Abstract
The spread of HIV in sub-Saharan Africa continues largely unabated. To improve prevention interventions, a better understanding of the determinants of HIV infection is required. Conceptual frameworks can guide epidemiological investigation and prevent a misguided focus on single risk factors in isolation. Existing frameworks of HIV infection focus on transmission. However, the transmitting individual is rarely known. By contrast, data on individual HIV acquisition are available from longitudinal studies and tests for recent HIV infection. From the perspective of individuals susceptible to HIV, it is important to distinguish between factors determining the individual’s biological disposition and sexual behavior and community-level factors, which can affect both HIV acquisition and the likelihood that a sex partner chosen from a community will be infected with HIV and transmit the infection. We propose a framework that takes the susceptible individual as a starting point and links distal, proximate and biological determinants of HIV infection at both the individual and the community level. We describe three necessary ingredients for the use of the framework (identification of the relevant community, multilevel analysis and methods for causal inference).
Keywords: community, epidemiology, HIV acquisition and transmission, multilevel
A total of 33 million people were living with HIV worldwide in 2007, with the majority (23 million) living in sub-Saharan Africa [1]. Antiretroviral treatment (ART) coverage in sub-Saharan Africa reached 30% in December 2007, an increase of 9 percentage points from the previous year [2]. By contrast. the spread of HIV continues largely unabated. UNAIDS estimates that 1.7 million new HIV infections occurred in sub-Saharan Africa in 2007 [1]. Recent data from South Africa, the country with the largest number of HIV-infected people worldwide [3], indicate that HIV incidence in some of the communities with the highest HIV prevalence in the country has remained stable at very high levels over the past 5 years (>three per 100 person-years) [4]. Effectiveness and reach of interventions to prevent HIV must urgently improve.
In order to develop and implement effective prevention interventions, it is crucial to understand the determinants of HIV infection. Many characteristics of individuals and their communities contribute to the risk of HIV acquisition and transmission, and multiple causal pathways link demographic, socioeconomic, behavioral and biological variables to infection [5]. Despite many insights gained from studies investigating individual risk factors for HIV, much is still unknown regarding which factors determine epidemic spread and why populations have experienced very different epidemics [6–13]. Population-level factors, such as demographic composition, fertility and mortality patterns, urbanization and migration, are well known to affect epidemic spread among individuals [14–16]. Recently, contradictory findings of three large, randomized, community-level trials investigating the effectiveness of treating sexually transmitted infections (STIs) in order to reduce HIV infection rates [17–19] were attributed to the complex multilevel nature of the HIV epidemic [20,21]. However, empirical studies of the complex relationships between factors that give rise to HIV infection remain rare [22–24]. The few such studies that do exist commonly focus on behavioral outcomes rather than biological end points [25]. Most other epidemiological studies of HIV infection focus on the individual (even though the community in which the individual is embedded may substantially affect risk of infection), are cross-sectional and ignore the evolution of STI epidemics over time, and fail to account for factors affecting transmission in addition to those determining acquisition of infection [26].
Importance of the local community in epidemic spread
In reality, the HIV epidemic is composed of a series of interlinked subepidemics that operate within different social and geographical spaces (all initiated at different times and progressing at different rates to different eventual saturation points) that make up the composite epidemic in any given area. Therefore, it is important to distinguish between risk factors that determine the individual’s own sexual behavior patterns, and risk factors that determine the level of infection (and the likelihood that a new infection is acquired in a sexual encounter) in the community of people from which the individual is likely to choose a sexual partner. Most risk and health-promoting behaviors tend to cluster in specific communities [27], and are associated with both context and composition of the communities to which individuals belong [21]. Community, place and ‘social fact’ [28] put individuals ‘at risk of risks’ [29]. As such, the community is increasingly being seen as critical to understanding the spread of the virus and key to prevention efforts [27,30–33]. For instance, a recent study in a circumscribed geographical area in rural South Africa finds ‘the existence of several localized HIV epidemics of varying intensity that are partly contained within geographically defined communities’ [27].
While interventions that focus on the individual can achieve some measure of success [34,35], evidence is increasing that intervention effectiveness can be substantially increased if the intervention is targeted to specific communities and the community is actively engaged in the prevention effort [9,31,36,37]. In settings where prevention efforts have had significant and sustained effects, for example, in Uganda, communities welcomed ‘open talk’ regarding HIV and were willing to support those infected with HIV [38]. In order to inform and focus interventions, a better understanding of community determinants of infection is critical.
Conceptual frameworks of HIV infection
To identify determinants of disease, epidemiological studies often include large numbers of variables [39,40]. Without a coherent conceptual framework, such studies can fail to shed light on the relative importance of the different variables and how they are connected to each other along causal pathways to health outcomes. This problem is likely in studies of HIV and other STIs because individual choices determine contacts between potential sexual partners, the social and gender norms governing sexual behaviors are complex and the distribution of risk factors is highly variable within populations [5]. As Garnett highlights in an editorial, a theoretical framework is required in order to systematically examine how different HIV risk factors are connected along pathways determining HIV infection [26]. It is ‘important that the relationship between social and cultural determinants, such as membership of a church, can be understood in terms of behaviors that directly determine risk’ [41].
In demography, proximate-determinants frameworks have been extensively used to structure analyses of fertility [42], following the work of Davis and Blake [43] and Bongaarts [44]. Essentially, these frameworks use knowledge of the biology of fertility to identify the ‘intermediate variables through which any social factors influencing the level of fertility must operate’ [43]. Therefore, poximate-determinants frameworks serve the important purpose of separating behavioral (or proximate) determinants from biological determinants and outcomes, on the one hand, and from socioeconomic and cultural (or distal) determinants, on the other hand. While such frameworks do not specify the individual causal chains from distal determinants to outcomes (which could run, for instance, from religious belief to sexual debut to fertility), they identify the order of sets of factors on such causal chains (i.e., religious belief must always operate through a defined set of behaviors, which include sexual debut, in order to influence fertility)1.
The power of this theoretical approach is apparent in the variety of purposes for which it has been used, which include studies of the determinants of fertility within a country, cross-country comparison of the contribution of different proximate determinants to fertility and analyses of the causes of fertility time trends [45]. Based on the proximate-determinants framework of fertility, Mosley and Chen developed a framework of child mortality [46], which was later modified by Van Norren et al. (to distinguish clearly between biological and mixed behavioral–biological determinants of child mortality) [47] and expanded by Mosley and Becker (to account for the interaction of multiple diseases affecting child survival) [48] and Becker and Black (to include the efficacy and coverage of interventions) [49].
Boerma and Weir proposed a proximate-determinants framework of HIV transmission, consisting of distal, proximate, and biological determinants [45]2. The biological determinants in this framework comprise the three factors that affect the basic reproductive number of HIV [45] (which is the average number of new infections resulting from one primary infection in a wholly susceptible population [50]): the average rate of sexual contacts of an HIV-infected individual with susceptible individuals, the probability of infecting a susceptible individual during one sexual contact and the average duration of the infectious period. Proximate determinants are either behavioral (such as coital frequency within a sexual relationship or ART uptake), or biological (such as the presence of other STIs that change HIV transmission risks, for example, infection with herpes simplex virus 2 or Trichomonas vaginalis) that link social, economic, demographic or cultural determinants to the three biological determinants of HIV infection.
Modified proximate-determinants framework of HIV infection
While the framework developed by Boerma and Weir is a useful starting point to structure the analysis of HIV infection, it has three limitations. First, it is a framework of HIV transmission. In the absence of comprehensive molecular databases that allow analyses of chains of HIV-transmission events [51,52]; however, data on HIV-transmitting individuals are rarely available, limiting the applicability of the framework for most epidemiological analyses. Most analyses of HIV incidence use data on HIV-acquiring individuals, for instance, from population-based HIV surveillance [4,13], prospective cohort studies [53,54], controlled trials [55,56] or applications of tests that distinguish between recent and nonrecent HIV infection in cross-sectional HIV surveys [57,58]. Second, the framework does not clearly differentiate between individual- and community-level effects, classifying all community-level effects as distal determinants. Third, the framework does not consider feedback effects from HIV infection to proximate determinants. However, it seems plausible that individuals adjust their behavior in response to knowledge about changes in HIV prevalence in the community [59,60].
In a modified proximate-determinants framework, we address the first limitation by taking an individual who is susceptible to HIV as the starting point. In a simple thought experiment, we introduce the susceptible individual into a community where he or she engages in random sexual acts with community members who can be either HIV infected or susceptible. Three biological variables determine the individual’s risk of HIV acquisition during the time he/she is present in the community and susceptible to HIV:
The duration of time in this community
The rate of sexual contact with infected individuals over time
The probability of acquiring HIV during one sexual contact with an infected individual
Summing the HIV infection risks across all susceptible individuals in a community in a period of time yields the expected number of HIV acquisitions.
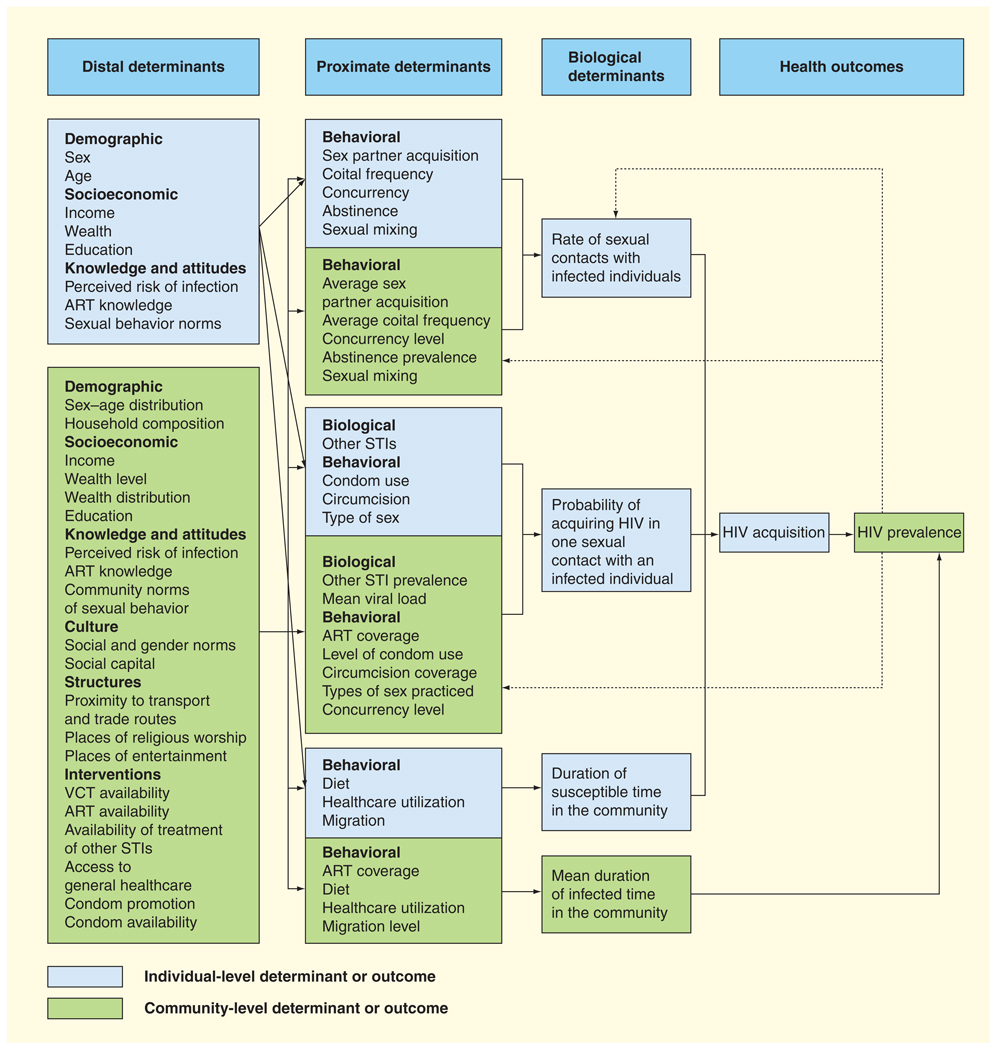
We address the second limitation by separating the individual-level determinants of the three biological variables from the community-level determinants (with blue indicating the individual level and green indicating the community level in Figure 1). The rate of sexual contacts with infected individuals and the probability of acquiring HIV in one sexual contact with an infected individual are functions of proximate determinants operating at both the individual and the community level. Many of the individual-level proximate determinants have community-level counterparts. These community variables are constructed by mathematically summarizing the characteristics of individuals that make up the local community. Under an assumption of random sexual acts by a susceptible individual with HIV-infected people, the community-level counterparts of the proximate determinants are the means of variables across all community members. If we relax the assumption that sexual acts between the newly introduced HIV-infected individual and the other community members are random and allow for preferential sexual mixing of the HIV-infected individual with certain strata of the community population, the mean can be replaced by a weighted average of the strata means, where the weights represent the probabilities of sexual mixing with individuals in the strata. Many of the distal determinants at the community level do not have individual-level counterparts (e.g., ART availability or condom promotion).
Figure 1. Proximate-determinants framework of HIV acquisition.
ART: Antiretroviral treatment; STI: Sexually transmitted infection; VCT: Voluntary counseling and testing.
Proximate determinants at the community level capture different aspects of HIV transmission. While the transmitting individual is usually not known in the data available, the proximate determinants of transmission can commonly be measured and aggregated to the community level in order to investigate transmission. For instance, the prevalence of STIs in (HIV-infected) community members can proxy for the influence of STI on HIV transmission (whereas the presence of an STI in an HIV-susceptible individual captures the influence on HIV acquisition). Concurrency levels in the community affect the average rate of sexual contact between susceptible and infected individuals (and, thus, the speed of epidemic spread), because concurrency ensures that ‘the infectious agent is no longer trapped in a monogamous relationship after transmission occurs, but can spread immediately beyond this relationship to infect others’ [61]. Concurrency in the community can also influence the probability of transmission because ‘under concurrency, the virus can jump across each concurrent connection available during the peak infectious period’ [61], which occurs shortly after HIV acquisition when the body’s immune response has not yet developed to effectively reduce viral load [62]. ART coverage in the community can influence both the probability of transmission (because ART reduces viral load levels in HIV-infected community members) and HIV prevalence (because ART prolongs the lives of HIV-infected community members and, thus, the duration of the infectious time in the community). All else equal, HIV prevalence affects the rate of sexual contacts that susceptible individuals have with HIV-infected members of their community (Figure 1). Note that Figure 1 shows only a selection of all the possible proximate and distal determinants of HIV infection at the individual and the community level. The reader can add further proximate determinants (e.g., the time gap between sexual partnerships [63] or the presence of ‘super spreader’ groups in the community [64]) by analyzing how the factor could affect the different biological determinants and add distal determinants (e.g., income distribution in the community) and by considering their influence on proximate determinants.
Last, we address the third limitation by suggesting feedback loops from HIV acquisition to proximate determinants of HIV acquisition (as dotted arrows in Figure 1). HIV acquisition increases HIV prevalence. It is possible that changes in HIV prevalence affect behavior. As HIV-susceptible individuals learn about increasing HIV prevalence in their community, they may decide to reduce their sexual risk of acquisition, for instance, by decreasing the number of sex partners [59] or increasing condom usage [60].
Using the proximate-determinants framework to analyze determinants of HIV infection: the ingredients
Several ingredients are necessary in order to apply the proximate-determinants framework to the analysis of causes of HIV acquisition. First, a community of potential sexual partners of HIV-susceptible individuals at risk of acquiring HIV needs to be identified. Second, multilevel analysis must be used because proximate and distal determinants exist at the level of both the individual and the community. Third, statistical approaches of causal inference should be used since the framework consists of multiple hypothesized pathways of causation running from distal determinants through proximate and biological determinants, to health outcome.
Identification of the relevant community
In our framework, the community is loosely defined as the group of people from which an individual is likely to choose a sexual partner. For practical reasons, in epidemiological analyses, communities are usually defined on the basis of administrative geographical boundaries (e.g., census areas and clinic catchments) because individuals are more likely to choose sexual partners from their immediate neighborhood in comparison to neighborhoods further away. For example, empirical analyses have shown that the STIs tend to spatially cluster in geographically defined communities [27,65–69], and ‘place’ effects in HIV epidemiology have been well documented [70–72]. In addition, recent work suggests that the geography of sexually highly active groups is relatively constant even though the composition of those groups is unstable, with individuals transitioning between groups [73,74]. While definitions of community based on administrative geographical boundaries may, in some situations, capture community-level phenomena, they may fail to do so in other situations, because the administrative boundaries of communities or neighborhoods differ from those within which individuals conduct their social and sexual lives. As Kawachi and Subramanian state ‘indeed, identifying ‘true’ neighborhood differences also requires identifying true neighborhoods, an aspect on which much of the applied work remains largely silent’ [75].
Moreover, in some settings meaningful communities cannot be created on the basis of geographical boundaries, requiring more nuanced approaches to identify communities. For instance, in one area in rural KwaZulu-Natal, South Africa, the population is widely dispersed over the landscape and not concentrated into villages or compounds as in many other parts of Africa [27]. In this setting, a novel geographical approach has therefore been applied to measure community-level variables. Community-level HIV prevalence was estimated using Gaussian-weighted average HIV status in a 3-km search radius around each individual. Thus, the weights given to each member of the ‘community’ in the radius decreased with distance from the index individual [27].
Multilevel analysis
Multilevel analysis is appropriate for data with nested sources of variability, such as data on individuals within communities [25]. It allows quantification of the clustering of outcomes at lower-levels (such as individual HIV acquisition) within higher-level units (such as geographical communities), and attribution of any such clustering to contextual factors (such as the socioeconomic status of the community or the presence of services in the community) or to individual composition of the higher-level units (such as the socioeconomic status of the individual members of a community). Multilevel analysis can be used further to examine pathways running across different levels [76]. Higher-level factors can modify or confound relationships between variables measured at lower-levels, and lower-level factors can be on pathways from higher-level influences to outcomes. Only when variables at different levels are brought together and the variability within and between higher-level units is taken into account, can fallacies in inference be avoided [77]. In HIV epidemiology, multilevel analysis can be used to examine how putative determinants of HIV acquisition at both the community and the individual level affect HIV acquisition, how determinants at both levels interact and how determinants at both levels contribute to community–community differences in HIV-acquisition rates.
Causal inferences
Our framework comprises of multiple hypothesized causal pathways from distal to proximate determinants and from proximate to biological determinants. An ideal approach to investigate causal effects is randomized, controlled trials (RCTs). A number of RCTs have been carried out or are currently underway in sub-Saharan Africa to investigate the effect on HIV acquisition of interventions at the level of proximate determinants (such as circumcision [56,78], use of microbicide gel during sexual intercourse [79] or STI treatment [80]) or distal determinants (such as a ‘microfinance program with a gender and HIV training curriculum’ [81] or ‘community-based HIV mobile voluntary counseling and testing, community mobilization and post-test support services’ [82]). In addition, RCTs in sub-Saharan Africa have examined whether interventions can influence proximate determinants of HIV acquisition [83].
However, RCTs investigating the effects of proximate or distal determinants on HIV acquisition are costly and complex to organize, and commonly require follow-up for several years before results are available. Moreover, it will be impossible to conduct RCTs studying those determinants that are beyond scientists’ sphere of influence (such as the sex–age composition of the population). Finally, unlike relationships uncovered in RCTs of proximate biological factors (such as circumcision), relationships identified in RCTs of proximate behavioral factors in one setting may not be generalizable to other settings, because such relationships are likely to be modified by many distal determinants that differ substantially across settings in sub-Saharan Africa (such as traditional practices, social and gender norms and religious beliefs). Therefore, it is unlikely that RCTs will ever examine more than a small subset of the determinants of HIV acquisitions relevant in any given setting in sub-Saharan Africa. Analyses of many of the causal pathways of HIV acquisition will have to use data generated in observational studies. In epidemiology, observational studies on the causes of HIV acquisition usually rely on observable variables to control for confounding, and on longitudinal follow-up of susceptible individuals to reduce the potential for reverse causality from HIV infection to the determinants under investigation [13,24,84]. In some situations, the strength of causal inferences from longitudinal observational data on HIV acquisition could be further increased with approaches that were developed in the past 30 years in statistics and economics [85–87], but are rarely used in epidemiological analyses – even though they fit in perfectly with the counterfactual model underlying epidemiological thinking. Such approaches include using selection models to control for sample selection on unobservable variables [88,89], and cause–effect estimation with instrumental variables to control for reverse causality, omitted covariates or error in the measurement of covariates [85,90].
Application
No previous study has used the proximate-determinants framework outlined here to analyze the relative importance of factors affecting HIV acquisition. Two recent studies (by Lewis et al. [5] and Lopman et al. [24]) have applied the proximate-determinants framework developed by Boerma and Weir. These two studies are noteworthy for a number of reasons; here, we discuss them for the following two reasons. First, they serve as examples of real-life applications of proximate-determinants approaches in general. Second, we use them to underline the fundamental differences – both in concept and method – between analyses based on the Boerma–Weir framework and the types of studies that we propose. Both studies use data from the Manicaland HIV/Sexually Transmitted Disease Prevention Project, a longitudinal, population-based cohort study. They include in their analyses similar sets of variables hypothesized to be proximate determinants of HIV infection (e.g., years of sexual activity, number of partners, partnership characteristics, STI, condom use and HIV prevalence among the opposite sex in the community) and distal determinants (e.g., age, marital status, religion, education, work status, socioeconomic status, beer hall visits, paying for sex, previous HIV test, migration and community type). Lewis et al. analyze the influence of the hypothesized determinants on HIV serostatus, while Lopman et al. examine the influence of the determinants on HIV acquisition. Certainly, in order to investigate potential determinants of HIV infection, it is the more powerful approach to study HIV acquisition over time in cohorts of individuals observed after an initial negative HIV test than to compare cross-sectional associations between HIV status and covariates, since the former approach avoids the reverse-causality biases that the latter is likely to suffer from because HIV status affects covariates, such as socioeconomic status, educational attainment or sexual behavior [13]. It is worth noting that while Lopeman et al. take the proximate-determinants framework of HIV transmission developed by Boerma and Weir as their starting point, they implicitly adopted the perspective of acquisition, as we propose. The most fundamental difference between the analyses we propose and the two studies based on the Boerma–Weir framework is the way in which community effects are incorporated. Conceptually, Lopman et al. do not consider that several of their variables measured at the individual level have community-level counterparts that could affect HIV acquisition over and above the effects of the individual-level variables. For instance, they could have aggregated their individual-level education variable to the level of the community of sex partners identified through the partnership characteristics of each individual, in order to capture the effect of education in persons who can potentially transmit HIV on the acquisition hazard.
Analytically, they use single-level regression analyses, even though they include variables measured at the community level (community type and HIV prevalence among the opposite sex in the community) [5,24]. This choice has several disadvantages. First, single-level analysis does not account for the clustering of HIV infection in communities and, thus, will lead to underestimates of the variance and false inferences regarding the significance of variables predicting HIV infection. Moreover, single-level analyses does not allow a quantification of the level of clustering of HIV in communities, which can inform on the role of context in epidemic spread. For instance, Lopman et al. could have quantified the level of clustering of HIV acquisition in communities and estimated the proportion of this clustering explained by community type and HIV prevalence, while adjusting for the observed differences in community composition. Finally, only multilevel analyses would have allowed a rigorous investigation of cross-level effects. For instance, Lopman et al. could have investigated whether the community or the community type modifies the relationship between age and HIV acquisition. As these examples demonstrate, adding a community layer to a proximate-determinants framework (as laid out schematically in Figure 1) can substantially improve the power of proximate-determinants frameworks in examining causal pathways of HIV acquisition.
Conclusion & future perspective
Without theoretical structure, the complex ‘web of causation’ [91] that determines health outcomes makes analyses difficult. Such structure is particularly important for the study of the causes of HIV acquisition because multiple pathways connect social norms and socioeconomic circumstances to the sexual behaviors and biological predispositions that lead to infection [5]. We propose a modified proximate-determinants framework of HIV infection, linking distal determinants at both the individual level (socioeconomic and demographic variables, knowledge and attitudes) and the community level (socioeconomic and demographic variables, knowledge and attitudes, structures and interventions) to proximate determinants. Distal determinants must operate through behavioral and biological proximate determinants in order to affect the biological variables that give rise to HIV acquisition. Proximate determinants exist at both the individual level (such as the STI of an individual susceptible to HIV) and the community level (such as the community prevalence of concurrency). The proposed framework can serve to structure epidemiological thinking and constitutes a useful tool to design analyses that include both individual risk factors of HIV acquisition and community-level risk factors of HIV transmission and spread.
Executive summary
Introduction
A total of 33 million people worldwide were living with HIV in 2007, with the majority (23 million) in sub-Saharan Africa.
In order to develop and implement effective HIV-prevention interventions, it is crucial to understand who is at risk and why. Much is still unknown regarding which factors determine epidemic spread and why populations have experienced very different epidemics.
A sound theoretical framework is vital in order to systematically examine how different HIV risk factors are connected along causal pathways that determine HIV infection. Existing conceptual frameworks that focus on HIV transmission as the outcome are of limited value since the individual who transmits the infection is rarely known.
Importance of the local community in epidemic spread
The HIV epidemic consists of interlinked subepidemics in different social and geographical communities.
From the perspective of individuals susceptible to HIV, it is particularly important in any framework to distinguish between risk factors that determine the individual’s own sexual behavior patterns and biological disposition (impacting on an individual’s risk of acquisition), and community-level risk factors that determine the likelihood that a partner chosen from that community will be HIV infected and transmit the infection in a sexual encounter.
Modified proximate-determinants framework of HIV infection
We propose a framework that takes the susceptible individual as a starting point and links distal determinants of HIV acquisition to proximate and biological determinants.
Community-level effects are incorporated at the distal, proximate and biological levels. Proximate determinants at the community level capture different aspects of HIV transmission.
The framework incorporates some behavioral feedback mechanisms in response to changes in HIV prevalence in the community.
Three ingredients are necessary to apply the proximate-determinants framework to the analysis of causes of HIV acquisition: identification of the relevant community of potential sexual partners of the susceptible individuals, multilevel analysis and statistical approaches of causal inference.
Conclusion
Our modified proximate-determinants framework of HIV infection constitutes a useful tool to design and structure causal pathway analyses that include individual-level risk factors of HIV acquisition and community-level factors that determine HIV transmission.
Footnotes
Different authors use different terminologies for the different types of determinants in proximate-determinants frameworks. In our usage, along causal pathways of HIV acquisition, ‘distal determinants’ are farther removed from the outcome of HIV acquisition than ‘proximate determinants’, and ‘proximate determinants’ are farther removed from the outcome than ‘biological determinants’. Other authors use the word ‘underlying’ instead of the word ‘distal’ [45].
Another framework, the ‘Social Epidemiology of Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome’ [92], conceptualizes risk factors of HIV at the individual, social and structural level. Individual factors include biologic, demographic, behavioral and socioeconomic variables that may influence the risk of HIV acquisition. Social-level factors include social capital, social networks, neighborhood effects and cultural context. Structural-level factors include demographic change, war, laws, policies, violence and discrimination. There are ‘extensive linkages between factors at all levels’, which lead to the observed epidemic patterns [93]. The social-epidemiology framework may be a useful tool for structuring the thinking about how different social and biological factors influence HIV infection [93]. However, its applicability in conceptualizing and identifying causal pathways of infection is limited. For example, all neighborhood influences are classified as social factors (along with cultural context, social capital and social networks) and feedback effects are not incorporated.
Financial & competing interests disclosure
Till Bärnighausen and Frank Tanser are supported by grant 1R01-HD058482-01 from the National Institute of Child Health and Human Development (NICHD). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.UNAIDS. Geneva, Switzerland: UNAIDS the Joint United Nations Programme on HIV/AIDS; 2007 AIDS Epidemic Update. 2007
- 2.WHO/UNAIDS/UNICEF. Geneva, Switzerland: WHO; Towards Universal Access: Scaling Up Priority HIV/ AIDS Interventions in the Health Sector. 2008
- 3.UNAIDS/WHO. Geneva, Switzerland: UNAIDS; Sub-Saharan Africa: AIDS Epidemic Update Regional Summary. 2008
- 4.Bärnighausen T, Tanser F, Newell M-L. Lack of a decline in HIV incidence in a rural community with high HIV prevalence in South Africa, 2003–2007. AIDS Res. Hum. Retroviruses. 2009;25(4):405–409. doi: 10.1089/aid.2008.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis JJ, Donnelly CA, Mare P, Mupambireyi Z, Garnett G, Gregson S. Evaluating the proximate determinants framework for HIV infection in rural Zimbabwe. Sex. Transm. Infect. 2007;83 Suppl. 1:i61–i69. doi: 10.1136/sti.2006.023671. [DOI] [PubMed] [Google Scholar]; ▪ Uses the Boerma–Weir proximate-determinants framework to analyze factors associated with HIV status.
- 6.Asamoah-Odei E, Garcia Calleja JM, Boerma JT. HIV prevalence and trends in sub-Saharan Africa: no decline and large subregional differences. Lancet. 2004;364(9428):35–40. doi: 10.1016/S0140-6736(04)16587-2. [DOI] [PubMed] [Google Scholar]
- 7.Auvert B, Buve A, Ferry B, et al. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. AIDS. 2001;15 Suppl. 4:S15–S30. doi: 10.1097/00002030-200108004-00003. [DOI] [PubMed] [Google Scholar]
- 8.Buve A, Bishikwabo-Nsarhaza K, Mutangadura G. The spread and effect of HIV-1 infection in sub-Saharan Africa. Lancet. 2002;359(9322):2011–2017. doi: 10.1016/S0140-6736(02)08823-2. [DOI] [PubMed] [Google Scholar]
- 9.Manhart LE, Holmes KK. Randomized controlled trials of individual-level, population-level, and multilevel interventions for preventing sexually transmitted infections: what has worked? J. Infect. Dis. 2005;191 Suppl. 1:S7–S24. doi: 10.1086/425275. [DOI] [PubMed] [Google Scholar]
- 10.Boerma JT, Gregson S, Nyamukapa C, Urassa M. Understanding the uneven spread of HIV within Africa: comparative study of biologic, behavioral, and contextual factors in rural populations in Tanzania and Zimbabwe. Sex. Transm. Dis. 2003;30(10):779–787. doi: 10.1097/01.OLQ.0000078820.62897.A6. [DOI] [PubMed] [Google Scholar]
- 11.Hayes R, Weiss H. Understanding HIV epidemic trends in Africa. Science. 2006;311(5761):620–621. doi: 10.1126/science.1124072. [DOI] [PubMed] [Google Scholar]
- 12.Morison L. The global epidemiology of HIV/AIDS. Br. Med. Bull. 2001;58(1):7–18. doi: 10.1093/bmb/58.1.7. [DOI] [PubMed] [Google Scholar]
- 13.Bärnighausen T, Hosegood V, Timaeus IM, Newell ML. The socioeconomic determinants of HIV incidence: evidence from a longitudinal, population-based study in rural South Africa. AIDS. 2007;21 Suppl. 7:S29–S38. doi: 10.1097/01.aids.0000300533.59483.95. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Good example of individual-level analysis of distal determinants of HIV acquisition.
- 14.Caldwell JC. Rethinking the African AIDS epidemic. Popul. Dev. Rev. 2000;26(1):117–135. [Google Scholar]
- 15.Quinn TC. Population migration and the spread of types 1 and 2 human immunodeficiency viruses. Proc. Natl Acad. Sci. USA. 1994;91(7):2407–2414. doi: 10.1073/pnas.91.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serwadda D, Wawer MJ, Musgrave SD, Sewankambo NK, Kaplan JE, Gray RH. HIV risk factors in three geographic strata of rural Rakai District, Uganda. AIDS. 1992;6(9):983–989. doi: 10.1097/00002030-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Grosskurth H, Mosha F, Todd J, et al. Impact of improved treatment of sexually transmitted diseases on HIV infection in rural Tanzania: randomised controlled trial. Lancet. 1995;346(8974):530–536. doi: 10.1016/s0140-6736(95)91380-7. [DOI] [PubMed] [Google Scholar]
- 18.Kamali A, Carpenter LM, Whitworth JA, Pool R, Ruberantwari A, Ojwiya A. Seven-year trends in HIV-1 infection rates, and changes in sexual behaviour, among adults in rural Uganda. AIDS. 2000;14(4):427–434. doi: 10.1097/00002030-200003100-00017. [DOI] [PubMed] [Google Scholar]
- 19.Wawer MJ, Sewankambo NK, Serwadda D, et al. Rakai Project Study Group. Control of sexually transmitted diseases for AIDS prevention in Uganda: a randomised community trial. Lancet. 1999;353(9152):525–535. doi: 10.1016/s0140-6736(98)06439-3. [DOI] [PubMed] [Google Scholar]
- 20.Korenromp EL, White RG, Orroth KK, et al. Determinants of the impact of sexually transmitted infection treatment on prevention of HIV infection: a synthesis of evidence from the Mwanza, Rakai, and Masaka intervention trials. J. Infect. Dis. 2005;191 Suppl. 1:S168–S178. doi: 10.1086/425274. [DOI] [PubMed] [Google Scholar]
- 21.Aral SO, Padian NS, Holmes KK. Advances in multilevel approaches to understanding the epidemiology and prevention of sexually transmitted infections and HIV: an overview. J. Infect. Dis. 2005;191 Suppl. 1:S1–S6. doi: 10.1086/425290. [DOI] [PubMed] [Google Scholar]; ▪▪ Important article reviewing the use of multilevel analysis in understanding the epidemiology of sexually transmitted infections and HIV.
- 22.Broome KM, Joe GW, Simpson DD. HIV risk reduction in outpatient drug abuse treatment: individual and geographic differences. AIDS Educ. Prev. 1999;11(4):293–306. [PubMed] [Google Scholar]
- 23.Rosel J, Oliver JC, Jara P, Caballer A. A multilevel time-series model for the incidence of AIDS cases in Spain. Health Place. 2000;6(4):309–317. doi: 10.1016/s1353-8292(00)00012-5. [DOI] [PubMed] [Google Scholar]
- 24.Lopman B, Nyamukapa C, Mushati P, et al. HIV incidence in 3 years of follow-up of a Zimbabwe cohort – 1998–2000 to 2001–2003: contributions of proximate and underlying determinants to transmission. Int. J. Epidemiol. 2008;37(1):88–105. doi: 10.1093/ije/dym255. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Uses the Boerma–Weir proximate-determinants framework to analyze factors determining HIV acquisition.
- 25.Diez Roux AV, Aiello AE. Multilevel analysis of infectious diseases. J. Infect. Dis. 2005;191 Suppl. 1:S25–S33. doi: 10.1086/425288. [DOI] [PubMed] [Google Scholar]; ▪▪ Provides an overview of the statistical theory and rationale for analyses that incorporate group-level effects in infectious disease epidemiology.
- 26.Garnett GP. The geographical and temporal evolution of sexually transmitted disease epidemics. Sex. Transm. Infect. 2002;78 Suppl. 1:i14–i19. doi: 10.1136/sti.78.suppl_1.i14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanser F, Bärnighausen T, Cooke GS, Newell M-L. Localized spatial clustering of HIV infections in a widely disseminated rural South African epidemic. Int. J. Epidemiol. 2009 doi: 10.1093/ije/dyp148. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Uses a novel spatial conceptualization of the local community to identify several localized HIV epidemics of varying intensity within a relatively small geographical area in rural South Africa.
- 28.Durkheim E. The Rules of Sociological Method. NY, USA: Free Press of Glencoe; 1964. [Google Scholar]
- 29.Link BG, Phelan J. Social conditions as fundamental causes of disease. J. Health Soc. Behav. 1995;35:80–94. [PubMed] [Google Scholar]
- 30.Padian NS, Aral SO, Holmes KK. Individual, group and population approaches to STD/HIV prevention. In: Wasserheit J, editor. Sexually Transmitted Diseases. NY, USA: McGraw-Hill; 1999. pp. 1231–1238. [Google Scholar]
- 31.Maticka-Tyndale E, Brouillard-Coylea C. The effectiveness of community interventions targeting HIV and AIDS prevention at young people in developing countries. World Health Organ. Tech. Rep. Ser. 2006;938:243–285. discussion 317–341. [PubMed] [Google Scholar]
- 32.Wegbreit J, Bertozzi S, DeMaria LM, Padian NS. Effectiveness of HIV prevention strategies in resource-poor countries: tailoring the intervention to the context. AIDS. 2006;20(9):1217–1235. doi: 10.1097/01.aids.0000232229.96134.56. [DOI] [PubMed] [Google Scholar]
- 33.Auerbach JD, Hayes RJ, Kandathil SM. Overview of effective and promising interventions to prevent HIV infection. World Health Organ. Tech. Rep. Ser. 2006;938:43–78. discussion 317–341. [PubMed] [Google Scholar]
- 34.Crepaz N, Lyles CM, Wolitski RJ, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20(2):143–157. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 35.Bonell C, Imrie J. Behavioural interventions to prevent HIV infection: rapid evolution, increasing rigour, moderate success. Br. Med. Bull. 2001;58:155–170. doi: 10.1093/bmb/58.1.155. [DOI] [PubMed] [Google Scholar]
- 36.Sikkema KJ, Kelly JA, Winett RA, et al. Outcomes of a randomized community-level HIV prevention intervention for women living in 18 low-income housing developments. Am. J. Public Health. 2000;90(1):57–63. doi: 10.2105/ajph.90.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kelly JA, Murphy DA, Sikkema KJ, et al. Community HIV Prevention Research Collaborative. Randomised, controlled, community-level HIV-prevention intervention for sexual-risk behaviour among homosexual men in US cities. Lancet. 1997;350(9090):1500–1505. doi: 10.1016/s0140-6736(97)07439-4. [DOI] [PubMed] [Google Scholar]
- 38.Kaleeba N, Kadowe JN, Lalinaki D, Williams BG. Open Secret: People Facing Up to HIV and AIDS in Uganda. London, UK: ACTIONAID; 2000. [Google Scholar]
- 39.Koopman JS. Emerging objectives and methods in epidemiology. Am. J. Public Health. 1996;86(5):630–632. doi: 10.2105/ajph.86.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koopman JS, Lynch JW. Individual causal models and population system models in epidemiology. Am. J. Public Health. 1999;89(8):1170–1174. doi: 10.2105/ajph.89.8.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garnett GP. Theory is critical in understanding the risks of acquiring HIV. Sex. Transm. Dis. 2007;34(10):737–738. doi: 10.1097/OLQ.0b013e3181559c5c. [DOI] [PubMed] [Google Scholar]
- 42.Menken J. Proximate determinants of fertility and mortality: a review of recent findings. Sociological Forum. 1987;2(4):697–717. [Google Scholar]
- 43.Davis K, Blake J. Social structure and fertility: an analytical framework. Econ. Dev. Cult. Change. 1956;4(3):211–235. [Google Scholar]
- 44.Bongaarts J. A framework for analyzing the proximate determinants of fertility. Popul. Dev. Rev. 1978;4(1):105–132. [Google Scholar]; ▪▪ One of the classic articles developing a proximate-determinants framework of fertility.
- 45.Boerma JT, Weir SS. Integrating demographic and epidemiological approaches to research on HIV/AIDS: the proximate-determinants framework. J. Infect. Dis. 2005;191 Suppl. 1:S61–S67. doi: 10.1086/425282. [DOI] [PubMed] [Google Scholar]; ▪▪ Individual-level proximate-determinants framework of HIV transmission.
- 46.Mosley WH, Chen LC. An analytical framework for the study of child survival in developing countries. Popul. Dev. Rev. 1984;10 Suppl.:25–45. [PMC free article] [PubMed] [Google Scholar]; ▪ One of the classic articles developing a proximate-determinants framework of child survival.
- 47.Van Norren B, Boerma JT, Sempebwa EK. Simplifying the evaluation of primary health care programmes. Soc. Sci. Med. 1989;28(10):1091–1097. doi: 10.1016/0277-9536(89)90393-6. [DOI] [PubMed] [Google Scholar]
- 48.Mosley WH, Becker S. Demographic models for child survival and implications for health interventions programmes. Health Policy Plan. 1991;6:6218–6133. [Google Scholar]
- 49.Becker S, Black RE. A model of child morbidity, mortality, and health interventions. Popul. Dev. Rev. 1996;22(3):431–456. [Google Scholar]
- 50.Anderson R, May R. Infectious Diseases of Humans: Dynamics and Control. NY, USA: Oxford University Press; 1991. [Google Scholar]
- 51.Hue S, Pillay D, Clewley JP, Pybus OG. Genetic analysis reveals the complex structure of HIV-1 transmission within defined risk groups. Proc. Natl Acad. Sci. USA. 2005;102(12):4425–4429. doi: 10.1073/pnas.0407534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Oliveira T, Pybus OG, Rambaut A, et al. Molecular epidemiology: HIV-1 and HCV sequences from Libyan outbreak. Nature. 2006;444(7121):836–837. doi: 10.1038/444836a. [DOI] [PubMed] [Google Scholar]
- 53.Borgdorff MW, Barongo LR, Klokke AH, et al. HIV-1 incidence and HIV-1 associated mortality in a cohort of urban factory workers in Tanzania. Genitourin. Med. 1995;71(4):212–215. doi: 10.1136/sti.71.4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bulterys M, Chao A, Habimana P, Dushimimana A, Nawrocki P, Saah A. Incident HIV-1 infection in a cohort of young women in Butare, Rwanda. AIDS. 1994;8(11):1585–1591. doi: 10.1097/00002030-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed S, Lutalo T, Wawer M, et al. HIV incidence and sexually transmitted disease prevalence associated with condom use: a population study in Rakai, Uganda. AIDS. 2001;15(16):2171–2179. doi: 10.1097/00002030-200111090-00013. [DOI] [PubMed] [Google Scholar]
- 56.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mermin J, Musinguzi J, Opio A, et al. Risk factors for recent HIV infection in Uganda. JAMA. 2008;300(5):540–549. doi: 10.1001/jama.300.5.540. [DOI] [PubMed] [Google Scholar]
- 58.Bärnighausen T, Wallrauch C, Welte A, et al. HIV incidence in rural South Africa: comparison of estimates from longitudinal surveillance and cross-sectional cBED assay testing. PLoS ONE. 2008;3(11):e3640. doi: 10.1371/journal.pone.0003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Auld MC. Estimating behavioral response to the AIDS epidemic. Contrib. Econ. Anal. Pol. 2006;5(1) (Article 12) [Google Scholar]
- 60.Philipson T, Ahituv A, Hotz J. The responsiveness of the demand for condoms to the local prevalence of AIDS. J. Hum. Resour. 1996;31(4):869–897. [Google Scholar]
- 61.Morris M, Kretzschmar M. Concurrent partnerships and the spread of HIV. AIDS. 1997;11(5):641–648. doi: 10.1097/00002030-199705000-00012. [DOI] [PubMed] [Google Scholar]
- 62.Mandell GL, Bennett JE, Dolin R. Principles and Practice of Infectious Diseases. Philadelphia, PA, USA: Elsevier; 2005. [Google Scholar]
- 63.Kraut-Becher JR, Aral SO. Gap length: an important factor in sexually transmitted disease transmission. Sex. Transm. Dis. 2003;30(3):221–225. doi: 10.1097/00007435-200303000-00009. [DOI] [PubMed] [Google Scholar]
- 64.Morris M, Kurth AE, Hamilton DT, Moody J, Wakefield S. Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am. J. Public Health. 2009;99(6):1023–1031. doi: 10.2105/AJPH.2008.147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jennings JM, Curriero FC, Celentano D, Ellen JM. Geographic identification of high gonorrhea transmission areas in Baltimore, Maryland. Am. J. Epidemiol. 2005;161(1):73–80. doi: 10.1093/aje/kwi012. [DOI] [PubMed] [Google Scholar]
- 66.Becker KM, Glass GE, Brathwaite W, Zenilman JM. Geographic epidemiology of gonorrhea in Baltimore, Maryland, using a geographic information system. Am. J. Epidemiol. 1998;147(7):709–716. doi: 10.1093/oxfordjournals.aje.a009513. [DOI] [PubMed] [Google Scholar]
- 67.Bernstein KT, Curriero FC, Jennings JM, Olthoff G, Erbelding EJ, Zenilman J. Defining core gonorrhea transmission utilizing spatial data. Am. J. Epidemiol. 2004;160(1):51–58. doi: 10.1093/aje/kwh178. [DOI] [PubMed] [Google Scholar]
- 68.Ellen JM, Hessol NA, Kohn RP, Bolan GA. An investigation of geographic clustering of repeat cases of gonorrhea and chlamydial infection in San Francisco, 1989–1993: evidence for core groups. J. Infect. Dis. 1997;175(6):1519–1522. doi: 10.1086/516491. [DOI] [PubMed] [Google Scholar]
- 69.Schleihauf E, Watkins RE, Plant AJ. Heterogeneity in the spatial distribution of bacterial STIs. Sex. Transm. Infect. 2009;85(1):45–49. doi: 10.1136/sti.2008.030197. [DOI] [PubMed] [Google Scholar]
- 70.Bloom SS, Urassa M, Isingo R, Ng’weshemi J, Boerma JT. Community effects on the risk of HIV infection in rural Tanzania. Sex. Transm. Infect. 2002;78(4):261–266. doi: 10.1136/sti.78.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Msisha WM, Kapiga SH, Earls FJ, Subramanian SV. Place matters: multilevel investigation of HIV distribution in Tanzania. AIDS. 2008;22(6):741–748. doi: 10.1097/QAD.0b013e3282f3947f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weir SS, Pailman C, Mahlalela X, Coetzee N, Meidany F, Boerma JT. From people to places: focusing AIDS prevention efforts where it matters most. AIDS. 2003;17(6):895–903. doi: 10.1097/01.aids.0000050809.06065.e0. [DOI] [PubMed] [Google Scholar]
- 73.Humblet O, Paul C, Dickson N. Core group evolution over time: high-risk sexual behavior in a birth cohort between sexual debut and age 26. Sex. Transm. Dis. 2003;30(11):818–824. doi: 10.1097/01.OLQ.0000097102.42149.11. [DOI] [PubMed] [Google Scholar]
- 74.Fichtenberg CM, Ellen JM. Moving from core groups to risk spaces. Sex. Transm. Dis. 2003;30(11):825–826. doi: 10.1097/01.OLQ.0000097141.29899.7F. [DOI] [PubMed] [Google Scholar]
- 75.Kawachi I, Subramanian SV. Neighbourhood influences on health. J. Epidemiol. Community Health. 2007;61(1):3–4. doi: 10.1136/jech.2005.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snijders T, Bosker R. Multilevel Analysis: an Introduction to Basic and Advanced Multilevel Modelling. London, UK: Sage; 1999. [Google Scholar]
- 77.Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am. J. Public Health. 1998;88(2):216–222. doi: 10.2105/ajph.88.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newell ML, Bärnighausen T. Male circumcision to cut HIV risk in the general population. Lancet. 2007;369(9562):617–619. doi: 10.1016/S0140-6736(07)60288-8. [DOI] [PubMed] [Google Scholar]
- 79.Van Damme L, Govinden R, Mirembe FM, et al. Lack of effectiveness of cellulose sulfate gel for the prevention of vaginal HIV transmission. N. Engl. J. Med. 2008;359(5):463–472. doi: 10.1056/NEJMoa0707957. [DOI] [PubMed] [Google Scholar]
- 80.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N. Engl. J. Med. 2008;358(15):1560–1571. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pronyk PM, Hargreaves JR, Kim JC, et al. Effect of a structural intervention for the prevention of intimate-partner violence and HIV in rural South Africa: a cluster randomised trial. Lancet. 2006;368(9551):1973–1983. doi: 10.1016/S0140-6736(06)69744-4. [DOI] [PubMed] [Google Scholar]
- 82.Khumalo-Sakutukwa G, Morin SF, Fritz K, et al. Project Accept (HPTN 043): a community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J. Acquir. Immune Defic. Syndr. 2008;49(4):422–431. doi: 10.1097/QAI.0b013e31818a6cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agha S, Van Rossem R. Impact of a school-based peer sexual health intervention on normative beliefs, risk perceptions, and sexual behavior of Zambian adolescents. J. Adolesc. Health. 2004;34(5):441–452. doi: 10.1016/j.jadohealth.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 84.Wawer MJ, Sewankambo NK, Berkley S, et al. Incidence of HIV-1 infection in a rural region of Uganda. BMJ. 1994;308(6922):171–173. doi: 10.1136/bmj.308.6922.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Imbens G, Angrist J, Rubin D. Identification of causal effects using instrumental variables. J. Econom. 1996;71(1–2):145–160. [Google Scholar]
- 86.Heckman JL. Causal parameters and policy analysis in economics: a twentieth century retrospective. Q. J. Econ. 2000;115(1):45–97. [Google Scholar]
- 87.Heckman JL, Leamer E. Handbook of Econometrics. Amsterdam, The Netherlands: Elsevier Science; 2001. [Google Scholar]
- 88.Heckman J. Sample selection bias as a specification error. Econometrica. 1979;47:153–161. [Google Scholar]
- 89.Winship C. Models for sample selection bias. Annu. Rev. Sociol. 1992;18:327–350. [Google Scholar]
- 90.Angrist JD, Krueger AB. Instrumental variables and the search for identification: from supply and demand to natural experiments. J. Econ. Perspect. 2001;15(4):69–85. [Google Scholar]
- 91.Susser M. Causal Thinking in the Health Sciences Concepts and Strategies of Epidemiology. NY, USA: Oxford University Press; 1973. [Google Scholar]
- 92.Poundstone KE, Strathdee SA, Celentano DD. The social epidemiology of human immunodeficiency virus/acquired immunodeficiency syndrome. Epidemiol. Rev. 2004;26(1):22–35. doi: 10.1093/epirev/mxh005. [DOI] [PubMed] [Google Scholar]
- 93.Zielhuis GA, Kiemeney LALM. Social epidemiology? No way. Int. J. Epidemiol. 2001;30(1):43–44. doi: 10.1093/ije/30.1.43. [DOI] [PubMed] [Google Scholar]