Abstract
The effects of maternal stress during pregnancy may depend, in part, on the timing in gestation of the occurrence of stress. The aim of the present study was to examine the effect of stage of gestation on maternal psychophysiological responses to stress using a standardized laboratory paradigm and on the cortisol response to awakening (CAR). A longitudinal design was employed to quantify maternal psychophysiological stress reactivity [changes in heart rate (HR), blood pressure, salivary cortisol, and psychological distress in response to the trier social stress test (TSST)] and the CAR at approximately 17 and 31 weeks gestation in a sample of 148 women. To account for the possible effects of habituation when being exposed to the same stress protocol twice, a non-pregnant comparison group (CG, N = 36) also underwent these assessments at two time points, with a comparable time interval between the assessments. In both groups, the TSST elicited significant changes in maternal HR, mean arterial pressure, and psychological distress levels but not a significant increase in cortisol levels. Among the pregnant women (pregnant group(PG)), the stressor-induced increases in HR, blood pressure, and psychological distress were significantly lower at the second (31 weeks gestation) compared to the first (17 weeks gestation) assessment of pregnancy (all p < 0.01). The maternal CAR was also significantly attenuated in later compared to earlier gestation (p = 0.003). In the CG, there were no significant differences in psychophysiological stress responses and in the CAR across the two assessments. Among pregnant women there is a progressive attenuation of psychophysiological stress responses with advancing gestation. This attenuation is unlikely to be attributable to habituation. Individual differences in the degree of attenuation of stress responses over gestation may represent a novel marker of stress susceptibility in human pregnancy.
Keywords: Cortisol awakening response (CAR) or rise, heart rate, mean arterial pressure, pregnancy, psychosocial stress, Trier Social Stress Test (TSST)
Introduction
Exposure to high levels of maternal stress during pregnancy has the potential to adversely impact fetal development, birth outcomes, and subsequent child and adult health outcomes (Van den Bergh et al. 2005; Wadhwa 2005; Entringer et al. 2008; Weinstock 2008; O’Donnell et al. 2009). There is, however, considerable variation in the effects of prenatal stress (Wadhwa 2005). One possible modifier of its effects is timing of the occurrence of stress. There may be critical time periods in human gestation of increased susceptibility to the effects of maternal stress. The notion of critical periods of susceptibility has been discussed previously, and its effects are usually attributed to the underlying ontogeny or time line of the development of the fetal brain and other organ systems (Rice and Barone 2000; Nijland et al. 2008), to suggest that a system may be most vulnerable to the effects of stress during its most rapid phase or phases of growth and development. We propose here another potential explanation for this phenomenon: as pregnancy advances there are progressive changes in maternal biology which, in turn, may result in alterations in physiological and psychological responses to stress and thereby produce variation in the effects of prenatal stress on the developing fetus.
It is well established that the state of pregnancy produces major alterations in physiological systems, including up-regulation of hormone production and cardiac output, that are crucial for supporting aspects of fetal growth and development (see Mastorakos and Ilias 2003 for an overview). Some evidence suggests that the state of pregnancy is associated with alterations in maternal physiological responsivity to a stimulus or stressor. Evoked physiological (cardiovascular and endocrine) responses have been studied in pregnant women in response to pharmacological and physical challenges. These studies generally suggest that maternal responses to challenge are dampened in pregnancy, particularly in the latter stages of gestation. For example, administration of exogenous corticotrophin-releasing hormone (CRH) in late pregnancy failed to evoke a significant pituitary or adrenal response (Sasaki et al. 1989; Schulte et al. 1990), and administration of dexamethasone produced less suppression of cortisol production (Odagiri et al. 1988). Similarly, autonomic responses [heart rate (HR), blood pressure] to a variety of challenges, including exercise, orthostatic challenge, and the cold pressure test, are attenuated in pregnancy (Ekholm and Erkkola 1996; Wolfe and Weissgerber 2003; de Weerth et al. 2005). Much less research, however, has been conducted regarding maternal responsivity to psychological stress during pregnancy. Studies in pregnant women that have employed laboratory-based challenge paradigms to induce psychological stress (e.g. cognitive challenge tests such as mental arithmetic, Stroop test, free speech, and mirror image tracing) are reviewed in deWeerth et al. (2005). The results of these studies generally suggest that blood pressure, HR, and cortisol responses to psychological stress appear to be attenuated in pregnancy. Two recent studies did not find differences in cortisol and HR responses to a laboratory stress protocol between pregnant and non-pregnant women (Nierop et al. 2006a; de Weerth et al. 2007). However, differences in experimental designs, the lack of adequate control groups, and other methodological limitations make it difficult to draw firm conclusions. Moreover, it is possible that the psychological appraisal of a stressful stimulus/experience also may be altered during pregnancy: some studies have suggested that women seem to become decreasingly sensitive to the psychological effects of stress as pregnancy advances (Glynn et al. 2001, 2004, 2008).
Thus, the aim of the present study was to systematically investigate whether there are changes over the course of human gestation in sympathoadrenal medullary (SAM), hypothalamic–pituitary–adrenal (HPA), and psychological responses to psychosocial stress. A prospective, longitudinal design with serial assessments was employed to assess maternal psychophysiological reactivity (HR, blood pressure, cortisol, and psychological appraisal of distress) in response to a standardized laboratory-based psychosocial stressor—the Trier Social Stress Test (TSST)—at two time points in the second and third trimester of pregnancy, respectively. The TSST was selected because it combines two critical elements of the experience of psychosocial stress—uncontrollability and social-evaluative threat—and has been shown in many different populations to reliably induce psychophysiological stress responses (Dickerson and Kemeny 2004). We also simultaneously assessed the maternal cortisol awakening response (CAR)—the rise in cortisol secretion following transition from sleep to wake in the morning. In addition, circadian regulation of cortisol production was assessed using the short diurnal cortisol profile. To control and distinguish the potential effects of habituation (Kirschbaum et al. 1995; Federenko et al. 2004) from those of pregnancy-related attenuation, a non-pregnant comparison group (CG) underwent the same protocol at two time points with a comparable time interval between assessments. We hypothesized (1) that the cardiovascular, endocrine, and psychological responses to the TSST as well as the CAR would be progressively attenuated as pregnancy advanced, and (2) that the magnitude of this attenuation would be greater than that of a possible habituation effect (as measured in the non-pregnant CG).
Materials and methods
Participants
A total of 148 pregnant women (PG) receiving prenatal care at university-affiliated obstetric clinics in Southern California were recruited as subjects for this study. Inclusion criteria were a singleton, intrauterine pregnancy < 20 weeks gestation. Exclusion criteria included tobacco, alcohol, or other drug use in pregnancy; uterine or cervical abnormalities; or presence of any conditions known to be associated with dysregulated cardiovascular or neuroendocrine function, such as endocrine, hepatic, or renal disorders or corticosteroid medication use. The mean age was 28.54 ± 0.49 (SEM) years; approximately half of the sample were nulliparous women (46.3%) of predominantly non-Hispanic White (39.2%), and Hispanic (30.7%) origin (see Table I).
Table I.
Study characteristics for age, race/ethnicity, education, marital status, BMI and parity for pregnant group (PG, n = 148) and (CG, n = 36) subjects, as well as p-values for comparisons between the two groups.
| PG | CG | P | |
|---|---|---|---|
| Age (years ± SD) | 28.5 ± 6.0 | 32.0 ± 7.0 | < 0.01 |
| Race/ethnicity | n.s. | ||
| Non-Hispanic White | 39% | 36% | |
| Hispanic White | 30% | 25% | |
| Hispanic other | 10% | 11% | |
| Non-Hispanic Black | 5% | 6% | |
| Non-Hispanic Asian | 7% | 11% | |
| Non-Hispanic other | 9% | 11% | |
| Years of school completed | 14.5 ± 2.5 | 14.0 ± 3.0 | n.s. |
| Married | 83% | 78% | n.s. |
| BMI (pre-pregnancy BMI for pregnant group) | 25.5 ± 6.1 | 25.9 ± 7.3 | n.s. |
| Nulliparous | 46% | 51% | n.s. |
n.s., non-significant.
For all subjects gestational age was determined by best obstetric estimate with a combination of last menstrual period and early uterine size, and was confirmed by obstetric ultrasonographic biometry using standard clinical criteria (O’Brien et al. 1981). The sample was predominantly low obstetric risk for adverse pregnancy outcomes; obstetric risk was abstracted from the medical record and coded as a dichotomous variable as previously described (Wadhwa et al. 2004).
A non-pregnant CG of women was recruited by presenting the study to university employees through e-mail announcements and placards. Exclusion criteria for the CG were smoking or presence of any conditions known to be associated with dysregulated cardiovascular or neuroendocrine function, such as endocrine, hepatic or renal disorders, or corticosteroid medication use. As presented in Table I, the CG was significantly older, but there were no significant differences regarding race/ethnicity, education, marital status, body mass index (BMI), and parity between the PG and the CG. All 36 CG women completed the first assessment, and 22 subjects completed both assessments. The CG women who had dropped from the study after the first assessment did not differ significantly from the CG women who completed both assessments. The study was approved by the Institutional Review Board and all study participants provided written informed consent.
Experimental procedures
Subjects reported to the research laboratory on two occasions. Of the 148 pregnant women, 118 subjects provided complete data at the first assessment during pregnancy [16.6 ± 0.14 (SEM) weeks gestation], 106 subjects provided complete data at the second assessment (31.5 ± 0.12 weeks gestation), and 76 subjects completed both visits†. Subjects that completed both visits did not differ at recruitment from subjects that only completed one of the two visits with respect to age, parity, race/ethnicity, levels of perceived stress (assessed using the Perceived Stress Scale, Cohen et al. 1983) and depressive symptoms (assessed using the Center for Epidemiological Studies Depression Scale, Radloff 1977; all p > 0.14). The mean time between the first and second assessments was 14.4 ± 0.19 weeks. These two time periods, around 17 and 30 weeks gestation, were selected because the change in endocrine parameters from early through late gestation is critical in characterizing the maternal–placental–fetal endocrine stress milieu with respect to its impact on parturition (Hobel et al. 1999; Wadhwa et al. 2001; Sandman et al. 2006). The CG of non-pregnant subjects also attended two laboratory sessions at a comparable time interval (15.8 ± 0.3 weeks). The time interval between the two sessions did not differ significantly between the PG and the CG.
All participants were instructed to refrain from physical exercise and non-prescription medication (e.g. antihistamines, antiinflammatory medications) 24 h prior to the assessment, refrain from caffeine intake in the preceding 4 h period, and only consume a light carbohydrate lunch on the day of the assessment, at least 60 min before the assessment. Each participant was exposed to the psychosocial stress protocol (TSST) in the afternoon at identical times across test days, commencing between 1430 and 1600 h. Time of day was controlled to avoid baseline level or reactivity differences due to the circadian rhythm of HPA axis activity.
Thirty minutes prior to the onset of the TSST, subjects were seated in a comfortable armchair in a recumbent position and were instrumented with Dinamap vital signs monitors (1846 SX, Critikon) for automated assessment of HR and blood pressure. Participants remained seated in this position and in the same room during the whole procedure. This was a modification to the standard TSST protocol (Kirschbaum et al. 1993) to minimize physical discomfort to the pregnant women. After a 30 min relaxation period, participants were exposed to the TSST, which consists of a free speech (5 min) and a mental arithmetic task (5 min) in front of an audience and a camera. Including the instruction and a short preparation period, the stressful situation lasted for 15 min. HR and blood pressure were assessed −30, −15, and −1 min prior to, as well as every +3 min up to +30 min after the onset of the TSST. Saliva samples were collected −15 and −1 min prior to and at +16, +30, +45, and + 60 min relative to the onset of the TSST.
To assess diurnal cortisol profiles, subjects were instructed to collect saliva samples at 0, 30, 45, and 60 min after awakening as well as at 1200, 1500, and 2000 h on the day before the TSST exposure. Exact time of saliva sampling was monitored using a Medication Event Monitoring System (MEMS®, Aardex group, Union City, CA, USA) that time stamped every opening of the plastic vial where the swabs to collect the saliva were stored. The use of an electronic monitoring device has been shown to increase compliance regarding the exact time of the sample collection, which, in turn, is critical for accurately characterizing an individual’s circadian profile (Kudielka et al. 2003). Accordingly, each swab was stored in a plastic tube labeled with the designated sampling time by the experimenter. Participants were instructed to refrain from brushing their teeth during the first hour after awakening and from eating 30 min before each saliva collection.
Psychological stress assessments
To obtain a measure of perceived distress associated with the task, participants were asked to rate how distressed they felt immediately before and after the task on a 5-point Likert scale ranging from 0 (not at all) to 4 (“extremely”, adapted from the “profile of mood states”, McNair et al. 1981).
Salivary cortisol assay
Saliva samples were collected using a Salivette sampling device (Sarstedt, Numbrecht, Germany). Saliva was recovered from each swab by centrifugation and stored at −70°C degrees until assayed. Thawed samples were centrifuged at 1700g for 15 min before assay. Salivary cortisol concentrations were determined by a competitive luminescence immunoassay (LIA; IBL-America, Minneapolis, MN, USA) with a detection limit of 0.015 μg/dl. The cross reactivity of the assay was < 2.5% with cortisone, prednisone, and corticosterone and < 0.1% with other naturally occurring steroids. The intra- and inter-assay coefficients of variance were 5.5 and 7.6%, respectively. Data reduction for the LIA assay was done by an automated four-parameter logistics program (software Mikro Win 2000; Berthold Microplate Luminometer). All samples were assayed in duplicate and averaged.
Statistical analyses
Hierarchical linear modeling (HLM) growth curve analyses (Raudenbush and Bryk 2002; Singer and Willett 2003) were conducted using the HLM 6.01 for Windows software package to characterize changes in HR, blood pressure, and cortisol concentrations in response to the TSST as well as cortisol responses to awakening and changes over the course of the day. The HLM procedure weights cases with complete data more heavily, but all cases are included in the estimation of effects. We used precise measures of time of day for sample collection (i.e., actual times of collection recorded by the MEMS® cap) and gestational age at testing rather than nominal estimates of assessment intervals in order to control for variability in the timing of collections or stage of pregnancy. All analyses were performed separately for pregnant women and the CG in order to control for pregnancy-related covariates among the pregnant group. All cortisol measures were log transformed by LnCort = ln (Cort + 1) to yield an unskewed response variable. Because mean arterial pressure (MAP) is a better predictor of pregnancy complications than either systolic or diastolic blood pressure (Cnossen et al. 2008), MAP was used and was calculated by the following formula: [(2 × diastolic) + systolic]/3 (diastole is weighted 2 × systole because 2/3 of the cardiac cycle is spent in diastole). The statistical significance level was set at alpha = 0.05.
Analyses of HR, MAP, and cortisol levels in response to the TSST over gestation
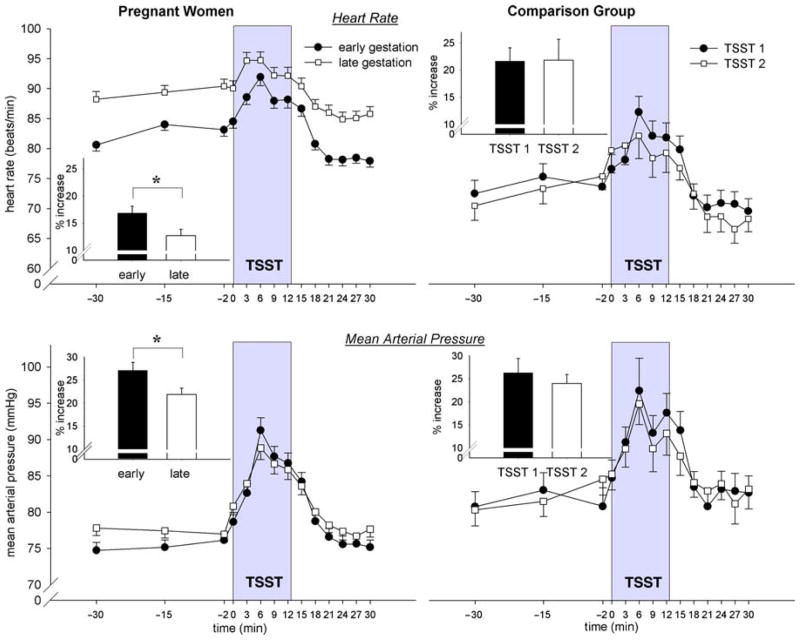
The assessments prior to the start of the TSST were averaged to obtain a baseline measure. As depicted in Figure 1, subjects, on average, returned to their baseline HR and MAP levels 21 min after the start of the TSST. The time interval from baseline until 21 min after the onset of the TSST was therefore considered the response period.
Figure 1.
Mean HR (±SEM) and MAP (±SEM) values before, during, and after the TSST in pregnant women (n = 148) and in the CG (n = 36) for both study assessments. To graphically illustrate the findings of the three-level HLM models, percent increase (±SEM) from baseline is depicted for HR and MAP for both groups. Significant differences (p < 0.05) are indicated with an asterisk.
Three-level HLM analyses were performed to evaluate stage-of-gestation related changes in maternal HR and MAP in response to the TSST. Level 1 captured parameters that change within an individual and within an assessment period i.e., time as random predictors of HR, MAP, and cortisol, respectively. Linear and quadratic effects of time were included in the final model, and comparison of deviance statistics showed that this model was superior to linear modeling (all p < 0.001). Time was centered at baseline so that the intercept represented the mean HR, MAP, or cortisol levels just prior to the start of the TSST.
Level 2 captured potential changes in stress physiology from one assessment to the next. For pregnant women, exact gestational age at testing (in weeks gestation) at each assessment was modeled (centered at mean gestational age at first visit) to capture change in stress physiology across pregnancy. For CG subjects, the first visit was set to zero to parallel the model for the pregnant women, and time in weeks captured the interval between visits.
Between individual differences predictors were introduced in level 3. Covariates of interest included age and BMI (pre-pregnancy BMI for the pregnant group) for both groups, and obstetric risk and fetal sex for the pregnant group. None of these variables influenced the changes in HR over gestation. There was, however, a significant effect of fetal sex on changes in maternal MAP responses to the TSST over gestation (p = 0.025). Hence, the results presented below are adjusted for the effects of fetal sex.
Analysis of psychological distress over pregnancy
Differences in distress levels from before to after the TSST at each assessment were calculated for each individual (Δ distress). Two-level models were constructed to model changes in Δ distress in response to the TSST over gestation (from first to second for the CG, respectively).
Analyses of diurnal cortisol over gestation
Three-level HLM models were performed to evaluate possible gestational-age related changes in the diurnal cortisol profile over pregnancy. Because the CAR (assessed at 0, 30, 45, and 60 min after awakening) and the slope over the day (day time profile, assessed at awakening, 1100, 1600, and 2000 h) are considered two different characteristics of HPA axis function (Pruessner et al. 1997; Wust et al. 2000; Wilhelm et al. 2007), level 1 included two time parameters, one for the CAR effect, and the second parameter for the short daytime profile to capture within-the-day changes in cortisol. Both the CAR and the diurnal slope were simultaneously modeled to account for the potential interdependence of these parameters and to ensure that associations with gestational age or individual differences were independently associated with cortisol levels, the CAR, and/or the diurnal slope. Based on known changes in the awakening and daytime pattern of cortisol production, linear and quadratic effects of time for both parameters were included, and this proved to be superior to linear modeling (all p < 0.001). Time was centered at awakening so that model intercept represents the mean log transformed cortisol levels at awakening. The two time parameters and the intercept were included as random factors since they are known to have substantial variability across individuals.
Exact gestational age at each assessment for the pregnant women (centered at mean gestational age at first visit) and time between visits for the CG were modeled at level 2. Not all participants awoke at the same time at each assessment period. To control for fluctuations in wake up time at each assessment, time of awakening was entered at level 2.
Level 3 captured between individual differences in predictors. Covariates of interest included maternal age, obstetric risk, pre-pregnancy BMI, and fetal sex. None of these variables influenced the change in cortisol concentrations and were therefore not included in the final models (all p > 0.15).
In order to examine the relative magnitude of the HR, MAP, and distress response attenuation across gestation in the pregnant women (and across time in the CG), we calculated the R2, the percent of session-by-session variance explained by gestational age (or time). The percentage of variance explained in an HLM model is complicated because variance at each different level of analyses can individually change with the inclusion of a predictor (Hox 2002). Consequently, for our purposes, the interpretation of the R2 described by Hox (2002) and Snidjers and Bosker (1994) is specific to the session-by-session level of analysis.
Results
Physiological and psychological responses to the TSST
The mean values (±SEM) of HR and MAP for pregnant women and the non-pregnant CG before, during, and after the TSST at both visits are depicted in Figure 1.
Heart rate
Table IIa shows results of the three-level HLM model for HR for pregnant women. Baseline HR was significantly higher in the later compared to earlier stage of pregnancy (p < 0.001). Basal HR increased by 0.52 beats per minute with each week of advancing gestation. The HR increase in response to the TSST was less steep as gestation advanced (p < 0.001) indicated by the significant stage of gestation-related changes in the linear and quadratic time slopes (all p < 0.001).
Table II.
Hierarchical linear model estimates for effects of time and gestational age at test predicting HR (a) and MAP (b) measurements.
| Parameter | SE | P | |
|---|---|---|---|
| (a) HR (beats/min) | |||
| Intercept | 78.97 | 0.838 | < 0.001 |
| Time | 2.207 | 0.165 | < 0.001 |
| Time2 | −0.1009 | 0.0075 | < 0.001 |
| Intercept × gestational age at test | 0.5151 | 0.0617 | < 0.001 |
| Time × gestational age at test | −0.0509 | 0.0097 | < 0.001 |
| Time2 × gestational age at test | 0.0021 | 0.0004 | < 0.001 |
| (b) MAP (mmHg) | |||
| Intercept | 76.85 | 1.052 | < 0.001 |
| Time | 2.299 | 0.188 | < 0.001 |
| Time2 | −0.1020 | 0.0091 | < 0.001 |
| Intercept × gestational age at test | 0.0764 | 0.0647 | 0.239 |
| Time × gestational age at test | −0.0431 | 0.0149 | 0.005 |
| Time2 × gestational age at test | 0.0018 | 0.0007 | 0.012 |
In contrast, the HR increase in response to the TSST in the CG was not different from the first to the second assessment, as indicated by no effect of weeks between visits on the intercept and the linear and quadratic slopes, all p > 0.88.
Mean arterial pressure
Table IIb shows the results of the three-level HLM model for MAP for pregnant women. There were no differences in baseline MAP across the two assessments in earlier and later gestation (p = 0.239). The increase in MAP in response to the TSST was, however, less steep as gestation advanced (p’s for interaction between linear and quadratic slopes and week of gestation < 0.005)‡.
In the CG, MAP responses to the TSST were not different across the two assessments (no significant effect of weeks between visits on the intercept, the linear slope or the overall shape of the curve, all p > 0.101).
Salivary cortisol concentration
In this population of subjects using the procedures described above, exposure to the TSST did not produce a significant increase in salivary cortisol concentration at either of the two assessments in either the pregnant and non-pregnant group (for linear slope, all p > 0.07). We, therefore, did not conduct any further HLM models to examine changes in the salivary cortisol response to the TSST across the two assessments.
Psychological distress
As expected, exposure to the TSST induced significant psychological distress in subjects in both groups. Among pregnant women, the extent of psychological distress evoked by the TSST was significantly attenuated as gestation advanced (B = −0.0348, SE = 0.0088, and p < 0.001, see Figure 2), whereas it did not differ in the CG across the two assessments (B = −0.0248, SE = 0.0144, and p = 0.092).
Figure 2.
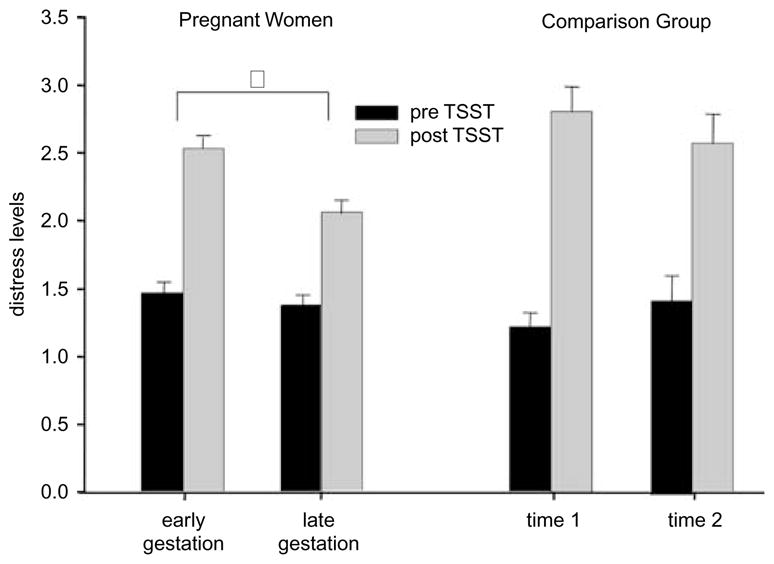
Mean distress levels (±SEM) before and after the TSST in pregnant women (n = 148) and in the CG (n = 36) for both study assessments. Significant differences (p < 0.05) are indicated with an asterisk.
Table III shows the percent of session-by-session variance explained by gestational age for the PG or time between sessions for the CG (R2). The proportion of variance in changes in the response magnitude from TSST1 to 2 explained by the state of pregnancy is higher (47% for HR, 10% for MAP, and 19% for distress) than the session-by-session variance explained by habituation to the task in the CG (0% for HR, 5% for MAP, and 4% for distress) indicating that the attenuation effect we observed in the PG is over and above the effect explained by habituation to the task in the CG.
Table III.
Explained session-by session variance (R2) for changes in HR, MAP, and distress from TSST 1 to 2 for the pregnant group (PG, n = 148) and the (CG, n = 36).
| R2 (for session-by-session variance) | PG | CG |
|---|---|---|
| HR | 0.47 | 0.00 |
| MAP | 0.10 | 0.05 |
| Distress | 0.19 | 0.04 |
Diurnal cortisol concentrations over the course of gestation
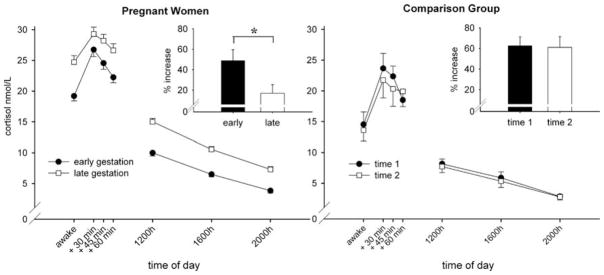
Mean cortisol concentrations (±SEM) in response to awakening and over the course of the day are presented for both study groups in Figure 3. The results of the final three-level HLM model on log-transformed salivary cortisol for pregnant women are summarized in Table IV. The main effects “Time” for CAR and course of day reflect changes in cortisol concentrations after awakening and over the course of the day, respectively. As indicated by the significant linear and quadratic time slope for the CAR (all p < 0.001), a significant increase in cortisol concentrations was observed in the first hour after awakening, and there was also a quadratic effect, as illustrated in Figure 3. Furthermore, from awakening to 20:00 h, cortisol concentrations decreased significantly reflected by the linear day time slope (p < 0.001). These results suggest that during pregnancy, the diurnal cortisol rhythmicity (increase in response to awakening and a general decrease over the course of the day) is preserved.
Figure 3.
Mean (±SEM) salivary cortisol concentrations in response to awakening (CAR) and over the course of the day in pregnant women (n = 148) and in the CG (n = 36) for both study assessments. To graphically illustrate the findings of the three-level HLM models, percent increase (±SEM) from awakening is depicted for both groups. Significant differences (p < 0.05) are indicated with an asterisk.
Table IV.
Hierarchical linear model estimates for effects of time (CAR and course of the day) and stage of gestation predicting salivary cortisol concentrations.
| Diurnal Cortisol [ln (nmol/L) + 1] |
|||
|---|---|---|---|
| Parameter | SE | P | |
| Intercept | 2.901 | 0.047 | < 0.001 |
| Time (CAR) | 0.9128 | 0.1359 | < 0.001 |
| Time2 (CAR) | −0.7087 | 0.1030 | < 0.001 |
| Time (course of day) | −0.1319 | 0.0140 | < 0.001 |
| Time2 (course of day) | 0.0012 | 0.0010 | 0.219 |
| Intercept × gestational age at test | 0.0188 | 0.0041 | < 0.001 |
| Time (CAR) × gestational age at test | −0.0321 | 0.0105 | 0.003 |
| Time2 (CAR) × gestational age at test | 0.0250 | 0.0079 | 0.002 |
| Time (day) × gestational age at test | 0.0023 | 0.0012 | 0.074 |
| Time2 (day) × gestational age at test | −0.0001 | 0.0001 | 0.605 |
The cross-level interactions between “Time” (CAR and day) and week of gestation (earlier vs. later in pregnancy) indicate how diurnal cortisol concentrations changed over the course of gestation. As expected, cortisol concentrations at awakening (intercept) significantly increased with gestational age (p < 0.001). The cortisol increase in response to awakening was, however, less steep and more platykurtic as gestation advanced indicated by significant gestation-related changes in the linear and quadratic CAR time slopes (all p ≤ 0.001).
The diurnal cortisol rhythm over the course of the day did not change significantly as gestation advances (as reflected by the linear day time slope (p = 0.07). Thus, the results suggest that after accounting for the changing baseline across gestation, there is a significantly smaller CAR with advancing gestation.
The CG also showed a significant cortisol increase in response to awakening as well as a decrease in cortisol concentrations over the course of the day at both assessments (for quadratic and linear CAR and day slopes, all p < 0.001). However, there were no significant changes across the two assessments in the magnitude of the CAR or the diurnal cortisol rhythm (no effect of weeks between visits on the intercept and the linear and quadratic time slopes, all p > 0.90).
Discussion
To the best of our knowledge, this is the first study to prospectively and longitudinally assess serial psychophysiological responses to a standardized laboratory-based psychosocial stress test as well as the CAR in pregnant women over the course of gestation and in a non-pregnant CG. As expected, baseline salivary cortisol and HR levels were significantly higher in the third compared to the second trimester of gestation. However, the CAR, as well as the increase in HR and MAP in response to the TSST were attenuated in the later compared to earlier stage of pregnancy. Moreover, the TSST-induced levels of psychological distress were also lower in later compared to earlier gestation. Thus, the findings of the present study not only replicate earlier reports suggesting physiological responses to challenge are dampened during pregnancy, but also suggest that there is a progressive attenuation of maternal physiological as well as psychological stress responses with advancing gestation. Because the non-pregnant CG did not exhibit significant differences in any of these parameters across the two assessments, we believe it is unlikely that the progressive attenuation of the stress responses in pregnant women is due to habituation to the task.
Although baseline levels increase over gestation (which is expected because pregnancy is associated with a progressive increase in hormone production), and although the law of initial values would postulate that the predicted decrease in response to a challenge is a function of the elevated baseline, our results suggest that even after accounting for baseline (which is done in the HLM model by examining level and change simultaneously, thereby partialing out the effects of one from the other at the appropriate level of analysis), the response to challenge (TSST, awakening) is attenuated with advancing gestation. Thus, because the observed attenuation of stress response is independent of baseline function, future studies in this cohort could examine whether individual differences in the degree of attenuation across gestation may serve as a marker of underlying susceptibility for prenatal stress-related outcomes in the offspring.
Our findings are consistent with those of Matthews and Rodin (1992), who reported that pregnancy was associated with a reduced diastolic blood pressure response across a variety of different stress tasks, and with those of DiPietro et al. (2003), who noted a decline in the maternal HR response to a psychological stressor from 24 to 36 weeks gestation. The use of the TSST, a standardized protocol to induce psychosocial stress, has so far been reported only twice in the context of human pregnancy (Nierop et al. 2006a,b; de Weerth et al. 2007). In contrast to our repeated measures longitudinal design, Nierop et al. (2006a) used a cross-sectional study design and did not observe any differences in HR response to the TSST in second compared to third trimester-pregnant women. They did, however, report smaller alpha-amylase responses to the TSST in third versus second trimester pregnant women. The activity of this enzyme in saliva is believed to reflect SAM reactivity (Ehlert et al. 2006); hence this aspect of their findings is consistent with our finding of a progressively attenuated cardiovascular response from mid to later pregnancy. Our findings of lower distress in response to the TSST over the course of gestation are consistent with previous reports suggesting a decline in perceived stress, state anxiety, and affective responses to specific life events during pregnancy (Glynn et al. 2001, 2004, 2008).
We are aware of two other studies examining the CAR in the context of pregnancy (de Weerth et al. 2007; Shea et al. 2007). Although Shea et al. (2007) reported an increase of about 44% from awakening to 30 min after awakening in their healthy pregnant group, they also state at the same time that the absolute increase of 5.3 nmol/l is much lower than the 9 nmol/l cortisol increase to awakening reported in healthy non-preganant women (Clow et al. 2004), which is in line with our findings. In addition, we note that they assessed the CAR on average at 28 weeks gestation, while in the present study the late pregnancy visits took place on average about 3 weeks later, at 31 weeks gestation. This could explain why the baseline awakening levels we observed at the second visit were on average higher than those reported by Shea et al. (2007), because cortisol levels increase progressively over gestation reaching levels 2–3 times as high at the end of pregnancy compared to non-pregnant women. In the de Weerth and Buitelaar study (2005), the CAR was assessed during pregnancy and 9 months postpartum in the same women. They report that the relative mean increase was comparable at both time points. As the authors point up in their discussion, the awakening response of mothers of 9-month-old-infants might be influenced by interruptions of sleep, and the women may not have been as compliant with the sampling protocol during motherhood. In addition, there are methodological differences regarding the sampling protocol and statistical analyses between our study and the two above-mentioned studies. Since it has been suggested that compliance and the accuracy of self-reported sampling records increases when participants are informed that an electronic monitoring device is recording their saliva collection activities (Kudielka et al. 2003), electronic monitoring devices (MEMS®, AAR-DEX) were used to time and date stamp instances of saliva collection, and exact time of sampling was used in the statistical models, while the de Weerth et al. (2005) and Shea et al. (2007) studies relied on self-reported sampling time.
A recent review of physiological stress reactivity in pregnancy by de Weerth and Buitelaar (2005) highlights several major methodological limitations in the existing literature and makes recommendations for future research. We note that our study design incorporated almost all of these recommendations i.e., investigating stress reactivity throughout pregnancy using a longitudinal design, and the inclusion of a non-pregnant CG. There are, however, some limitations to our study. Although the psychosocial stress protocol used by us (the TSST) successfully stimulated the SAM system at all assessments (as indicated by significant increases in HR and blood pressure), we did not observe a significant endocrine (cortisol) response to the task. We speculate that the failure of the task to produce a significant cortisol response in not only the pregnant but also the non-pregnant women may be a consequence of our modification of the standard TSST protocol. Because we used the stress protocol twice during pregnancy (second and third trimester), we tried to find a balance between choosing a stressor that was potent enough to reliably elicit a stress response, but that did not induce any physical discomfort in the third trimester pregnant women. Our modification of the TSST had the subjects comfortably seated in the same room during the anticipation, stress, and recovery periods, and we speculate this may have decreased the novelty, uncertainty, and unpredictability of the situation, which are important situational factors for stress activation (Mason 1968; Dickerson and Kemeny 2004). In addition to those factors, the degree of social evaluative threat experienced in a stressful situation seems to be an important factor in eliciting a cortisol response (Gruenewald 2004; Dickerson 2008). Gruenwald et al. (2004) reported that not all physiological systems are activated in parallel or at the same threshold by a psychological stressor. In their experiment, they manipulated the degree of social evaluative threat that the subjects were exposed to during a speech and mental arithmetic stress task (two conditions: high and low evaluative threat). In the low social evaluative threat condition, participants did not mount a significant cortisol response, while HR and BP showed large increases in both conditions, and Dickerson et al. (2008) reported similar findings. The fact that the subjects were reclined in an armchair in front of the jury may have made the situation more informal and familiar, and therefore may have reduced the experience of social evaluative threat. This may explain while we did not observe a cortisol response, while the task was still potent enough to stimulate the SAM system and evoke a feeling of distress at all assessments.
The sample size of our CG was smaller than the sample size of the pregnant group. However, we do not believe that the fact that the attenuation of the response magnitude of HR, MAP and distress was significant in the PG and non-significant in CG was due to lack of statistical power in the CG, since the percent of session-by-session variance explained by gestational age (R2) is much larger than the percent of session-by-session variance explained by time between sessions for the CG. Direct comparison of the pregnant versus control participants in a single statistical model was not possible given our pregnancy specific covariates, so one limitation is that the comparison is primarily descriptive. Addressing this limitation in future work will be important, yet difficult because pregnancy represents a unique physiological state of a woman’s life.
Changes in neuroendocrine function during human pregnancy include a progressive increase in placental CRH and maternal adenocrticotropic hormone (ACTH) and cortisol levels over the course of gestation. A consequence of the increase in baseline levels may be downregulation of receptor sensitivity and therefore reduced responsiveness of the system to challenge. One implication of our findings of attenuated physiological as well as psychological responsivity to stress with advancing gestation supports the importance of the timing of the occurrence of stress during pregnancy in terms of its potentially detrimental effects. Thus, the effects of stress on the developing fetus may be more detrimental earlier than later in pregnancy. In addition, the activity of the placental enzyme, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2), which oxidizes cortisol to its inactive form cortisone, increases during pregnancy, thereby resulting in a reduction of the proportion of maternal cortisol passing into the fetal compartment (Murphy et al. 2006). The moderating effect of timing of stress in pregnancy also may depend, as discussed earlier, on the ontogeny of fetal development. Systems may be most vulnerable to the effects of maternal stress when undergoing rapid development suggesting that systems that develop earlier in gestation may be more vulnerable to the effects of maternal stress. For these reasons, it is possible that individual differences in the degree of dampening of stress responses over the course of gestation may represent a marker of stress susceptibility.
What drives these changes in maternal responses to stress over gestation and hence potentially alters its putative effects on the developing fetus? An implicit assumption regarding the direction of causality is that it is unidirectional in nature, in that potentially unfavorable circumstances in a pregnant woman’s life that are perceived or appraised by the maternal brain as stressful may then influence maternal physiology, which, in turn, may impact the developing embryo/fetus via direct or indirect biological mechanisms, as discussed above. However, the alterations in maternal physiology associated with pregnancy are known to originate from the fetal compartment (the placenta is an organ of fetal origin). Because these observed variations in maternal physiology (that have consequences for maternal stress responses and fetal susceptibility to maternal stress exposure) originate in and are sustained by the developing fetus over gestation, there is the intriguing possibility of reciprocal, bidirectional causality, in which variations in processes that underlie fetal growth, maturation, and development result in variations in maternal physiology which, in turn, influence or moderate the effects of maternal stress exposure on the developing fetus, including perhaps, subsequent changes in maternal physiology and fetal susceptibility to maternal stress. The object (fetus) of the influence (effects of maternal stress) is the cause of the process that produces the influence (changes in maternal stress responsivity); this process is dynamic, recursive, and bidirectional between the fetal and maternal compartment over the course of gestation.
Taken together, the findings of the present study establish that maternal responsivity to stress is attenuated as pregnancy advances and thereby suggest that the timing of the occurrence of stress in pregnancy may matter in terms of its potential impact on fetal development and subsequent health outcomes. More empirical research is needed to examine the role of individual differences in the degree of attenuation as a marker of underlying fetal pathophysiology, in terms of its effects on pregnancy, fetal development, and subsequent birth, child, and adult health outcomes.
Acknowledgments
This study was supported, in part, by US PHS (NIH) grants HD-33506, HD-041696, and HD-47609 to PDW.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Our final models included all 148 subjects. However, analyses on only the subgroup of subjects that completed both visits (n = 76) provided similar results (significant effects with comparable effect sizes), albeit with reduced statistical power.
Running the same models for systolic and diastolic blood pressure separately yielded similar results.
References
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cnossen JS, Vollebregt KC, de Vrieze N, ter Riet G, Mol BW, Franx A, Khan KS, vam der Post JA. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: Systematic review and meta-analysis. BMJ. 2008;336:1117–1120. doi: 10.1136/bmj.39540.522049.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy–A review. Neurosci Bio behav Rev. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- de Weerth C, Wied CC, Jansen LM, Buitelaar JK. Cardiovascular and cortisol responses to a psychological stressor during pregnancy. Acta Obstet Gynecol Scand. 2007:1–12. doi: 10.1080/00016340701547442. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Gurewitsch ED. Fetal response to induced maternal stress. Early Hum Dev. 2003;74:125–138. doi: 10.1016/j.earlhumdev.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Mycek PJ, Zaldivar F. Negative social evaluation, but not mere social presence, elicits cortisol responses to a laboratory stressor task. Health Psychol. 2008;27(1):116–21. doi: 10.1037/0278-6133.27.1.116. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Erni K, Hebisch G, Nater U. Salivary alpha-amylase levels after yohimbine challenge in healthy men. J Clin Endocrinol Metab. 2006;91:5130–5133. doi: 10.1210/jc.2006-0461. [DOI] [PubMed] [Google Scholar]
- Ekholm EM, Erkkola RU. Autonomic cardiovascular control in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:29–36. doi: 10.1016/0301-2115(95)02255-4. [DOI] [PubMed] [Google Scholar]
- Entringer S, Wust S, Kumsta R, Layes IM, Nelson EL, Hellhammer DH, Wadhwa PD. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008;199:498, e491–e497. doi: 10.1016/j.ajog.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federenko IS, Nagamine M, Hellhammer DH, Wadhwa PD, Wust S. The heritability of hypothalamus pituitary adrenal axis responses to psychosocial stress is context dependent. J Clin Endocrinol Metab. 2004;89:6244–6250. doi: 10.1210/jc.2004-0981. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Wadhwa PD, Dunkel-Schetter C, Chicz-Demet A, Sandman CA. When stress happens matters: Effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Wadhwa PD, Sandman CA. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56:47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Kemeny ME, Aziz N, Fahey JL. Acute threat to the social self: Shame, social self-esteem, and cortisol activity. Psychosom Med. 2004;66(6):915–24. doi: 10.1097/01.psy.0000143639.61693.ef. [DOI] [PubMed] [Google Scholar]
- Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:S257–S263. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- Hox JJ. Multilevel analyses: Techniques and applications. Mahwah, New Jersey: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “trier social stress test”–A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, FederenkoI, Gaab J, Lintz D, Schommer N, Hellhammer DH. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosom Med. 1995;57:468–474. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Mason JW. A review of psychoendocrine research on the sympathetic-adrenal medullary system. Psychosom Med. 1968;30(Suppl):631–653. doi: 10.1097/00006842-196809000-00022. [DOI] [PubMed] [Google Scholar]
- Mastorakos G, Ilias I. Maternal and fetal hypothalamic–pituitary–adrenal axes during pregnancy and postpartum. Ann N Y Acad Sci. 2003;997:136–149. doi: 10.1196/annals.1290.016. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Rodin J. Pregnancy alters blood pressure responses to psychological and physical challenge. Psychophysiology. 1992;29:232–240. doi: 10.1111/j.1469-8986.1992.tb01691.x. [DOI] [PubMed] [Google Scholar]
- McNair DM, Loor M, Droppleman LF. Profile of mood states. San Diego, CA: Educational and Industrial Testing Service; 1981. [Google Scholar]
- Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: The role of the mother, placenta, and fetus. Endocr Rev. 2006;27:141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Klinkenberg A, Nater UM, Zimmermann R, Ehlert U. Prolonged salivary cortisol recovery in second-trimester pregnant women and attenuated salivary alpha-amylase responses to psychosocial stress in human pregnancy. J Clin Endocrinol Metab. 2006a;91:1329–1335. doi: 10.1210/jc.2005-1816. [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosom Med. 2006b;68:931–937. doi: 10.1097/01.psy.0000244385.93141.3b. [DOI] [PubMed] [Google Scholar]
- Nijland MJ, Ford SP, Nathanielsz PW. Prenatal origins of adult disease. Curr Opin Obstet Gynecol. 2008;20:132–138. doi: 10.1097/GCO.0b013e3282f76753. [DOI] [PubMed] [Google Scholar]
- O’Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139:540–545. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, O’Connor TG, Glover V. Prenatal stress and neurodevelopment of the child: Focus on the HPA axis and role of the placenta. Dev Neurosci. 2009;31:285–292. doi: 10.1159/000216539. [DOI] [PubMed] [Google Scholar]
- Odagiri E, Ishiwatari N, Abe Y, Jibiki K, Adachi T, Demura R, Demura H, Shizume K. Hypercortisolism and the resistance to dexamethasone suppression during gestation. Endocrinol Jpn. 1988;35:685–690. doi: 10.1507/endocrj1954.35.685. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ Health Perspect. 2000;108(Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): Priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Shinkawa O, Yoshinaga K. Placental corticotropin-releasing hormone may be a stimulator of maternal pituitary adrenocorticotropic hormone secretion in humans. J Clin Invest. 1989;84:1997–2001. doi: 10.1172/JCI114390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte HM, Weisner D, Allolio B. The corticotrophin releasing hormone test in late pregnancy: Lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf) 1990;33:99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: Preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- Snijders TAB, Bosker RJ. Modeled variance in two-level models. Sociol Methods Res. 1994;22:342–363. [Google Scholar]
- Van den Bergh BR, Mulder EJ, Mennes M, Glover V. Antenatal maternal anxiety and stress and the neurobehavioural development of the fetus and child: Links and possible mechanisms. A review. Neurosci Biobehav Rev. 2005;29:237–258. doi: 10.1016/j.neubiorev.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Garite TJ. The neurobiology of stress in human pregnancy: Implications for prematurity and development of the fetal central nervous system. Prog Brain Res. 2001;133:131–142. doi: 10.1016/s0079-6123(01)33010-8. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Garite TJ, Porto M, Glynn L, Chicz-DeMet A, Dunkel-Schetter C, Sandman CA. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. Am J Obstet Gynecol. 2004;191:1063–1069. doi: 10.1016/j.ajog.2004.06.070. [DOI] [PubMed] [Google Scholar]
- Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Born J, Kudielka BM, Schlotz W, Wust S. Is the cortisol awakening rise a response to awakening? Psychoneuroendocrinology. 2007;32:358–366. doi: 10.1016/j.psyneuen.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Wolfe LA, Weissgerber TL. Clinical physiology of exercise in pregnancy: A literature review. J Obstet Gynaecol Can. 2003;25:473–483. doi: 10.1016/s1701-2163(16)30309-7. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]