Abstract
Objective
The aim of the present study is to compare the accuracy in using laboratory data or clinical factors, or both, in predicting probability of dying within 7 days of hospice admission in terminal cancer patients.
Methods
We conducted a prospective cohort study of 727 patients with terminal cancer. Three models for predicting the probability of dying within 7 days of hospice admission were developed: (i) demographic data and laboratory data (Model 1); (ii) demographic data and clinical symptoms (Model 2); and (iii) combination of demographic data, laboratory data and clinical symptoms (Model 3). We compared the models by using the area under the receiver operator curve using stepwise multiple logistic regression.
Results
We estimated the probability dying within 7 days of hospice admission using the logistic function, P = Exp(βx)/[1 + Exp(βx)]. The highest prediction accuracy was observed in Model 3 (82.3%), followed by Model 2 (77.8%) and Model 1 (75.5%). The log[probability of dying within 7 days/(1 − probability of dying within 7 days)] = −6.52 + 0.77 × (male = 1, female = 0) + 0.59 × (cancer, liver = 1, others = 0) + 0.82 × (ECOG score) + 0.59 × (jaundice, yes = 1, no = 0) + 0.54 × (Grade 3 edema = 1, others = 0) + 0.95 × (fever, yes = 1, no = 0) + 0.07 × (respiratory rate, as per minute) + 0.01 × (heart rate, as per minute) − 0.92 × (intervention tube = 1, no = 0) − 0.37 × (mean muscle power).
Conclusions
We proposed a computer-assisted estimated probability formula for predicting dying within 7 days of hospice admission in terminal cancer patients.
Keywords: hospice, palliative, computer-assisted estimated probability, survival, advanced cancer
INTRODUCTION
‘How much longer will my relative live, can he (she) pass this festival, doctor?’ is a question often raised by family caregivers in hospice. Knowing how long one will live allows the individual to bring closure to personal and family matters. An accurate prognostication can also help physicians in planning for appropriate care options those respect the wishes of the patients and their families. Duration of patients' survival after hospice enrollment is an important outcome indicator in end-of-life care because it is relevant to the cost of care and quality of patients received (1). It was also associated with families' perception of helpfulness and responsiveness from hospice services. Furthermore, late hospice referral could increase the risk of a major depressive disorder during the first year of bereavement (2).
In the present study, late referral was defined as initiation of hospice care at ≤7 days before death (3). In Taiwan, late referral for inpatient hospice care was reported to be 32.5% in 2004 (4), which is similar to the 30.8% reported in the US national statistics in 2007 (5), 29–36% reported by Virnig et al. (6) and 35.1% reported by Farnon and Hofmann (7).
When patients were enrolled in hospice with <7 days, hospice team often did not have enough time to become familiar with patients and their home situation. The goal for comprehensive care such as patients' wish to die at home might be difficult to be fulfilled (8). Part of the explanation for late referral can be attributed to difficulties in establishing an accurate prognosis (9).
Clinicians are usually optimistic in estimating survival prognosis (10–12). A number of prognostic scales are available to help improve the estimation of survival in terminal cancer patients. They can be grouped into two categories according to the parameters of scales. The first category focuses on clinical variables and performance status including the Morita's Palliative Prognostic Index (PPI) (13), Stone's PPI (14) and Chuang's Prognostic Score (CPS) (15). The second category focuses on clinical variables, performance status, clinical prediction of survival and laboratory data. They include the Pirovano's Palliative Prognostic Score (PaP) (12) and the Bozcuk's Intrahospital Cancer Mortality Risk Model (ICMRM) (16).
Clinical variables have been considered as better predictors of time to death than quality of end-of-life evaluation for terminal patients (17). However, few scales based solely on the laboratory data have been described in literature. Comparison of prediction accuracy between clinical factors and laboratory data was seldom discussed. The purpose of our study is to compare the accuracy in using laboratory data or clinical factors, or both, in predicting dying within 7 days of hospice admission for terminal cancer patients and to develop a computer-assisted model for prediction.
PATIENTS AND METHODS
We conducted a prospective, observational cohort study of 727 terminal cancer patients in a hospice ward at the Buddhist Dalin Tzu Chi General Hospital, Chiayi, Taiwan, from November 2004 to May 2007. Patients with incurable cancer were referred from other wards of the same hospital, other hospitals or from patients' homes. The decision to admit a patient was based on an initial assessment according to the government regulations for hospice and palliative care. For the purpose of respecting the medical wishes of patients at the terminal stage of an incurable illness and safeguarding their rights, the ‘Hospice-Palliative Care Act’ was promulgated in Taiwan on 7 June 2000. Patient at terminal stage may establish will of consent in choice of hospice-palliative care. One of the main points of the Act is to allow a competent patient to refuse resuscitation attempts (18). The Bureau of the National Health Insurance also issued new reimbursement regulations effective from 1 July 2000 to provide inpatient hospice care to cancer patients who are recognized as incurable and are willing to receive hospice care. Recruitment of patients and design of the present study were approved by the Institutional Review Board of Buddhist Dalin Tzu Chi General Hospital (Nos B09303011 and B09502017). Written informed consents were obtained.
Data on demographic characteristics, the presence and severity of clinical symptoms and signs, laboratory measurement and survival were collected by a team of experienced staff comprising physicians and senior nurses. All data were collected within 24 h of hospital admission and the accuracy of the data was rechecked in weekly team meeting. Eighteen symptoms and signs identified from previous studies (19–21) were assessed. Symptoms noted included pain, dyspnea, fatigue/tiredness (fatigue is perceived as unusual, abnormal or excessive whole-body tiredness, disproportionate to or unrelated to activity or exertion) (22), nausea, vomiting and constipation were graded according to the patients or caregiver descriptions, as follows: 0, never happened; 1, mild and seldom happened; 2, moderate or sometimes happened; 3, severe or continuously happened. Clinical signs for weight loss in the past 3 months, edema, ascites, jaundice and cognitive status, and the degree of severity were graded according to the clinical examination results: weight loss in the past 3 months (score as 0, no; 1, ≤5%; 2, 5–10%; 3, ≥10% as recalled by the patient or caregiver), edema (score as 0, no; 1, less than 1/2 finger breadth; 2, 1/2–1 finger breadth; 3, ≥1 finger breadth), ascites (score as 0, no; 1, only by ultrasound; 2, shifting dullness by physical examination; 3, umbilical protrusion), jaundice (score as 0, no; 1, slightly yellowish; 2, remarkably yellow; 3, deeply yellow or greenish) and cognitive status (score as 0, clear; 1, lethargy; 2, confusion or delirium; 3, comatose) (23–25). Other clinical signs including heart rhythm, poor appetite, medication for insomnia, fever, pressure sore, intervention tube placement and muscle power were evaluated according to their operating definitions: heart rhythm (irregular vs. regular), poor appetite (yes vs. no; yes defined as <500 cc of milk or <2 bowls of porridge by mouth or tube feeding within 24 h of admission), medication for insomnia (yes vs. no), fever (yes vs. no; yes defined as core temperature ≥37.5°C), intervention tube placement [yes vs. no; yes defined as had the intervention tube, e.g. percutaneous nephrostomy (PCN), percutaneous transhepatic cholangiodrainage (PTCD), pig tail for pleural effusion or ascites drainage, and feeding tube except nasogastric (NG) tube], and muscle power was calculated as the sum of muscle power of each extremity divided by four, muscle powers are graded using the Medical Research Council (MRC) scale of 0–5: 5, normal power; 4, moderate movement against resistance; 3, movement against gravity but not against resistance; 2, movement with gravity eliminated; 1, flicker of movement; 0, no movement. An additional 13 laboratory variables were examined, including white blood cell count, differential cell percentages, hemoglobin, blood urea nitrogen (BUN), creatinine, serum glutamic oxaloacetic transaminase (SGOT), serum glutamic pyruvate transaminase (SGPT), total bilirubin, albumin, serum sodium, serum potassium, corrected calcium and blood sugar. Time to death in days of subjects was recorded. When there was difficulty in verbal communication with patients, their status was obtained from their caregivers.
Statistical Analysis
Statistical analyses were conducted using the SAS® software, Version 9.1.3 (SAS Institute Inc., Cary, NC, USA) and R 2.6.1 (R Foundation for Statistical Computing, Vienna, Austria) (http://www.r-project.org). Log-rank test was used for different group survival comparison. Univariate logistic regression was used for selecting significant variables associated with dying within 7 days of hospice admission. Model-fitting techniques for multiple logistic regression analysis, including (i) stepwise variable selection, (ii) the Hosmer–Lemeshow goodness-of-fit test and (iii) regression diagnostics including variance inflation factor were applied to assure the quality of analyses. Receiver operating characteristic (ROC) curves were employed for comparing the different models. Three prediction models for dying within 7 days were developed: (i) demographic data and laboratory data (Model 1); (ii) demographic data and clinical symptoms (Model 2); and (iii) combination of demographic data, laboratory data and clinical symptoms (Model 3). All statistical assessments were two-sided and evaluated at the 0.05 level of significant difference.
RESULTS
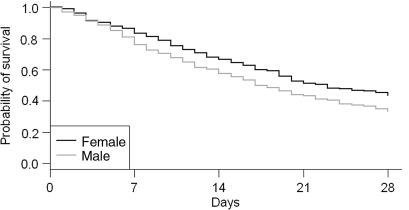
The median time to death of the 727 terminal cancer patients enrolled in the study was 17 days. Male had poorer survival than female (P = 0.002). Time to death of ≤7 days occurred in 103 (24.0%) males and 49 (16.8%) females. The survival probability at 1 week after admission was 79% (Fig. 1). The demographic characteristics of the subjects are shown in Table 1. There was no difference in time to death between different age groups (P = 0.767). Bone (P = 0.009) and liver (P < 0.001) metastases significantly reduced time to death (Table 1). The different severities of clinical symptoms and signs are listed in Table 2 and the P values of log-rank tests were all <0.05. Sex, liver cancer, respiratory rate, heart rate, Grade 3 edema, muscle power score, jaundice, intervention tube, ECOG score, BUN, creatinine, albumin, SGOT and SGPT were significant factors for predicting dying within 7 days of hospice admission by univariate logistic analysis (Table 3).
Figure 1.
The Kaplan–Meier survival curve.
Table 1.
Characteristics of the study population (n = 727)
| Variable | n (%) | P |
|---|---|---|
| Survival days, median (mean ± SD) | 17 (30.3 ± 42) | |
| Admission days, median (mean ± SD) | 10 (12.4 ± 9) | |
| Sex | ||
| Female | 294 (40.4) | 0.002 |
| Male | 433 (59.6) | |
| Age (years) | ||
| 40 | 32 ( 4.4) | 0.767 |
| 40–64 | 285 (39.2) | |
| ≥65 | 410 (56.4) | |
| Diabetes | 212 (29.2) | 0.714 |
| Hypertension | 299 (41.1) | 0.889 |
| Admitted from | ||
| Emergency Room | 272 (37.4) | 0.158 |
| Outpatient department | 216 (29.7) | |
| Oncology department | 113 (15.5) | |
| Other outpatient department | 126 (17.3) | |
| Cancer | ||
| Lung | 132 (18.2) | <0.001 |
| Liver | 140 (19.3) | |
| Colon | 83 (11.4) | |
| Stomach | 41 ( 5.6) | |
| Head Neck cancer | 97 (13.4) | |
| Pancreas | 29 ( 4.0) | |
| Male genitourinary | 24 ( 3.3) | |
| Female genitourinary | 46 ( 6.3) | |
| Breast | 25 ( 3.4) | |
| Esophagus | 19 ( 2.6) | |
| Unknown and others | 91 (12.5) | |
| Metastasis | ||
| Bone | 189 (26.0) | 0.009 |
| Lung | 124 (17.1) | 0.822 |
| Liver | 140 (19.3) | <0.001 |
| Brain | 67 ( 9.2) | 0.244 |
| Operation | 319 (43.9) | 0.015 |
| Chemotherapy | 381 (52.4) | 0.677 |
| Radiotherapy | 259 (35.6) | <0.001 |
P, P value of log-rank test; SD, standard deviation.
Table 2.
Prevalence of significant clinical signs by the symptoms/signs severity
| Clinical signs | Prevalence by severity (0/1/2/3) | P |
|---|---|---|
| Cognitive function | 501/120/59/47 | <0.001 |
| Edema | 378/108/82/159 | <0.001 |
| Jaundice | 517/108/42/60 | <0.001 |
| ECOG score | 12/181/405/129a | <0.001 |
| Body weight loss | 40/260/252/174 | 0.003 |
| Ascites | 483/123/66/55 | <0.001 |
P, P value of log-rank test.
aECOG score is 1–4.
Table 3.
Univariate logistic regression for the probability of dying within 7 days of hospice admission in terminal cancer patients
| Variable | P | OR | 95% CI |
|---|---|---|---|
| Age (per year) | 0.084 | 1.01 | 1.00–1.03 |
| Sex (male vs. female) | 0.020 | 1.57 | 1.07–2.29 |
| Liver cancer vs. other cancer | <0.001 | 2.21 | 1.47–3.32 |
| Lung cancer vs. other cancer | 0.553 | 1.15 | 0.73–1.79 |
| Diabetes history (yes vs. no) | 0.674 | 0.91 | 0.58–1.42 |
| Hypertension history (yes vs. no) | 0.226 | 0.77 | 0.50–1.18 |
| ECOG score (per score) | <0.001 | 2.46 | 1.85–3.28 |
| Respiratory rate (per 1/min) | <0.001 | 1.08 | 1.04–1.12 |
| Heart rate (per 1/min) | <0.001 | 1.02 | 1.01–1.03 |
| Edema (Grade 3 vs. others) | <0.001 | 2.03 | 1.36–3.03 |
| Mean muscle power (per score) | <0.001 | 0.59 | 0.49–0.70 |
| Fever (yes vs. no) | 0.534 | 1.14 | 0.75–1.74 |
| Jaundice (yes vs. no) | <0.001 | 2.37 | 1.63–3.44 |
| Intervention tube (yes vs. no) | 0.029 | 0.43 | 0.20–0.92 |
| WBC (per 103/μl) | 0.609 | 1.001 | 0.996–1.006 |
| Hemoglobin (per mg/dl) | 0.305 | 1.05 | 0.96–1.14 |
| Glucose (per mg/dl) | 0.810 | 1.000 | 0.997–1.002 |
| BUN (per mg/dl) | <0.001 | 1.03 | 1.02–1.04 |
| Creatinine (per mg/dl) | <0.001 | 1.43 | 1.22–1.67 |
| Albumin (per g/dl) | 0.008 | 0.65 | 0.47–0.89 |
| SGOT (per 10 IU/l) | <0.001 | 1.04 | 1.02–1.05 |
| SGPT (per 10 IU/l) | <0.001 | 1.04 | 1.02–1.05 |
OR, odds ratio; WBC, white blood cell; BUN, blood urea nitrogen; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvate transaminase.
From laboratory variables and demographic data, four significant factors were identified to form Model 1 through stepwise logistic regression. The factors were hemoglobin, BUN, SGOT and albumin. From clinical symptoms and signs and demographic data, 10 significant prognostic clinical factors were identified to form Model 2. The factors were sex, hepatocellular carcinoma, fever, Grade 3 edema, jaundice, intervention tubes, ECOG scale, mean muscle power, heart rate and respiratory rate. The 10 significant factors identified to form Model 3 were sex, intervention tubes, Grade 3 edema, ECOG score, mean muscle power, hemoglobin, BUN, SGOT, respiratory rate and heart rate (Table 4).
Table 4.
Three computer-assisted estimated probability models for the prediction of dying within 7 days of hospice admission in terminal cancer patients
| Variable | Model 1 |
Model 2 |
Model 3 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| β | P | OR | β | P | OR | β | P | OR | |
| Intercept | −2.20 | 0.001 | −6.52 | <0.001 | −7.76 | <0.001 | |||
| Hemoglobin (per mg/dl) | 0.11 | 0.028 | 1.12 | 0.14 | 0.006 | 1.15 | |||
| BUN (per mg/dl) | 0.03 | <0.001 | 1.03 | 0.03 | <0.001 | 1.03 | |||
| Albumin (per g/dl) | −0.50 | 0.009 | 0.61 | ||||||
| SGOT (per 10 IU/l) | 0.03 | 0.001 | 1.03 | 0.03 | <0.001 | 1.03 | |||
| Sex (male vs. female) | 0.77 | 0.001 | 2.17 | 0.68 | 0.004 | 1.98 | |||
| Intervention tube (yes vs. no) | −0.92 | 0.024 | 0.40 | −0.93 | 0.027 | 0.40 | |||
| Edema (Grade 3 vs. others) | 0.54 | 0.019 | 1.72 | 0.61 | 0.013 | 1.83 | |||
| ECOG (per score) | 0.82 | <0.001 | 2.27 | 0.76 | <0.001 | 2.14 | |||
| Muscle power (per score) | −0.37 | 0.001 | 0.69 | −0.30 | 0.009 | 0.74 | |||
| Cancer (liver vs. others) | 0.59 | 0.023 | 1.81 | ||||||
| Fever (yes vs. no) | 0.95 | 0.040 | 2.59 | ||||||
| Jaundice (yes vs. no) | 0.59 | 0.011 | 1.81 | ||||||
| Respiratory rate (per 1/min) | 0.07 | 0.005 | 1.07 | 0.06 | 0.019 | 1.06 | |||
| Heart rate (per beat/min) | 0.01 | 0.034 | 1.01 | 0.01 | 0.024 | 1.01 | |||
According to the logistic model:
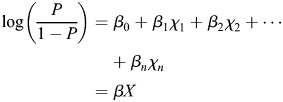 |
(Function 1) |
| (Function 2) |
where P is the probability of event, β0 the intercept, βn the parameter and χn the variable.
We proposed a computer-assisted estimated probability (CEP) for predicting dying within 7 days of hospice admission in terminal cancer patients. The formula based on Model 2 is:
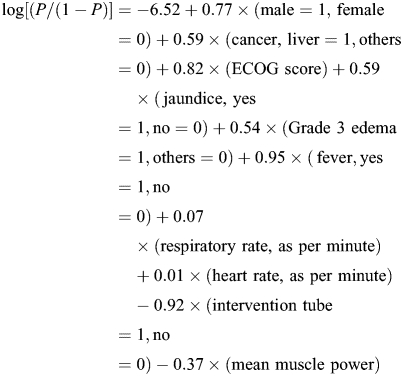 |
When the cut-off score (P) was >0.6, the positive predictive value and the negative predictive value for patients dying within 7 days of hospice admission were 0.74 and 0.83.
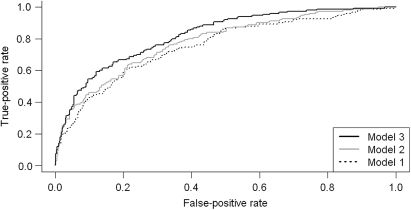
We compared the accuracy of these three models by ROC curves (Fig. 2). The area under the curve for Model 1 was 75.5%, Model 2 was 77.8% and Model 3 was 82.3%. Model 3 exhibited the best predictor value in comparison with the other two models (P = 0.005) and the trend was also significant (P = 0.002). The programming code for probability calculation based on the fitted model in the R environment (http://www.r-project.org/) is provided in Appendix 1.
Figure 2.
The receiver operating characteristic curve of three computer-assisted estimated probability models for prediction dying within 7 days of hospice admission in terminal cancer patients: Model 1, laboratory data and demographic data; Model 2, clinical factors and demographic data; Model 3, clinical factors, laboratory data and demographic data.
Validations were performed using split data sets, in which the model was trained on a randomly selected subset of half of the data and tested on the remaining data. Validation tests were repeated 10 times for different selections of training and test data. The models produced were similar to the original and performed nearly as well on test data as on training data.
DISCUSSION
The probability of dying within 7 days of hospice admission was 20.9%, which is better than the findings of 33.5% in Taiwan in 2004. Part of the reason is the new policy of integrating hospice service into acute care wards issued by the Bureau of Health Promotion, Department of Heath, Taiwan, in 2005. The new policy has a potential to expand the utilization of hospice care by cancer decedents. Barriers to accessing hospice care are complex and often overlapping, and some factors are related to physicians. For example, physicians often delay patients' referral to hospice because of their often over-optimistic view of their patients' prognosis shortly before death (26). By improving the accuracy of prediction of dying within 7 days of hospice admission, we hope to assist physicians in making a more realistic survival prediction in their patients.
The accuracy of predicting probability of dying within 7 days of hospice admission by the three models was significantly different. Model 2 (clinical factors and demographic data) was more accurate than Model 1 (laboratory tests and demographic data). The laboratory data were derived from the biochemical and blood tests of admission routine and it could supplement the prognostic power of clinical and demographic variables.
Previous studies have identified many putative prognostic factors in patients with advanced cancer, including clinical estimates of survival, demographic and clinical variables and laboratory parameters (27,28). Some groups have constructed prognostic scales using different combinations of these variables (12,16). Model 3 was the best predictive model and included performance status (ECOG score), five clinical variables (edema with degree 3 severity, mean score of muscle power, heart rate, respiratory rate and intervention tube), sex and three laboratory parameters (hemoglobin, BUN and SGOT). The factors of ECOG, edema with a degree 3 severity, heart rate and sex were significant predictors in previous studies (15–17,29–32). We identified five useful prognostic factors in this study: (i) the mean score of muscle power can express the weakness or energy level of a patient. A lower muscle power score correlated with a shorter predicted survival. (ii) For the basic vital signs of respiratory and heart rate, higher rates were significantly correlated with increased probability of mortality within 1 week, similar to an earlier study (33). (iii) Intervention tube, e.g. PCN, PTCD, pig tail drainage, feeding tube excluding NG tube, indicated that the patients were receiving aggressive interventions before being admitted to the palliative care unit and was associated with longer survival. Patients with placement of intervention tube had significantly lower risk for death in 7 days after admission in our study. The placement of intervention tube might prolong the survival days of the patients after the clinical issues had solved by the placement of the tube or that the placement of intervention tube was able to help the patients to live better. (iv) One unique finding in this study was that the higher hemoglobin indicated a higher probability of within 7-day survival, whereas the low hemoglobin group had a worse survival after 2 weeks. Anemia was a predictive factor for shorter survival in most studies, as measured in weeks to months survival (27). (v) BUN was also identified as a predictor in the previous study (34). Terminal azotemia refers to the dehydration status and acute renal failure involved in the dying process. (vi) SGOT is the prognostic factor in patients with hepatocellular carcinoma (35), which is the leading cause of death in Taiwan for more than 20 years; and it is also identified in the other study (36).
Previous studies have discussed prognostic tools for prediction of survival from weeks to months in advanced cancer patients with disparate results (37). However, prediction of dying within 7 days of hospice admission has rarely been discussed. The method of CEP can easily be calculated within 24 h of patient admission and can serve as a useful tool to assist estimation of survival prediction.
Limitations of this study include recall bias and misclassification error. When the patients could not accurately recall their body weights 3 months before the study, calculation of weight loss had to be based on the information provided by patients' family members. Moreover, misclassification error may be present in the grading of the clinical signs such as severity of ascites, jaundice and cognitive status. In addition, data regarding symptoms on the regular chart such as extremity cyanosis, self-conscious dying and biologic parameters such as serum electrolytes, B12/C-reactive protein (38), serum lactate dehydrogenase and alkaline phosphatase were not included in data analysis.
In conclusion, a CEP that utilized clinical factors, demographic factors and laboratory data were developed for patients with advanced cancer. We suggested using Model 2 as a readily accessible tool for making prediction and using Model 3 if laboratory data are available. It is hope that the CEP prognostic scale can assist clinicians in making accurate survival prediction and thus able to form treatment decisions that minimize harm and discomfort in patients.
Funding
This project was supported by grants from Buddhist Dalin Tzu Chi General Hospital (Project Nos DTCRD94-10 and DTCRD96-18).
Conflict of interest statement
None declared.
Appendix 1. Programming code in R for calculating probability of dying within 7 days after hospice admission in patients with terminal cancer
Substitute the values for the variables X1 to X10 in the regression equation to calculate the probability of dying within 7 days after hospice admission.
- yhat <− (−6.52
# constant
- +0.77*X1
# X1 = sex (male = 1, female = 0)
- +0.59*X2
# X2 = cancer (liver cancer = 1, others = 0)
- +0.82*X3
# X3 = ECOG score
- +0.59*X4
# X4 = jaundice (yes = 1, no = 0)
- +0.54*X5
# X5 = edema (1 if edema = grade 3, 0 if otherwise)
- +0.95*X6
# X6 = fever (yes = 1, no = 0)
- +0.07*X7
# X7 = respiratory rate per minute
- +0.01*X8
# X8 = heart rate, beat per minute
- −0.92*X9
# X9 = intervention tube (yes = 1, no = 0)
- −0.37*X10
# X10 = mean muscle power score
- phat <- 1/(exp(-(yhat)) + 1)
- phat
# copy these syntax and paste on the R console
References
- 1.Miller SC, Kinzbrunner B, Pettit P, Williams JR. How does the timing of hospice referral influence hospice care in the last days of life? J Am Geriatr Soc. 2003;51:798–806. doi: 10.1046/j.1365-2389.2003.51253.x. [DOI] [PubMed] [Google Scholar]
- 2.Bradley EH, Prigerson H, Carlson MDA, Cherlin E, Johnson-Hurzeler R, Kasl SV. Depression among surviving caregivers: does length of hospice enrollment matter? Am J Psychiatry. 2004;161:2257–62. doi: 10.1176/appi.ajp.161.12.2257. [DOI] [PubMed] [Google Scholar]
- 3.Christakis NA, Iwashyna TJ. Impact of individual and market factors on the timing of initiation of hospice terminal care. Med Care. 2000;38:528–41. doi: 10.1097/00005650-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Tang ST, Chen ML, Huang EW, Koong SL, Lin GL, Hsiao SC. Hospice utilization in Taiwan by cancer patients who died between 2000 and 2004. J Pain Symptom Manage. 2007;33:446–53. doi: 10.1016/j.jpainsymman.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 5.National Hospice and Palliative Care Organization. NHPCO's 2008 Facts and Figures: Hospice Care in America. http://www.nhpco.org/files/public/Statistics_Research/NHPCO_facts-and-figures_2008.pdf. (22 July 2009, date last accessed) [Google Scholar]
- 6.Virnig BA, Persily NA, Morgan RO, Devito CA. Do Medicare HMOs and Medicare FFS differ in their use of the Medicare hospice benefit? Hosp J. 1999;14:1–12. doi: 10.1080/0742-969x.1999.11882910. [DOI] [PubMed] [Google Scholar]
- 7.Farnon C, Hofmann M. Factors contributing to late hospice admission and proposals for change. Am J Hosp Palliat Care. 1997;14:212–8. doi: 10.1177/104990919701400504. [DOI] [PubMed] [Google Scholar]
- 8.Miller SC, Weitzen S, Kinzbrunner B. Factors associated with the high prevalence of short hospice stays. J Palliat Med. 2003;6:725–36. doi: 10.1089/109662103322515239. [DOI] [PubMed] [Google Scholar]
- 9.Glare P, Virik K, Jones M, Hudson M, Eychmuller S, Simes J, et al. A systematic review of physicians’ survival predictions in terminally ill cancer patients. BMJ. 2003;327:195–200. doi: 10.1136/bmj.327.7408.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone PC, Lund S. Predicting prognosis in patients with advanced cancer. Ann Oncol. 2007;18:971–6. doi: 10.1093/annonc/mdl343. [DOI] [PubMed] [Google Scholar]
- 11.Chow E, Harth T, Hruby G, Finkelstein J, Wu J, Danjoux C. How accurate are physicians' clinical predictions of survival and the available prognostic tools in estimating survival times in terminally ill cancer patients? A systematic review. Clin Oncol (R Coll Radiol) 2001;13:209–18. doi: 10.1053/clon.2001.9256. [DOI] [PubMed] [Google Scholar]
- 12.Pirovano M, Maltoni M, Nanni O, Marinari M, Indelli M, Zaninetta G, et al. A new palliative prognostic score: a first step for the staging of terminally ill cancer patients. Italian Multicenter and Study Group on Palliative Care. J Pain Symptom Manage. 1999;17:231–9. doi: 10.1016/s0885-3924(98)00145-6. [DOI] [PubMed] [Google Scholar]
- 13.Morita T, Tsunoda J, Inoue S, Chihara S. The Palliative Prognostic Index: a scoring system for survival prediction of terminally ill cancer patients. Support Care Cancer. 1999;7:128–33. doi: 10.1007/s005200050242. [DOI] [PubMed] [Google Scholar]
- 14.Stone CA, Tiernan E, Dooley BA. Prospective validation of the palliative prognostic index in patients with cancer. J Pain Symptom Manage. 2008;35:617–22. doi: 10.1016/j.jpainsymman.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Chuang RB, Hu WY, Chiu TY, Chen CY. Prediction of survival in terminal cancer patients in Taiwan: constructing a prognostic scale. J Pain Symptom Manage. 2004;28:115–22. doi: 10.1016/j.jpainsymman.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Bozcuk H, Koyuncu E, Yildiz M, Samur M, Ozdogan M, Artac M, et al. A simple and accurate prediction model to estimate the intrahospital mortality risk of hospitalised cancer patients. Int J Clin Pract. 2004;58:1014–9. doi: 10.1111/j.1742-1241.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- 17.Toscani P, Brunelli C, Miccinesi G, Costantini M, Gallucci M, Tamburini M, et al. Predicting survival in terminal cancer patients: clinical observation or quality-of-life evaluation? Palliat Med. 2005;19:220–7. doi: 10.1191/0269216305pm1000oa. [DOI] [PubMed] [Google Scholar]
- 18.Department of Health, Executive Yuan, Taiwan. Hospice-Palliative Care Act. [Internet] Version: June 2004, http://www.doh.gov.tw/ufile/doc/200406_Hospce-Palliative%20Care%20Act.pdf. (cited 21 November 2009) [Google Scholar]
- 19.Chiu TY, Hu WY, Chen CY. Prevalence and severity of symptoms in terminal cancer patients: a study in Taiwan. Support Care Cancer. 2000;8:311–3. doi: 10.1007/s005209900112. [DOI] [PubMed] [Google Scholar]
- 20.Conill C, Verger E, Henriquez I, Saiz N, Espier M, Lugo F, et al. Symptom prevalence in the last week of life. J Pain Symptom Manage. 1997;14:328–31. doi: 10.1016/s0885-3924(97)00263-7. [DOI] [PubMed] [Google Scholar]
- 21.Vainio A, Auvinen A. Prevalence of symptoms among patients with advanced cancer: an international collaborative study. Symptom Prevalence Group. J Pain Symptom Manage. 1996;12:3–10. doi: 10.1016/0885-3924(96)00042-5. [DOI] [PubMed] [Google Scholar]
- 22.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in the healthy individuals. J Support Care Cancer. 1996;4:82–96. doi: 10.1007/BF01845757. [DOI] [PubMed] [Google Scholar]
- 23.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method: a new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorder. 4th edn. Washington, DC: American Psychiatric Press; 2002. Text revision. DSM IV-TR. [Google Scholar]
- 25.Frances A, Pincus HA, First MB. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4th edn. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 26.Friedman BT, Hardwood MK, Shields M. Barriers and enablers to hospice referrals: an expert overview. J Palliat Med. 2002;5:73–84. doi: 10.1089/10966210252785033. [DOI] [PubMed] [Google Scholar]
- 27.Maltoni M, Caraceni A, Brunelli C, Broeckaert B, Christakis N, Eychmueller S, et al. Prognostic factors in advanced cancer patients: evidence-based clinical recommendations—a study by the Steering Committee of the European Association for Palliative Care. J Clin Oncol. 2005;23:6240–8. doi: 10.1200/JCO.2005.06.866. [DOI] [PubMed] [Google Scholar]
- 28.Maltoni M, Amadori D. Prognosis in advanced cancer. Hematol Oncol Clin N Am. 2002;16:715–29. doi: 10.1016/s0889-8588(02)00024-2. [DOI] [PubMed] [Google Scholar]
- 29.Vigano A, Dorgan M, Buckingham J, Bruera E, Suarez-Almazor ME. Survival prediction in terminal cancer patients: a systematic review of the medical literature. Palliat Med. 2000;14:363–74. doi: 10.1191/026921600701536192. [DOI] [PubMed] [Google Scholar]
- 30.den Daas N. Estimating length of survival in end-stage cancer: a review of the literature. J Pain Symptom Manage. 1995;10:548–55. doi: 10.1016/0885-3924(95)00103-6. [DOI] [PubMed] [Google Scholar]
- 31.Maltoni M, Pirovano M, Nanni O, Scarpi E, Indelli M, Martini C, et al. Biological indices predictive of survival in 519 Italian terminally ill cancer patients. Italian Multicenter Study Group on Palliative Care. J Pain Symptom Manage. 1997;13:1–9. doi: 10.1016/s0885-3924(96)00265-5. [DOI] [PubMed] [Google Scholar]
- 32.Faris M. Clinical estimation of survival and impact of other prognostic factors on terminally ill cancer patients in Oman. Support Care Cancer. 2003;11:30–4. doi: 10.1007/s00520-002-0401-0. [DOI] [PubMed] [Google Scholar]
- 33.de Miguel Sanchez C, Elustondo SG, Estirado A, Sanchez FV, de la Rasilla Cooper CG, Romero AL, et al. Palliative performance status, heart rate and respiratory rate as predictive factors of survival time in terminally ill cancer patients. J Pain Symptom Manage. 2006;31:485–92. doi: 10.1016/j.jpainsymman.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Ho SY, Guo HR, Chen HHW, Peng CJ. Nutritional predictors of survival in terminally ill cancer patients. J Formos Med Assoc. 2003;102:544–50. [PubMed] [Google Scholar]
- 35.Attali P, Prod'Homme S, Pelletier G, Papoz L, Ink O, Buffet C, et al. Prognostic factors in patients with hepatocellular carcinoma. Cancer. 1987;59:2108–11. doi: 10.1002/1097-0142(19870615)59:12<2108::aid-cncr2820591225>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 36.Graf W, Bergstrom R, Pahlman L, Glimelius B. Appraisal of a model for prediction of prognosis in advanced colorectal cancer. Eur J Cancer. 1994;30A:453–7. doi: 10.1016/0959-8049(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 37.Glare PA, Sinclair CT. Palliative medicine review: prognostication. J Palliat Med. 2008;11:84–103. doi: 10.1089/jpm.2008.9992. [DOI] [PubMed] [Google Scholar]
- 38.Kelly L, White S, Stone PC. The B12/CRP index as a simple prognostic indicator in patients with advanced cancer: a confirmatory study. Ann Oncol. 2007;18:1395–9. doi: 10.1093/annonc/mdm138. [DOI] [PubMed] [Google Scholar]