Abstract
Minimizing loss to follow-up (LTFU) in long-term cohort studies is essential for reducing bias and maintaining statistical stability. However, factors associated with attrition of children in observational studies have received little attention. The authors used survival analysis methods to evaluate the association of participant and site characteristics with time to LTFU in a multicenter cohort study conducted in the United States of 2,693 human immunodeficiency virus (HIV)-infected and 1,370 HIV-exposed-but-uninfected children enrolled in 2000–2004. As of 2004, 91% of HIV-infected and 86% of uninfected children had been retained in the study. Among the HIV infected, factors associated with higher risk of LTFU included site prohibition of participant compensation, low caregiver educational level, age >15 years, and higher viral load, whereas death of a family member was associated with better retention. Among uninfected children, sites accruing low numbers of subjects, social worker responsible for retention, young age (1–2 years), and birth abnormalities were associated with higher risk of LTFU. Occurrences of certain stressful life events, such as a death in the family or financial instability, were associated with higher retention, but risk of LTFU increased when children started school or mothers began employment. Although participant characteristics are difficult to modify, the authors identified several potentially modifiable site practices that could be targeted to improve retention.
Keywords: Adolescent, caregivers, child, cohort studies, HIV, multicenter study, patient participation, proportional hazards model
Minimizing loss to follow-up (LTFU) in long-term cohort studies is essential for reducing bias and maintaining both study validity and sufficient power to address study objectives. It has been well established that differential LTFU by treatment arm in clinical trials can bias estimates of treatment effects (1–5). Effect measures from observational studies may be similarly biased if LTFU is associated with both exposures and outcomes of interest (6, 7). Even if LTFU is not differential, if subjects who are lost differ from those who remain in the study, then results may apply to only a narrower subset of children, thus reducing the generalizability of the study (8–10). LTFU must be considered in study design, and larger expected losses require increased sample sizes (11, 12).
The issue of LTFU has been specifically addressed in the context of human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome clinical trials and observational studies. Seage et al. (13) evaluated factors associated with LTFU in the context of an HIV vaccine preparedness study, and de Bruyn et al. (14) investigated predictors of LTFU in clinical trials of candidate HIV vaccines. Amato (15) noted that the primary limitation of long-term trials of antiretroviral therapy in the HIV-infected population is the high rates of voluntary withdrawal. More recently, it has been suggested that improvement in the health status of acquired immunodeficiency syndrome patients occurring with the advent of highly active antiretroviral therapy may reduce the willingness of such patients to participate and remain in longitudinal studies (2).
Retaining subjects in observational cohort studies of children infected by or exposed to HIV is particularly challenging. Studies of HIV-infected and HIV-exposed children face issues of possible stigmatization and concern regarding disclosure of the HIV status of either the child or the caregiver. Relatively high mortality rates of mothers with HIV infection often lead to changes in family structure that may complicate participation in research studies, and maternal morbidity due to HIV infection may increase the difficulty of caring for their child. In studies of HIV-exposed-but-uninfected children, the risk of losing participants over time is particularly high because of long gaps between visits for uninfected subjects and less motivation to attend study visits, whereas infected children often obtain their primary care through these clinics and often develop close personal connections with site staff. As is true in all research studies, offering monetary or other incentives for children to participate in observational studies may be effective, but such incentives are sometimes explicitly prohibited by site institutional review boards and, when offered, vary widely across research sites (16–19).
Factors associated with attrition of children from observational studies have received little attention. In this paper, we report on the impact of participant, family, and site characteristics associated with time to LTFU for subjects in a long-term pediatric cohort study conducted in HIV-infected and HIV-exposed uninfected subjects.
MATERIALS AND METHODS
Study population and conduct
This analysis was based on data collected as part of the Pediatric AIDS Clinical Trials Group (PACTG) 219C cohort study, which enrolled and has followed both HIV-infected and uninfected perinatally exposed children in the United States since September 2000 to study long-term effects of in utero and postnatal antiretroviral therapy and complications of HIV infection. PACTG 219C was a revised version of PACTG 219, which was a protocol initiated in 1993 to study effects of HIV infection in children, as described in greater detail elsewhere (20, 21). The original PACTG 219 study allowed enrollment of only those children coenrolled in another PACTG treatment trial or children whose mothers participated in such a trial. The revised version, PACTG 219C, extended enrollment to any HIV-infected or perinatally exposed child aged 21 years or younger; the majority of uninfected children were born to HIV-infected mothers participating in perinatal treatment trials, and they were enrolled at birth or soon afterward. Children were enrolled at one of 89 participating PACTG 219C sites, representing a mix of urban and rural sites across the United States and Puerto Rico. The study protocol for PACTG 219C was reviewed and approved by the institutional review board at each participating site, and written informed consent was obtained from each child’s parent/guardian or from older participants who could self-consent. Written assent was obtained from children aged 12 years or older when appropriate or as specified by the local institutional review board.
At entry into PACTG 219C, clinical records were abstracted to obtain medical and clinical histories, including a complete history of antiretroviral therapy medications, clinical diagnoses, and birth history. At entry and at scheduled follow-up visits, CD4-positive T-lymphocyte percentages and HIV viral loads were measured, changes in antiretroviral therapy and new diagnoses were collected, and quality-of-life information was obtained as reported by the child or caregiver. Quality-of-life questionnaires collected information on 18 potential “stressful life events,” including changes in household financial status, changes in family structure, changes in environment (child starting school, mother/caregiver beginning employment), and illness or death of family members.
If a site was unable to maintain a subject in the study or lost contact with a subject, personnel completed an “off-study” form that provided the reason for study discontinuation. We defined the outcome “lost to follow-up” as off study for any reason other than site closure, death, or study completion at age 24 years.
Statistical analysis
We examined participant-related and site-related factors and evaluated their association with LTFU by fitting a Cox proportional hazards model for time to LTFU for each characteristic of interest. Factors with p values of <0.20 in the univariate models were considered in a full multivariate Cox model, which was then reduced by using robust model selection procedures to a final model. Separate models were initially fit for participant-specific factors and for site-specific factors, and each model was fit separately for HIV-infected and uninfected subjects.
We considered participant-specific factors to be those collected on an individual participant, relating to personal as well as maternal, family, and caregiver characteristics for that child (table 1). Maternal characteristics included risk factors during pregnancy or delivery and for HIV exposure. Primary caregiver characteristics included type of caregiver (biologic parent or not) and educational level. Child characteristics included demographic factors, risk factors at delivery, HIV exposure risks, and, for HIV-infected subjects, immunologic and virologic health characteristics. Because the relation of age with LTFU may not be linear, we classified age as 0–<3, 3–<8, 8–<15, or ≥15 years for HIV-infected; and 0–1, 1–<2, –<4, or ≥4 years for HIV-exposed uninfected participants. The age distribution for HIV-infected children is similar to the general distribution in the US population, reflecting the dramatic decline in perinatal transmission of HIV in the last decade, whereas the age distribution for uninfected children reflects the need to evaluate safety of prenatal antiretroviral therapy exposure from birth.
TABLE 1.
Participant-specific characteristics (demographic and birth characteristics, maternal and caregiver characteristics, and stressful life events), by HIV* status, in a US-based multisite cohort study, PACTG* 219C, 2000–2004
| HIV-infected subjects (N = 2,693) |
HIV-exposed uninfected subjects (N = 1,370) |
|||
|---|---|---|---|---|
| No. | % | No. | % | |
| Child (participant) characteristics | ||||
| Age (years) at enrollment (median (IQR*)) | 9.9 (6.7–13.0) | 0.9 (0.5–2.7) | ||
| Age (years) at latest visit (median (IQR)) | 12.4 (9.2–15.6) | 3.0 (1.5–5.1) | ||
| Female | 1,396 | 52 | 693 | 51 |
| Perinatal exposure to HIV | 2,428 | 90 | 1,370 | 100 |
| Sexual abuse as a risk factor for HIV | 65 | 2 | 5 | <1 |
| Sexual partner with HIV as a risk factor | 127 | 5 | 0 | 0 |
| Rollover from the PACTG 219 study | 1,326 | 49 | 489 | 36 |
| Race/ethnicity | ||||
| White/other | 422 | 16 | 164 | 12 |
| Black non-Hispanic | 1,569 | 58 | 781 | 57 |
| Hispanic | 702 | 26 | 425 | 31 |
| Low birth weight | 483 | 18 | 212 | 15 |
| Birth by cesarean section | 370 | 14 | 587 | 43 |
| Any perinatal infections | 203 | 8 | 58 | 4 |
| Any birth abnormalities | 83 | 3 | 63 | 5 |
| CD4%* <15% at entry | 286 | 11 | NA* | NA |
| HIV-1 RNA >10,000 copies/ml at entry | 745 | 27 | NA | NA |
| Maternal and caregiver characteristics | ||||
| Primary caregiver | ||||
| Biologic parent | 1,121 | 42 | 1,223 | 89 |
| Relative or other adult | 1,353 | 50 | 134 | 10 |
| Shelter/home/other | 52 | 2 | 13 | 1 |
| Self-care | 167 | 6 | 0 | 0 |
| Change in caregiver since enrollment | 287 | 11 | 64 | 5 |
| Educational level of primary caregiver | ||||
| Grades 1–11 | 630 | 23 | 427 | 31 |
| High school graduate | 772 | 29 | 409 | 30 |
| Some college/technical school | 589 | 22 | 289 | 21 |
| College graduate or higher | 319 | 12 | 98 | 7 |
| Other/not reported | 383 | 14 | 147 | 11 |
| Age (years) of mother at delivery | ||||
| Median (IQR) | 27 (23–31) | 27 (23–32) | ||
| <20 | 204 | 8 | 161 | 12 |
| Maternal substance use during pregnancy | 858 | 32 | 338 | 24 |
| STD* in mother during pregnancy | 362 | 14 | 284 | 21 |
| Hepatitis (any type) during pregnancy | 97 | 4 | 131 | 10 |
| Complications during pregnancy | 309 | 12 | 308 | 22 |
| Maternal intravenous drug use as an HIV risk factor | 585 | 22 | 109 | 8 |
| Family/household characteristics | ||||
| Stressful life events | ||||
| Change in financial status† | 1,254 | 47 | 724 | 53 |
| Change in family structure‡ | 1,061 | 39 | 570 | 42 |
| Change in home environment§ | 1,212 | 45 | 541 | 39 |
| Family member sick or died | 1,454 | 54 | 589 | 43 |
| Death of family member | 815 | 30 | 334 | 24 |
| No. of stressful life events (median (IQR)) | 3 (1–5) | 3 (1–6) | ||
HIV, human immunodeficiency virus; PACTG, Pediatric AIDS Clinical Trials Group; IQR, interquartile range; CD4%, CD4-positive T-lymphocyte percentage; NA, not applicable; STD, sexually transmitted disease.
Parent lost job, moved or lost housing, loss of entitlement (e.g., food stamps), loss of health insurance, or other change in parents’ financial standing.
Family member left home, change of caregiver, separation/divorce/marriage of parents, jail sentence of parent, birth of sibling.
Child began school or mother/caregiver began working.
The site-specific factors included the size of the site (in terms of total patient accrual), the percentage of accrued subjects who were HIV infected, and self-reported policies and practices of the site from a 2003 Site Retention Survey. The Site Retention Survey included three sections: 1) retention policies and access to personnel with related expertise, 2) conduct of visit reminders and postvisit contact, and 3) personnel at the site responsible for tracking participants. Twenty-one sites lost funding prior to 2003; these sites were not asked to complete the site survey (participation of all subjects remaining in the study at these sites was discontinued because of site closure).
Generalized estimating equation models were initially used to evaluate both the participant- and site-specific factors and their association with LTFU, while taking into account within-site clustering. However, after adjustment for site and participant characteristics, the estimated within-site correlation was negligible (<0.05), suggesting that these factors had adequately captured potential sources of correlation in LTFU. In addition, results of the generalized estimating equation and survival analysis approaches were similar; thus, we present only the Cox proportional hazards model results in this paper. Joint models of participant and site factors initially included all covariates from the final multivariate Cox models described above. These models were then reduced by using robust model selection procedures to a final model within each HIV infection status. Variables were included in final models on the basis of hazard ratios and corresponding confidence intervals, evaluation of changes in effect estimates resulting from removing or adding other variables, and use of Akaike’s Information Criterion.
All analyses were conducted by using SAS 9.1 software (SAS Institute, Inc., Cary, North Carolina) and included data submitted to our data management center by July 2004. Two-sided p values of <0.05 were considered statistically significant.
RESULTS
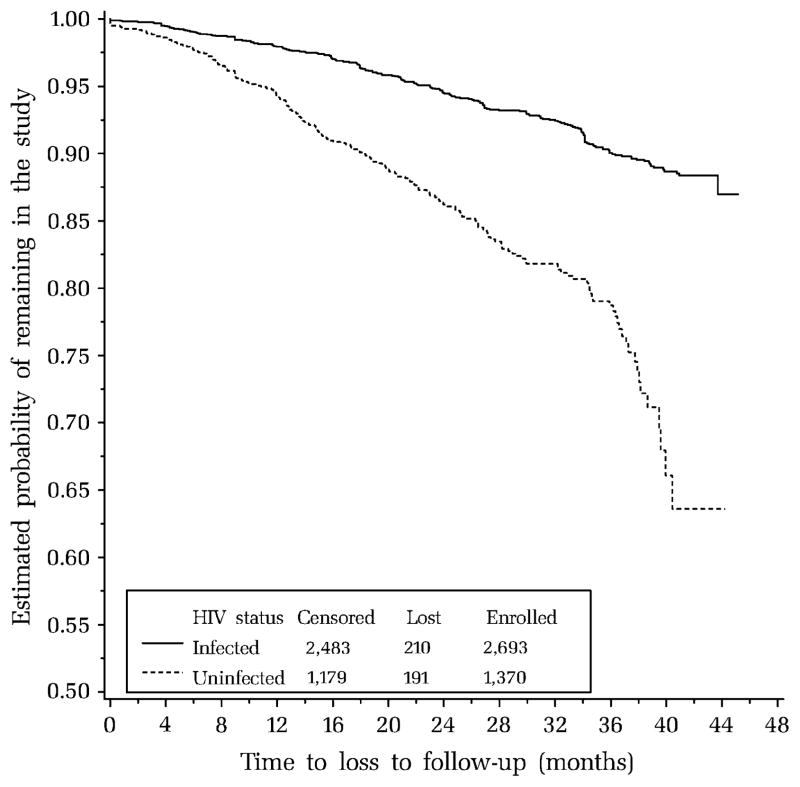
As of July 2004, 2,693 HIV-infected and 1,370 HIV-exposed uninfected children and adolescents had enrolled in PACTG 219C, and 81 percent of the HIV-infected and 84 percent of the uninfected participants remained in the study. However, some had discontinued the study for unavoidable reasons, including site closure because of loss of funding for 21 sites (n = 318 participants) and death of 42 participants; in addition, five subjects completed the study at age 24 years. Among the remaining subjects, 9 percent of the infected (n = 210 of 2,362) and 14 percent of the uninfected (n = 191 of 1,336) were lost to follow-up. Kaplan-Meier estimates of LTFU over time, censoring unavoidable losses at study discontinuation, indicate approximately 3–4 percent LTFU per year for HIV-infected subjects and 6–8 percent for uninfected subjects (figure 1).
FIGURE 1.
Kaplan-Meier estimates of probability of loss to follow-up over time by human immunodeficiency virus (HIV) infection status, for 2,693 HIV-infected and 1,370 HIV-exposed uninfected children and adolescents enrolled between 2000 and 2004 in a US-based multisite cohort study, Pediatric AIDS Clinical Trials Group (PACTG) 219C. Participants who died, completed the study, or were lost to follow-up because of site closure were censored as of their last study visit, along with those who remained in the study as of July 2004.
The enrollment characteristics of the subjects are summarized in table 1 by HIV status. The median age at enrollment was 9.9 years for HIV-infected and 0.9 years for uninfected subjects, and 58 percent were Black and more than 25 percent were Hispanic. Among the HIV-infected subjects, 11 percent had CD4-positive T-lymphocyte percentages of <15 percent, and 27 percent had a high HIV-1 viral load (>10,000 copies/ml). Differences between the HIV-infected and uninfected subjects are clear: HIV-infected subjects were much older, less likely to have a biologic parent as primary caregiver, and less likely to be born by cesarean section.
A summary of participant-specific factors associated with LTFU of HIV-infected subjects based on univariate Cox proportional hazards models is provided in table 2. Factors associated with an increase in the risk of LTFU included older age, advanced HIV infection (based on low CD4 count or percentage or high HIV-1 viral load), and low educational level of the primary caregiver. Surprisingly, occurrence of stressful events in the family was associated with improved retention, particularly in the case of death of a family member. However, after adjustment for age, many of the univariate predictors shown in table 2 were no longer significant. The final multivariate model for participant-specific predictors of LTFU in HIV-infected children (table 2, bottom panel) indicated twice the risk of LTFU for those aged 15 years or older (hazard ratio (HR) = 2.16) and higher risk for those with a high HIV-1 viral load (HR = 1.36) and those whose caregiver had less than a high school education (HR = 1.60). Participants who had experienced a death in the family had less than half the risk of LTFU (HR = 0.45).
TABLE 2.
Participant-specific effects on time to loss to follow-up, with p < 0.20 from univariate Cox proportional hazards models, among 2,693 HIV*-infected children and adolescents enrolled in 2000–2004 in a US-based multisite cohort study, PACTG* 219C, and the final multivariate Cox model for participant-specific effects
| Characteristic | No. | Estimated HR*,† | 95% CI* | p value‡ |
|---|---|---|---|---|
| Univariate predictors | ||||
| Participant-related characteristics, including health related | ||||
| Age ≥15 years at entry | 2,693 | 2.51 | 1.85, 3.41 | <0.001 |
| Perinatal exposure to HIV | 2,693 | 0.58 | 0.39, 0.86 | 0.01 |
| Rollover from the PACTG 219 study | 2,693 | 0.83 | 0.63, 1.09 | 0.19 |
| Sexual abuse as a risk factor for HIV | 2,450 | 2.05 | 1.08, 3.88 | 0.03 |
| Sexual partner with HIV as a risk factor | 2,570 | 3.12 | 1.96, 4.96 | <0.001 |
| Low CD4%* (<15%) at entry | 2,678 | 1.38 | 0.92, 2.08 | 0.13 |
| CD4 count <200 cells/mm3 at entry | 2,657 | 1.76 | 1.14, 2.71 | 0.01 |
| HIV-1 RNA >10,000 copies/ml at entry | 2,675 | 1.31 | 0.98, 1.76 | 0.07 |
| Maternal and caregiver characteristics | ||||
| Subject as primary caregiver | 2,682 | 2.43 | 1.61, 3.66 | <0.001 |
| Low caregiver educational level (<12th grade) | 2,693 | 1.76 | 1.32, 2.33 | <0.001 |
| Mother aged <20 years at birth of subject | 2,472 | 1.38 | 1.03, 1.84 | 0.03 |
| Maternal complications during pregnancy | 2,457 | 0.63 | 0.38, 1.06 | 0.08 |
| Family/household characteristics (stressful life events) | ||||
| No. of stressful life events | 2,582 | 0.94 | 0.89, 0.99 | 0.01 |
| Change in financial status of household | 2,582 | 0.66 | 0.46, 0.93 | 0.02 |
| Family member sick or died | 2,582 | 0.63 | 0.47, 0.83 | 0.001 |
| Death of family member | 2,591 | 0.44 | 0.31, 0.62 | <0.001 |
| Final multivariate model (n = 2,572) | ||||
| Age ≥15 years | 2.16 | 1.55, 3.01 | <0.001 | |
| HIV-1 RNA >10,000 copies/ml at entry | 1.36 | 1.01, 1.84 | 0.04 | |
| Low caregiver educational level (< 12th grade) | 1.60 | 1.21, 2.13 | 0.001 | |
| Death of family member | 0.45 | 0.31, 0.64 | <0.001 | |
HIV, human immunodeficiency virus; PACTG, Pediatric AIDS Clinical Trials Group; HR, hazard ratio; CI, confidence interval; CD4%, CD4-positive T-lymphocyte percentages.
Values above 1 indicate higher risk of loss to follow-up.
Two-sided p values from Wald tests of effects in Cox proportional hazards models.
Among HIV-exposed uninfected participants (table 3), age between 1 and 2 years at entry, certain birth abnormalities (including perinatal infections), and maternal risk factors for HIV such as having a sexual partner with a history of intravenous drug use or of unknown HIV status were associated with decreased retention in univariate models. Occurrence of certain stressful life events was again associated with reduced risk of LTFU. However, a change in family environment (child starting school or mother beginning employment) was associated with an increased risk of LTFU. The final multivariate model for uninfected participants indicated a significantly higher risk of LTFU for those aged 1–2 years compared with other age groups and for those with reported birth abnormalities, and a 30 percent reduction in risk of loss for children who previously participated in PACTG 219 (HR = 0.69). Two types of stressful life events—change in financial status or death of a family member—were associated with reductions in risk of LTFU (HR = 0.62 and HR = 0.57, respectively), but starting a new school or employment of the mother was associated with increased risk of loss (HR = 1.45).
TABLE 3.
Participant-specific effects on time to loss to follow-up, with p < 0.20 from univariate Cox proportional hazards models, among 1,370 HIV*-exposed uninfected children and adolescents enrolled in 2000–2004 in a US-based multisite cohort study, PACTG* 219C, and the final multivariate Cox model for participant-specific effects
| Characteristic | No. | Estimated HR*,† | 95% CI* | p value‡ |
|---|---|---|---|---|
| Univariate predictors | ||||
| Participant-related characteristics | ||||
| Age 1–2 years | 1,370 | 1.38 | 0.96, 1.98 | 0.08 |
| Rollover from the PACTG 219 study | 1,370 | 0.61 | 0.45, 0.82 | 0.001 |
| Birth abnormalities or perinatal infections | 1,355 | 2.31 | 1.58, 3.38 | <0.001 |
| Maternal and caregiver characteristics | ||||
| Mother’s partner with HIV | 1,095 | 0.76 | 0.53, 1.08 | 0.12 |
| Mother’s partner with history of intravenous drug use | 998 | 1.37 | 0.93, 2.00 | 0.11 |
| Mother’s partner of unknown HIV status | 1,097 | 1.50 | 1.08, 2.09 | 0.02 |
| Family/household characteristics (stressful life events) | ||||
| No. of stressful life events | 1,272 | 0.84 | 0.79, 0.89 | <0.001 |
| Family member sick or died | 1,272 | 0.45 | 0.33, 0.62 | <0.001 |
| Change in financial status | 1,272 | 0.50 | 0.37, 0.69 | 0.001 |
| New school/mother began working | 1,272 | 1.79 | 1.30, 2.46 | <0.001 |
| Death of family member | 1,272 | 0.49 | 0.34, 0.73 | 0.001 |
| Final multivariate model (n = 1,266) | ||||
| Age 1–2 years | 1.71 | 1.15, 2.53 | 0.01 | |
| Rollover from the PACTG 219 study | 0.69 | 0.50, 0.97 | 0.03 | |
| Birth abnormalities or perinatal infections | 2.53 | 1.67, 3.81 | <0.001 | |
| New school/mother began working | 1.45 | 1.03, 2.05 | 0.03 | |
| Change in financial status | 0.62 | 0.44, 0.86 | 0.005 | |
| Death of family member | 0.57 | 0.40, 0.80 | 0.001 | |
HIV, human immunodeficiency virus; PACTG, Pediatric AIDS Clinical Trials Group; HR, hazard ratio; CI, confidence interval.
Values above 1 indicate higher risk of loss to follow-up.
Two-sided p values from Wald tests of effects in Cox proportional hazards models.
The 2003 Site Retention Survey was completed by 63 of the 67 sites actively participating in PACTG 219C at that time, and survey responses are summarized in table 4 along with the distribution of site sizes in terms of total patient accrual. The highest LTFU for both HIV-infected and uninfected subjects was observed at the “small” sites (those accruing <20 subjects), with about twice the risk of LTFU compared with sites accruing more subjects. Some of the site-related policies seemed to have unexpected results in terms of retention. For example, sites that either called or sent a visit reminder, rather than both calling and mailing reminders, had more than twice the risk of LTFU for both infected and uninfected participants; note, however, that only three sites sent no reminders. Sites with three or more retention specialists had a lower risk of LTFU of HIV-infected participants (HR = 0.40) but a higher risk of LTFU of uninfected participants (HR = 2.87) compared with sites with no retention specialist.
TABLE 4.
Site-specific characteristics of responding sites and association with loss to follow-up among HIV*-infected and HIV-exposed uninfected children and adolescents enrolled in 2000–2004 in a US-based multisite cohort study, PACTG* 219C
| Sites in category |
LTFU* in HIV infected† (n = 2,314) |
LTFU in uninfected† (n= 1,311) |
||||||
|---|---|---|---|---|---|---|---|---|
| No. | % | % Lost | HR*,‡ | p value§ | % Lost | HR‡ | p value§ | |
| Size of site | ||||||||
| Total no. of subjects accrued (HIV infected and HIV-exposed uninfected) | ||||||||
| 1–19 | 14 | 22 | 17.2 | 1.00 | Referent | 28.1 | 1.00 | Referent |
| 20–49 | 12 | 19 | 9.0 | 0.42 | 0.003 | 14.0 | 0.36 | 0.01 |
| 50–99 | 28 | 44 | 6.5 | 0.31 | <0.001 | 14.9 | 0.42 | 0.01 |
| ≥100 | 9 | 14 | 8.6 | 0.44 | 0.002 | 9.4 | 0.26 | <0.001 |
| Retention policies and expertise | ||||||||
| Site has contact/tracking form | ||||||||
| Yes | 26 | 41 | 8.5 | 1.14 | 0.37 | 13.6 | 1.26 | 0.15 |
| No | 37 | 59 | 7.5 | 1.00 | Referent | 12.8 | 1.00 | Referent |
| No. of contacts listed | ||||||||
| 0 | 37 | 59 | 7.5 | 1.00 | Referent | 12.8 | 1.00 | Referent |
| 1–2 | 15 | 24 | 9.2 | 1.14 | 0.45 | 14.0 | 1.12 | 0.55 |
| ≥3 | 11 | 17 | 7.7 | 1.15 | 0.49 | 13.1 | 1.57 | 0.04 |
| Site has standard retention policy | ||||||||
| Yes | 9 | 14 | 9.6 | 1.04 | 0.85 | 10.3 | 0.69 | 0.10 |
| No | 54 | 86 | 7.6 | 1.00 | Referent | 13.7 | 1.00 | Referent |
| Access to retention specialist | ||||||||
| Yes | 13 | 21 | 6.1 | 0.73 | 0.15 | 18.5 | 1.51 | 0.02 |
| No/unknown | 50 | 79 | 8.3 | 1.00 | Referent | 12.1 | 1.00 | Referent |
| No. of retention specialists 1 | ||||||||
| 0 | 50 | 79 | 8.3 | 1.00 | Referent | 12.1 | 1.00 | Referent |
| 1–2 | 8 | 13 | 9.2 | 0.94 | 0.79 | 12.6 | 0.95 | 0.84 |
| ≥3 | 5 | 8 | 2.7 | 0.40 | 0.04 | 30.1 | 2.87 | <0.001 |
| Site IRB* prohibits incentives | ||||||||
| Yes | 8 | 13 | 13.8 | 1.77 | 0.005 | 10.8 | 0.75 | 0.19 |
| No | 55 | 87 | 7.3 | 1.00 | Referent | 13.6 | 1.00 | Referent |
| Responsibility for tracking subjects at site | ||||||||
| Responsible staff member(s)¶ | ||||||||
| Study nurse | 57 | 90 | 7.7 | 0.76 | 0.26 | 13.2 | 1.11 | 0.76 |
| Principal investigator | 4 | 6 | 9.8 | 1.23 | 0.53 | 14.0 | 1.01 | 0.98 |
| Social or outreach worker | 27 | 43 | 6.8 | 0.80 | 0.15 | 15.8 | 1.71 | 0.001 |
| Case manager | 13 | 21 | 7.4 | 1.12 | 0.56 | 13.0 | 1.13 | 0.48 |
| Office manager/office staff | 12 | 19 | 5.9 | 0.68 | 0.05 | 16.4 | 1.20 | 0.26 |
| Study coordinator | 10 | 16 | 14.0 | 1.62 | 0.006 | 8.4 | 0.50 | 0.02 |
| Data manager/coordinator | 5 | 8 | 3.3 | 0.43 | 0.01 | 19.9 | 1.71 | 0.01 |
| Research assistant | 3 | 5 | 11.4 | 1.28 | 0.44 | 7.7 | 0.52 | 0.04 |
| Retention the primary responsibility of certain site staff members | 18 | 29 | 8.3 | 1.05 | 0.77 | 16.5 | 1.61 | 0.01 |
| No. of responsible staff | ||||||||
| 1 | 24 | 38 | 8.8 | 1.00 | Referent | 12.0 | 1.00 | Referent |
| 2 | 19 | 30 | 7.7 | 0.89 | 0.51 | 11.9 | 1.13 | 0.56 |
| ≥3 | 20 | 32 | 7.1 | 0.84 | 0.34 | 15.1 | 1.30 | 0.15 |
| Visit reminders and postvisit contacts | ||||||||
| Site sends visit reminders | ||||||||
| Yes | 60 | 95 | 8.2 | 2.04 | 0.12 | 13.3 | 1.67 | 0.19 |
| No | 3 | 5 | 3.6 | 1.00 | Referent | 9.6 | 1.00 | Referent |
| Type of visit reminders | ||||||||
| Call or mail reminder | 32 | 51 | 8.5 | 2.20 | 0.09 | 16.6 | 2.31 | 0.03 |
| Call and mail reminder | 28 | 44 | 7.9 | 1.89 | 0.16 | 11.0 | 1.31 | 0.50 |
| No reminders sent | 3 | 5 | 3.6 | 1.00 | Referent | 9.6 | 1.00 | Referent |
| Contact after missed visit | ||||||||
| Within hours | 22 | 35 | 7.9 | 1.00 | Referent | 15.5 | 1.00 | Referent |
| Within days | 35 | 56 | 7.9 | 1.12 | 0.48 | 12.0 | 0.78 | 0.11 |
| Within weeks | 6 | 9 | 8.0 | 1.17 | 0.58 | 9.9 | 0.67 | 0.28 |
HIV, human immunodeficiency virus; PACTG, Pediatric AIDS Clinical Trials Group; LTFU, loss to follow-up; HR, hazard ratio; IRB, institutional review board.
Because they were at defunded sites without a completed site survey, 379 of the 2,693 HIV infected (14%) and 59 of the 1,370 uninfected (4%) could not be included in this analysis.
Hazard ratio from Cox proportional hazards model. Because HRs reflect differences in time to event rather than percentage with the event, ordering of HRs may differ from ordering of percentage lost.
Two-sided p values from Wald tests of effects in Cox proportional hazards models.
Sites may report that multiple staff members are responsible for subject tracking at their sites, so percentages may add to more than 100; each HR represents the risk of LTFU for the specified staff member reported as responsible for tracking follow-up versus that staff member not being reported as responsible.
Many of the types of site staff reported to be responsible for tracking study participants had strong associations with retention rates, but often in opposite directions for HIV-infected and HIV-exposed uninfected participants. Among the HIV-infected subjects, lower risks of LTFU were observed when a social worker/outreach worker, office manager/office staff, or data manager/data coordinator was responsible, while higher risk of loss was observed when a study coordinator was responsible. The opposite pattern was observed among the HIV-exposed uninfected participants.
The final multivariate Cox models for time to LTFU incorporating both participant-specific factors (tables 2 and 3) and site-specific factors (table 4) are summarized in table 5. The final models for time to LTFU of HIV-infected and uninfected participants included both child- and family-specific characteristics as well as site-specific characteristics. Smaller sites had more than twice the risk of loss of HIV-infected participants and almost three times the risk of loss of HIV-exposed children. In addition, sites whose institutional review boards did not allow monetary incentives to be provided had over twice the risk of LTFU for the HIV infected (HR = 2.41). Small sites were also most likely to have institutional review boards that prohibited monetary incentives, and thus two models were obtained for the HIV infected that provided very similar fit: one included site size and the other included the institutional review board compensation prohibition, with other factors relatively unaffected. Sites with a larger percentage of HIV-infected subjects among their enrolled participants had increased risk of LTFU of both infected and uninfected children.
TABLE 5.
Final multivariate Cox proportional hazards models for time to loss to follow-up among HIV*-infected and HIV-exposed children enrolled in 2000–2004 in a US-based multisite cohort study, PACTG* 219C, incorporating both participant-specific and site-specific characteristics
| Characteristic | Estimated HR*,† | 95% CI* | p value‡ |
|---|---|---|---|
| Final multivariate model for the HIV infected (n = 2,572) | |||
| Age ≥15 years at entry | 2.15 | 1.54, 3.01 | <0.001 |
| HIV-1 RNA >10,000 copies/ml at entry | 1.32 | 0.98, 1.79 | 0.07 |
| Low caregiver educational level (<12th grade) | 1.63 | 1.22, 2.16 | 0.001 |
| Death of family member | 0.45 | 0.32, 0.65 | <0.001 |
| Percentage enrolled who are HIV infected§ | 1.02 | 1.01, 1.03 | <0.001 |
| Site accrual <20 subjects in the study | 2.05 | 1.35, 3.12 | 0.001 |
| Alternate final multivariate model for the HIV infected (n = 2,203) | |||
| Age ≥15 years at entry | 2.35 | 1.65, 3.36 | <0.001 |
| HIV-1 RNA >10,000 copies/ml at entry | 1.29 | 0.93, 1.79 | 0.12 |
| Low caregiver educational level (<12th grade) | 1.55 | 1.15, 2.11 | 0.005 |
| Death of family member | 0.47 | 0.32, 0.68 | <0.001 |
| Percentage enrolled who are HIV infected§ | 1.03 | 1.02, 1.04 | <0.001 |
| Site IRB* prohibits compensation | 2.41 | 1.55, 3.76 | <0.001 |
| Final multivariate model for the HIV-exposed uninfected (n = 1,225) | |||
| Age 1–2 years | 1.89 | 1.27, 2.83 | 0.002 |
| Birth abnormalities or perinatal infections | 2.27 | 1.47, 3.49 | <0.001 |
| New school/mother began working | 1.72 | 1.22, 2.43 | 0.002 |
| Change in financial status | 0.61 | 0.43, 0.85 | 0.004 |
| Death of family member | 0.55 | 0.39, 0.79 | 0.001 |
| Percentage enrolled who are HIV infected§ | 1.03 | 1.02, 1.04 | <0.001 |
| Site accrual <20 subjects in the study | 2.98 | 1.39, 6.37 | 0.005 |
| Social or outreach worker responsible | 1.81 | 1.30, 2.53 | <0.001 |
| Study coordinator responsible | 0.33 | 0.17, 0.64 | 0.001 |
| Only one type of reminder sent (mail or phone) | 2.07 | 1.47, 2.93 | <0.001 |
| Reminder sent within weeks (vs. earlier) | 0.37 | 0.16, 0.90 | 0.03 |
HIV, human immunodeficiency virus; PACTG, Pediatric AIDS Clinical Trials Group; HR, hazard ratio; CI, confidence interval; IRB, institutional review board.
Hazard ratio from Cox proportional hazards model.
Two-sided p values from Wald tests of effects in Cox proportional hazards model.
These hazard ratios correspond to a one-unit increase in the percentage of subjects enrolled at the site in PACTG 219C who are HIV infected.
Age at study entry strongly influenced retention of both HIV-infected and uninfected participants, but older children (aged >15 years) were at greater risk of loss for the HIV infected while those aged 1–2 years at entry were at greatest risk of loss for the uninfected. Stressful life events, including death of a family member and changes in household financial status, were associated with reduced risk of LTFU in both the HIV infected and HIV uninfected. However, there was a 72 percent increase in risk of LTFU if the child began school or the mother/caregiver began employment. Other site personnel and retention policies appeared to primarily affect LTFU among the HIV-exposed uninfected children: there was an 80 percent higher risk of loss if a social or outreach worker was responsible for retention, while the risk was reduced by two thirds (HR = 0.33) if a study coordinator was responsible.
DISCUSSION
The PACTG 219C cohort study was able to limit LTFU to about 3–4 percent per year for the HIV infected and 6–8 percent for the uninfected. These attrition rates compare favorably with those of many other long-term observational studies, which range from 14 percent to 30 percent (2–4) over study durations of up to 2 years. Despite this study’s relative success in maintaining subjects in the study, ongoing attrition reduces the ability to evaluate rare adverse events and may threaten the validity of certain planned analyses. Costagliola and Mary-Krause (22) recommend questioning the results of a study whenever more subjects are lost than those with the event of interest. Other recommendations include using a cutoff of 10 percent or 15 percent LTFU (5, 23) to either institute site restrictions or question trial results. Given the clear declines in participation rates in epidemiologic studies noted by Galea and Tracy (24), great efforts should be expended to retain those who do volunteer to participate in research studies.
We found several factors related to the participant, the caregiver, or the family to be associated with the risk of LTFU. These factors included poorer health status (higher viral load in HIV-infected and birth abnormalities in HIV-uninfected participants), age at entry (older for the HIV infected, younger for the HIV uninfected), recent stressful life events, educational level of the caregiver, and participation in previous trials. Other studies have reported fairly consistent increases in attrition for those with low educational levels (3, 4, 8, 13, 25) and poorer health status (2, 8, 25, 26). Age has been demonstrated across numerous studies to affect retention, with younger age typically associated with higher LTFU (13, 14, 27, 28); however, these studies have addressed adult rather than adolescent populations. Our finding of higher LTFU among HIV-infected adolescents is consistent with other research indicating poorer medication adherence in this age group (29).
Stressful life events have been reported to be associated with an increased risk of mortality, and other measures of impaired quality of life have been associated with reduced retention (26). However, we found that youth experiencing more stressful life events were more likely to remain in the study. It may be that occurrence of such events leads to a greater reliance of the child and/or his or her family on the health care team for social and emotional support. Unstable housing has often been reported to be associated with increased LTFU (1, 3, 4, 13, 30). However, we found no association with loss of housing in itself, although the number of stressful life events (which includes loss of housing) was related to improved retention, as described above.
In addition to participant-related factors associated with LTFU, we identified several clinic or site-level policies and factors that were associated with retention, even after control for subject-specific characteristics. For example, sites accruing more subjects to the study had lower rates of loss than “small” sites of both HIV-infected and uninfected children, perhaps because of their increased staffing and ability to hire or designate retention specialists. The type of staff member responsible for retention appeared to be particularly important for retaining uninfected children, and multiple reminders (by mail and phone) helped reduce loss. Other researchers have also evaluated site-specific characteristics and their association with retention, and they noted that unsatisfactory interactions with study physicians or long waits for appointments tended to increase attrition (8) and that LTFU was related to clinical setting characteristics, patient provider characteristics, and provision of site incentives (1). These results suggest that multisite networks may improve retention efforts by including fewer numbers of larger sites and training both administrative and other staff at these sites on key factors noted to improve retention.
Among HIV-infected children, we found that site institutional review board prohibition against monetary compensation for participants’ time and effort was associated with more than twice the risk of LTFU. Some site institutional review boards have argued that offering monetary reimbursement or incentives may remove, to a certain extent, the voluntary nature of study participation and may result in unintended targeting of vulnerable populations of lower socioeconomic status, in essence resulting in undue inducement (16, 17, 31). However, the wide variability noted across institutional review boards and investigators both in allowing payment and in amounts of payment (16, 17) may, in practice, create greater inequities among research subjects, especially children (18, 19). Bagley et al. (31) recommend offering children older than age 9 years an appropriate compensation, or “wage,” citing their developmental understanding of the monetary value of research participation; for younger children, the parents/caregivers would primarily make decisions regarding participation. We urge site institutional review boards to develop guidelines for standardizing monetary compensation appropriate for both adult and pediatric populations. A reasonable and noncoercive place to start is to compensate participants the median hourly wage for their community.
A limitation of our analysis is that the site survey was completed while the study was ongoing, rather than prior to opening the study, so it may have reflected more recent policies adopted in response to poor retention rather than causally related to subsequent poor retention. For example, sites may have hired retention specialists in response to high LTFU at their site. In addition, because it was not possible to obtain completed surveys from sites that had already closed, subjects at these sites could not be included in the joint models of site- and participant-specific factors.
There are several reasons why identifying factors associated with LTFU is important. First, if specific participants are considered at high risk of LTFU, then sites could identify these subjects early in the study and increase retention efforts for them (28). Retention strategies can be developed that appropriately consider the context of the family and household characteristics of participants. Second, if certain site practices are found to be related to retention, such practices can be recommended or discouraged as appropriate. In addition, sites with expertise in areas identified to improve retention can be encouraged and supported to share such strategies with other sites.
Our results indicate that, while short-term retention of HIV-infected and HIV-exposed but uninfected children was high, particular challenges make longer-term studies problematic. More work will be needed to modify and improve retention efforts. These efforts should engage the responsible staff members’ full cooperation across study sites to enhance participant-site connections. Multisite study teams should strongly encourage appropriate participant compensation to improve retention, thus limiting study bias.
Acknowledgments
This work was supported by the Statistical and Data Analysis Center at the Harvard School of Public Health, under National Institute of Allergy and Infectious Diseases cooperative agreements 5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and 1 U01 AI068616 with the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Group. Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (U01 AI068632).
Abbreviations
- HIV
human immunodeficiency virus
- HR
hazard ratio
- LTFU
loss to follow-up
- PACTG
Pediatric AIDS Clinical Trials Group
APPENDIX
Participating Institutions and Investigators
The following institutions and investigators participated in the US-based multisite cohort study, PACTG Protocol 219C, between 2000 and 2004.
University of New Jersey Medical and Dental School–Department of Pediatrics, Division of Allergy, Immunology and Infectious Diseases: Dr. Arlene Bardeguez, Dr. Arry Dieudonne, Linda Bettica, and Juliette Johnson; Boston Medical Center, Division of Pediatric Infectious Diseases: Dr. Stephen I. Pelton, Dr. Ellen R. Cooper, Lauren Kay, and Ann Marie Regan; Med, Children’s Hospital LA–Department of Pediatrics, Division of Clinical Immunology and Allergy: Dr. Joseph A. Church and Theresa Dunaway; Long Beach Memorial Medical Center, Miller Children’s Hospital: Dr. Audra Deveikis, Dr. Jagmohan Batra, Susan Marks, and Ilaisanee Fineanganofo; Harbor-UCLA Medical Center–Department of Pediatrics, Division of Infectious Diseases: Dr. Margaret A. Keller, Dr. Nasser Redjal, Spring Wettgen, and Sheryl Sullivan; Johns Hopkins Hospital and Health System–Department of Pediatrics, Division of Infectious Diseases: Dr. Nancy Hutton, Beth Griffith, Mary Joyner, and Carolyn Keifer; University of Maryland Medical Center, Division of Pediatric Immunology and Rheumatology: Dr. Douglas Watson and Dr. John Farley; Texas Children’s Hospital, Allergy and Immunology Clinic: Dr. Mary E. Paul, Chivon D. Jackson, Faith Minglana, and Dr. Heidi Schwarzwald; Cook County Hospital: Dr. Kenneth M. Boyer, Dr. Jamie Martinez, Dr. James B. McAuley, and Maureen Haak; Children’s Hospital of Columbus, Ohio: Dr. Michael Brady, Dr. Katalin Koranyi, Jane Hunkler, and Charon Callaway; University of Miami Miller School of Medicine, Division of Pediatric Immunology and Infectious Disease: Dr. Gwendolyn B. Scott, Dr. Charles D. Mitchell, Dr. Claudia Florez, and Joan Gamber; University of California San Francisco School of Medicine, Department of Pediatrics: Dr. Diane W. Wara, Dr. Ann Petru, Nicole Tilton, and Mica Muscat; Children’s Hospital and Research Center Oakland, Pediatric Clinical Research Center and Research Lab: Dr. Ann Petru, Teresa Courville, Karen Gold, and Katherine Eng; University of California San Diego Mother, Child and Adolescent HIV Program: Dr. Stephen A. Spector, Dr. Rolando M. Viani, Mary Caffery, and Kimberly Norris; Duke University School of Medicine–Department of Pediatrics, Children’s Health Center: Margaret Donnelly, Dr. Kathleen McGann, Carole Mathison, and John Swetnam; University of North Carolina at Chapel Hill School of Medicine–Department of Pediatrics, Division of Immunology and Infectious Diseases: Dr. Tom Belhorn, Jean Eddleman, and Betsy Pitkin; Schneider Children’s Hospital: Dr. Vincent R. Bonagura, Dr. Susan Schuval, Dr. Blanka Kaplan, and Dr. Constance Colter; Harlem Hospital Center: Dr. Elaine J. Abrams, Maxine Frere, and Delia Calo; New York University School of Medicine, Division of Pediatric Infectious Diseases: Dr. William Borkowsky, Nagamah Deygoo, Maryam Minter, and Seham Akleh; Children’s National Medical Center, ACT: Diana Dobbins, Deidre Wimbley, Dr. Lawrence D’Angelo, and Hans Spiegel; University of Washington School of Medicine–Children’s Hospital and Regional Medical Center: Dr. Ann J. Melvin, Kathleen M. Mohan, Michele Acker, and Suzanne Phelps; University of Illinois College of Medicine at Chicago, Department of Pediatrics: Dr. Kenneth C. Rich, Dr. Karen Hayani, and Julia Camacho; Yale University School of Medicine–Department of Pediatrics, Division of Infectious Disease: Dr. Warren A. Andiman, Leslie Hurst, Dr. Janette de Jesus, and Donna Schroeder; SUNY at Stony Brook School of Medicine, Division of Pediatric Infectious Diseases: Denise Ferraro, Jane Perillo, and Michele Kelly; Howard University Hospital, Department of Pediatrics and Child Health: Dr. Sohail Rana, Dr. Helga Finke, Patricia Yu, and Dr. Jhoanna Roa; LA County/University of Southern California Medical Center: Dr. Andrea Kovacs, Dr. James Homans, Dr. Michael Neely, and Dr. LaShonda Spencer; University of Florida Health Science Center Jacksonville, Division of Pediatric Infectious Disease and Immunology: Dr. Mobeen H. Rathore, Dr. Ayesha Mirza, Kathy Thoma, and Almer Mendoza; North Broward Hospital District, Children’s Diagnostic and Treatment Center: Dr. Ana M. Puga, Dr. Guillermo Talero, James Blood, and Stefanie Juliano; University of Rochester Medical Center, Golisano Children’s Hospital: Dr. Geoffrey A. Weinberg, Barbra Murante, Susan Laverty, and Dr. Francis Gigliotti; Medical College of Virginia: Dr. Suzanne R. Lavoie and Tima Y. Smith; St. Jude Children’s Research Hospital, Department of Infectious Diseases: Dr. Aditya Gaur, Dr. Katherine Knapp, Dr. Nehali Patel, and Marion Donohoe; University of Puerto Rico, U. Children’s Hospital AIDS: Dr. Irma L. Febo, Dr. Licette Lugo, Ruth Santos, and Ibet Heyer; Children’s Hospital of Philadelphia, Center for Pediatric and Adolescent AIDS: Dr. Steven D. Douglas, Dr. Richard M. Rutstein, Carol A. Vincent, and Patricia C. Coburn; St. Christopher’s Hospital for Children/Drexel University College of Medicine: Dr. Jill Foster, Dr. Janet Chen, Dr. Daniel Conway, and Dr. Roberta Laguerre; Bronx-Lebanon Hospital Center, Infectious Diseases: Dr. Emma Stuard, Caroline Nubel, Dr. Stefan Hagmann, and Dr. Murli Purswani; New York Medical College/Metropolitan Hospital Center: Dr. Mahrukh Bamji, Dr. Indu Pathak, Dr. Savita Manwani, and Dr. Ekta Patel; University of Massachusetts Memorial Children’s Medical School, Department of Pediatrics: Dr. Katherine Luzuriag and Dr. Richard Moriarty; Baystate Health, Baystate Medical Center: Dr. Barbara W. Stechenberg, Dr. Donna J. Fisher, Dr. Alicia M. Johnston, and Maripat Toye; Connecticut Children’s Medical Center: Dr. Juan C. Salazar, Kirsten Fullerton, and Gail Karas; Medical College of Georgia School of Medicine, Department of Pediatrics, Division of Infectious Disease: Dr. Stuart Foshee, Dr. Chitra S. Mani, Dr. Dennis L. Murray, and Dr. Christopher White; University of South Alabama College of Medicine, Southeast Pediatric ACTU: Dr. Mary Y. Mancao and Dr. Benjamin Estrada; LSU Health Sciences Center: Dr. Ronald D. Wilcox; Tulane University Health Sciences Center: Dr. Margarita Silio, Dr. Thomas Alchediak, Cheryl Borne, and Shelia Bradford; St. Josephs Hospital and Medical Center; Cooper University Hospital–Children’s Hospital Boston, Division of Infectious Diseases; David Geffen School of Medicine at UCLA–Department of Pediatrics, Division of Infectious Diseases; Children’s Hospital of Orange County; Children’s Memorial Hospital–Department of Pediatrics, Division of Infectious Disease; University of Chicago–Department of Pediatrics, Division of Infectious Disease; Mt. Sinai Hospital Medical Center–Chicago, Women’s and Children’s HIV Program; Columbia University Medical Center, Pediatric ACTU; Incarnation Children’s Center; Cornell University, Division of Pediatric Infectious Diseases and Immunology; University of Miami Miller School of Medicine–Jackson Memorial Hospital; Bellevue Hospital (Pediatric); San Francisco General (Pediatric); Phoenix Children’s Hospital; Metropolitan Hospital Center (New York); University of Cincinnati; SUNY Downstate Medical Center, Children’s Hospital at Downstate; North Shore University Hospital, Jacobi Medical Center; University of South Florida–Department of Pediatrics, Division of Infectious Diseases; Cornell University; Oregon Health and Science University–Department of Pediatrics, Division of Infectious Diseases; Children’s Hospital of the King’s Daughters, Infectious Disease; Lincoln Medical and Mental Health Center; Mt. Sinai School of Medicine, Division of Pediatric Infectious Diseases; Emory University Hospital; San Juan City Hospital; UMDNJ–Robert Wood Johnson; Ramon Ruiz Arnau University Hospital; Medical University of South Carolina; SUNY Upstate Medical University, Department of Pediatrics; Wayne State University School of Medicine; Children’s Hospital of Michigan; Children’s Hospital at Albany Medical Center; Children’s Medical Center of Dallas; Children’s Hospital–University of Colorado at Denver and Health Sciences Center, Pediatric Infectious Diseases; Columbus Children’s Hospital; University of Florida College of Medicine–Department of Pediatrics, Division of Immunology, Infectious Diseases and Allergy; University of Mississippi Medical Center; Palm Beach County Health Department; Children’s Hospital LA–Department of Pediatrics, Division of Adolescent Medicine; Vanderbilt University Medical Center, Division of Pediatric Infectious Diseases; Washington University School of Medicine at St. Louis; St. Louis Children’s Hospital; Children’s Hospital and Medical Center, Seattle ACTU; Oregon Health Sciences University; St. Luke’s-Roosevelt Hospital Center; Montefiore Medical Center–Albert Einstein College of Medicine; Children’s Hospital, Washington, DC; Children’s Hospital of the King’s Daughters; University of Alabama at Birmingham, Department of Pediatrics, Division of Infectious Diseases; Columbus Regional Health-Care System; The Medical Center; Sacred Heart Children’s Hospital/CMS of Florida; and Bronx Municipal Hospital Center/Jacobi Medical Center.
Footnotes
For permissions, please: journals.permissions@oxfordjournals.org.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
The institutions and investigators who participated in PACTG Protocol 219C between 2000 and 2004 are provided in the Appendix.
Conflict of interest: none declared.
References
- 1.Conwell DS, Mosher A, Khan A, et al. Factors associated with loss to follow-up in a large tuberculosis treatment trial (TBTC Study 22) Contemp Clin Trials. 2007;28:288–94. doi: 10.1016/j.cct.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Brown DM, Thorne JE, Foster GL, et al. Factors affecting attrition in a longitudinal study of patients with AIDS. AIDS Care. 2006;18:821–9. doi: 10.1080/09540120500466747. [DOI] [PubMed] [Google Scholar]
- 3.Dudley J, Jin S, Hoover D, et al. The Multicenter AIDS Cohort Study: retention after 9½ years. Am J Epidemiol. 1995;142:323–30. doi: 10.1093/oxfordjournals.aje.a117638. [DOI] [PubMed] [Google Scholar]
- 4.Hessol NA, Schneider M, Greenblatt RM, et al. Retention of women enrolled in a prospective study of human immunodeficiency virus infection: impact of race, unstable housing, and use of human immunodeficiency virus therapy. Am J Epidemiol. 2001;154:563–73. doi: 10.1093/aje/154.6.563. [DOI] [PubMed] [Google Scholar]
- 5.Matts JP, Launer CA, Nelson ET, et al. A graphical assessment of the potential impact of losses to follow-up on the validity of study results. The Terry Beirn Community Programs for Clinical Research on AIDS Stat Med. 1998;16:1943–54. doi: 10.1002/(sici)1097-0258(19970915)16:17<1943::aid-sim631>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 6.Hunt JR, White E. Retaining and tracking cohort study members. Epidemiol Rev. 1998;20:57–70. doi: 10.1093/oxfordjournals.epirev.a017972. [DOI] [PubMed] [Google Scholar]
- 7.Neill KM, Chessa F. Recruitment and retention of women in nontherapeutic clinical trials. Appl Nurs Res. 1998;3:148–51. doi: 10.1016/s0897-1897(98)80152-3. [DOI] [PubMed] [Google Scholar]
- 8.Orr PR, Blackhurst DW, Hawkins BS. Patient and clinic factors predictive of missed visits and inactive status in a multi-center clinical trial. Control Clin Trials. 1992;13:40–9. doi: 10.1016/0197-2456(92)90028-x. [DOI] [PubMed] [Google Scholar]
- 9.Morse EV, Simon PM, Besch CL, et al. Issues of recruitment, retention, and compliance in community-based clinical trials with traditionally underserved populations. Appl Nurs Res. 1995;1:8–14. doi: 10.1016/s0897-1897(95)80240-1. [DOI] [PubMed] [Google Scholar]
- 10.Friedland DJ. Guide for assessing the validity of a study. In: Friedland DJ, editor. Evidence-based medicine: a framework for clinical practice. Stamford, CT: Appleton and Lange; 1998. [Google Scholar]
- 11.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42:507–19. [PubMed] [Google Scholar]
- 12.Palta M, McHugh R. Planning the size of a cohort study in the presence of both losses to follow-up and non-compliance. J Chronic Dis. 1980;33:501–12. doi: 10.1016/0021-9681(80)90075-2. [DOI] [PubMed] [Google Scholar]
- 13.Seage GR, III, Holte SE, Metzger D, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness. Study Am J Epidemiol. 2001;153:619–27. doi: 10.1093/aje/153.7.619. [DOI] [PubMed] [Google Scholar]
- 14.de Bruyn G, Hudgens MG, Sullivan PS, et al. Participant retention in clinical trials of candidate HIV vaccines. J Acquir Immune Defic Syndr. 2005;39:499–501. doi: 10.1097/01.qai.0000148532.12329.df. [DOI] [PubMed] [Google Scholar]
- 15.Amato DA. Selection of endpoints for assessment of treatment efficacy in an AIDS trial. In: Finkelstein DM, Schoenfeld DA, editors. AIDS clinical trials. New York, NY: Wiley; 1995. [Google Scholar]
- 16.Dickert N, Grady C. What’s the price of a research subject? Approaches to payment for research participation. N Engl J Med. 1999;341:198–203. doi: 10.1056/NEJM199907153410312. [DOI] [PubMed] [Google Scholar]
- 17.Grady A, Dickert N, Jawetz T, et al. An analysis of U.S. practices of paying research participants. Contemp Clin Trials. 2005;26:365–75. doi: 10.1016/j.cct.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Iltis AS, DeVader S, Matsuo H. Payments to children and adolescents enrolled in research: a pilot study. Pediatrics. 2006;118:1546–52. doi: 10.1542/peds.2006-0821. [DOI] [PubMed] [Google Scholar]
- 19.Kimberly MB, Hoehn KS, Feudtner C, et al. Variation in standards of research compensation and child assent practices: a comparison of 69 institutional review board-approved informed permission and assent forms for 3 multicenter pediatric clinical trials. Pediatrics. 2006;117:1706–11. doi: 10.1542/peds.2005-1233. [DOI] [PubMed] [Google Scholar]
- 20.Gortmaker S, Hughes M, Cervia J, et al. Effect of combination therapy including protease inhibitors on mortality among children and adolescents infected with HIV-1. N Engl J Med. 2001;345:1522–8. doi: 10.1056/NEJMoa011157. [DOI] [PubMed] [Google Scholar]
- 21.Brogly S, Williams P, Seage G, III, et al. Antiretroviral treatment in pediatric HIV infection in the United States: from clinical trials to clinical practice. JAMA. 2005;293:2213–20. doi: 10.1001/jama.293.18.2213. [DOI] [PubMed] [Google Scholar]
- 22.Costagliola D, Mary-Krause M. Zidovudine in patients with HIV infection. (Letter) Ann Intern Med. 1996;124:372. doi: 10.7326/0003-4819-124-3-199602010-00024. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers TC, Smith H, Jr, Blackburn B, et al. A method for assessing the quality of a randomized control trial. Control Clin Trials. 1981;2:31–49. doi: 10.1016/0197-2456(81)90056-8. [DOI] [PubMed] [Google Scholar]
- 24.Galea S, Tracy M. Participation rates in epidemiologic studies. Ann Epidemiol. 2007;17:643–54. doi: 10.1016/j.annepidem.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Ioannidis JPA, Taha TE, Kumwenda N, et al. Predictors and impact of losses to follow-up in an HIV-1 perinatal transmission cohort in Malawi. Int J Epidemiol. 1999;28:769–75. doi: 10.1093/ije/28.4.769. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson DL, Wu AW, Feinberg J, et al. Health-related quality of life predicts survival, cytomegalovirus disease, and study retention in clinical trial participants with advanced HIV disease. J Clin Epidemiol. 2003;56:874–9. doi: 10.1016/s0895-4356(03)00062-3. [DOI] [PubMed] [Google Scholar]
- 27.Goldman AI, Holcomb R, Perry HM, Jr, et al. Can dropout and other noncompliance be minimized in a clinical trial? Control Clin Trials. 1982;3:75–89. doi: 10.1016/0197-2456(82)90037-x. [DOI] [PubMed] [Google Scholar]
- 28.Cramer JA, Collins JF, Mattson RH. Can categorization of patient background be used to determine early termination in a clinical trial? Control Clin Trials. 1988;9:47–63. doi: 10.1016/0197-2456(88)90008-6. [DOI] [PubMed] [Google Scholar]
- 29.Williams PL, Storm D, Montepiedra G, et al. Predictors of adherence to antiretroviral medications in children and adolescents with HIV infection. Pediatrics. 2006;118:e1745–57. doi: 10.1542/peds.2006-0493. [DOI] [PubMed] [Google Scholar]
- 30.Poole WK, Perritt R, Shah KB, et al. A characterization of patient drop-outs in a cohort of HIV-positive homosexual/bisexual men and intravenous drug users. J Epidemiol Community Health. 2001;55:66–7. doi: 10.1136/jech.55.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagley SJ, Reynolds WW, Nelson RB. Is a wage-payment model for research participation appropriate for children? Pediatrics. 2007;119:46–51. doi: 10.1542/peds.2006-1813. [DOI] [PubMed] [Google Scholar]