Abstract
Purpose
Copper transporter 2 (CTR2) is known to mediate the uptake of Cu+1 by mammalian cells. Several other Cu transporters, including the influx transporter CTR1 and the two efflux transporters ATP7A and ATP7B, also regulate sensitivity to the platinum-containing drugs. We sought to determine the effect of CTR2 on influx, intracellular trafficking, and efflux of cisplatin and carboplatin.
Experimental Design
The role of CTR2 was examined by knocking down CTR2 expression in an isogenic pair of mouse embryo fibroblasts consisting of a CTR1+/+ line and a CTR1−/− line in which both CTR1 alleles had been deleted. CTR2 levels were determined by quantitative reverse transcription-PCR and Western blot analysis. Cisplatin (DDP) was quantified by inductively coupled plasma mass spectrometry and 64Cu and [14C]carboplatin (CBDCA) accumulation by γ and scintillation counting.
Results
Deletion of CTR1 reduced the uptake of Cu, DDP, and CBDCA and increased resistance to their cytotoxic effects by 2- to 3-fold. Knockdown of CTR2 increased uptake of Cu only in the CTR1+/+ cells. In contrast, knockdown of CTR2 increased whole-cell DDP uptake and DNA platination in both CTR1+/+ and CTR1−/− cells and proportionately enhanced cytotoxicity while producing no effect on vesicular accumulation or efflux. A significant correlation was found between CTR2 mRNA and protein levels and sensitivity to DDP in a panel of six ovarian carcinoma cell lines.
Conclusions
CTR2 is a major determinant of sensitivity to the cytotoxic effects of DDP and CBDCA. CTR2 functions by limiting drug accumulation, and its expression correlates with the sensitivity of human ovarian carcinoma cell lines to DDP.
Platinum-based chemotherapeutic agents have been among the most widely used and effective anticancer agents since the 1970s (1). However, patients often develop resistance during the course of treatment (2–7) and the mechanisms that account for such resistance have not been fully identified. Changes in drug influx and efflux, deficiencies in the mismatch repair pathway, and down-regulation of the apoptotic cascade have all been proposed (8–12). To complicate matters, little is known about the intracellular metabolism of platinum-based agents. It is believed that these drugs kill cells through formation of adducts on the purine bases of nuclear DNA (9, 13, 14); however, it is not clear how the drugs traffic through the cell to reach the nucleus. There is a strong correlation between drug sensitivity and drug accumulation, with DDP-resistant cells uniformly accumulating less drug (4, 15–21).
Several studies have provided evidence that the platinum-containing drugs are taken up, shuttled around the cell, and exported by transporters and chaperones belonging to the Cu homeostasis system. Cells selected for resistance to cisplatin (DDP) are cross-resistant to Cu and vice versa (22–25). CTR1 is a high-affinity plasma membrane Cu transporter. Cu associates with the hCTR1 metal binding motif in the NH2-terminal domain and is transported into the cytosol through a channel formed by the hCTR1 trimer (26, 27). Cu is then handed off to the various chaperones to be trafficked throughout the cell. Deletion of the gene coding for CTR1 was shown to render yeast resistant to the cytotoxic effects of DDP, and this was correlated with decreased uptake of drug (28, 29). Deletion of both CTR1 alleles in mouse embryo fibroblasts also markedly reduced accumulation of all three Pt-containing drugs and increased resistance to cell killing (30). However, this did not completely eliminate the accumulation of DDP, suggesting that DDP can enter the cell through transporters other than CTR1.
Copper transporter 2 (CTR2) is a copper transport protein with substantial structural homology to CTR1. Although sharing only 41% amino acid homology and having a shorter NH2-terminal domain, CTR2 is similar to CTR1 in having an essential methionine ~20 amino acids away from the first of three transmembrane domains, as well as an MXXXM motif in the highly conserved second transmembrane domain that is required for Cu transport in CTR1 (31, 32). In yeast, Ctr2 and its Schizosaccharomyces pombe orthologue Ctr6 are localized in vacuoles with the COOH-terminal tail oriented toward the cytosol (31, 33). It has been shown that Ctr2 releases Cu from intercellular vacuolar stores under conditions of Cu starvation and delivers Cu to various chaperones (31, 34, 35). The Ctr2-1 mutant of yeast Ctr2 partially mislocalizes to the plasma membrane in a position in which it mediates Cu transport similar to that of Ctr1 (31). Less is known about the function of mammalian CTR2. In mammalian cells, CTR2 is primarily localized to late endosomes and lysosomes, although it also has reportedly been found on the plasma membrane in some cells (36, 37). Like CTR1, CTR2 forms multimers, some of which colocalize with CTR1 (37). Mammalian CTR2 has been shown to increase Cu influx in cells in which it localizes to the plasma membrane (36). Although its affinity is less than that of CTR1 (36, 37), it is apparent that CTR2 plays an important role in copper homeostasis, and it is now the subject of intense study in this field.
In the current study, we sought to determine whether CTR2, like CTR1, functions as a transporter for the platinum-containing chemotherapeutic agents and whether it modulates sensitivity to their cytotoxic effects. We report here that knockdown of CTR2 expression increases the cellular accumulation of DDP, the extent of DNA adduct formation, and the cytotoxicity of DDP in both CTR1-proficient and CTR1-deficient mouse embryo fibroblasts. In addition, an association was found between DDP sensitivity and CTR2 expression in a panel of six ovarian cancer cell lines.
Materials and Methods
Drugs and reagents
Platinol AQ was a gift from Bristol-Myers Squibb; it contains DDP at a concentration of 3.33 mmol/L in 0.9% NaCl. [14C]CBDCA was purchased from Amersham Biosciences. The drugs were diluted into Opti-MEM reduced serum medium (Life Technologies) to produce final concentrations of 10, 30, and 100 μmol/L. Bradford reagent was purchased from Bio-Rad Laboratories, Inc.; sulforhodamine B was obtained from Sigma-Aldrich; and 0.4% sulforhodamine B (w/v) was solubilized in 1% (v/v) acetic acid solution.
Cell types, culture, and engineering
Parental mouse embryonic fibroblasts containing wild-type alleles of CTR1 (CTR1+/+) and an isogenic line in which both copies of CTR1 had been somatically knocked out (CTR1−/−) were a gift from Dr. Dennis Thiele (Duke University Medical Center, Department of Pharmacology and Cancer Biology, Durham, NC) (38). The CTR2kd sublines were constructed by infecting the CTR1+/+ and CTR1−/− cells with lentivirus expressing a short hairpin RNA targeting mouse CTR2 mRNA purchased from Sigma-Aldrich. The short hairpin RNA sequences used were CCGGGCCTTGGAACACATGAGGATTCTCGAGAATCCTCATGTGTTCCAAGGCTTTTTG and CCGGCCCACTTCTCAACATGACTTACTCGAGTAAGTCATGTTGAGAAGTGGGTTTTTG. Knockdowns were selected in medium containing 5 μmol/L puromycin. Cell survival following exposure to increasing concentrations of drugs was assayed using the sulforhodamine B assay system (39). Five thousand cells were seeded into each well of a 96-well tissue culture plate. Cells were incubated overnight at 37°C, 5% CO2 and then exposed to varying drug concentrations in 200 μL complete medium. Cells were allowed to grow for 5 d, after which the medium was removed and the protein was precipitated with 50% trichloroacetic acid and stained using 100 μL of 0.4% sulforhodamine B in 1% acetic acid at room temperature for 15 min. Following washing, the absorbance of each well at 515 nm was recorded using a Versamax Tunable Microplate Reader (Molecular Devices). All experiments were repeated at least thrice using three cultures for each drug concentration.
Western blotting
Whole-cell lysates were dissolved in lysis buffer [150 mmol/L NaCl, 5 mmol/L EDTA, 1% Triton X-100, and 10 mmol/L Tris (pH 7.4)] and subjected to electrophoresis on 4% to 15% gels using ~30 μg of protein per lane. Protein levels were first determined by Bradford assay (Bio-Rad). A Bio-Rad Trans-Blot system was used to transfer the proteins to Immobilon-P membranes (Millipore). Blots were incubated overnight at 4°C in 4% dry nonfat milk in TBS [150 mmol/L NaCl, 300 mmol/L KCl, 10 mmol/L Tris (pH 7.4), 0.01% Tween 20]. Blots were incubated for 1 h at room temperature in CTR2 antibody at 1:400 dilution (generous gift from Dr. Jessie Bertinato; Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch, Health Canada, Ottawa, Ontario, Canada). A horseradish peroxidase–conjugated secondary antibody (GE Healthcare) was dissolved in 4% milk in the TBS buffer and incubated with the blot for 1 h at room temperature. After four 5-min washes, blots were exposed to the Pierce enhanced chemiluminescence reagent (Thermo Scientific) and detected on X-ray films (HyBlot CL, Denville Scientific, Inc.).
Quantitative reverse transcription-PCR
CTR2 mRNA levels were measured using a quantitative PCR (qPCR) method of detection of relative amounts of first-strand cDNA. cDNA was generated from mRNA isolated using Trizol (Invitrogen). Purified mRNA was converted to cDNA using oligo(dT)20 priming and the SuperScript III First-Strand kit (Invitrogen). qPCR was done on a Bio-Rad MyIQ qPCR machine. The forward and reverse primers for hCTR2, mCTR2, and mouse β-actin were as follows: mCTR2, tccaggtagtcatcagct (forward) and tggcagtgctctgtgatgtc (reverse); β-actin, aggtgacagattgcttctg (forward) and gctgcctcaacacctcaac (reverse). Reactions were prepared using iQ SYBR Green Supermix (Bio-Rad) according to the manufacturer’s recommendations. Samples were prepared in quadruplicate with three independent sample sets being analyzed. Analysis was done using the Bio-Rad iQ5 system software.
Measurement of drug accumulation into whole cells, vesicles, and DNA
CTR1+/+/CTR2+/+, CTR1+/+/CTR2kd, CTR1−/−/CTR2+/+, and CTR1−/−/CTR2kd cells were grown to 90% confluence in T-150 tissue culture flasks. Cells were then harvested using trypsin, and 7.5 × 105 cells were placed into each well of six-well tissue culture plates and allowed to grow overnight in 2.5 mL of medium at 37°C in 5% CO2. The next day, medium was removed by aspiration and the cells were exposed to 500 μL of drug-containing Opti-MEM medium (Invitrogen) at 37°C for either 0, 5, or 60 min, after which the drug-containing medium was removed and the plates were washed thrice with ice-cold PBS and then placed on ice. In the case of the time zero samples, the drug-containing medium was aspirated within 15 s of the start of drug exposure. Concentrated (50–70%) nitric acid (215 μL) was added to each well and the plate was incubated overnight at room temperature. The following day, the acid was moved into Omni-vials (Wheaton) and incubated at room temperature overnight to thoroughly dissolve all cellular debris. The following day, the nitric acid was diluted with 3 mL of buffer (0.1% Triton X-100, 1.4% nitric acid, 1 ppb In in double-distilled water). Pt concentration was measured using a Perkin-Elmer Element 2 inductively coupled plasma mass spectrometry (ICP-MS) located at the Analytical Facility at Scripps Institute of Oceanography at the University of California, San Diego. As a method of normalization, total sulfur was measured using a Perkin-Elmer inductively coupled plasma optical emission spectroscopy (ICP-OES) also located at Scripps Institute of Oceanography at the University of California, San Diego. Samples that were previously prepared for the ICP-MS were then introduced into the ICP-OES, where total ppb of sulfur was measured. All data presented are the means of at least three independent experiments each done with six wells per concentration tested.
For measurement of accumulation into vesicles, drug-exposed cells were harvested using trypsin and centrifuged at 2,000 rpm for 10 min. Medium was removed and cell pellets were combined. Vesicles isolated from lysed cells were separated by sucrose gradient subcellular fractionation as described by Tjelle et al. (40). For measurement of Pt in DNA, cells were lysed and DNA was harvested using DNAzol (Invitrogen) according to the manufacturer’s protocol. As a method of normalization, DNA was measured before addition of nitric acid using a NanoDrop 1000 spectrophotometer (Thermo Scientific). The microsome and DNA samples were digested in nitric acid before measurement of Pt by ICP-MS as described above.
Measurement of [14C]CBDCA and 64Cu accumulation
Cells were seeded at 7.5 × 105 per well in six-well tissue culture plates and allowed to grow overnight in 2.5 mL of medium at 37°C in 5% CO2. For measurement of [14C]CBDCA accumulation, 500 μL of 50 μmol/L [14C]CBDCA were added to the cells and incubated at 37°C in 5% CO2 for 60 min. At the end of the incubation period, the plates were placed on ice, and the wells were rinsed thrice with 3 mL of ice-cold PBS. Cells were harvested in 200 μL lysis buffer [150 mmol/L NaCl, 5 mmol/L EDTA, 1% Triton X-100, and 10 mmol/L Tris (pH 7.4)] and transferred to tubes containing 3 mL of scintillation buffer (National Diagnostics). [14C]CBDCA was quantified by scintillation counting. Total protein as measured by Bradford assay was used for normalization of values. For measurement of 64Cu accumulation, 2 μmol/L 64CuSO4 was added to the plates and incubated at 37°C in 5% CO2 for 60 min. At the end of the incubation period, the plates were placed on ice and the wells were rinsed thrice with 3 mL of ice-cold PBS. Cells were harvested in 215 μL concentrated nitric acid and transferred to tubes containing 3 mL of buffer as described above for γ counting on a Beckman Gamma 5500B (Beckman Coulter). Total sulfur was used for normalization as described earlier. All data presented are the means of at least three independent experiments each done with six wells per concentration tested.
Measurement of drug export
Cells were grown in six-well plates as described above. One day after seeding, the medium was removed by aspiration and the cells were exposed to 500 μL of 30 μmol/L DDP-containing Opti-MEM medium at 37°C for 60 min and immediately rinsed with room temperature PBS, after which the drug-containing medium was replaced with drug-free medium for 0, 0.5, 1, 2, 8, 30, or 240 min. The plates were washed thrice with ice-cold PBS and then placed on ice. Whole-cell drug accumulation was determined as described above.
Statistical analysis
All data points represent the mean ± SE of at least three independent experiments, each using a minimum of three cultures per concentration tested. The significance of differences was determined using the Student’s t test with the assumption of unequal variances.
Results
Knockdown of mCTR2 in mouse embryo fibroblasts
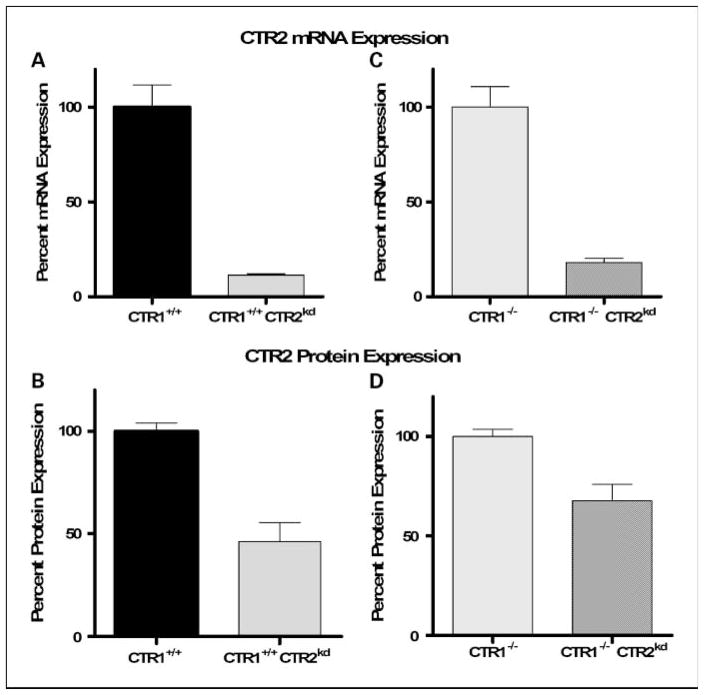
A wild-type (CTR1+/+) mouse embryo fibroblast cell line and an isogenic line in which both alleles of CTR1 had been knocked out (CTR1−/−) were used to examine the effect of disabling the function of CTR2 in cells that were either proficient or deficient in CTR1 function. The CTR1+/+ and CTR1−/− cells were infected with lentivirus expressing a short hairpin RNA targeted to mCTR2, and individual colonies were selected using 5 μmol/L puromycin. Knockdown of mCTR2 expression was analyzed by quantitative reverse transcription-PCR (qRT-PCR) and Western blot analysis, and individual clones were chosen for further study. As shown in Fig. 1A and B, CTR2 mRNA and protein expression was reduced by 88.5% and 55%, respectively, in the CTR1+/+/CTR2kd subline. CTR2 knockdown did not affect CTR1 levels as measured by qRT-PCR (data not shown). Figure 1C and D shows that CTR2 mRNA and protein expression was reduced by 81.8% and 33%, respectively, in CTR1−/−/CTR2kd cells.
Fig. 1.
Relative CTR2 mRNA and protein levels in parental and knockdown cells measured by qRT-PCR andWestern blot analysis. Relative CTR2 mRNA (A) and protein (B) levels in CTR1+/+/CTR2+/+ and CTR1+/+/CTR2kd cells. Relative CTR2 mRNA (C) and protein (D) levels in CTR1−/−/CTR2+/+ and CTR1−/−/CTR2kd cells. Bars, SE.
Reduction of CTR2 expression increases sensitivity to DDP and CBDCA
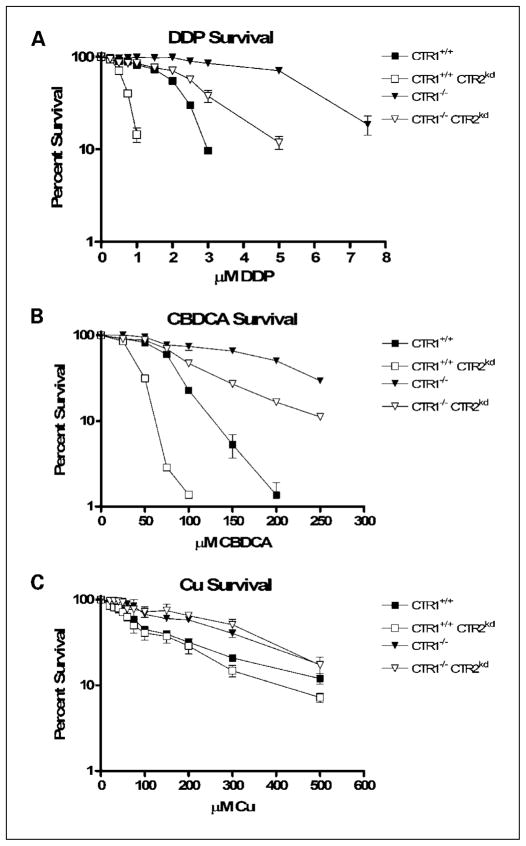
The CTR1+/+/CTR2+/+, CTR1+/+/CTR2kd, CTR1−/−/CTR2+/+, and CTR1−/−/CTR2kd cells were exposed to increasing concentrations of DDP for 5 days, and the change in growth rate was quantified by staining the remaining cells with sulforhodamine B. Figure 2A shows the concentration-survival curves for each of the cell lines. Loss of CTR1 function rendered the CTR1−/−/CTR2+/+ cells 2.6-fold more resistant to DDP relative to the CTR1+/+/CTR2+/+ cells. The mean (±SE) IC50 values were 2.1 ± 0.02 μmol/L and 5.5 ± 0.2 μmol/L for the two cell lines, respectively (P = 0.002). In contrast, the knockdown of CTR2 rendered cells hypersensitive to DDP irrespective of whether CTR1 was expressed or not. Knockdown of CTR2 in the CTR1+/+/CTR2+/+ cells reduced the IC50 by 69% to 0.7 ± 0.01 μmol/L (P = 0.0001). Likewise, knockdown of CTR2 in the CTR1−/−/CTR2+/+ cells reduced the DDP IC50 by 51% to 2.7 ± 0.2 μmol/L (P = 0.0002). Stated another way, loss of mCTR2 expression caused a 3.2-fold increase in DDP sensitivity in wild-type cells and a 2.0-fold increase in cells lacking mCTR1.
Fig. 2.
Inhibition of growth as a function of concentration. A, DDP. B, CBDCA. C, Cu. Bars, SE.
A similar effect on cell growth was observed when the knockdown cells were exposed to CBDCA (Fig. 2B). The mean ± SE IC50 values for CBDCA were as follows: CTR1+/+/CTR2+/+ cells, 79.6 ± 0.3 μmol/L; CTR1+/+/CTR2kd cells, 38.4 ± 1.1 μmol/L; CTR1−/−/CTR2+/+ cells, 197.8 ± 7.1 μmol/L; and CTR1−/−/CTR2kd cells, 95.9 ± 2.4 μmol/L. Thus, reduction of mCTR2 expression caused a 1.9-fold increase in CBDCA sensitivity in the parental wild-type cells (P = 0.0006) and a 2.1-fold increase in cells lacking mCTR1 (P = 0.002). In contrast, as shown in Fig. 2C, whereas loss of CTR1 function rendered the cells 2.6-fold resistant to Cu (P = 0.009), reduction in the expression of CTR2 had no discernable effect on the sensitivity to Cu in either the CTR1+/+ or CTR1−/− background. The mean ± SE IC50 values for Cu were as follows: CTR1+/+/CTR2+/+ cells, 243.9 ± 20.9 μmol/L; CTR1+/+/CTR2kd cells, 309.6 ± 23.0 μmol/L; CTR1−/−/CTR2+/+ cells, 93.1 ± 9.2 μmol/L; and CTR1−/−/CTR2kd cells, 95.8 ± 25.6 μmol/L. Thus, knockdown of CTR2 produced a similar effect on sensitivity to DDP and CBDCA; however, there was a clear difference in the effect of knocking down CTR2 expression on sensitivity to these two platinum-containing drugs and the effect on sensitivity to Cu.
Reduction of CTR2 expression increases whole-cell platinum drug accumulation
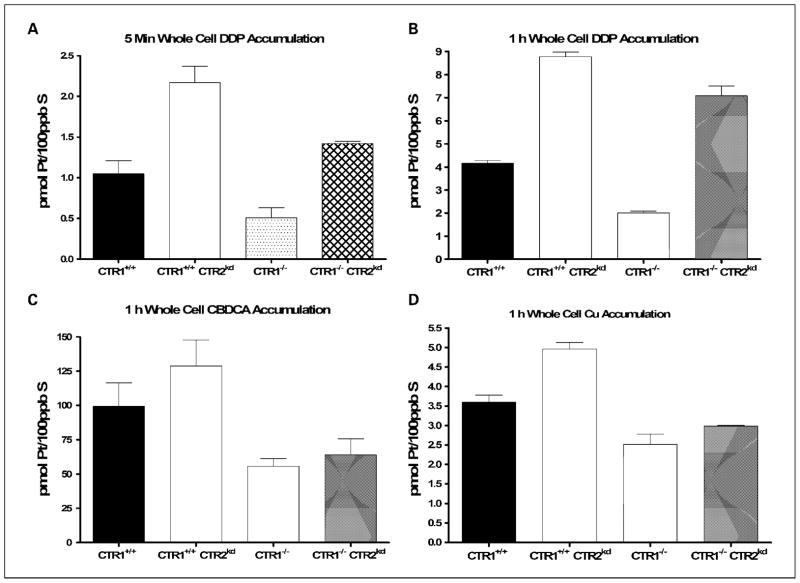
To determine whether the change in sensitivity to DDP was linked to changes in drug accumulation, total whole-cell Pt accumulation was measured following either a 5-minute or 1-hour exposure to 30 μmol/L DDP in all four cell lines by ICP-MS. The data, normalized to the content of sulfur as measured by ICP-OES as a surrogate for total cellular protein, are presented in Table 1. Figure 3A shows that, in both the CTR1+/+ and CTR1−/− backgrounds, reduction in the expression of CTR2 increased the whole-cell accumulation of DDP. Reduction of CTR2 expression in the CTR1+/+ background increased initial accumulation, determined by 5-minute exposure, by 2.2-fold (P = 0.004), whereas in the CTR1−/− background it increased it by 2.8-fold (P = 0.006). After 1 hour of DDP exposure, the accumulation was 2.1-fold higher in the CTR1+/+/CTR2kd cells than in the CTR1+/+/CTR2+/+ cells (P = 0.003); likewise, the uptake was 3.5-fold higher in the CTR1−/−/CTR2kd cells than in the CTR1−/−/CTR2+/+ cells (P = 0.03; Fig. 3B). As shown in Fig. 3C, a similar although more muted change in accumulation was observed for CBDCA after a 1-hour period of drug exposure.
Table 1.
Accumulation of DDP, CBDCA, and Cu
| CTR1+/+/CTR2+/+ | CTR1+/+/CTR2kd | CTR1−/−/CTR2+/+ | CTR1−/−/CTR2kd | |
|---|---|---|---|---|
| DDP uptake at 5 min* | 1.05 ± 0.16 | 2.17 ± 0.20 | 0.51 ± 0.12 | 1.43 ± 0.03 |
| DDP uptake at 1 h* | 4.17 ± 0.11 | 8.78 ± 0.20 | 2.01 ± 0.08 | 7.08 ± 0.43 |
| CBDCA uptake at 1 h† | 99.5 ± 17.7 | 128.7 ± 27.8 | 55.5 ± 5.6 | 63.9 ± 11.8 |
| Cu uptake at 1 h* | 3.60 ± 0.18 | 4.96 ± 0.17 | 2.52 ± 0.26 | 2.98 ± 0.03 |
| DNA adduct formation, pmol/L Pt/μg DNA | 0.14 ± 0.02 | 0.30 ± 0.01 | 0.10 ± 0.01 | 0.32 ± 0.03 |
| Vesicle accumulation, pmol/L Pt/100 ng sulfur | 0.70 ± 0.04 | 0.73 ± 0.05 | 0.70 ± 0.04 | 0.70 ± 0.08 |
pmol/L/100 ng sulfur.
cpm/μg protein.
Fig. 3.
Whole-cell accumulation of DDP in CTR1+/+/CTR2+/+, CTR1+/+/CTR2kd, CTR1−/−/CTR2+/+, and CTR1−/−/CTR2kd cells. Whole-cell Pt accumulation following 5-min (A) and 1-h (B) exposure to 30 μmol/L DDP as measured by ICP-MS. C, whole-cell [14C]CBDCA accumulation following 1-h exposure to 50 μmol/L [14C]CBDCA as measured by scintillation counting. D, whole-cell 64Cu accumulation following 1-h exposure to 2 μmol/L 64CuSO4 as measured by γ counting. Bars, SE.
As expected, deletion of CTR1 reduced the Cu accumulation at 1 hour to 70% of control. Knockdown of CTR2 expression in the CTR1+/+ background caused 1.4-fold increase in Cu uptake (P = 0.01; Fig. 3D). Knockdown of CTR2 in the CTR1−/− background had little effect. These results indicate that CTR2 has greater effects on the cellular pharmacology of the platinum-containing drugs than Cu. In wild-type cells, knockdown of CTR2 increased Cu uptake, suggesting that CTR2 functions to efflux Cu. Under circumstances where Cu uptake was severely impaired due to loss of CTR1 function, knockdown of CTR2 had little further effect. In contrast, knockdown of CTR2 substantially increased DDP uptake irrespective of the status of CTR1. This indicates that the interaction of DDP with CTR2 is independent of the function of CTR1. The fact that similar effects were observed on both the initial and subsequent phases of uptake indicates that either CTR2 functions to suppress influx or it affects an efflux system that operates much more rapidly than previously appreciated.
Loss of mCTR2 expression increases DNA adduct formation
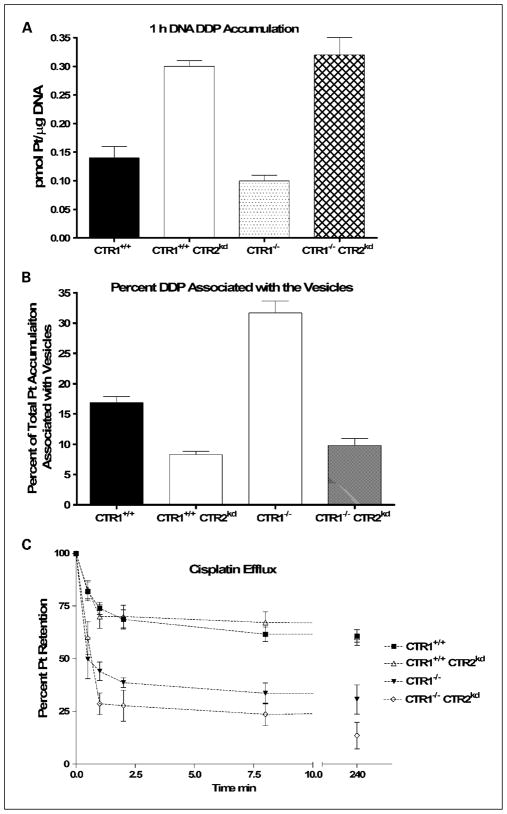
In a prior study conducted in human ovarian cancer cells, forced overexpression of CTR1 increased DDP accumulation but failed to increase cytotoxicity or DNA adduct formation (41). To determine whether the increased influx of DDP that accompanies the knockdown of CTR2 led to more drug reaching the nucleus and the critical targets that mediate cytotoxicity, the extent of DNA adduct formation was measured in each of the four cell lines after a 1-hour exposure to 30 μmol/L DDP. Figure 4A shows that knockdown of CTR2 in both the CTR1+/+ and CTR1−/− background increased DNA adduct formation. Knockdown of CTR2 in the CTR1+/+ cells increased DNA adduct formation by 2.1-fold (P = 0.0002), whereas in the CTR1−/− cells it increased adduct formation by 3.2-fold (P = 0.001). The fact that the increase in DNA adduct formation closely paralleled the increase in whole-cell accumulation indicates that the enhancement of drug accumulation was not simply due to drug being sequestered in intracellular vesicles. Instead, the increased Pt represented a pool of drug available for trafficking to the nucleus and reacting with DNA.
Fig. 4.
Pt accumulation in DNA and vesicles following exposure to DDP. DNA Pt (A) and vesicle accumulation (B) following 1-h exposure to 30 μmol/L DDP as measured by ICP-MS. C, Pt content as a function of efflux time. Bars, SE.
Increased platinum accumulation in CTR2 knockdown cells is not due to enhanced vesicular accumulation
In yeast, CTR2 is expressed in the vacuolar membrane (31, 33), and in mammalian cells, it is predominantly localized to the mammalian equivalent of the yeast vacuole, which consists of the late endosomal and lysosomal compartments (31, 36, 37). Currently available data indicate that CTR2 functions primarily to efflux Cu from these structures under conditions of low environmental Cu (37). To determine whether the changes in whole-cell accumulation of DDP accompanying knockdown of CTR2 could be accounted for by enhanced accumulation in intracellular vesicles, all four cell lines were exposed to 30 μmol/L DDP for 1 hour. Intracellular vesicles were then isolated by sucrose gradient enrichment and their platinum content was measured by ICP-MS. There was no difference in the concentration of Pt in the vesicles of any of the four cell types; the mean concentration ranged from 0.70 to 0.72 pmol Pt/100 ppb sulfur. However, as shown in Fig. 4B, both loss of CTR1 and CTR2 produced quite large changes in the fraction of total intracellular Pt associated with the vesicles. Loss of CTR1 decreased whole-cell DDP uptake but nearly doubled the fraction of DDP in the vesicular fraction from 16.9 ± 0.9% (SE) to 31.7 ± 1.9% (SE). In contrast, knockdown of CTR2 increased whole-cell DDP uptake but decreased the fraction in the vesicles in both the CTR1+/+ and CTR1−/− cells. Knockdown of CTR2 in the CTR1+/+ cells reduced the percentage by half to 8.3 ± 0.6% (SE); in the CTR1−/− cells, it reduced the fraction by two thirds to 9.8 ± 1.1% (SE). These results indicate that, whereas both CTR1 and CTR2 modulated whole-cell DDP uptake, neither had a significant effect on the absolute amount of DDP resident in the vesicular fraction.
Increased platinum accumulation in CTR2 knockdown cells is not due to a change of drug export
In addition to changes in initial uptake, increased drug accumulation may be caused by a loss of export. ATP7A and ATP7B can regulate drug accumulation through the regulation of export. To determine if loss of drug export was the mechanism behind the increased Pt accumulation observed in CTR2 knockdown cells, both the initial and subsequent phases of drug export were examined. Cells were pretreated with 30 μmol/L DDP for 1 hour followed by an immediate replacement of medium not containing drug. The total whole-cell Pt levels were measured by ICP-MS following 0, 0.5, 1, 2, 8, 30, and 240 minutes in drug-free medium. Figure 4C shows the percentage of Pt remaining in each type of cell as a function of efflux time. Whereas loss of CTR1 enhanced efflux, no change was detectable in either the early or late phases of DDP efflux when CTR2 was knocked down in either the CTR1+/+ or CTR1−/− cells.
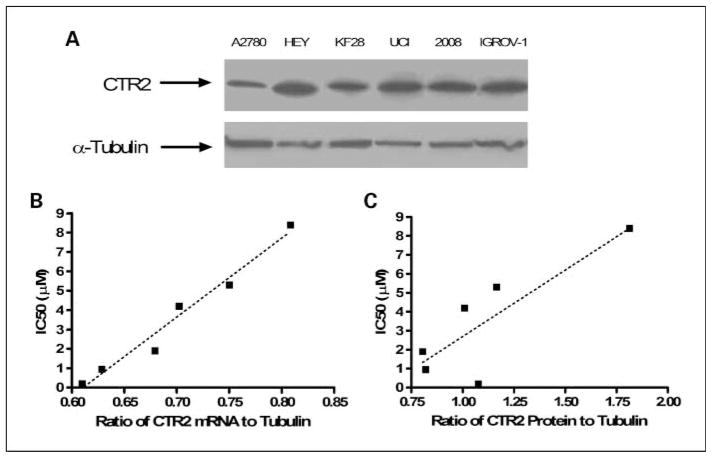
Relationship between hCTR2 expression and DDP sensitivity in ovarian cancer
Given that just a 55% to 33% reduction in CTR2 protein level was associated with a 2.0- to 3.2-fold increase in drug sensitivity in the mouse embryo CTR1+/+ and CTR1−/− fibroblasts, it was of interest to determine whether human ovarian cell lines that vary in sensitivity to DDP differ in their expression of CTR2. CTR2 protein expression levels were analyzed in six established human ovarian carcinoma cell lines of varying sensitivity to DDP. The IC50 for DDP was determined for each cell line by quantifying inhibition of growth by staining with sulforhodamine B. CTR2 mRNA expression was quantified using qRT-PCR with β-actin as a loading control. CTR2 expression was quantified by densitometry on triplicate Western blots on which α-tubulin expression was used as a loading control (Fig. 5A). Figure 5B and C shows that there was a significant correlation between CTR2 expression at both the mRNA (r2 = 0.97, P = 0.0003) and protein levels (r2 = 0.71, P < 0.04) and resistance to the cytotoxic effect of DDP. The higher the expression of hCTR2, the greater was the observed DDP IC50. This suggests that CTR2 expression may be one of the parameters that determine differences in DDP sensitivity in human ovarian carcinomas.
Fig. 5.
Relationship between CTR2 expression and DDP sensitivity in ovarian carcinoma cell lines. A, Western blot analysis of CTR2 in A2780, HEY, KF 28, 2008, and IGROV-1 cells. B, correlation between CTR2 mRNA levels and DDP IC50 of the six ovarian carcinoma cell lines as measured by qRT-PCR (r2 = 0.97, P = 0.0003). C, correlation between CTR2 protein levels and IC50 of the six ovarian carcinoma cell lines as determined byWestern blot analysis (r2 = 0.71, P < 0.04).
Discussion
Resistance to the cytotoxic effect of DDP is closely linked to reduced drug accumulation in human tumor cell lines (4, 15–21). This decrease in accumulation must be the result of either impaired drug influx, reduced intracellular sequestration, enhanced efflux, or a combination of these. Previous studies have documented that the Cu transporters can regulate all of these processes. Elimination of CTR1 reduces initial influx and results in substantial degrees of DDP resistance when measured both in vitro and in vivo (30, 42). Increased expression of the Cu efflux transporters ATP7A and ATP7B enhances resistance to DDP (43). ATP7A seems to function primarily to sequester DDP intracellularly (43–45), whereas ATP7B mediates platinum drug efflux (44) via a process that involves its transport into vesicles involved in the secretory pathway (45). The results of the current study indicate that CTR2 is also an important determinant of both sensitivity to the cytotoxic effect of DDP and its intracellular pharmacology.
To study the effect of CTR2 on the cellular pharmacology of the platinum drugs, we took advantage of a very powerful model and knocked down the expression of CTR2 in both CTR1+/+ and CTR1−/− mouse embryo fibroblasts. Elimination of CTR1 resulted in a substantial increase in resistance to DDP and CBDCA, as we have previously reported (30, 42). Whereas CTR2 mRNA levels were reduced by 82% to 88%, this resulted in a more modest 33% to 55% reduction in the CTR2 protein level. These sublines were selected in a medium that did not contain additional Cu, and it is possible that a more severe reduction in CTR2 is lethal, particularly in the absence of CTR1 function. These relatively modest reductions in CTR2 protein level led to a 2.0- to 3.2-fold increase in sensitivity to DDP irrespective of the presence or absence of CTR1. A similar result was observed for CBDCA. Thus, the effect of reducing CTR2 expression was not dependent on the CTR1 status of the cells. This is in contrast to its effect on sensitivity to the cytotoxic effect of Cu. Elimination of the expression of CTR1 produced the anticipated decrease in sensitivity to Cu; however, knockdown of CTR2 in the CTR1−/− cells had no further effect on sensitivity. These results support two conclusions. First, in the case of the platinum drugs, the effect of knocking down CTR2 seems to be independent of the status of CTR1. Second, CTR2 functions differently with respect to Cu and the platinum drugs.
To explore the mechanism by which loss of CTR2 increased cell sensitivity to the platinum drugs, we measured whole-cell drug accumulation at 5 minutes and 1 hour, the extent of DNA adduct formation, and the amount of DDP associated with the vesicle fraction of the cells by ICP-MS. Consistent with our prior studies (30), deletion of both alleles of CTR1 reduced the influx of DDP when measured at both 5 minutes and 1 hour, and this was accompanied by a proportional decrease in DNA adduct formation. Reduction of CTR2 expression had the opposite effect. Knockdown of CTR2 led to a ~2.1 to 3.5-fold increase in whole-cell Pt accumulation and DNA adduct formation, and it did so irrespective of whether CTR1 was expressed or not. The increase in whole-cell platinum accumulation and DNA adduct formation was similar to the magnitude of the change in cytotoxicity, suggesting that the hypersensitivity caused by loss of CTR2 was directly linked to increased accumulation. Knockdown of CTR2 produced a very similar change in cytotoxicity and drug accumulation for CBDCA, indicating that, despite the differences in the structure of DDP and CBDCA and their rates of aquation and reaction with nucleophilic targets, these drugs are affected similarly by CTR2.
As noted with respect to cytotoxicity, the knockdown of CTR2 had somewhat different effects on the cellular accumulation of DDP and CBDCA versus Cu. Several points are noteworthy. First, complete loss of CTR1 expression only reduced whole-cell Cu accumulation at 1 hour by 31%, indicating that there is another route of Cu accumulation other than just CTR1. Second, unlike the situation for DDP, the effect of knocking down CTR2 on Cu accumulation was dependent on the status of CTR1. Knockdown of CTR2 increased Cu accumulation only when CTR1 was also expressed. In the absence of CTR1 expression, the reduction in CTR2 produced only a small additional increase in uptake. Interestingly, the increase in DDP accumulation that accompanied knockdown of CTR2 was associated with an increase in cytotoxicity, whereas this was not true for Cu. These observations indicate that CTR2 interacts differently with DDP than with Cu.
Knockout of CTR1 has been shown to reduce the rate of DDP accumulation in both yeast and mammalian cells (28–30, 42). Because a reduction in uptake was observed at the earliest measurable time (5 minutes), this was interpreted as indicating an effect on influx. However, the detailed measurements of efflux made using ICP-MS in the current study reveal that there is a very rapid phase of DDP efflux, an observation that confirms results obtained using less sensitive atomic absorption spectroscopic measurements many years ago (46). The observation that loss of CTR1 resulted in a substantial increase in the rate of initial efflux indicates that CTR1 functions to retain DDP in the cell and raises the question of whether the impaired influx observed in CTR1−/− cells is actually due to an increased rate of efflux.
How can the effects of knocking down CTR2 on Cu uptake be explained in light of what is already known about the function of CTR1 and CTR2 in mammalian cells? CTR2 is primarily localized to the late endosome and lysosome compartments (36, 37) where, by inference from the results obtained in yeast, it is thought to participate in the export of Cu to the cytoplasm. However, in Cu-starved COS-7 cells transfected with a CTR2 expression vector, some CTR2 was observed at the plasma membrane and these cells exhibited enhanced Cu uptake but no change in Cu efflux (36), indicating that either CTR2 transports Cu across the plasma membrane or it enhances intracellular sequestration. We observed that knockdown of CTR2 in the CTR1+/+ cells increased Cu accumulation by 78% at 1 hour, indicating that CTR2 normally functions to limit the accumulation of Cu. The fact that this was not observed when CTR2 was knocked down in the CTR1−/− cells indicates that it was specifically dependent on CTR1. This suggests that CTR2 either regulates the level of CTR1 or its ability to mediate Cu influx. CTR2 has been reported to partially colocalize with CTR1 on intracellular vesicles of mammalian cells (37), consistent with possible regulatory role for the former by the latter.
The results reported here provide only an outline of how CTR1 and CTR2 modulate the accumulation of DDP and CBDCA. Because even DDP, the smaller of these two drugs, is too large to fit through the pore that trimeric CTR1 has been reported to form in the plasma membrane (47), and because DDP seems to trigger rapid macropinocytosis of CTR1 (48), our current hypothesis is that DDP and CBDCA bind to the extracellular domain of CTR1 and enter via an endocytotic process that delivers them to intracellular vesicles. One possibility is that CTR2 functions sequentially with CTR1 in the drug influx process by transporting DDP out of vesicles and into the cytoplasm. However, there are several problems with this model. First, one would expect knockdown of CTR2 to have little effect when CTR1 was not expressed and that a limited amount of DDP would enter the cell by endocytosis, whereas the actual observation was that the CTR2 knockdown increased DDP uptake in both CTR1+/+ and CTR1−/− cells. Second, knockdown of CTR2 had no effect (at all) on the absolute amount of DDP that accumulated in intracellular vesicles; changes in the fraction of DDP in the vesicular compartment were due to changes in uptake in other parts of the cell. Finally, how CTR2 might export DDP from intracellular vesicles is uncertain. Like CTR1, CTR2 may also form trimers (37) whose pore size is likely to be too small to accommodate DDP so that one would have to hypothesize some other mechanism by which CTR2 transfers DDP out of vesicles. The finding that knockdown of CTR2 enhanced the accumulation of DDP, at even just 5 minutes, suggests that CTR2 is restraining initial influx in some manner either through regulation of the uptake at the level of the plasma membrane or by regulating trafficking to intracellular sites. The fact that CTR2 has no effect on drug efflux lends credence to the possibility that CTR2 acts to mediate DDP influx rather than to restrain efflux.
Given that small changes in CTR2 expression produced relatively large changes in sensitivity due to the cytotoxic effect of DDP, we were interested in whether differences in CTR2 expression in human tumor cell lines were linked to differences in intrinsic sensitivity to DDP. In a panel of six human ovarian carcinoma cell lines, we found a significant correlation between DDP sensitivity and both CTR2 mRNA level (r2 = 0.97) and CTR2 protein level (r2 = 0.71). This provides the impetus for further studies focused on how CTR2 levels change during the acquisition of resistance that accompanies repeated exposure to DDP both in vitro and in vivo. The fact that modest reductions in the level of CTR2 protein were accompanied by quite large increases in sensitivity to both DDP and CBDCA suggests that CTR2 expression may also be a useful biomarker of initial responsiveness to therapy with these drugs in patients with ovarian cancer and that strategies directed at selectively modulating the expression of this transporter may be therapeutically useful.
Translational Relevance
Cisplatin (DDP) and carboplatin (CBDCA) are two of the most widely used chemotherapeutic agents. Despite years of clinical use, the mechanism by which these drugs enter tumor cells, are distributed within, and exported from cells remains poorly understood. We and others have previously documented that the copper transporters CTR1, ATP7A, and ATP7B and the intracellular chaperone ATOX1 have major effects on the cellular pharmacology of DDP and CBDCA. In this study, we show that another copper transporter, copper transporter 2 (CTR2), also regulates accumulation and cytotoxicity of these drugs. The magnitude of the effect is quite large. This result provides further evidence that the platinum-containing drugs use the copper homeostasis proteins to enter tumor cells and access key targets. Our findings also provide the basis for enhancing the efficacy of DDP and CBDCA through pharmacologic regulation of CTR2 expression.
Acknowledgments
Grant support: NIH grant CA095298 and Department of Defense grant W81XWH-08-1-0135.
We thank Dr. Dennis Thiele for generously providing the CTR1+/+ and CTR1−/− mouse embryo fibroblasts, Dr. Jessie Bertinato for the anti-CTR2 antibody, Angela Robles and Erin M. Drenning for editorial assistance, and Sakura Moua for technical assistance.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niedner H, Christen R, Lin X, Kondo A, Howell SB. Identification of genes that mediate sensitivity to cisplatin. Mol Pharmacol. 2001;60:1153–60. [PubMed] [Google Scholar]
- 3.Walker EM, Jr, Walker SM. Evolution of chemotherapy with platinum compounds. Ann Clin Lab Sci. 1999;29:263–74. [PubMed] [Google Scholar]
- 4.Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993;67:1171–6. doi: 10.1038/bjc.1993.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muggia FM, Los G. Platinum resistance: laboratory findings and clinical implications. Stem Cells. 1993;11:182–93. doi: 10.1002/stem.5530110304. [DOI] [PubMed] [Google Scholar]
- 6.Andrews PA, Howell SB. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990;2:35–43. [PubMed] [Google Scholar]
- 7.Schabel FM, Jr, Skipper HE, Trader MW. Concepts for controlling drug-resistant tumor cells. In: Mouridsen HT, Palshof T, editors. Breast cancer: experimental and clinical aspects. Oxford: Pergamon Press; 1980. pp. 199–212. [PubMed] [Google Scholar]
- 8.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 9.Sedletska Y, Giraud-Panis MJ, Malinge JM. Cisplatin is a DNA-damaging antitumour compound triggering multifactorial biochemical responses in cancer cells: importance of apoptotic pathways. Curr Med Chem Anti-Canc Agents. 2005;5:251–65. doi: 10.2174/1568011053765967. [DOI] [PubMed] [Google Scholar]
- 10.Manic S, Gatti L, Carenini N, Fumagalli G, Zunino F, Perego P. Mechanisms controlling sensitivity to platinum complexes: role of p53 and DNA mismatch repair. Curr Cancer Drug Targets. 2003;3:21–9. doi: 10.2174/1568009033333727. [DOI] [PubMed] [Google Scholar]
- 11.Crul M, Schellens J, Beijnen J, Maliepaard M. Cisplatin resistance and DNA repair. Cancer Treat Rev. 1997;23:341–66. doi: 10.1016/s0305-7372(97)90032-3. [DOI] [PubMed] [Google Scholar]
- 12.Chu G. Cellular responses to cisplatin. J Biol Chem. 1994;269:787–90. [PubMed] [Google Scholar]
- 13.Zorbas H, Keppler BK. Cisplatin damage: are DNA repair proteins saviors or traitors to the cell? Chembio-chem. 2005;6:1157–66. doi: 10.1002/cbic.200400427. [DOI] [PubMed] [Google Scholar]
- 14.Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. 1997;57:850–6. [PubMed] [Google Scholar]
- 15.Song IS, Savaraj N, Siddik ZH, et al. Roles of copper transporter ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and resistant cells. Mol Cancer Ther. 2004;3:1543–9. [PubMed] [Google Scholar]
- 16.Twentyman PR, Wright KA, Mistry P, Kelland LR, Murrer BA. Sensitivity to novel platinum compounds of panels of human lung cancer cell lines with acquired and inherent resistance to cisplatin. Cancer Res. 1992;52:5674–80. [PubMed] [Google Scholar]
- 17.Waud WR. Differential uptake of cis-diamminedichloro-platinum(II) in sensitive and resistant murine l1210 leukemia cell lines. Cancer Res. 1987;46:6549–55. [PubMed] [Google Scholar]
- 18.Teicher BA, Holden SA, Herman TS, et al. Characteristics of five human tumor cell lines and sublines resistant to cis-diamminedichloroplatinum(II) Int J Cancer. 1991;47:252–60. doi: 10.1002/ijc.2910470214. [DOI] [PubMed] [Google Scholar]
- 19.Oldenburg J, Begg AC, van Vugt MJH, et al. Characterization of resistance mechanisms to cis-diamminedichloroplatinum (II) in three sublines of the cc531 colon adenocarcinoma cell line in vitro. Cancer Res. 1994;54:487–93. [PubMed] [Google Scholar]
- 20.Kelland LR, Mistry P, Abel G, et al. Establishment and characterization of an in vitro model of acquired resistance to cisplatin in a human testicular nonseminomatous germ cell line. Cancer Res. 1992;52:1710–6. [PubMed] [Google Scholar]
- 21.Metcalfe SA, Cain K, Hill BT. Possible mechanisms for differences in sensitivity to cis-platinum in human prostate tumor cell lines. Cancer Lett. 1986;31:163–9. doi: 10.1016/0304-3835(86)90007-8. [DOI] [PubMed] [Google Scholar]
- 22.Fukuda M, Ohe Y, Kanzawa F, Oka M, Hara K, Saijo N. Evaluation of novel platinum complexes, inhibitors of topoisomerase I and II in non-small cell lung cancer (NSCLC) sublines resistant to cisplatin. Anticancer Res. 1995;15:393–8. [PubMed] [Google Scholar]
- 23.Rixe O, Ortuzar W, Alvarez M, et al. Oxaliplatin, tetraplatin, cisplatin, and carboplatin: spectrum of activity in drug-resistant cell lines and in the cell lines of the National Cancer Institute’s anticancer drug screen panel. Biochem Pharmacol. 1996;52:1855–65. doi: 10.1016/s0006-2952(97)81490-6. [DOI] [PubMed] [Google Scholar]
- 24.Naredi P, Heath DD, Enns RE, Howell SB. Cross-resistance between cisplatin and antimony in a human ovarian carcinoma cell line. Cancer Res. 1994;54:6464–8. [PubMed] [Google Scholar]
- 25.Shen D-W, Pastan I, Gottesman MM. Cross-resistance to methotrexate and metals in human cisplatin-resistant cell lines results from a pleiotropic defect in accumulation of these compounds associated with reduced plasma membrane binding proteins. Cancer Res. 1998;58:268–75. [PubMed] [Google Scholar]
- 26.Nose Y, Rees EM, Thiele DJ. Structure of the ctr1 copper trans’pore’ter reveals novel architecture. Trends Biochem Sci. 2006;31:604–7. doi: 10.1016/j.tibs.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Aller SG, Unger VM. Projection structure of the human copper transporter ctr1 at 6-A resolution reveals a compact trimer with a novel channel-like architecture. Proc Natl Acad Sci U S A. 2006;103:3627–32. doi: 10.1073/pnas.0509929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishida S, Lee J, Thiele DJ, Herskowitz I. Uptake of the anticancer drug cisplatin mediated by the copper transporter ctr1 in yeast and mammals. Proc Natl Acad Sci U S A. 2002;99:14298–302. doi: 10.1073/pnas.162491399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin X, Okuda T, Holzer A, Howell SB. The copper transporter ctr1 regulates cisplatin uptake in Saccharomyces cerevisiae. Mol Pharmacol. 2002;62:1154–9. doi: 10.1124/mol.62.5.1154. [DOI] [PubMed] [Google Scholar]
- 30.Larson CA, Blair BG, Safaei R, Howell SB. The role of the mammalian copper transporter 1 in the cellular accumulation of platinum-based drugs. Mol Pharmacol. 2009;75:324–30. doi: 10.1124/mol.108.052381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rees EM, Lee J, Thiele DJ. Mobilization of intracellular copper stores by the ctr2 vacuolar copper transporter. J Biol Chem. 2004;279:54221–9. doi: 10.1074/jbc.M411669200. [DOI] [PubMed] [Google Scholar]
- 32.Puig S, Lee J, Lau M, Thiele DJ. Biochemical and genetic analyses of yeast and human high affinity copper transporters suggest a conserved mechanism for copper uptake. J Biol Chem. 2002;277:26021–30. doi: 10.1074/jbc.M202547200. [DOI] [PubMed] [Google Scholar]
- 33.Bellemare DR, Shaner L, Morano KA, Beaudoin J, Langlois R, Labbe S. Ctr6, a vacuolar membrane copper transporter in Schizosaccharomyces pombe. J Biol Chem. 2002;18:18. doi: 10.1074/jbc.M206444200. [DOI] [PubMed] [Google Scholar]
- 34.Kampfenkel K, Kushnir S, Babiychuk E, Inze D, Van Montagu M. Molecular characterization of a putative Arabidopsis thaliana copper transporter and its yeast homologue. J Biol Chem. 1995;270:28479–86. doi: 10.1074/jbc.270.47.28479. [DOI] [PubMed] [Google Scholar]
- 35.Portnoy ME, Schmidt PJ, Rogers RS, Culotta VC. Metal transporters that contribute copper to metallochapersones in Saccharomyces cerevisiae. Mol Genet Genomics. 2001;265:873–82. doi: 10.1007/s004380100482. [DOI] [PubMed] [Google Scholar]
- 36.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L’Abbe MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in cos-7 cells. Biochem J. 2007;409:731–40. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 37.van den Berghe PV, Folmer DE, Malingre HE, et al. Human copper transporter 2 is localized in late endosomes and lysosomes and facilitates cellular copper uptake. Biochem J. 2007;407:49–59. doi: 10.1042/BJ20070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Petris MJ, Thiele DJ. Characterization of mouse embryonic cells deficient in the ctr1 high affinity copper transporter. J Biol Chem. 2002;277:40253–9. doi: 10.1074/jbc.M208002200. [DOI] [PubMed] [Google Scholar]
- 39.Monks A, Scudiero D, Skehan P, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83:757–65. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 40.Tjelle TE, Brech A, Juvet LK, Griffiths G, Berg T. Isolation and characterization of early endosomes, late endosomes and terminal lysosomes: their role in protein degradation. J Cell Sci. 1996;109:2905–14. doi: 10.1242/jcs.109.12.2905. [DOI] [PubMed] [Google Scholar]
- 41.Holzer A, Samimi G, Katano K, Naedermann W, Howell SB. The role of human copper transporter hctr1 in cisplatin uptake in human ovarian carcinoma cells. Proc Am Assoc Cancer Res. 2003;44:923. [Google Scholar]
- 42.Holzer AK, Manorek GH, Howell SB. Contribution of the major copper influx transporter ctr1 to the cellular accumulation of cisplatin, carboplatin, and oxaliplatin. Mol Pharmacol. 2006;70:1390–4. doi: 10.1124/mol.106.022624. [DOI] [PubMed] [Google Scholar]
- 43.Samimi G, Varki NM, Wilczynski S, Safaei R, Alberts DS, Howell SB. Increase in expression of the copper transporter ATP7A during platinum drug-based treatment is associated with poor survival in ovarian cancer patients. Clin Cancer Res. 2003;9:5853–9. [PubMed] [Google Scholar]
- 44.Katano K, Safaei R, Samimi G, Naerdemann W, Holzer A, Howell SB. Copper-transport P-type adenosine triphosphatase (ATP7B) modifies cisplatin resistance of carcinoma cells. Proc Am Assoc Cancer Res. 2002;43:423. [Google Scholar]
- 45.Safaei R, Larson BJ, Otani S, Rasmussen ML, Howell SB. Transport of cisplatin by the copper efflux transporter ATP7B. Mol Pharmacol. 2008;73:461–8. doi: 10.1124/mol.107.040980. [DOI] [PubMed] [Google Scholar]
- 46.Mann SC, Andrews PA, Howell SB. Short-term cis-diamminedichloroplatinum(II) accumulation in sensitive and resistant human ovarian carcinoma cells. Cancer Chemother Pharmacol. 1990;25:236–40. doi: 10.1007/BF00684878. [DOI] [PubMed] [Google Scholar]
- 47.Aller SG, Eng ET, De Feo CJ, Unger VM. Eukaryotic ctr copper uptake transporters require two faces of the third transmembrane domain for helix packing, oligomerization, and function. J Biol Chem. 2004;279:53435–41. doi: 10.1074/jbc.M409421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holzer AK, Howell SB. The internalization and degradation of human copper transporter 1 following cisplatin exposure. Cancer Res. 2006;66:10944–52. doi: 10.1158/0008-5472.CAN-06-1710. [DOI] [PubMed] [Google Scholar]