Abstract
Intracellular pH (pHi) was measured in isolated Xenopus single myofibers at the onset of contractions, with and without glycolytic blockade, to investigate the time course of glycolytic activation. Single myofibers (n=8; CON) were incubated in BCECF-AM (10 µM; for fluorescence measurement of pHi) and stimulated for 15 sec at 0.67 Hz in anoxia in the absence (control condition; CON) and presence of a glycolytic inhibitor (1 mM iodoacetic acid; IAA). Intracellular pHi and tension were continuously recorded and the differences in pHi between conditions were used to estimate the activation time of glycolysis. An immediate and steady increase in pHi (initial alkalosis) at the onset of contractions was similar between CON and IAA trials for the first 9 seconds of the contractile bout. However, from 6 contractions (~10 sec) through the remainder of the bout, IAA demonstrated a continued rise in pHi in contrast to a progressive decrease in pHi in CON (p<0.05). These results demonstrate, with high temporal resolution, that glycolysis is activated within 6 contractions (10 sec at 0.67 Hz) in single Xenopus skeletal muscle fibers.
Keywords: metabolism, glycolysis, skeletal muscle
Introduction
During a step increase in work rate, energetic demand increases instantly. However, it can take 45–90 seconds for oxidative phosphorylation to achieve the new steady state required by the increased rate of work. Therefore, the increased demand for ATP initially is met through substrate level phosphorylation (PCr breakdown and anaerobic glycolysis). It is well accepted that PCr breakdown occurs immediately following the onset of an increased rate of work to buffer [ATP]. Indeed, our laboratory and others have shown that blocking PCr breakdown (creatine kinase inhibition; CKi) significantly impairs contractile function of a single contracting muscle fiber immediately after the initial contraction (Dahlstedt et al., 2000; Kindig et al., 2005), demonstrating the importance of PCr splitting at the onset of contractions. Given the relatively limited reserve of PCr, it has been thought (Hultman & Sjoholm, 1983; Henriksson et al., 1986; Sahlin, 2005) that glycolysis also needs to be rapidly activated in order to supplement anaerobic ATP rephosphorylation (anaerobic glycolysis) as well as to supply substrate for oxidative phosphorylation. However, while the time course of glycolytic activation will certainly depend on contractile intensity, with higher work rates leading to more rapid PCr depletion and accumulation of the factors that activate glycolysis, the time course of activation of glycolysis has been a subject of debate.
This assertion of rapid glycolytic activation is supported by biochemical measures of lactate in muscle following brief periods (5 – 10 seconds) of exercise (Jacobs et al., 1983; Connett, 1987a, b; Howlett et al., 1999). Although biochemical analysis of muscle samples have provided a wealth of information regarding muscle metabolism, they require the disruption of the muscle fibers, thus allowing only one sample time to be measured in a sample typically composed of numerous fibers. In contrast, magnetic resonance spectroscopy (MRS) imaging of skeletal muscle offers a noninvasive alternative to study muscle metabolism over multiple sampling times, and has been used to study muscle glycolytic activation during a variety of contraction regimens. Using MRS, Crowther et al. (Crowther et al., 2002) estimated that during moderate intensity voluntary contractions glycolysis was not activated for 27 contractions, regardless of the time required to complete these 27 contractions. Although these data, and others (Yamada & Sugi, 1987; Yamada et al., 1993), support the idea that glycolysis is activated in response to both overall metabolite accumulation and contraction number, they also imply that glycolytic flux is not required to maintain contractile function for a considerable period of time (e.g., 27 seconds at 1 Hz voluntary contractions (Crowther et al., 2002)) during moderate intensity exercise.
MRS measurements of glycolytic activation often rely on indirect estimates of muscle pH. The rationale for these measurements is based on differences in cellular pH resulting from PCr- vs. anaerobic glycolysis-supported ATP resphosphorylation. Although the acid-base balance in the cell during contractile activity is dependent on a number of factors (see (Hultman & Sahlin, 1980; Robergs et al., 2004; Lindinger et al., 2005)), it is accepted that PCr splitting results in a net increase in pH, while ATP turnover supported by anaerobic glycolysis results in a net decrease in pH. While whole muscle MRS estimates of pH (Conley et al., 1997) appear to be quite accurate at rest (Constantin-Teodosiu et al., 1997), there are concerns about the validity of these measurements during high rates of work (Sahlin, 1992; Constantin-Teodosiu et al., 1997). In addition, MRS measurements of pH are derived from multiple acquisitions, thus reducing the temporal resolution of the measurements. Finally, both MRS and biochemical estimates of muscle pH require a relatively large sample of muscle fibers and are susceptible to fiber type recruitment/heterogeneity uncertainties. Thus, MRS and biochemical measurements of whole muscle are unable to accurately discern, with high temporal resolution, the time course and magnitude of changes in intracellular pH at the onset of contractions at the level of the single muscle fiber.
The purpose of the present study was twofold: 1) to use internally calibrated fluorescence measurements of intracellular pH (pHi) in intact single skeletal muscle fibers to ascertain the time course of changes in muscle pH at the onset of contractile activity; and 2) test the hypothesis that glycolysis is activated rapidly at the onset of contractions(i.e., during the initial few contractions). The use of isolated muscle fibers removes confounding influences of other cells and allows the extracellular environment to be precisely controlled. Furthermore, the use of a fluorescent probe (BCECF) allows for internal calibration for accurate measurement of pHi in each intact muscle fiber with high temporal resolution. Measurements of pHi during contractions were performed in the presence and absence of an inhibitor of glycolysis. Due to the nature of [H+] changes between PCr splitting and anaerobic glycolysis, differences in pHi were used to identify the time course of glycolytic activation at the onset of contractions.
Methods
Female adult Xenopus laevis were used in this investigation. Skeletal muscle fibers from Xenopus laevis have previously been used to study muscle energetics in our laboratory (Howlett & Hogan, 2003; Kindig et al., 2005; Stary & Hogan, 2005; Walsh et al., 2006) and others (Lannergren & Westerblad, 1988; Nagesser et al., 1993; Westerblad & Lannergren, 1995), and have been demonstrated to behave in a similar manner to mammalian fibers (Nagesser et al., 1992). All procedures were approved by the University of California-San Diego animal care and use committee and conform to National Institutes of Health standards.
We did not attempt to distinguish the fiber type that was isolated, although the common fiber type isolated from the frog muscle is similar to the mammalian type IIa. Biochemically and metabolically, there are no differences between these vertebrate frog fibers and mammalian muscle types (Edman, 2005). They operate at different temperatures in vivo and the frog fibers do not have myoglobin, but otherwise the fiber types are very similar in most respects between amphibians and mammals. In fact, the frog fibers have at least the same mitochondrial content as mammalian fibers (see (Stary et al., 2004)) and the maximal oxygen uptake in the different fiber types are similar (van der Laarse et al., 1989) between frog muscle and mammalian muscle.
Single Skeletal Muscle Fiber Preparation
Single muscle cells (n=15) were isolated and prepared as described previously (Hogan, 1999). Briefly, frogs were doubly pithed and the lumbrical muscles (II–IV) were removed from the hind feet. Single myocytes were dissected with tendons intact in a chamber of physiological Ringer’s solution consisting of (in mM) 116.5 NaCl, 2.0 KCl, 1.9 CaCl2, 2.0 Na2HPO4, 0.1 EGTA, pH = 7.0. Following isolation, the fibers were incubated in Ringer’s solution containing BCECF-AM (10 µM) for 1 hour to allow fluorescence measurement of intracellular pH (pHi).
Experimental Protocol
Platinum clips were attached to the tendons of each myocyte to facilitate fiber positioning within the Ringer’s solution-filled chamber. One tendon was fixed, whereas the contralateral was attached to an adjustable force transducer (model 400A, Aurora Scientific, Aurora, Ontario, Canada), allowing the muscle to be set at optimum muscle length (i.e., length at which maximal tetanic force was produced). The analog signal from the force transducer was recorded via a data acquisition system (AcqKnowledge, Biopac Systems, Santa Barbara, CA, USA) for subsequent analysis. Fibers were superfused in Ringer’s solution at 22°C throughout the experiment. Immediately prior to each contractile bout fibers were superfused with anoxic Ringer’s solution (equilibrated with 3% CO2 and 0% O2 in N2 balance; verified with an O2 electrode immediately prior to the contractile protocol) to avoid the influence of oxidative phosphorylation on muscle pH (i.e., oxidation of pyruvate instead of forming lactate). Constant superfusion was maintained throughout the protocol to reduce the occurrence of an unstirred layer surrounding the cell. Tetanic contractions were elicited using direct (9 V) stimulation of the muscle from end to end (model S48, Grass Instruments, Warwick, RI, USA). The stimulation protocol consisted of ~250 ms trains of 70-Hz impulses of 1-ms duration. Myocytes were subjected to trials of 15 s at a stimulation frequency of 0.67 Hz with a 60 min rest period between trials.
One group of fibers (experimental group; n=8) was subjected to a 15 s contraction protocol (0.67 Hz) in anoxia (CON). Following an hour of rest in normoxia, fibers were subjected to an identical contraction protocol in the presence of anoxia and 1 mM iodoacetic acid (IAA; inhibitor of glycolysis at glyceraldehyde 3-phosphate dehydrogenase). Peak tension and intracellular pH were monitored throughout the bouts. Because of the irreversible nature of IAA, a blocked order design was not possible. Therefore, in order to determine whether an order effect occurred, a second group of fibers (n=7) performed two identical bouts of contraction (i.e., no IAA) at 0.67 Hz in the presence of anoxia with 1 hour of rest between bouts (CONa and CONb), and data were analyzed in the same manner as the CON vs. IAA trials.
pHi fluorescence
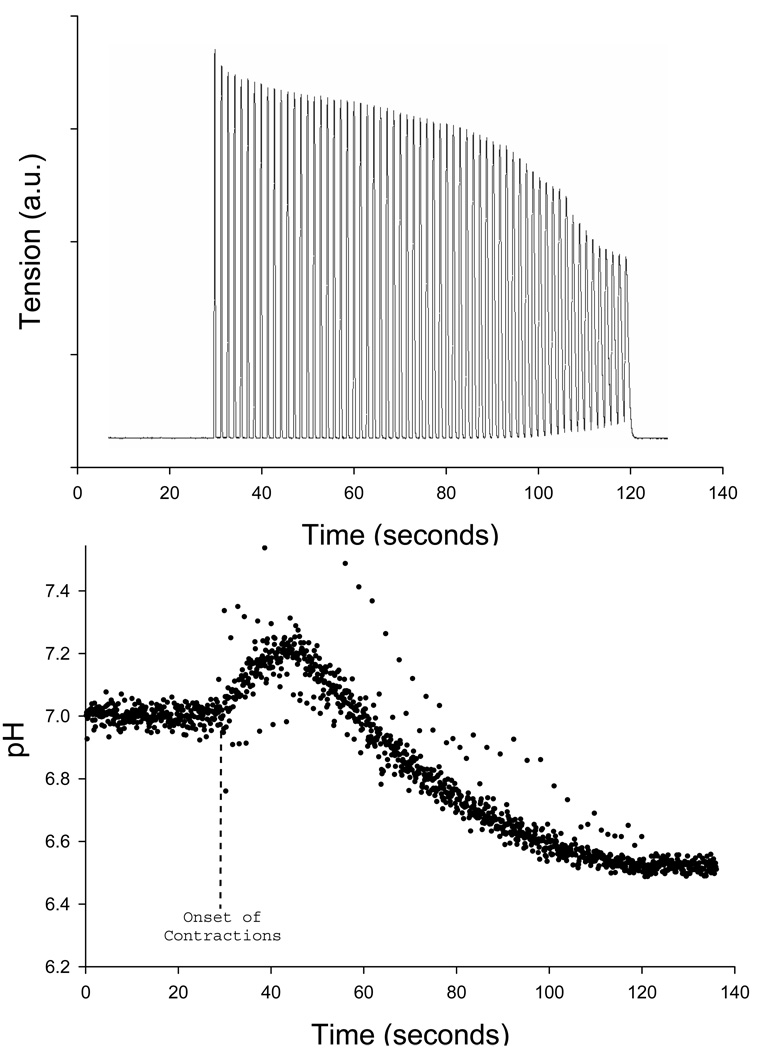
Relative changes in pHi were obtained by use of pHi-dependent fluorescence spectroscopy. Fibers were incubated for 15 minutes with 10 µM of the membrane-permeant acetoxymethyl ester form of the [H+] indicator 2',7'-bis-(2-carboxyethyl)−5(6)-carboxyfluorescein (BCECF; Molecular Probes). Following the incubation period, fibers were rinsed with normal Ringer’s solution (the acetoxymethyl ester form of BCECF is cleaved by intracellular esterases and becomes trapped inside of the cell and fluoresces). Incubated fibers were illuminated with two rapidly alternating (20 Hz) excitation wavelengths of 440 and 490 nm, and the resulting fluorescence emission intensities at 535 nm were divided (490 nm/440 nm) to obtain the pHi-dependent signal (Westerblad & Allen, 1992). Following the contraction protocol, absolute pHi was determined by superperfusing the fiber with 10 µM of the K+/H+ ionophore nigericin in KCl-buffered (140 mM) Ringer and calibrating with three pH-standardized solutions in series: pH 6.5, 7.0, and 7.5 (Westerblad & Allen, 1992). Fluorescence was measured with a Photon Technology International illumination and detection system (DeltaScan model), integrated with a Nikon inverted microscope with a ×40 Fluor objective. Previously, our lab (Stary & Hogan, 2005) determined, by monitoring fluorescence in noncontracting cells, that relative changes in the BCECF fluorescence ratio were physiological and not due to a spectroscopic artifact such as photobleaching. This method for measuring pHi in intact single fibers has previously been used by our laboratory (Stary & Hogan, 2005) and others (Westerblad & Allen, 1992; Westerblad et al., 1997). Figure 1 demonstrates a representative trace of BCECF fluorescence in a single fiber contracting at 0.67 Hz in normoxia for 90 seconds. Similar to our previous study (Stary & Hogan, 2005), pHi (BCECF fluroscence) demonstrates a transient alkalinization at the onset of contractions, followed by acidification.
Figure 1.
Representative traces of tension development and pHi in a single muscle fiber during 90 seconds of contractions at 0.67 Hz in normoxia. Tension is presented in arbitrary units, while pH was assessed via fluorescence microscopy (BCECF fluorescence; each point on the graph represents 1 measurement) and internally calibrated after the contractile bout. Due to the prolonged time course of this representative contractile bout (90 seconds), these data are presented for descriptive purposes only and were not included in the experimental cohort of muscle fibers.
Statistical Analysis
Data were normalized to the average starting pH (7.02 ±0.02; not significantly different between the 4 groups) in order to compare the change in pH between groups during contractions. Data are presented as mean pH ± SE. Differences between trials were tested via a repeated measures 1-way ANOVA. When significant F-values were present, the Tukey post-hoc test was employed for determination of between-group differences. Statistical significance was accepted at p < 0.05.
Results
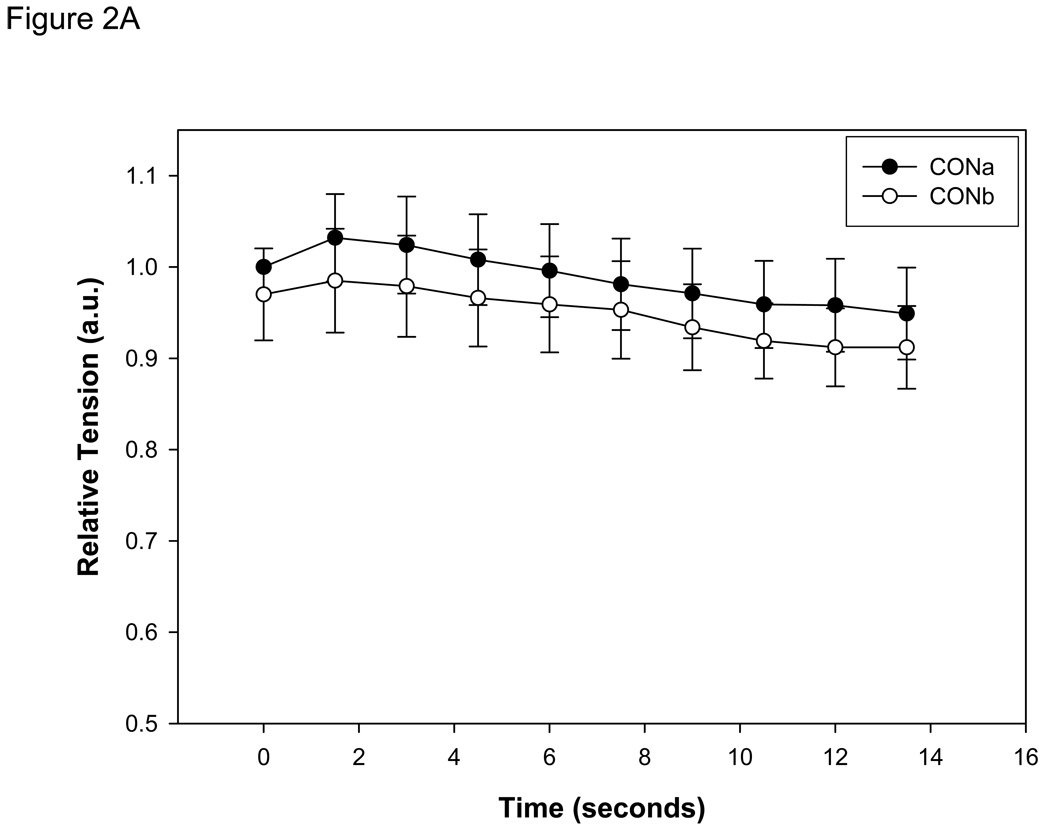
Initial pHi, prior to contractions, averaged 7.02 (±0.02) for all fibers and did not differ among any of the four trials. In one group of fibers (n=7), the contraction protocol was performed twice in the absence of IAA (CONa and CONb; two control trials separated by 60 min) to determine the existence of an order effect. The results demonstrated no order effect in either tension development or pHi (Figures 2 A & B) during contractions.
Figure 2.
A: Tension development (mean ± SE) measured in single muscle fibers (n=7) during 15 seconds of contractions at 0.67 Hz in control conditions (CONa) and repeated after 1 hour of recovery (CONb). Absolute tension development was not different at any sample time between conditions. Tension is presented relative to the initial contraction of the CONa bout. B: Intracellular pH (mean ± SE) measured in the same single muscle fibers. pHi was not significantly different between CONa and CONb at any sample time during the contractile bout.
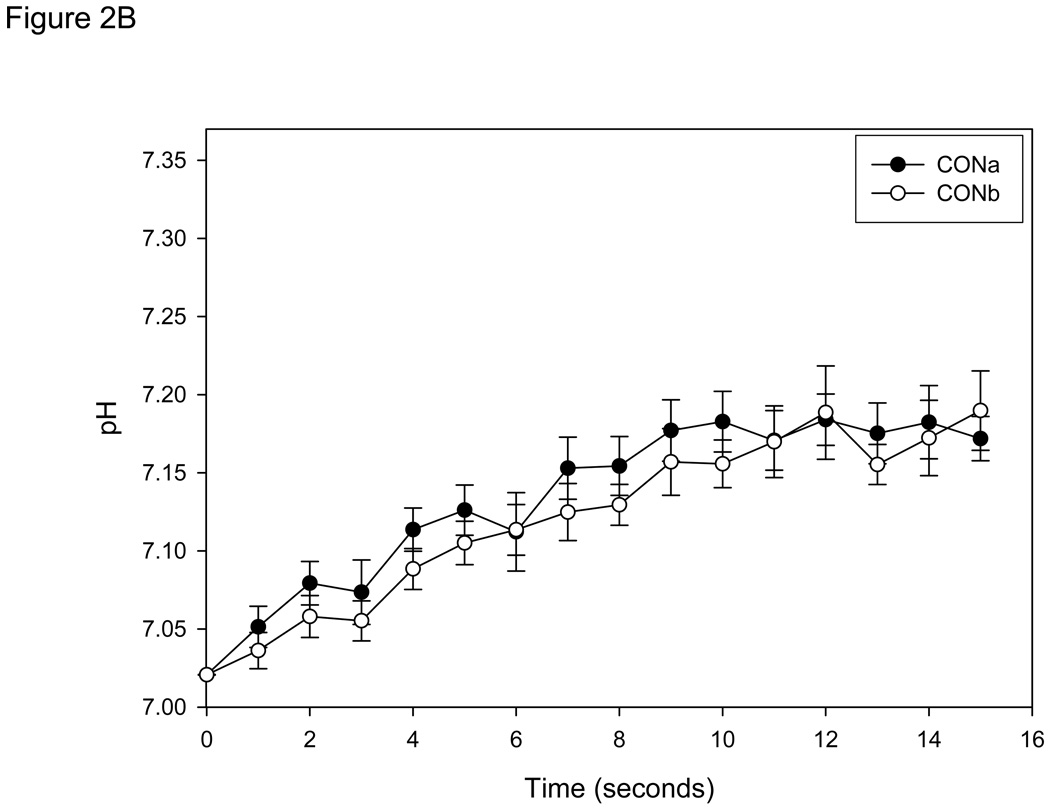
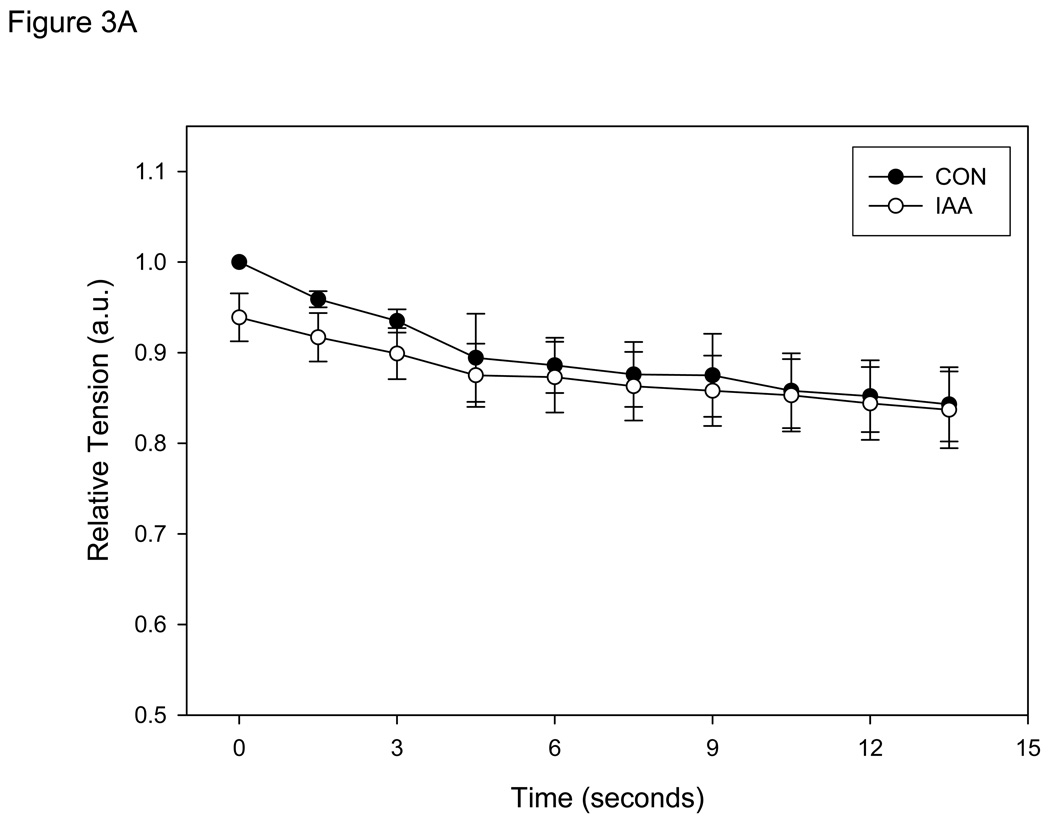
Peak Tension
Absolute tension development was not significantly different between CON and IAA for the entire contraction bout (Figure 3A), demonstrating that during 15 seconds of contraction in anoxia at 0.67 Hz exposure to IAA had no significant influence on contractile function or the rate of ATP hydrolysis (assuming a constant efficiency) . However, it should be noted that if the contraction protocol was continued, some of the fibers in IAA demonstrated rigor at ~25 seconds (data not shown). Since this was not observed in the control condition, it suggests significant energetic depletion in the cell (PCr stores exhausted) and indicates that glycolysis was indeed inhibited in the cell.
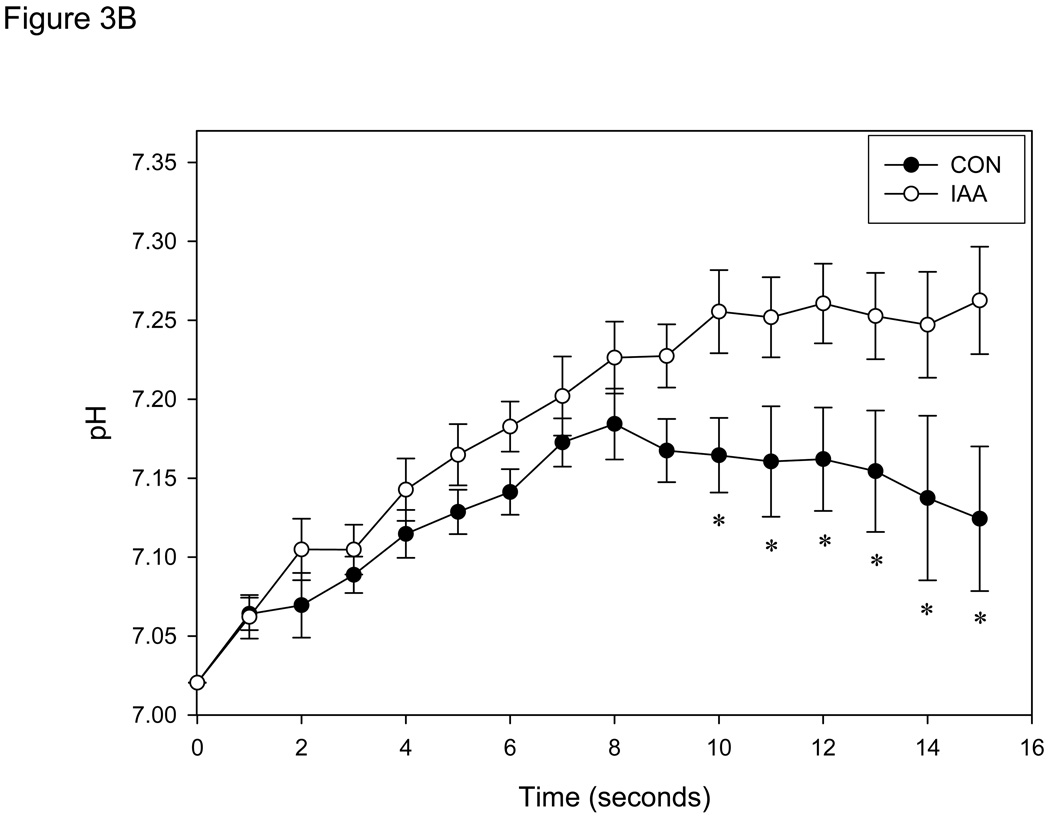
Figure 3.
A: Tension development (mean ± SE) measured in single muscle fibers (n=8) during 15 seconds of contractions at 0.67 Hz in control conditions (anoxia; CON) and conditions in which glycolysis was inhibited (anoxia + iodoacetic acid; IAA). Absolute tension development was not different at any sample time between conditions. Tension is presented relative to the initial contraction of the CON bout for each fiber. B: Intracellular pH (mean ± SE) measured in same single muscle fibers. * = P<0.05 vs.CON.
pHi and Glycolytic Activation
In control conditions, fibers demonstrated an initial increase in pHi followed by a leveling off or decrease as the contractile bout progressed (Fig. 3B), similar to that described in normoxic conditions (Figure 1) as well as previous biochemical, MRS, and fluorescence estimates of pHi (Connett, 1987a; Haseler et al., 1998; Hogan et al., 1999; Stary & Hogan, 2005) in whole muscle. In contrast, fibers exposed to IAA continued to exhibit an increase in pHi throughout the experiment. A significant difference in pHi between conditions was achieved after 6 contractions (10 sec), and this difference was sustained for the remainder of the contraction bout (P < 0.05; Fig. 3B).
Discussion
The results of the present study demonstrate a rapid increase in pHi in single muscle fibers immediately following the onset of contractions, irrespective of whether glycolysis was inhibited (see Figure 3B). The identical pHi and tension development in CON and IAA during the first 5 contractions suggest that PCr splitting at the onset of contractions was not accelerated when glycolysis was inhibited and thereby demonstrates that high energy phosphates (ATP and PCr) are the predominant source of energy for the first few seconds of high intensity contractions. However, after 6 contractions pHi was significantly more acidic in the condition in which glycolysis was not inhibited, demonstrating in these single myofibers a relatively rapid activation (< 10 seconds; 6 contractions) of glycolysis in the anoxic conditions of this study.
Energetics at the Onset of Contractile Activity
At the onset of an elevated rate of work, the rate of ATP hydrolysis can increase several hundred fold (Hultman & Sjoholm, 1983). A substantial fall in [ATP] can lead to severe energetic problems within the cell and cell death. However, cellular [ATP] is remarkably maintained over a broad range of metabolic rates, such that situations in which severe ATP depletion occur are rare. This maintenance of [ATP] is achieved by the integration of a number of metabolic pathways to rephosphorylate ADP.
One of the initial pathways activated to maintain [ATP] at the onset of increased rates of work involves the breakdown of another high energy phosphate group in the form of PCr. This reversible reaction, catalyzed by creatine kinase, rephosphorylates ADP and consumes a hydrogen ion (equation 1).
| (equation 1) |
Under most conditions, a relatively large net change in [PCr] occurs at the onset of contractions in order to maintain [ATP] and results in an immediate net intracellular alkalinization (Hultman & Sahlin, 1980; Haseler et al., 1998; Hogan et al., 1999; Stary & Hogan, 2005; Chance et al., 2006), as seen in the present study. Given the relatively low concentration of PCr present in the cell (~ 15–23 mmol kg w.wt.−1; depending on fiber type (Greenhaff et al., 1994; Brault et al., 2003)) to buffer the ATP hydrolysis (up to ~ 3 mmol ATP kg w.wt.−1 s−1 (Hultman & Sjoholm, 1983)), PCr will not be sufficient to rephosphorylate ADP for more than a few seconds of high intensity work.
In addition to providing substrate for oxidative phosphorylation, glycolysis also directly resphosphorylates ADP. Although the ATP produced per glucose/glycogen molecule is relatively small compared to the subsequent pathways of oxidative phosphorylation, this ATP generating pathway can occur in the absence of oxidative phosphorylation and may be critical during moderate to high rates of work. Anaerobic glycolysis results in net decrease in pH (Hultman & Sahlin, 1980; Meyer & Foley, 1996) and will therefore have an opposing effect on intracellular pH to that of PCr breakdown during transitions between workloads. Although creatine kinase inhibition results in near immediate impairment of contractile function (e.g., tension development is severely impaired within 2 contractions; (Kindig et al., 2005)), this does not necessarily exclude the contribution of anaerobic glycolysis during the initial seconds of contraction (i.e., it is possible that both pathways were activated, but anaerobic glycolysis could not compensate for the absence of PCr breakdown). Therefore, while there is little doubt that net PCr breakdown occurs almost immediately at the onset of contractions, it is less clear how quickly anaerobic glycolysis is activated to supplement the maintenance of [ATP] by PCr splitting.
Time course of glycolytic activation at the onset of contractions
Data collected using MRS during muscle contractions have supported the theory that glycolysis is activated by a “dual control” model (Yamada & Sugi, 1987; Crowther et al., 2002). According to this model, glycolysis is activated by both a signal related directly to contraction (e.g., Ca2+ at the site of glycogen phosphorylase) and a signal related to metabolic demand / metabolite accumulation (e.g., ADP, AMP, and Pi at the level of phosphofructokinase as well as AMP and Pi at glycogen phosphorylase) (Connett & Sahlin, 1996). It has been suggested that a threshold of metabolite accumulation must be achieved before contraction-related signals can increase glycolytic flux (Yamada & Sugi, 1987; Yamada et al., 1993; Crowther et al., 2002). Therefore, the rate of glycolytic activation will partly be a function of the rate of work (i.e., metabolite accumulation). However, there has been discrepancy among measurements attempting to ascertain the extent of metabolic activation required to activate glycolysis. Several studies using biochemical methods have suggested that glycolysis is rapidly activated after the onset of increased rates of work (Hultman & Sjoholm, 1983; Jacobs et al., 1983; Henriksson et al., 1986; Connett, 1987a, b Howlett et al., 1999; Sahlin, 2005). Indeed, Howlett et al. have demonstrated a 10-fold increase in muscle lactate after only 10 seconds of maximal exercise (Howlett et al., 1999). In contrast to muscle biopsy studies, MRS has generally demonstrated a slower glycolytic activation time course during a variety of contraction types ranging from a prolonged tetanus to individual twitch contractions (Yamada & Sugi, 1988; Sugi & Yamada, 1989; Conley et al., 1997; Conley et al., 1998). Using a more physiologically relevant contraction paradigm of voluntary ballistic exercise in humans, Crowther et al. have shown that ~27 contractions at 1 Hz in anoxia (i.e., 27 seconds) are required prior to glycolytic activation (Crowther et al., 2002). Although MRS data have provided important information regarding the control of glycolysis, they rely on indirect measurements of pH (calculated from the Pi and PCr peaks in MRS spectra) and there are concerns about the validity of these measurements during high rates of work (Sahlin, 1992; Constantin-Teodosiu et al., 1997); although not all studies have agreed with this contention (Sullivan et al., 1994). In addition, MRS measurements require averaging of acquisitions, which reduces the temporal sensitivity of the measurements, and both MRS and muscle biopsy data are potentially susceptible to fiber type recruitment/heterogeneity uncertainties.
In the present study, an alternative technique (fluorescence microcopy) was used to measure real time changes in pHi. Because of the difference in proton handling between ATP resynthesis from PCr (decrease in proton concentration) and anaerobic glycolysis (increase in proton concentration), measurements of pHi can be used to estimate glycolytic activity in contracting intact single muscle fibers. To allow comparison with previous studies (Yamada et al., 1993; Crowther et al., 2002), and to remove the confounding influences of oxidative phosphorylation activation, experiments were performed in anoxia. Thus, it should be noted that, similar to previous studies in anoxia, the rate of activation of glycolysis may be more rapid than that which occurs in a system in which oxidative phosphorylation is allowed to occur. However, comparison of normoxic measurements of pHi from the current study (Figure 1), as well as a previous study in our laboratory (Stary & Hogan, 2005), suggests that the time course of the pHi response appears to be quite similar between these anoxic conditions and normoxic conditions (i.e., pHi reached a peak value at ~6 contractions in both conditions). Thus, it appears that differences in glycolytic activation between anoxic and normoxic conditions are minimal during the initial 15 seconds of contractions, as would be expected from the relatively slow activation of mitochondrial oxidative phosphorylation.
Tension development was identical between CON and IAA conditions, which suggests that the metabolic rate was not different between the conditions. Therefore, the identical pHi response during the initial 5 contractions in CON and IAA indicates that the rate of PCr breakdown was unaffected by the inhibition of glycolysis during this time period. However, after 6 contractions, pHi was significantly different when glycolysis was allowed to occur compared to conditions in the absence of glycolysis (IAA), suggesting energetic supplementation with anaerobic glycolysis. In the absence of glycolysis, pHi continued to increase and tension development was maintained, implying that PCr was not exhausted at the onset of anaerobic glycolysis. However, the development of rigor in some fibers in which the contraction protocol was extended (~25 seconds) demonstrates that further exclusive reliance on PCr as a sole source of energy is not sustainable and results in severe metabolic perturbations.
These data suggest a considerably more rapid activation of glycolysis than that observed using MRS. However, the wide range of contraction protocols and techniques used in previous investigations, ranging from electrically stimulated twitch and tetanic contractions to voluntary contractions, make direct comparisons with the present study difficult. Nevertheless, our results appear to be in line with those obtained from muscle biopsy data, including the greater than 10 fold increase in muscle lactate within 10 seconds of initiation of maximal voluntary exercise (Howlett et al., 1999) and glycolytic activation within 5 seconds of 4 Hz stimulation in whole muscle even under aerobic conditions (Connett, 1987b).
As with any technique that estimates glycolytic activity through measurement of pHi, assumptions should be made with caution. Although Adams et al. (Adams et al., 1990) have demonstrated that the alkalinization at the start of exercise can be entirely accounted for by PCr hydrolysis, changes in muscle pH do not always coincide with rates of substrate level phosphorylation because intracellular pH ultimately depends on a number of additional factors such as buffering capacity (Roussel et al., 2003). However, both control and experimental conditions were performed in the same fiber in the present study. As such, complications arising from inherent differences between fibers were avoided, suggesting the predominant cause of differences in pHi between experimental conditions were factors related to glycolytic activation. Finally, it is possible that glycolysis was activated earlier in the contractile period than the time in which a difference in pH was detectable (i.e., 6th contraction), and the present estimate of glycolytic activation may be considered a conservative estimate.
In conclusion, our data demonstrate with high temporal resolution that pHi increases in single myocytes immediately at the onset of contractions and is unaffected by inhibition of anaerobic glycolysis for the first 5 contractions. This suggests that initial PCr breakdown was not altered between the two conditions of the study and that anaerobic glycolysis is not significantly activated during this time period. However, after 6 contractions pHi deviated significantly between CON and IAA conditions. Although these data can not discern the extent to which the glycolysis-related alterations in pHi that occurred after 6 contractions were the result of a slowing of PCr breakdown and/or increase in the rate of anaerobic glycolysis, they nevertheless demonstrate a relatively rapid activation of glycolysis during contractions in these isolated muscle fibers.
Acknowledgements
This study was supported by NIH AR40155.
References
- Adams GR, Foley JM, Meyer RA. Muscle buffer capacity estimated from pH changes during rest-to-work transitions. J Appl Physiol. 1990;69:968–972. doi: 10.1152/jappl.1990.69.3.968. [DOI] [PubMed] [Google Scholar]
- Brault JJ, Abraham KA, Terjung RL. Phosphocreatine content of freeze clamped muscle: influence of creatine kinase inhibition. J Appl Physiol. 2003;94:1751–1756. doi: 10.1152/japplphysiol.01070.2002. [DOI] [PubMed] [Google Scholar]
- Chance B, Im J, Nioka S, Kushmerick M. Skeletal muscle energetics with PNMR: personal views and historic perspectives. NMR Biomed. 2006;19:904–926. doi: 10.1002/nbm.1109. [DOI] [PubMed] [Google Scholar]
- Conley KE, Blei ML, Richards TL, Kushmerick MJ, Jubrias SA. Activation of glycolysis in human muscle in vivo. Am J Physiol Cell Physiol. 1997;273:C306–C315. doi: 10.1152/ajpcell.1997.273.1.C306. [DOI] [PubMed] [Google Scholar]
- Conley KE, Kushmerick MJ, Jubrias SA. Glycolysis is independent of oxygenation state in stimulated human skeletal muscle in vivo. J Physiol (Lond) 1998;511:935–945. doi: 10.1111/j.1469-7793.1998.935bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connett RJ. Cytosolic pH during a rest-to-work transition in red muscle: application of enzyme equilibria. J Appl Physiol. 1987a;63:2360–2365. doi: 10.1152/jappl.1987.63.6.2360. [DOI] [PubMed] [Google Scholar]
- Connett RJ. Glycolytic regulation during an aerobic rest-to-work transition in dog gracilis muscle. J Appl Physiol. 1987b;63:2366–2374. doi: 10.1152/jappl.1987.63.6.2366. [DOI] [PubMed] [Google Scholar]
- Connett RJ, Sahlin K. Control of glycolysis and glycogen metabolism. In: Rowell Lb, Shepherd Jt., editors. Exercise: Regulation and Integration of Multiple Systems. Ney York: Oxford Univ. Press; 1996. pp. 870–911. [Google Scholar]
- Constantin-Teodosiu D, Greenhaff PL, McIntyre DB, Round JM, Jones DA. Anaerobic energy production in human skeletal muscle in intense contraction: a comparison of 31P magnetic resonance spectroscopy and biochemical techniques. Exp Physiol. 1997;82:593–601. doi: 10.1113/expphysiol.1997.sp004049. [DOI] [PubMed] [Google Scholar]
- Crowther GJ, Carey MF, Kemper WF, Conley KE. Control of glycolysis in contracting skeletal muscle. I. Turning it on. Am J Physiol Endocrinol Metab. 2002;282:E67–E73. doi: 10.1152/ajpendo.2002.282.1.E67. [DOI] [PubMed] [Google Scholar]
- Dahlstedt AJ, Katz A, Wieringa B, Westerblad H. Is creatine kinase responsible for fatigue? Studies of isolated skeletal muscle deficient in creatine kinase. Faseb J. 2000;14:982–990. doi: 10.1096/fasebj.14.7.982. [DOI] [PubMed] [Google Scholar]
- Edman KA. Contractile properties of mouse single muscle fibers, a comparison with amphibian muscle fibers. J Exp Biol. 2005;208:1905–1913. doi: 10.1242/jeb.01573. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Nevill ME, Soderlund K, Bodin K, Boobis LH, Williams C, Hultman E. The metabolic responses of human type I and II muscle fibres during maximal treadmill sprinting. J Physiol. 1994;478(Pt 1):149–155. doi: 10.1113/jphysiol.1994.sp020238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseler LJ, Richardson RS, Videen JS, Hogan MC. Phosphocreatine hydrolysis during submaximal exercise: the effect of FIO2. J Appl Physiol. 1998;85:1457–1463. doi: 10.1152/jappl.1998.85.4.1457. [DOI] [PubMed] [Google Scholar]
- Henriksson J, Katz A, Sahlin K. Redox state changes in human skeletal muscle after isometric contraction. J Physiol (Lond) 1986;380:441–451. doi: 10.1113/jphysiol.1986.sp016296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC. Phosphorescence quenching method for measurement of intracellular PO2 in isolated skeletal muscle fibers. J Appl Physiol. 1999;86:720–724. doi: 10.1152/jappl.1999.86.2.720. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999;86:1367–1373. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Heigenhauser GJ, Spriet LL. Skeletal muscle metabolism during high-intensity sprint exercise is unaffected by dichloroacetate or acetate infusion. J Appl Physiol. 1999;87:1747–1751. doi: 10.1152/jappl.1999.87.5.1747. [DOI] [PubMed] [Google Scholar]
- Howlett RA, Hogan MC. Dichloroacetate accelerates the fall in intracellular PO2 at onset of contractions in Xenopus single muscle fibers. Am J Physiol Regul Integr Comp Physiol. 2003;284:R481–R485. doi: 10.1152/ajpregu.00078.2002. [DOI] [PubMed] [Google Scholar]
- Hultman E, Sahlin K. Acid-base balance during exercise. Exerc Sport Sci Rev. 1980;8:41–128. [PubMed] [Google Scholar]
- Hultman E, Sjoholm H. Substrate availability. Biochem. Exer. 1983;13:63–75. [Google Scholar]
- Jacobs I, Tesch PA, Bar-Or O, Karlsson J, Dotan R. Lactate in human skeletal muscle after 10 and 30 s of supramaximal exercise. J Appl Physiol. 1983;55:365–367. doi: 10.1152/jappl.1983.55.2.365. [DOI] [PubMed] [Google Scholar]
- Kindig CA, Howlett RA, Stary CM, Walsh B, Hogan MC. Effects of acute creatine kinase inhibition on metabolism and tension development in isolated single myocytes. J Appl Physiol. 2005;98:541–549. doi: 10.1152/japplphysiol.00354.2004. [DOI] [PubMed] [Google Scholar]
- Lannergren J, Westerblad H. The effect of temperature and stimulation scheme on fatigue and recovery in Xenopus muscle fibres. Acta Physiol Scand. 1988;133:73–82. doi: 10.1111/j.1748-1716.1988.tb08382.x. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Kowalchuk JM, Heigenhauser GJ. Applying physicochemical principles to skeletal muscle acid-base status. Am J Physiol Regul Integr Comp Physiol. 2005;289:R891–R894. doi: 10.1152/ajpregu.00225.2005. author reply R904–910. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Foley JM. Handbook of Physiology. Bethesda, MD: Am. Physiol. Soc.; 1996. Cellular processes integrating the metabolic response to exercise; pp. 841–869. [Google Scholar]
- Nagesser AS, van der Laarse WJ, Elzinga G. Metabolic changes with fatigue in different types of single muscle fibres of Xenopus laevis. J Physiol. 1992;448:511–523. doi: 10.1113/jphysiol.1992.sp019054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesser AS, Van der Laarse WJ, Elzinga G. ATP formation and ATP hydrolysis during fatiguing, intermittent stimulation of different types of single muscle fibres from Xenopus laevis. J Muscle Res Cell Motil. 1993;14:608–618. doi: 10.1007/BF00141558. [DOI] [PubMed] [Google Scholar]
- Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287:R502–R516. doi: 10.1152/ajpregu.00114.2004. [DOI] [PubMed] [Google Scholar]
- Roussel M, Mattei JP, Le Fur Y, Ghattas B, Cozzone PJ, Bendahan D. Metabolic determinants of the onset of acidosis in exercising human muscle: a 31P-MRS study. J Appl Physiol. 2003;94:1145–1152. doi: 10.1152/japplphysiol.01024.2000. [DOI] [PubMed] [Google Scholar]
- Sahlin K. Non-invasive measurements of O2 availability in human skeletal muscle with near-infrared spectroscopy. Int J Sports Med. 1992;13 Suppl 1:S157–S160. doi: 10.1055/s-2007-1024625. [DOI] [PubMed] [Google Scholar]
- Sahlin K. Determination of muscle pH and glycolytic flux by magnetic resonance spectroscopy in contracting human skeletal muscle may have systematic errors. Am J Physiol Cell Physiol. 2005;289:C230. doi: 10.1152/ajpcell.00069.2005. [DOI] [PubMed] [Google Scholar]
- Stary CM, Hogan MC. Intracellular pH during sequential, fatiguing contractile periods in isolated single Xenopus skeletal muscle fibers. J Appl Physiol. 2005;99:308–312. doi: 10.1152/japplphysiol.01361.2004. [DOI] [PubMed] [Google Scholar]
- Stary CM, Mathieu-Costello O, Hogan MC. Resistance to fatigue of individual Xenopus single skeletal muscle fibres is correlated with mitochondrial volume density. Exp Physiol. 2004;89:617–621. doi: 10.1113/expphysiol.2004.027763. [DOI] [PubMed] [Google Scholar]
- Sugi H, Yamada T. Nuclear magnetic resonance studies on the mechanism of regulation of glycogenolysis in contracting skeletal muscle. Biomed Biochim Acta. 1989;48:S335–S340. [PubMed] [Google Scholar]
- Sullivan MJ, Saltin B, Negro-Vilar R, Duscha BD, Charles HC. Skeletal muscle pH assessed by biochemical and 31P-MRS methods during exercise and recovery in men. J Appl Physiol. 1994;77:2194–2200. doi: 10.1152/jappl.1994.77.5.2194. [DOI] [PubMed] [Google Scholar]
- van der Laarse WJ, Diegenbach PC, Elzinga G. Maximum rate of oxygen consumption and quantitative histochemistry of succinate dehydrogenase in single muscle fibres of Xenopus laevis. J Muscle Res Cell Motil. 1989;10:221–228. doi: 10.1007/BF01739812. [DOI] [PubMed] [Google Scholar]
- Walsh B, Howlett RA, Stary CM, Kindig CA, Hogan MC. Measurement of Activation Energy and Oxidative Phosphorylation Onset Kinetics in Isolated Muscle Fibers in the Absence of Crossbridge Cycling. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1701–R1713. doi: 10.1152/ajpregu.00687.2005. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol. 1992;449:49–71. doi: 10.1113/jphysiol.1992.sp019074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lannergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lannergren J. Reduced maximum shortening velocity in the absence of phosphocreatine observed in intact fibres of Xenopus skeletal muscle. J Physiol. 1995;482:383–390. doi: 10.1113/jphysiol.1995.sp020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Kikuchi K, Sugi H. 31P nuclear magnetic resonance studies on the glycogenolysis regulation in resting and contracting frog skeletal muscle. J Physiol. 1993;460:273–286. doi: 10.1113/jphysiol.1993.sp019471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Sugi H. 31P-NMR study of the regulation of glycogenolysis in living skeletal muscle. Biochim Biophys Acta. 1987;931:170–174. doi: 10.1016/0167-4889(87)90203-5. [DOI] [PubMed] [Google Scholar]
- Yamada T, Sugi H. 31P NMR study of the regulation of glycogenolysis in iodoacetate-treated skeletal muscle. Adv Exp Med Biol. 1988;226:449–456. [PubMed] [Google Scholar]