Abstract
In Rakai, Uganda, HIV+ men were randomized to immediate (intervention) or delayed circumcision (controls). Penile swabs were assayed for high risk human papillomavirus (HR-HPV) by Roche HPV Linear Array at enrollment and 24 months (intervention n=103, control n=107). Rate ratios (RR) of HR-HPV were estimated by Poisson regression. At 24 months, HR-HPV prevalence was intervention 55.3% and control 71.7% (RR=0.77, 95%CI 0.62–0.97). Multiple HR-HPV infections were intervention 22.4% and controls 42.5% (RR=0.53, 95%CI 0.33–0.83). New HR-HPV genotypes were acquired by 42.0% of intervention and 57.0% of control arm men (RR=0.74, 95%CI 0.54–1.01, p=0.06). Multiple new HR-HPV genotypes were acquired by 9.9% intervention and 24.7% control arm men (RR = 0.40, 95%CI 0.19–0.84, p = 0.01). Circumcision did not affect the acquisition of single HR-HPV infections (RR=1.00, 95%CI 0.65–1.53) or clearance of HR-HPV (RR=1.09, 95%CI 0.94–1.27). Circumcision of HIV+ men reduced the prevalence and incidence of multiple HR-HPV infections.
Introduction
Three randomized trials in South Africa, Kenya and Uganda have shown that male circumcision reduces HIV acquisition in men by 50–60%[1–3], and WHO now recommends that circumcision be provided as a component of HIV prevention programs.[4] It is inevitable that as male circumcision services become widely available, HIV-infected men will request the procedure, and WHO recommends that they should be provided with circumcision unless there are medical contraindications for surgery.[4] We previously reported that surgery-related complications were comparable in HIV-infected and uninfected men,[5] and that circumcised HIV-positive men had reduced rates of genital ulcer disease [6].
Two trials of male circumcision have shown a reduced prevalence of penile high risk human papillomavirus (HR-HPV) infection in circumcised HIV-negative men.[7, 8] However, the effects of circumcision on HR-HPV infection in men with HIV are unknown. HIV infection is associated with high rates of HR-HPV infection and cervical neoplasia in women,[9, 10] and with penile and anal HR-HPV infection and cancers in men.[11–14] Therefore, if circumcision reduces penile carriage of HR-HPV in HIV-positive men, it may provide a health benefit to these men and potentially to their female sexual partners. In this paper we report on the efficacy of circumcision for prevention of penile high risk human papillomavirus infections in a randomized trial of HIV-infected men in Uganda.
Methods
We conducted two parallel trials of male circumcision in HIV-negative and HIV-positive men in Rakai district of southwestern Uganda from 2003–2007. The design and conduct of the trials has been described previously.[3, 15] In brief, both HIV-infected and uninfected men were informed of the trial objectives and procedures, and consenting men were then screened for eligibility. Criteria for enrollment included being uncircumcised, aged 15–49 years, and having no indications for or contraindication to surgery. Men found to have genital infections or a hemoglobin level ≥ 8 grams/dL were treated and rescreened. All screened participants were offered voluntary HIV counseling and testing, health education on HIV/STI prevention and free condoms.
HIV status at enrollment was assessed by two enzyme immunoassays: Vironostika HIV-1 (Organon Teknika, Charlotte, North Carolina, USA] and Cambridge Biotech [Worcester, Massachusetts, USA). Discordant EIA results were confirmed by Western blot (Calypte Biomedcial Corparation, Rockville, MD, USA).
Because the safety of surgery in HIV-infected men was unknown, we excluded HIV-positive men from enrollment if they had evidence of immunosuppression as indicated by a CD4 cell count below 350 cells/mm3 or WHO clinical stage 3 or 4 disease. All HIV-infected men were referred for management of their HIV disease by the Rakai Health Sciences Program’s HIV care services supported by the President’s Emergency Fund for AIDS Relief. All HIV-infected men received a basic care package of cotrimoxazole prophylaxis, insecticide impregnanted bed nets and hypochlorite water disinfection packets. Men with a CD4 cell count below 250 cells/mm3 or WHO stage 4 disease were advised to initiate antiretroviral therapy which was available free of charge through the Rakai Program.
Eligible participants provided written informed consent for enrollment which described study procedures, potential risks and benefits and the voluntary nature of participation. Men who were randomized to the intervention arm received circumcision within two weeks of enrollment using the sleeve procedure under local anesthesia and were followed postoperatively at 1–2 and 7–9 days and 4–6 weeks. All intervention and control participants were followed at 6, 12 and 24 months. At enrollment and at each follow up visit, participants completed an interview to ascertain sociodemographic characteristics, sexual risk behaviors and symptoms suggestive of STIs or AIDS.
At each visit, clinical officers examined the men’s genitalia recording any abnormality and took a penile swab for HPV detection. Moistened Dacron swabs were taken from the subpreputial cavity of uncircumcised men and from the coronal sulcus/glans of circumcised men, placed in Digene specimen transport medium (STM), and stored at −80° C until assay. HPV genotyping was performed using the Roche HPV Linear Array (Roche Diagnostics, Indianapolis, IN) as previously described [16–18]. HPV genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were considered the primary high risk HPV (HR-HPV) carcinogenic viral genotypes. Genotypes 6, 11, 26, 40, 42, 43,53, 54, 55, 61, 67, 70, 71 72, 73, 81 82, 83, 84, and 108 were considered low risk HPV (LR HPV) genotypes. To ensure the adequacy of penile swabs for PCR detection we restricted analyses to samples with amplifiable DNA, defined as swabs with the detection of any high or low risk HPV DNA and/or detectable human beta-globin control amplification. Penile swabs negative for both HPV and the beta-globin internal control were considered to be insufficient for HR-HPV detection and were excluded from HPV analyses. [19, 20]
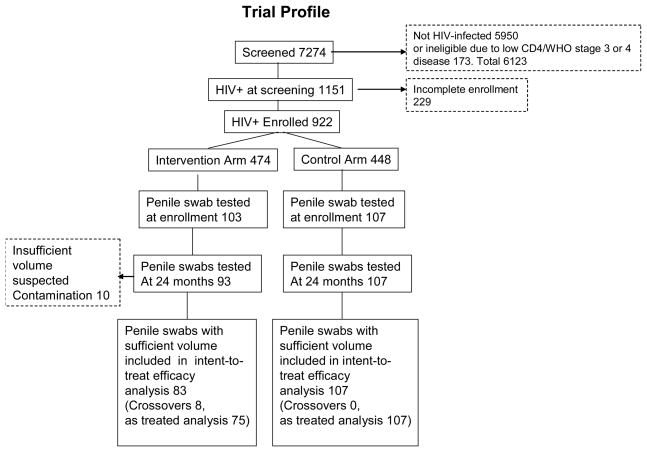
The trial profile is shown in Figure 1. There were 1151 HIV-infected men identified at screening of whom 922 (80.1%) were enrolled and randomized to the intervention (n = 474) or control (n = 448) arms. The lack of balance between study arms was due to the fact that enrollment and randomization was decentralized in these rural communities and took place at 10 sites. Also, all men (HIV-infected and uninfected) were randomized at time of enrollment men. Thus, imbalances due to chance occurred in the numbers allocated to intervention and control arms. Due to financial constraints and assay costs, we only tested a random sample of HIV-positive participants who provided penile swabs at enrollment and at 24 months follow up. Resources were insufficient to assay samples at the 6 and 12 month visits. There were 103 enrollment samples tested in the intervention arm (21.7% of men enrolled) and 107 in the control arm (23.9% of men enrolled). At 24 months follow up, assay results were available for 93 intervention arm men (90.3% of those tested at enrollment). Ten intervention arm samples were excluded due to insufficient volume or suspected contamination. There were 8 crossovers in the intervention arm, defined as men who failed to accept circumcision by six months post-randomization. Follow up assays were conducted for all 107 control arm men at follow up and there were no control crossovers.
Figure 1.
Trial profile.
The trials were approved by two IRBs in Uganda (The Scientific and Ethics Committee of the Uganda Virus Research Institute and the Uganda National Council of Research and Technology), and in the United States (The Johns Hopkins University, Bloomberg School of Public Health, Committee for Human Research, and Western Institutional Review Board, Olympia, WA). The trial in HIV-infected men was registered with ClinicalTrials.gov, number NCT00124878
Statistical analysis
Characteristics, behaviors and HR-HPV infections were assessed by arm of assignment at enrollment. We assessed the efficacy of circumcision for reduction of prevalent HR-HPV using an intention-to-treat analysis, based on two classifications of HR-HPV outcomes: 1) the prevalence of any (one or more HR-HPV infections), and 2) the prevalence of single or multiple (more than one) HR-HPV infections at 24 months follow up. Similar analyses were conducted for low risk HPV infections (LR-HPV). An as treated analysis classified intervention arm crossovers as uncircumcised if they failed to accept surgery by the six month visit. There were no control arm crossovers in this analytic sample. The units of observation were individual study participants. The prevalence risk ratio (PRR) of HR-HPV in the intervention relative to the control arm was estimated by comparing the two binomial proportions.
The incidence of new HR-HPV infections at 24 months was determined among men who were either HR-HPV negative at enrollment, or who were HR-HPV positive for a given genotype but acquired a new HR-HPV genotype at follow up. The denominator for estimation of incidence consisted of men with valid samples at both enrollment and 24 months follow up. Incidence was estimated as the proportion of men with a new HR-HPV infection at the 24 months follow up. We assessed acquisition of any (ie., one or more new HR-HPV infection), as well as single and multiple infections per person. The proportion of new genotype-specific infections was estimated from the number of new genotypes detected at 24 month follow up among men with samples negative for the newly acquired genotypes at enrollment. The incidence rate ratio (IRR) of new HR-HPV infections acquired over 24 months was estimated by Poisson log linear regression. Adjusted IRRs were estimated by multivariable Poisson regression and included covariates for age, marital status, number of sex partners, condom use and alcohol consumption with sex.
The clearance of pre-existing genotype-specific HR-HPV infections over 24 months was estimated from the proportions of prevalent genotype-specific infections detected at enrollment which were not detected at follow up, among samples with amplifiable viral or cellular DNA at both time points. The clearance ratio of any HR-HPV genotype infection was estimated using a log binomial model with robust variance estimates, assuming an exchangeable correlation structure between the multiple clearances observed on the same individual.
Analyses were performed in R 2.8.1 (R Foundation for Statistical Computing: Vienna, Austria, 2007.).
Results
At enrollment, the two study groups were comparable with respect to age, education, condom use, numbers of sexual partners and alcohol use with sex within the prior year (Table 1). The percentage of currently married men in the intervention arm (23.5%) was lower than that in the control arm (33.6%), but this difference was not statistically significant (p=0.11.)
Table 1.
Participant characteristics at enrollment
| Characteristic | Intervention | Control | p-value | ||
|---|---|---|---|---|---|
| Number | % | Number | % | ||
| All | 103 | 100.0 | 107 | 100.0 | |
| Age | |||||
| 15–24 | 15 | 14.6 | 19 | 17.8 | |
| 25–29 | 28 | 27.2 | 26 | 24.3 | |
| 30–34 | 28 | 27.2 | 26 | 24.3 | |
| 35+ | 32 | 31.1 | 36 | 33.6 | 0.86 |
| Education | |||||
| No Education | 4 | 3.9 | 8 | 7.5 | |
| Primary | 85 | 83.3 | 78 | 72.9 | |
| Secondary+ | 13 | 12.7 | 21 | 19.6 | 0.18 |
| Marital status | |||||
| Currently married | 78 | 76.5 | 71 | 66.4 | |
| Not married | 24 | 23.5 | 36 | 33.6 | 0.11 |
| Condom use | |||||
| Used Condom | 50 | 51.0 | 60 | 58.8 | |
| Not Use | 48 | 49.0 | 42 | 41.2 | 0.27 |
| Alcohol with sex | |||||
| No | 25 | 24.5 | 31 | 29.0 | |
| Yes | 77 | 75.5 | 76 | 71.0 | 0.47 |
| Number of sex partners | |||||
| 0 | 4 | 3.9 | 5 | 4.7 | |
| 1 | 48 | 47.1 | 48 | 44.9 | |
| 2 | 28 | 27.5 | 34 | 31.8 | |
| 3+ | 22 | 21.6 | 20 | 18.7 | 0.88 |
A high proportion of these HIV-positive men had HR-HPV infections at enrollment (Table 2), but the proportions with HR-HPV were comparable between the intervention arm (72.2%) and the control arm (76.6%). The proportion of men infected with two or more HR-HPV genotypes at enrollment was 49.5% in the intervention arm and 47.9% in the control arm. At the 24 month follow up, the proportion of HIV+ men with one or more HR-HPV genotypes was 55.3% in the intervention arm and 71.7% in the control arm (PRR = 0.77, 95%CI 0.62–0.97). The proportion of men with one or more HR-HPV among intervention arm men declined from enrollment to follow up (72.2% and 55.3%, respectively, p = 0.02), whereas there was no change over time in proportion of controls infected with one or more HR-HPV genotypes (76.6% at enrollment and 71.7% at follow up, respectively, p = 0.52). The prevalence of single HR-HPV genotype infections did not differ significantly between study arms at the 24 month follow up (PRR = 1.13, 95%CI 0.74–1.72). However, the prevalence of multiple (i.e., two or more) HR-HPV genotype infections at 24 months follow up was markedly lower in the intervention arm (22.4%), than in the control arm (42.5%, PRR = 0.53, 95%CI 0.33–0.83).
Table 2.
HR-HPV Prevalence at Enrollment and Follow up
| Study visit and number of HR-HPV infections | Intervention | Control | Prevalence risk ratio of HR- HPV infection, intervention/control (referent no HR-HPV) (95%CI) | ||
|---|---|---|---|---|---|
| HR-HPV | % | HR-HPV | % | ||
| Enrollment | N = 97* | N = 94* | |||
| No HR-HPV | 27 | 23.4 | 22 | 27.8 | |
| One or more HR-HPV infection | 70 | 72.2 | 72 | 76.6 | 0.94 (0.80–1.11) |
| Single HR-HPV infection | 22 | 22.7 | 27 | 28.7 | 0.79 (0.49–1.28) |
| Multiple HR-HPV infections | 48 | 49.5 | 45 | 47.9 | 1.03 (0.77–1.38) |
| 24 Months | N = 85* | N = 106* | |||
| No HR-HPV | 38 | 44.7 | 30 | 28.3 | |
| One or more HR-HPV infection | 47 | 55.3 | 76 | 71.7 | 0.77 (0.62–0.97) |
| Single HR-HPV infection | 28 | 32.9 | 31 | 29.2 | 1.13 (0.74–1.72) |
| Multiple HR-HPV infections | 19 | 22.4 | 45 | 42.5 | 0.53 (0.33–0.83) |
The denominators are samples will amplifiable cellular or viral DNA. At enrollment, 6 intervention and 13 control samples had non-amplifiable viral and cellular DNA, and at 24 months, 8 intervention and 1 control samples had unamplifiable DNA.
The frequency of new HR-HPV infections is shown in Table 3. In an intention to treat analysis, the proportion of men acquiring any (i.e., one or more) new HR-HPV genotype infection was lower in the intervention (42.0%) than the control arm (57.0%), and this was of borderline statistical significance (IRR=0.74, 95%CI 0.57–1.01). After multivariate adjustment for marital status and number of sex partners, the adjusted IRR was 0.68, 95%CI 0.44–1.04). There was no effect of circumcision on the proportions acquiring a single new HR-HPV genotype infection (IRR=1.00, 95%CI 0.65–1.53). However, the proportions of men acquiring multiple new HR-HPV genotype infections was markedly lower in the intervention arm (9.9%) than the control arm (24.7%, IRR=0.40, 95%CI 0.19–0.84). The adjusted IRR was 0.37, 95%CI 0.16–0.83). In an as treated analysis, the incidence of one or more HR-HPV infections was 20.8/100 py (29/117 py) in circumcised men and 40.3/100 py (58/144 py) in uncircumcised men with an IRR = 0.61 (95%CI 0.39–0.96). The as treated incidence of multiple (two or more) HPV infections was 5.0/100 py (7/139 py) in circumcised and 13.5/100 py (24/178 py) in uncircumcised men with an IRR = 0.37 (0.16–0.87).
Table 3.
Incidence of single and multiple HR-HPV infections over 24 months by study arm
| New HR-HPV Infections | Intervention | Control | Incidence Risk Ratio (95%CI) | ||
|---|---|---|---|---|---|
| Incident/N* | % | Incident/N* | % | Intervention vs control | |
| Proportion | |||||
| One or more HR-HPV genotype infection | 34/81 | 42.0 | 53/93 | 57.0 | 0.74 (0.54– 1.01) |
| Single HR-HPV genotype infection | 26/81 | 32.1 | 30/93 | 32.2 | 1.00 (0.65– 1.53) |
| Multiple HR-HPV genotype infections | 8/81 | 9.9 | 23/93 | 24.7 | 0.40 (0.19– 0.84) |
Table 4 shows the proportion of men who acquired a new HR-HPV genotypic infection among men who were negative for each specific genotype at enrollment. The acquisition of new HR-HPV genotypes was lower in the intervention than the control arm for thirteen of the fourteen HR-HPV genotypes, but none of these differences between study arms were statistically significant.
Table 4.
HR-HPV incident infections from enrollment to 24 months follow up, by study arm
| HR-HPV genotype | Intervention | Control | IRR (95%CI) | ||
|---|---|---|---|---|---|
| Incident/N* | % | Incident/N* | % | Intervention vs control | |
| 16 | 4/69 | 5.8 | 11/74 | 14.9 | 0.39 (0.12–1.22) |
| 18 | 3/70 | 4.3 | 9/81 | 11.1 | 0.39 (0.10–1.42) |
| 31 | 4/73 | 5.5 | 5/86 | 5.8 | 0.94 (0.25–3.51) |
| 33 | 2/69 | 2.9 | 7/80 | 8.8 | 0.33 (0.07–1.59) |
| 35 | 4/66 | 6.1 | 5/80 | 6.3 | 0.97 (0.26–3.61) |
| 39 | 1/73 | 1.4 | 5/86 | 5.8 | 0.24 (0.03–2.02) |
| 45 | 3/73 | 4.1 | 8/78 | 10.3 | 0.40 (0.11–1.51) |
| 51 | 5/68 | 7.4 | 10/72 | 13.9 | 0.53 (0.18–1.55) |
| 52 | 7/69 | 10.1 | 7/85 | 8.2 | 1.23 (0.43–3.51) |
| 56 | 2/76 | 2.6 | 5/84 | 6.0 | 0.44 (0.09–2.28) |
| 58 | 2/66 | 3.0 | 10/77 | 13.0 | 0.23 (0.05–1.06) |
| 59 | 1/69 | 1.4 | 5/78 | 6.4 | 0.23 (0.03–1.94) |
| 66 | 2/76 | 2.6 | 5/81 | 6.2 | 0.43 (0.08–2.20) |
| 68 | 6/70 | 8.6 | 8/78 | 10.3 | 0.84 (0.29–2.41) |
The denominators are samples which had amplifiable cellular or viral DNA at both enrollment and follow up, and were negative for the specific genotype at enrollment.
At enrollment, the prevalence of low risk HPV (LR-HPV) genotypes was 85.6% (83/97) in the intervention arm and 83.0% (78/94) in the control arm (PRR = 1.03, 95%CI 0.91–1.17). At the 24 month follow up, the prevalence of LR-HPV genotypes declined to 49.4% (42/85) the intervention arm men, but remained relatively stable at 77.4% (82/106) in control arm men, and the prevalence rate ratio was significantly reduced (PRR = 0.64, 95%CI 0.50–0.81).
Table 5 shows the genotype specific HPV clearance rates by study arm. In the intervention arm, a total of 147 HR-HPV genotype infections were detected at enrollment and 112 (76.2%) of these had cleared by 24 months. Among controls, there were a total of 182 HR-HPV genotype infections detected at enrollment, of which 131 (71.0%) had cleared at follow up. This difference was not statistically significant (RR=1.09, 95% CI: 0.94–1.27), after adjusting for correlation between clearance events observed within the same individual.
Table 5.
Clearance of HR_HPV infection over 24 months by study arm
| HR-HPV genotype | Intervention | Control | RR (95%CI) | ||
|---|---|---|---|---|---|
| Cleared/N* | % | Cleared/N* | % | Intervention vs control | |
| 16 | 8/12 | 66.7 | 12/19 | 63.2 | 1.06 (0.43–2.58) |
| 18 | 8/11 | 72.7 | 11/12 | 91.7 | 0.79 (0.32–1.97) |
| 31 | 6/8 | 75.0 | 6/7 | 85.7 | 0.88 (0.21–2.71) |
| 33 | 12/12 | 100.0 | 10/13 | 76.9 | 1.30 (0.56–3.01) |
| 35 | 12/15 | 80.0 | 11/13 | 84.6 | 0.95 (0.42–2.14) |
| 39 | 8/8 | 100.0 | 3/7 | 42.9 | 2.33 (0.62–8.80) |
| 45 | 5/8 | 62.5 | 11/15 | 73.3 | 0.85 (0.30–2.45) |
| 51 | 8/13 | 61.5 | 12/21 | 57.1 | 1.08 (0.44–2.63) |
| 52 | 10/12 | 83.3 | 5/8 | 62.5 | 1.33 (0.46–3.90) |
| 56 | 5/5 | 100.0 | 6/9 | 66.7 | 1.50 (0.54–2.44) |
| 58 | 14/15 | 93.3 | 13/16 | 81.3 | 1.15 (0.54–2.44) |
| 59 | 7/12 | 58.3 | 11/15 | 73.3 | 0.80 (0.31–2.05) |
| 66 | 2/5 | 40.0 | 7/12 | 58.3 | 0.69 (0.14–3.30) |
| 68 | 7/11 | 63.6 | 13/15 | 86.7 | 0.73 (0.29–1.84) |
| Total | 112/147 | 76.2 | 131/182 | 71.0 | 1.09 (0.94–1.27)** |
The denominators are samples which had amplifiable cellular or viral DNA at both enrollment and follow up, and were positive for the genotype at enrollment.
Clearance ratio after adjusting for correlation between multiple clearances observed within the same individual.
Discussion
Circumcision of HIV-infected men with CD4 counts >350 cells/mL3 and no evidence of AIDS related illnesses reduced the prevalence and incidence of infection with multiple HR-HPV and LR-HPV genotypes over 24 months, but did not affect acquisition of single HR-HPV genotype infections or clearance of pre-existing HR-HPV infections.
We are not aware of other studies which have assessed the effects of male circumcision in HIV-infected men, so our findings need to be replicated. Nevertheless, these observations in HIV-infected men are compatible with the protective effects of circumcision observed in trials of HIV-negative men, among whom circumcision was found to have an efficacy of 35–36% for prevention of prevalent HR-HPV infection [7, 8] compared with the 23% efficacy observed in HIV-positive men (Table 2). Also, circumcision was found to have a more marked effect on the prevalence of multiple HR-HPV infections in both HIV-negative men (65% efficacy),[8] and HIV-infected men (efficacy of 47%, Table 2). Similarly, with respect to HR-HPV incidence, the efficacy for prevention of any (i.e., one or more) HR-HPV genotype infection was 33%, in both HIV-negative and HIV-positive men (Table 3). The efficacy for prevention of multiple HR-HPV genotypes was also comparable in HIV-uninfected (35%) and HIV-infected men (37%, Table 3). It is noteworthy that circumcision did not affect the incidence of single HR-HPV infections in either HIV-negative or positive men (Table 3). Additionally, circumcision reduced the prevalence of LR-HPV infections in both HIV-infected and uninfected men. Thus, circumcision appears to have comparable efficacy for the prevention of HR-HPV infections irrespective of HIV co-infection.
Our findings suggest that male circumcision may provide a direct benefit to HIV-positive men by preventing penile HR-HPV infection and thus potentially averting penile cancer. In addition, couples studies show that HIV/HPV co-infection is strongly associated with increased HPV infection in sexual partners,[13] so it is possible that circumcision of HIV-infected men may protect female partners from infection and potentially from cervical neoplasia.[12]
There are limitations to this study. Due to resource constraints, the number of samples assayed was small and this limited study power. The 24 month interval between repeat HPV testing was long, and it is likely that we missed incident HR-HPV infections which cleared prior to the 24 months follow up visit. Because we observed no difference in HR-HPV clearance between study arms in the HIV-infected men, any failure to detect incident HR-HPV infections which cleared prior to the 24 month follow up visit is likely to be non-differential between study arms and thus should not bias estimates of efficacy. As noted above, the efficacy of circumcision for prevention of any HR-HPV infection was similar in HIV-positive (65%) and HIV-negative men (63%), suggesting that any under-ascertainment did not introduce serious bias. The protracted follow up interval did not allow precise estimation of clearance (and thus duration of infection) in these HIV-infected men and we observed no effect of circumcision on HR-HPV clearance (Table 5). Nevertheless, the rates of clearance over 24 months in the present study are similar to those observed in the trial of HIV-negative men, although in the latter, we did observe significantly increased clearance rates in the intervention arm.
A further limitation is the HR-HPV assays were done on swabs from the coronal sulcus/glans and some studies of multiple anatomical sampling sites suggest lower detection of HPV from this area, compared to the shaft,[19, 20] However, other studies found somewhat higher rates of HPV detection from the coronal sulcus/glans than from the shaft.[21] Therefore, it is unlikely that the sampling site used here led to serious under-ascertainment of infection. We have no explanation for the finding circumcision reduced the incidence of multiple HR-HPV genotypes, but had no effect on acquisition of single genotype infections.
Further studies are needed to confirm that circumcision results in an overall lower burden of genital tract HR-HPV in HIV-infected men. We cannot assess whether the observed effects of circumcision on reducing multiple HR-HPV genotype infections has implications for the development of penile or cervical neoplasia. Nor can we determine whether circumcision of men with CD4 counts <350 or with AIDS defining illnesses will affect HR-HPV carriage.
Finally, we are not able to determine whether the effect of circumcision on reducing the incidence of HR-HPV reflects protection against acquisition of new HR-HPV genotypes or protection against reactivation of a latent infections. Whatever the mechanism, the marginal reduction in HPV detectability is likely to reflect a decrease in viral shedding (at least from the coronal sulcus/glans), which may be expected to decrease transmission risk to female partners.
In summary, we conclude that circumcision of HIV-infected men significantly reduced the incidence and prevalence of multiple high and low risk HPV infections and thus provides a direct health benefit to these individuals and potentially a benefit to their sexual partners.
Acknowledgments
This study was funded by a grant (22006) from The Bill and Melinda Gates Foundation. Additional support for laboratory analyses and training were provided, respectively, by the Division of Intramural Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health and the Fogarty International Center (grants 5D43TW001508 and D43TW00015) Support for HPV assays was provided by Dr. Charles Rabkin under Subcontract # BRC 55502-23 from the National Cancer Institute.
We thank the Rakai Community Advisory Board and particularly wish to express our gratitude to the study participants whose commitment and cooperation made the study possible.
Footnotes
Clinical Trials.gov Protocol Registration System NCT00124878
All authors declare that they have no conflicts of interest.
References
- 1.Auvert B, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bailey RC, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 3.Gray RH, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. New data on male circumcision and HIV prevention: policy and programme implications. UNAIDS; Montreux: 2007. [Google Scholar]
- 5.Kigozi G, et al. The Safety of Adult Male Circumcision in HIV-Infected and Uninfected Men in Rakai, Uganda. PLoS Med. 2008;5(6):e116. doi: 10.1371/journal.pmed.0050116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wawer M, et al. Trial of male circumcision in HIV+men, Rakai, Uganda: effects in HIV+men and in women partners. Fifteenth Conference on Retroviruses and Opportunistic Infections; 2008. Abstract 33LB. [Google Scholar]
- 7.Auvert B, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of randomized controlled trial conducted in orange farm, South Africa. J Infect Dis. 2009;199(1):14–9. doi: 10.1086/595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tobian AAR, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med. 2009;360(13):1298–309. doi: 10.1056/NEJMoa0802556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Safaeian M, et al. Prevalence and risk factors for carcinogenic human papillomavirus infections in rural Rakai, Uganda. Sex Transm Infect. 2008;84(4):306–11. doi: 10.1136/sti.2007.027318. [DOI] [PubMed] [Google Scholar]
- 10.Safaeian M, et al. Determinants of incidence and clearance of high-risk human papillomavirus infections in rural Rakai, Uganda. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1300–7. doi: 10.1158/1055-9965.EPI-07-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bosch FX, et al. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellsague X, et al. Male circumcision, penile human papillomavirus infection, and cervical cancer in female partners. N Engl J Med. 2002;346(15):1105–12. doi: 10.1056/NEJMoa011688. [DOI] [PubMed] [Google Scholar]
- 13.Mbulawa ZZ, et al. Genital human papillomavirus prevalence and human papillomavirus concordance in heterosexual couples are positively associated with human immunodeficiency virus coinfection. J Infect Dis. 2009;199(10):1514–24. doi: 10.1086/598220. [DOI] [PubMed] [Google Scholar]
- 14.de Pokomandy A, et al. Prevalence, clearance, and incidence of anal human papillomavirus infection in HIV -infected men: the HIPVIRG cohort study. J Infect Dis. 2009;199(7):965–73. doi: 10.1086/597207. [DOI] [PubMed] [Google Scholar]
- 15.Wawer M, et al. Circumcision of HIV -infected men and its effect on HIV transmission to female partners in rakai, Uganda: randomized controlled trial. Lancet. 2009;373 doi: 10.1016/S0140-6736(09)60998-3. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gravitt PE, et al. Evaluation of self-collected cervicovaginal cell samples for human papillomavirus testing by polymerase chain reaction. Cancer Epidemiol Biomarkers Prev. 2001;10(2):95–100. [PubMed] [Google Scholar]
- 17.Gravitt PE, et al. Improved amplification of genital human papillomavirues. J Clin Microbiol. 2000;38(1):357–61. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gravitt PE, et al. Genotyping of 27 human papillomavirus types by using L1 consensus PCR products by a single -hybridization, reverse line blot detection method. J Clin Microbiol. 1998;36(10):3020–7. doi: 10.1128/jcm.36.10.3020-3027.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giuliano AR, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis. 2007;196(8):1146–52. doi: 10.1086/521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver BA, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis. 2004;189(4):677–85. doi: 10.1086/381395. [DOI] [PubMed] [Google Scholar]
- 21.Smith JS, et al. Human papillomavirus detection by penile site in young men from Kenya. Sex Transm Dis. 2007;34(11):928–34. doi: 10.1097/OLQ.0b013e318065b8ef. [DOI] [PMC free article] [PubMed] [Google Scholar]