ pool of water molecules in the interstitial spaces between individual gadofullerenes which exchanges rapidly with the bulk water increasing again the relaxivity. (24) Depending on the PEG length and aggregate sizes, different restricted pools of water would be created as mentioned above influencing the measured overall relaxivities.
In the limit of τM ≪ T1M, T2M for equations (2) and (3), we obtain
| (7) |
At low field, ωH2τC2≪1 and the ratio approaches the value 7/6=1.17. . Our measured ratios of r2/r1 are listed in Table 3. Based on equation 7, the ratios at 0.35T are consistent with the predicted value. Another result of the SBM equations is ωHτC ≈ 1 at maximum r1. According to equation (7) this leads to r2/r1≈11/6=1.83 for the peak. The ratios for the 750 and 350 PEGs at 2.4 T are close to 11/6 = 1.83 and hence the maximum r1 values should occur near 100 MHz (2.4 T). We can also see that at 2.4 T the 750 Da species has the maximum r1 of all the samples. Hence at 2.4 T we obtain approximately for the 750/350 sample: τC=1/ΩH=1.59ns, which is significantly longer than the commercial contrast agent, e.g. τR=66 ps for the [Gd(DTPA-BMA)] (1). This assumes that τC is dominated by a rotational component.
TABLE 3.
The r2/r1 ratios of the pegylated/hydroxylated samples
| Sample | r2/r1 (0.35T) | r2/r1 (2.4T) | r2/r1 (9.4T) |
|---|---|---|---|
| 5000 | 1.19 | 1.59 | 3.54 |
| 2000 | 1.14 | 1.58 | 5.20 |
| 750 | 1.11 | 1.72 | 4.33 |
| 350 | 1.18 | 1.94 | 6.42 |
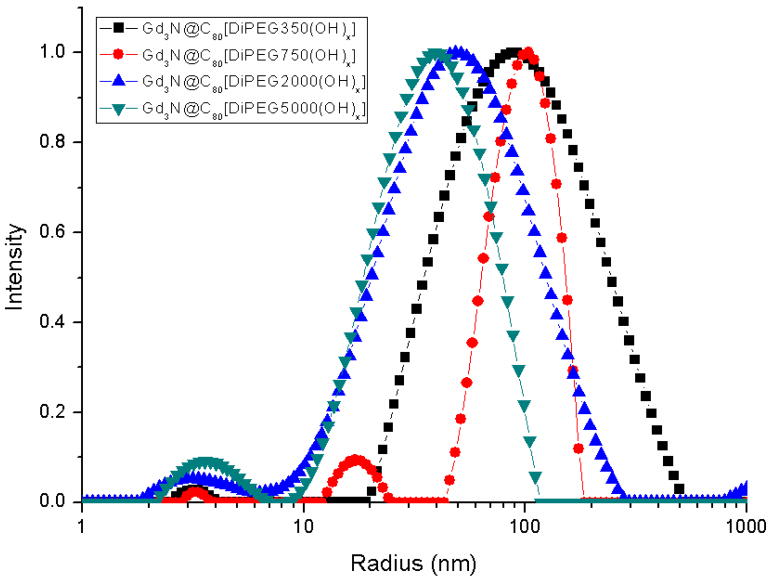
The aggregation behavior was studied by DLS experiment. The DLS results of all Gd3N@C80[DiPEG(OH)x] samples are shown in Figure 3. The mean peak positions are 75nm, 76nm, 58nm and 37nm for Gd3N@C80[DiPEG350(OH)x], Gd3N@C80[DiPEG750(OH)x], Gd3N@C80[DiPEG2000(OH)x] and Gd3N@C80[DiPEG5000(OH)x] respectively. The 350PEG and 750PEG samples have the largest average aggregation size (~75nm) while the 5000PEG sample is much smaller (~37nm). The trend of decreasing aggregation size with increasing molecular weight is consistent with the relaxivities trend. It should also be noted that these pegylated particles clearly deviate from spherical shape and the ones with longer PEG chains might exhibit internal flexibility. This could be an important additional factor for the observed reduced relaxivities for these larger molecules due to increased anisotropic rotation described by a fast local motion component that controls relaxivity and a slow global motion component. (25)
Figure 3.
Size distribution function of Gd3N@C80[DiPEG(OH)x] from DLS experiment. The mean peak position is 75nm, 76nm, 58nm and 37nm for Gd3N@C80[DiPEG350(OH)x], Gd3N@C80[DiPEG750(OH)x], Gd3N@C80[DiPEG2000(OH)x] and Gd3N@C80[DiPEG5000(OH)x] respectively.
Animal Infusion Study
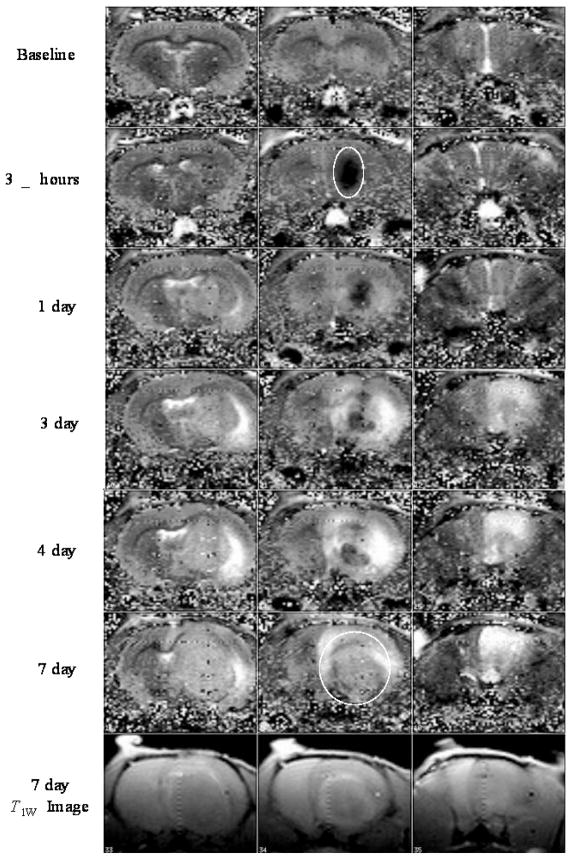
Pure T1 computed images of three consecutive brain slices of a live rat brain following infusion of 0.0235 mM Gd3N@C80[DiPEG350(OH)x] into the brain tumor are shown in Figure 4. The middle column indicates the slice with the tumor, while the left and the right columns are slices without tumor used as a control. Immediately after the infusion is terminated (“3.5 hrs”), the Gd3N@C80[DiPEG350(OH)x] nanoparticles have been distributed throughout the tumor, reducing the tissue T1 values. The tumor appears as a dark area as the white circle indicates. As time progresses, the brain tumor grows and the Gd3N@C80[DiPEG350(OH)x] nanoparticles appear to be pushed out into the tumor periphery. This is illustrated at day 7 in the T1 map which shows aggregates on the surface and the T1-weighted image that shows an enhancement ring around the tumor. These results illustrate the enhanced in vivo relaxivity of this optimized Gd3N@C80[DiPEG350(OH)x] system at low molar concentrations. It should also be noted that after 7 days the nanoparticles can still enhance the MRI contrast which indicates that these metallofullerenes agents can be used for long-term imaging diagnostic applications. In addition, these metallofullerenes agents provide a platform for effective brachytherapy applications when Gd is replaced and/or combined with other radioactive agents (177Lu).
Figure 4.
T1 computed images and T1w image of a live rat brain after direct infusion into the tumor of 0.0235 mM Gd3N@C80[DiPEG350(OH)x].
Conclusions
We synthesized hydroxylated and pegylated Gd-containing TNT EMFs, Gd3N@C80[DiPEG(OH)x], with different chain lengths of PEG. The relaxivities were measured at three magnetic field strengths. All the samples show significantly higher r1 and r2 compared to commercially available Gd-chelates and show considerable promise as a new class of contrast agents. They represent some of the highest relaxivities reported in the literature for any commercial or investigational MRI contrast agents. Analyzing the field dependence of r1, r2 and r2/r1 indicates reasonable agreement with the predictions of the SBM theory under the assumption that the rotational correlation timeτR dominates the relaxation process. Direct infusion of these particles into the live tumor-bearing rat brain demonstrates an initial uniform distribution and hence the potential for effective brachytherapy applications when Gd is replaced with radioactive 177Lu.
Supplementary Material
References
- 1.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 2.Lauffer RB. Paramagnetic Metal Complexes as Water Proton Relaxation Agents for NMR Imaging: Theory and Design. Chem Rev. 1987;87:901–927. [Google Scholar]
- 3.Sieber MA, Pietsch H, Walter J, Haider W, Frenzel T, Weinmann HJ. A preclinical study to investigate the development of nephrogenic systemic fibrosis: A possible role for gadolinium-based contrast media. Invest Radiol. 2008;43:65–75. doi: 10.1097/RLI.0b013e31815e6277. [DOI] [PubMed] [Google Scholar]
- 4.Grobner T. Gadolinium - a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol, Dial, Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 5.Shu CY, Zhang EY, Xiang JF, Zhu CF, Wang CR, Pei XL, Han HB. Aggregation studies of the water-soluble gadofullerene magnetic resonance imaging contrast agent: [Gd@C82O6(OH)16(NHCH2CH2COOH)8]x. J Phys Chem B. 2006;110:15597–15601. doi: 10.1021/jp0615609. [DOI] [PubMed] [Google Scholar]
- 6.Shu CY, Gan LH, Wang CR, Pei XL, Han HB. Synthesis and characterization of a new water-soluble endohedral metallofullerene for MRI contrast agents. Carbon. 2006;44:496–500. [Google Scholar]
- 7.Fatouros PP, Corwin FD, Chen ZJ, Broaddus WC, Tatum JL, Kettenmann B, Ge Z, Gibson HW, Russ JL, Leonard AP, Duchamp JC, Dorn HC. In vitro and in vivo imaging studies of a new endohedral metallofullerene nanoparticle. Radiology. 2006;240:756–764. doi: 10.1148/radiol.2403051341. [DOI] [PubMed] [Google Scholar]
- 8.Toth E, Bolskar RD, Borel A, Gonzalez G, Helm L, Merbach AE, Sitharaman B, Wilson LJ. Water-soluble gadofullerenes: Toward high-relaxivity, pH-responsive MRI contrast agents. J Am Chem Soc. 2005;127:799–805. doi: 10.1021/ja044688h. [DOI] [PubMed] [Google Scholar]
- 9.Sitharaman B, Bolskar RD, Rusakova I, Wilson LJ. Gd@C60[C(COOH)2]10 and Gd@C60(OH)x: Nanoscale Aggregation Studies of Two Metallofullerene MRI Contrast Agents in Aqueous Solution. Nano Lett. 2004;4:2373–2378. [Google Scholar]
- 10.Kato H, Kanazawa Y, Okumura M, Taninaka A, Yokawa T, Shinohara H. Lanthanoid Endohedral Metallofullerenols for MRI Contrast Agents. J Am Chem Soc. 2003;125:4391–4397. doi: 10.1021/ja027555+. [DOI] [PubMed] [Google Scholar]
- 11.Bolskar RD, Benedetto AF, Husebo LO, Price RE, Jackson EF, Wallace S, Wilson LJ, Alford JM. First soluble M@C60 derivatives provide enhanced access to metallofullerenes and permit in vivo evaluation of Gd@C60[C(COOH)2]10 as a MRI contrast agent. J Am Chem Soc. 2003;125:5471–5478. doi: 10.1021/ja0340984. [DOI] [PubMed] [Google Scholar]
- 12.Mikawa M, Kato H, Okumura M, Narazaki M, Kanazawa Y, Miwa N, Shinohara H. Paramagnetic water-soluble metallofullerenes having the highest relaxivity for MRI contrast agents. Bioconjugate Chem. 2001;12:510–514. doi: 10.1021/bc000136m. [DOI] [PubMed] [Google Scholar]
- 13.Shu CY, Corwin FD, Zhang JF, Chen ZJ, Reid JE, Sun MH, Xu W, Sim JH, Wang CR, Fatouros PP, Esker AR, Gibson HW, Dorn HC. Facile Preparation of a New Gadofullerene-Based Magnetic Resonance Imaging Contrast Agent with High H-1 Relaxivity. Bioconjugate Chem. 2009;20:1186–1193. doi: 10.1021/bc900051d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shu CY, Wang CR, Zhang JF, Gibson HW, Dorn HC, Corwin FD, Fatouros PP, Dennis TJS. Organophosphonate functionalized Gd@C-82 as a magnetic resonance imaging contrast agent. Chem Mater. 2008;20:2106–2109. [Google Scholar]
- 15.Shu CY, Ma XY, Zhang JF, Corwin FD, Sim JH, Zhang EY, Dorn HC, Gibson HW, Fatouros PP, Wang CR, Fang XH. Conjugation of a water-soluble gadolinium endohedral fulleride with an antibody as a magnetic resonance imaging contrast agent. Bioconjugate Chem. 2008;19:651–655. doi: 10.1021/bc7002742. [DOI] [PubMed] [Google Scholar]
- 16.MacFarland DK, Walker KL, Lenk RP, Wilson SR, Kumar K, Kepley CL, Garbow JR. Hydrochalarones: A novel endohedral metallofullerene platform for enhancing magnetic resonance imaging contrast. J Med Chem. 2008;51:3681–3683. doi: 10.1021/jm800521j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang EY, Shu CY, Feng L, Wang CR. Preparation and characterization of two new water-soluble endohedral metallofullerenes as magnetic resonance imaging contrast agents. J Phys Chem B. 2007;111:14223–14226. doi: 10.1021/jp075529y. [DOI] [PubMed] [Google Scholar]
- 18.Stevenson S, Rice G, Glass T, Harich K, Cromer F, Jordan MR, Craft J, Hadju E, Bible R, Olmstead MM, Maitra K, Fisher AJ, Balch AL, Dorn HC. Small-bandgap endohedral metallofullerenes in high yield and purity. Nature. 1999;401:55–57. [Google Scholar]
- 19.Ge ZX, Duchamp JC, Cai T, Gibson HW, Dorn HC. Purification of endohedral trimetallic nitride fullerenes in a single, facile step. J Am Chem Soc. 2005;127:16292–16298. doi: 10.1021/ja055089t. [DOI] [PubMed] [Google Scholar]
- 20.Krause M, Dunsch L. Gadolinium nitride Gd3N in carbon cages: The influence of cluster size and bond strength. Angewandte Chemie-International Edition. 2005;44:1557–1560. doi: 10.1002/anie.200461441. [DOI] [PubMed] [Google Scholar]
- 21.Yang SF, Kalbac M, Popov A, Dunsch L. Gadolinium-based mixed metal nitride clusterfullerenes GdxSC3-xN@C-80 (x=1, 2) Chemphyschem. 2006;7:1990–1995. doi: 10.1002/cphc.200600323. [DOI] [PubMed] [Google Scholar]
- 22.Zhang S, Sun D, Li X, Pei F, Liu S. Synthesis and solvent enhanced relaxation property of water-soluble endohedral metallofullerenols. Fullerene Sci Technol. 1997;5:1635–1643. [Google Scholar]
- 23.Qian MC, Ong SV, Khanna SN, Knickelbein MB. Magnetic endohedral metallofullerenes with floppy interiors. Phys Rev B: Condens Matter. 2007;75:104424. [Google Scholar]
- 24.Laus S, Sitharaman B, Toth E, Bolskar RD, Helm L, Wilson LJ, Merbach AE. Understanding paramagnetic relaxation phenomena for water-soluble gadofullerenes. J Phys Chem C. 2007;111:5633–5639. [Google Scholar]
- 25.Lipari G, Szabo A. Model-Free Approach to the Interpretation of Nuclear Magnetic-Resonance Relaxation in Macromolecules. 1 Theory and Range of Validity. J Am Chem Soc. 1982;104:4546–4559. [Google Scholar]
- 26.Laus S, Sitharaman B, Toth E, Bolskar RD, Helm L, Asokan S, Wong MS, Wilson LJ, Merbach AE. Destroying gadofullerene aggregates by salt addition in aqueous solution of Gd@C60(OH)x and Gd@C60[C(COOH)2]10. J Am Chem Soc. 2005;127:9368–9369. doi: 10.1021/ja052388+. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.