Abstract
Natural killer (NK) cells are innate immune lymphocytes that can react to cells lacking self-MHC class I. However, NK cells that cannot engage self-MHC through an inhibitory receptor are resistant to stimulation through their activation receptors. To become licensed, i.e. functionally competent to be triggered through its activation receptors, an NK cell must engage host MHC class I via a MHC class I-specific inhibitory receptor, such as a member of the murine Ly49 family. To explore potential determinants of NK cell licensing on a single Ly49 receptor, we have investigated the relative licensing impacts of the b, d, k, q, r, and s H2 haplotypes on Ly49A+ NK cells. The results indicate that licensing is essentially analog but is saturated by moderate-binding MHC class I ligands. Interestingly, licensing exhibited a strong inverse correlation with a measure of cis engagement of Ly49A. Finally, licensing of Ly49A+ NK cells was found to be less sensitive to MHC class I engagement than Ly49A-mediated effector inhibition, suggesting that licensing establishes a margin of safety against NK cell autoreactivity.
Introduction
Natural killer (NK) cells are innate immune lymphocytes that produce pro-inflammatory cytokines and kill transformed and virally infected cells. Traditionally, NK cells are thought to recognize “missing self,” the lack of normal expression of MHC class I molecules (1). For this purpose, murine NK cells express inhibitory receptors of the Ly49 C-type lectin superfamily, such as Ly49A, that dampen effector responses (2). Like other inhibitory Ly49 receptors, Ly49A is expressed on a subset of NK cells. It is specific for some but not all MHC class I alleles, e.g. H2Dd and H2Dk and not H2Kb (3, 4). That Ly49A interacts with H2Dd is particularly well supported, including crystallographic information (5), whereas Ly49A binding to other MHC class I alleles has been less thoroughly examined.
According to the missing-self hypothesis, NK cells in MHC class I-deficient mice should be autoreactive, as host MHC class I is unable to engage NK cell inhibitory receptors. However, NK cells that develop in MHC class I-deficient hosts, e.g. β2-microglobulin−/− (β2m−/−) mice, are hypo-responsive, i.e. they fail to produce cytokines or release cytotoxic effectors in response to activation receptor stimuli that elicit responses from wild-type NK cells (6). NK cells require engagement of an “inhibitory” receptor with MHC class I, such as Ly49A with H2Dd, to attain functional competence (7, 8). This process, termed licensing, allows NK cells to be activated through activation receptors to detect and kill cells lacking self-MHC class I. NK cells without self-MHC-specific inhibitory receptors remain unlicensed and hence are unable to react against MHC class I-deficient cells, thus avoiding autoreactivity. Licensing requires the cytoplasmic tail and specifically the immunoreceptor tyrosine-based inhibitory motif (ITIM) of the self-MHC-specific inhibitory Ly49 receptor (7). Thus, the NK cell inhibitory receptors have a second function in licensing or education of NK cells in self-tolerance.
More detailed knowledge of the contribution of individual NK cell inhibitory receptors to licensing in different MHC contexts may have clinical implications. Human NK cells display properties consistent with licensing in that NK cells expressing a killer immunoglobulin-like receptor (KIR) with specificity for self-HLA molecules exhibit more robust responsiveness than NK cells without self-HLA specific receptors in the same individual (9–11). With increasing numbers of studies reporting a connection of NK cell inhibitory receptors and licensing with human anti-viral and anti-cancer responses (e.g., (12–14)), a better understanding of licensing in mice should provide insight into human NK cell biology and its effects on human health and disease.
Recent studies addressed the potency of NK cell subsets in mice expressing one, two, or three MHC class I alleles (15, 16). Joncker et al reported that NK cell potency increases with the number of self-specific inhibitory receptors: NK cells expressing two different self-specific inhibitory receptors respond more robustly to stimulation than NK cell subsets expressing only one self-specific inhibitory receptor (16). Brodin et al used flow cytometry to assess the NK cell potency of Ly49-monopositive populations in mice expressing selected MHC class I alleles (15). They found that the Ly49A-monopositive NK cell subset of mice expressing only H2Dd, a known licensing ligand for Ly49A (7), exhibited a more robust response to NKG2D stimulation than the corresponding cells from mice expressing only H2Db, which in turn responded more strongly than cells from mice lacking MHC class I. Based upon these and other data, Brodin et al and Joncker et al propose a quantitative (“rheostat”) model of licensing, in which the potency of a given NK cell subpopulation is modulated by the nature and number of Ly49-MHC class I interactions. Data from studies of human NK cells also support a quantitative model of licensing. Yu et al and Yawata et al both report that NK cell potency was higher among NK cells that expressed two self-specific KIRs than among NK cells expressing only one self-specific KIR (11, 17). Thus, the available data support quantitative modulation of potency of an individual NK cell based upon the number of self-MHC-specific inhibitory receptors that it expresses.
On the other hand, evidence of the effect of engagement of a single inhibitory receptor by different MHC alleles is limited, although the simplest prediction of the rheostat model is that stronger interactions should produce more potent NK cells. However, most studies of murine NK cells have been confined to just two H2 haplotypes, H2b and H2d. The most detailed study, by Brodin et al, used H2Kb, Db, and Dd as model MHC class I molecules. Whereas Ly49A does not bind H2Kb, and has occasionally but not consistently been reported to bind weakly to H2Db in older binding studies (e.g., (4, 18)), Ly49A binds strongly to H2Dd. Thus, only a very limited selection of MHC class I alleles has been used to study the putative quantitative nature of licensing through a single self-MHC-specific receptor, such as Ly49A.
To more thoroughly address whether a particular Ly49 receptor obeys a quantitative model of licensing, a larger and more diverse group of MHC class I alleles should be investigated. MHC-congenic mice on a C57BL/10 background represent an ideal model system in which to study NK cells in a variety of MHC class I contexts. These mice should all possess the same genetic background, including the same haplotype of the Ly49 gene family in the NK gene complex, which otherwise can display profound genetic differences between inbred strains of mice (19). Moreover, these mice allow study of MHC class I alleles that have generally not been studied in detail in NK cell biology.
Interpretation of Ly49 function with respect to self-MHC alleles requires understanding of Ly49 specificities. Binding assays of Ly49A with an array of MHC class I molecules have been published, but these studies feature several important limitations that were initially unappreciated. For example, Ly49A specificity was previously determined using MHC class I tetramers refolded with human β2m to bind Ly49A-transfected cells (3). As the Ly49A binding site on MHC class I is now known to include β2m contact sites that are disrupted by human β2m (4, 20), these results must be interpreted with caution. Cell-cell adhesion assays of Ly49A-transfected cells with MHC-congenic Con A blasts have been used to assess binding of Ly49A to cells of more unusual MHC haplotypes (3). However, cell-cell adhesion assays are less quantitative; these data deserve reassessment with more precise molecular tools, like Ly49A tetramer binding. Indeed, Ly49A tetramers have been used to stain naïve splenocytes from a number of different mouse strains (18). However, these studies were limited to only a handful of MHC-congenic strains, with the remaining MHC haplotypes tested on cells from inbred strains with different genetic backgrounds, raising the possibility that unknown non-MHC-associated loci could influence the interaction. Regardless, these additional MHC alleles have not been examined in the context of licensing. Thus, while previous studies provided important groundwork on the MHC-specificities of Ly49A, new studies are warranted to reassess Ly49A specificity and to correlate these specificities with licensing of Ly49A+ NK cell populations.
Herein we have investigated licensing of Ly49A+ NK cells in MHC-congenic hosts in parallel with studies of Ly49A tetramer binding to the same MHC haplotypes. The strength of licensing of Ly49A+ NK cells varied with MHC haplotype and was saturated by a relatively low level of Ly49A-MHC class I binding affinity. Interestingly, licensing of Ly49A+ NK cells has a higher affinity/avidity threshold than effector inhibition mediated by Ly49A. This difference in threshold may represent a safeguard against autoimmunity similar to the difference in threshold between T cell negative selection in the thymus and activation in the periphery.
Materials and Methods
Mice
MHC-congenic mice, including C57BL/10 (H2b), B10.D2 (H2d), B10.BR (H2k), B10.D1 (H2q), B10.RIII (H2r), and B10.S (H2s), were purchased from The Jackson Laboratory (Bar Harbor, ME). H2Kb−/− H2Db−/− (Kb−/− Db−/−) mice were purchased from Taconic. Dd-transgenic Kb−/− Db−/− mice were obtained by crossing Dd-transgenic mice (D8, expressing a Dd genomic construct with transgenic Dd expression comparable to H2d mice) provided by D. Margulies (NIAID, Bethesda, MD) to Kb−/− Db−/− mice. To generate offspring homozygous, hemizygous, or nullizygous for the Dd transgene, Kb−/− Db−/− mice homozygous for the Dd transgene were bred to Kb−/− Db−/− mice, and F1 mice were then bred together. Similarly, B10.BR mice were bred to Kb−/− Db−/− mice to produce mice hemizygous for the H2k class I locus. These mice were then compared to commercially obtained parental strains. For the B10.RIII F2 hybrid mouse experiments, we mated B10.RIII mice to C57BL/6 mice. The subsequent (B10.RIII × C57BL/6)F1 hybrid mice were sibling mated to produce F2 hybrid mice which were then screened with microsatellite markers for the H2 locus and chromosome 10 from B10.RIII mice. Microsatellite markers used are described in supplemental Table I. All mice were used in accordance with institutional guidelines for animal experimentation.
Antibodies and flow cytometry
The following antibodies and reagents were purchased from BD Biosciences (San Jose, CA): Pacific Blue and PerCP Cy5.5 anti-CD3 (145-2C11); PerCP Cy5.5 anti-CD19 (1D3); APC and PE Cy7 anti-NK1.1 (PK136); Alexa 488, PE, or PE Cy7 anti-IFNγ (XMG1.2); FITC anti-H2Dk (15-5-5); PE anti-H2Dd (34-5-8S); and PE and APC streptavidin. Anti-Ly49A clones A1 and JR9 were produced from hybridomas as previously detailed (21, 22), purified, and labeled with biotin or FITC using standard protocols. For gating on Ly49A-monopositive NK cells, the following antibody clones were used to exclude other inhibitory NK cell receptors: 4LO33 (anti-Ly49C; produced from a hybridoma kindly provided by Suzanne Lemieux); 5E6 (anti-Ly49C/I; BD); HBF-719 (anti-Ly49F; BD); 4D11 (anti-Ly49G2; BD or hybridoma from ATCC); YLI-90 (anti-Ly49I; BD); 16a11 (anti-NKG2A; eBioscience). The commercially available antibodies were purchased in PE-conjugated form, except 4D11 (APC). Homemade 4LO33 and 4D11 were used in biotinylated form with streptavidin-PE. Anti-NK1.1 clone PK136 (ATCC) was grown from the hybridoma and purified according to standard protocols. For flow cytometric analysis, RBC-lysed single-cell suspensions of splenocytes were stained in the presence of 2.4g2 supernatants. Samples were analyzed on a FACSCanto (BD Biosciences), and the data were analyzed using FlowJo (TreeStar, Inc., Ashland, OR)
Cytokine assays
Splenocytes were harvested and stimulated with PK136 (anti-NK1.1) monoclonal antibody essentially as previously described (7). Briefly, 6-well tissue culture-treated plates were coated with 2 µg or 5 µg purified PK136 in 1 mL PBS. 107 naïve splenocytes were added to the washed plates and incubated at 37 C and 5% CO2 for 1 hour, and then further incubated in the presence of brefeldin A (GolgiPlug, BD Biosciences) for an additional 7 hours. IFNγ was detected by intracellular cytokine staining and flow cytometry as described previously (23).
Ly49A+ LAK cell preparation
Splenocytes were harvested from H2Dd-transgenic Kb−/− Db−/− mice, and nylon wool column non-adherent cells were isolated and cultured in R10 media supplemented with 800 U/mL IL-2. On day 6, Ly49A+ and Ly49A− LAK cells were isolated as previously described (21). Cells were used on Day 9. Ly49A+ LAK cell purity (Ly49A+ NK1.1+ CD3−) ranged from 79.1 to 93.4% (mean ± SD: 87.3 ± 5.5%), and Ly49A− LAK cell purity (Ly49A− NK1.1+ CD3−) ranged from 88.7 to 95.5% (mean ± SD: 92.6 ± 2.3%).
Cytotoxicity assays
Four-hour 51Cr-release assays were performed according to standard protocols using Day 9 Ly49A+ or Ly49A− LAK cells as effectors. Con A blast target cells were produced by culturing MHC-congenic splenocytes in R10 plus 6 µg/mL concanavalin A for two days.
Ly49A tetramer synthesis and binding assays
Recombinant soluble BirA-tagged Ly49A monomers were synthesized, purified, and refolded as previously described from a construct generously provided by N. Matsumoto (University of Tokyo, Tokyo, Japan) (18). The monomers were tetramerized with APC-conjugated streptavidin (BD Biosciences) immediately prior to cell staining.
Statistical analysis
Statistical calculations were performed using GraphPad Prism software (Treestar, Inc.). Statistical significance of differences between two groups was calculated using the unpaired two-tailed t-test. Correlations between groups of values were analyzed using two-tailed correlation regression.
Results
Licensing of Ly49A+ NK cells in MHC-congenic mice
The licensing status of NK cells must be determined functionally, as no molecular marker of licensed NK cells has yet been identified. Herein, we used a target cell-free system of ex vivo stimulation of naïve splenocytes with immobilized monoclonal antibodies against NK1.1, an activation receptor expressed on all NK cells of mice with the C57BL/6 and C57BL/10 backgrounds (23). Stimulation through NK1.1 allowed us to compare the activation of individual NK cells through a universally expressed NK cell activation receptor, unlike target cell-based stimulation assays, which activate NK cells through multiple receptors and pathways that are incompletely defined and may differ from NK cell to NK cell. Furthermore, NK1.1 expression was not affected by MHC haplotype (data not shown). In the ex vivo NK1.1 stimulation assay, a higher frequency of licensed NK cells produce IFNγ, whereas few unlicensed NK cells produce IFNγ (7).
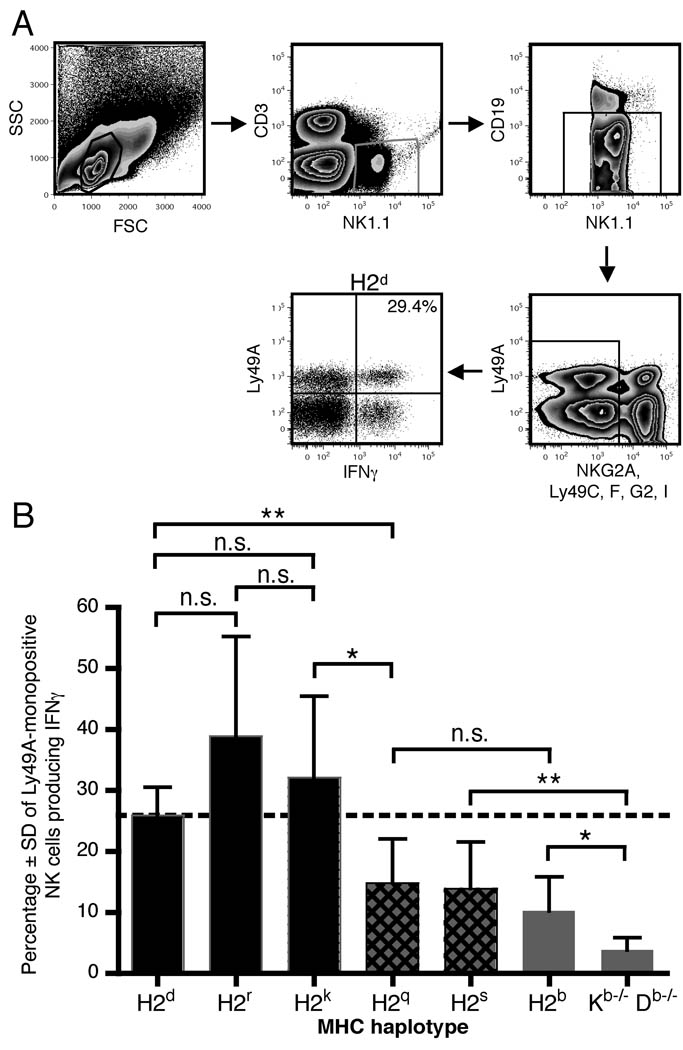
IFNγ production by bulk Ly49A+ NK cells as well as Ly49A-monopositive (Ly49A+ C− F− G2− I− NKG2A−) NK cells from six MHC-congenic strains and MHC class I-deficient (Kb−/− Db−/−) mice was measured by intracellular cytokine staining and flow cytometry (Fig. 1, supplemental Fig. 1A, and supplementary table). (For comparison, IFNγ production by the corresponding NK cell populations lacking Ly49A in addition to other inhibitory Ly49 and NKG2A receptors is shown in supplemental Fig. 1B.) Three MHC haplotypes (H2q, r, s) were included in which the function of Ly49A on NK cells had not previously been analyzed. Ly49A+ NK cells from H2r mice produced IFNγ at high frequencies, suggesting that this haplotype encodes an as-yet unidentified MHC molecule(s) able to engage Ly49A. In contrast, Ly49A+ NK cells of the H2q and H2s MHC haplotypes produced IFNγ at lower frequencies, indicating weak licensing. In H2b mice, which express H2Db, a putative weak ligand of Ly49A, Ly49A+ NK cells produced IFNγ at a similarly low frequency, confirming that the H2b haplotype poorly licenses Ly49A+ NK cells (15). As shown previously (7), NK1.1 crosslinking leads to a high frequency of IFNγ production by Ly49A+ NK cells from H2d mice, which express the Ly49A ligand H2Dd, but by very few Ly49A+ NK cells from MHC class I-deficient mice. NK cells from H2k mice, which express H2Dk, a known ligand for Ly49A, also produced IFNγ at a high frequency (24, 25).
Figure 1. IFNγ production by Ly49A-monopositive NK cells varies with MHC haplotype.
(A) Representative gating scheme for flow cytometric analysis of intracellular IFNγ production by Ly49-Amonopositive NK cells (Ly49A+ NK1.1+ CD3− CD19− NKG2A− Ly49C− Ly49F− Ly49G2− Ly49I−). The number in the final dot plot represents the percent IFNγ+ cells among the Ly49A+ population. (B) Average frequency ± SD of IFNγ production by naïve Ly49A-monopositive NK cells (Ly49A+ NK1.1+ CD3− CD19− NKG2A− Ly49C− Ly49F− Ly49I−; Ly49G2+ NK cells were also gated out in two of three experiments) incubated with plate-bound anti-NK1.1 antibody (5 µg PK136). The dotted line indicates the mean IFNγ production frequency of Ly49A+ NK cells of the H2d haplotype, which is known to strongly license Ly49A+ NK cells. N=6 or 7 per group, pooled results from three independent experiments. See Materials and Methods for antibody clones used. For each MHC haplotype, the data are shown in the same rank order in Figs. 1, 3, 5, and supplemental Figs 1 and 2, according to the MFI of anti-Ly49A staining depicted in Fig. 3B. Numerical values for the data shown in these figures is displayed in the Table. *: p<0.05; **: p<0.01.
The dotted lines in Fig. 1B and supplemental Fig. 1A indicate the mean IFNγ production by Ly49A+ NK cells from mice of the H2d haplotype. As H2Dd is known to clearly license Ly49A+ NK cells, MHC haplotypes with IFNγ production frequencies above this level can be considered strongly licensing MHC haplotypes for Ly49A. Three MHC haplotypes, H2q, H2s, and H2b, fall below this line but exhibit stronger IFNγ responses by Ly49A+ NK cells than MHC class I-deficient cells. The low level of licensing of Ly49A+ cells of the H2b haplotype likely represents the same weak licensing impact observed by Brodin et al in mice expressing H2Db either alone or in combination with H2Kb (15). By extension, the H2s and H2q MHC haplotypes also offer weak licensing environments to Ly49A+ NK cells.
Notably, the Ly49A+ NK cell populations of the H2q, H2s, and H2b MHC haplotypes do not produce IFNγ at a higher frequency than the corresponding Ly49A− populations (Fig. 1B and supplemental Fig. 1B). Conventionally, this would argue against a licensing impact of Ly49A in these MHC haplotypes. However, recent work indicates that unengaged Ly49A receptors actually suppress NK cell responsiveness to stimulation (26). Our observations support this view, as Ly49A-monopositive NK cells from MHC class I-deficient mice produced IFNγ at a lower frequency than the corresponding Ly49- and NKG2A-negative population. Thus, our findings of similar IFNγ responses by Ly49A+ and Ly49A− populations of the H2q, H2s, and H2b haplotypes is consistent with the presence of a weak licensing interaction for Ly49A.
While the MHC-congenic strains used herein have a long history as tools for the study of lymphocyte biology, the potential for interference from incidental genetic contamination remains. Although we do not know of genetic contamination in the other MHC-congenic strains, the B10.RIII (H2r) strain contains a large MHC-donor RIII-derived region on chromosome 10 that correlates with increased susceptibility to models of arthritis, independent of the MHC locus (27, 28). To test whether this genetic contamination was responsible for the observed high frequency of IFNγ production by B10.RIII NK cells, we crossed B10.RIII mice with C57BL/6 mice to produce F2 hybrid mice that were homozygous for RIII-derived genetic segments on either chromosome 10 or at the MHC locus. Such mice were identified by genotyping of microsatellite markers. In the PK136 stimulation assay, Ly49A+ NK cells from mice of the H2r MHC haplotype but lacking the RIII-derived chromosome 10 region produced IFNγ at a similar frequency to mice encoding both RIII-derived regions (Fig. 2). In contrast, Ly49A+ NK cells from mice of the H2b MHC haplotype that contained the RIII-derived chromosome 10 segment were not licensed beyond the level of H2b mice lacking the RIII-derived region on chromosome 10. Thus, the licensing phenotype of Ly49A+ NK cells in B10.RIII mice is not affected by the genetic contamination on chromosome 10 of this strain.
Figure 2. IFNγ production by NK cells from B10.RIII mice is not influenced by a known region of genetic contamination.
IFNγ production by Ly49A+ NK cells from mice expressing RIII- or C57BL/6 (B6)-derived regions on chromosome 10 or at the MHC locus. B10.RIII mice were crossed with B6 mice, and the F2 pups were typed by microsatellite analysis to select for mice homozygous for the RIII- or B6-derived segments on chromosome 10 and chromosome 17, as indicated. Bars represent average frequency ± SD of IFNγ production by naïve Ly49A+ NK cells (NK1.1+ CD3− CD19−) incubated with plate-bound anti-NK1.1 antibody (2 µg PK136). N=9 per group. Pooled results from three independent experiments. *: p<0.05; ***: p<0.001
Correlations of NK cell licensing with measures of Ly49A-MHC class I interactions in cis and trans
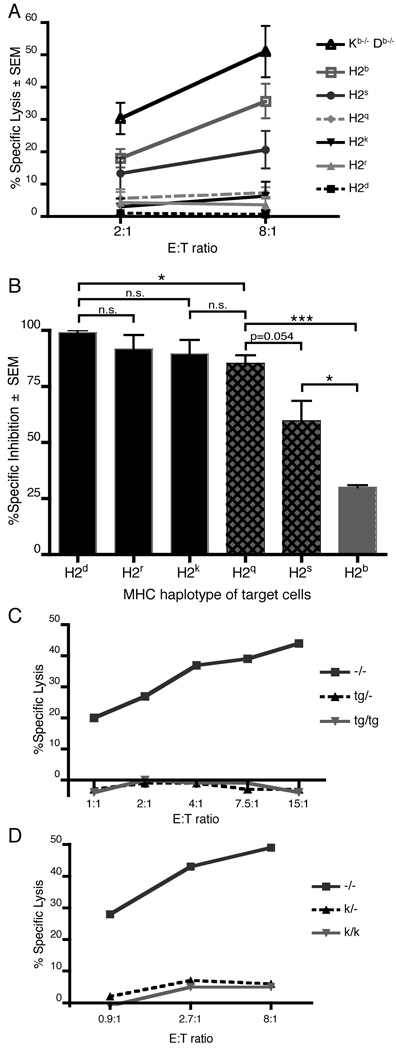
Using this panel of MHC-congenic mice that differentially license Ly49A+ NK cells, we next investigated whether the affinity of Ly49A engagement by these MHC haplotypes correlated with NK cell licensing. We measured relative Ly49A affinities by binding of soluble Ly49A tetramers to naïve splenocytes from MHC-congenic and Kb−/− Db−/− mice. As expected, MHC haplotypes that exhibited strong licensing of Ly49A+ NK cells bound more Ly49A tetramer than cells of the haplotypes with weak licensing (Fig. 3A). Most surprisingly, cells of the H2d haplotype bound much more Ly49A tetramer than any of the other MHC haplotypes, yet its level of licensing of Ly49A+ NK cells was not higher than H2r and H2k. These results suggest that licensing is saturated by a certain threshold of Ly49A engagement with self-MHC.
Figure 3. Correlations between licensing, soluble Ly49A tetramer binding, and putative cis binding of Ly49A.
(A) Naïve splenocytes from MHC-congenic and Kb−/− Db−/− mice were stained with soluble Ly49A tetramers. Mean fluorescence intensity (MFI) ± SEM is shown. N=3 for each group. Representative of three independent experiments. (B) Naïve splenocytes from MHC-congenic mice were stained for Ly49A (mAb A1), NK1.1, CD3, and CD19. MFI ± SEM of Ly49A staining of Ly49A+ NK cells (NK1.1+ CD3− CD19−) is shown. N=3 for each group. Representative of two independent experiments. (C) Correlation analysis of frequency of IFNγ production by Ly49A-monopositive NK cells from and Ly49A tetramer staining of MHC-congenic and MHC class I-deficient mice. For details on data, see Figs. 1B and 3A. (D) Correlation analysis of frequency of IFNγ production by and anti-Ly49A staining MFI of Ly49A-monopositive NK cells from MHC-congenic mice. For details on data, see Figs. 1B and 3B. *: p<0.05; **: p<0.01; ***: p<0.001.
Ly49A and MHC class I molecules can also interact in cis, i.e. on the surface of the same cell (29, 30). The extent of cis engagement can be measured indirectly by the MFI of anti-Ly49A monoclonal antibodies such as A1: cis engagements block binding of the antibody and result in a lower MFI (30). The MFI of anti-Ly49A staining of Ly49A+ NK cells from MHC-congenic mice produces a spectrum of values, with H2d, H2r, and H2k approaching saturation (Fig. 3B). Similar results were obtained with a second anti-Ly49A monoclonal antibody, JR9, that is partially blocked by cis engagement of Ly49A and MHC class I (supplemental Fig. 2) (30). With either antibody, the results suggest that cis interactions of Ly49A with various MHC haplotypes are analog in character because, like tetramer binding, nearly all pairwise comparisons of MHC haplotypes produce a statistically significant difference. However, the differences between the MHC haplotypes with the lowest Ly49A surface accessibility were very small in magnitude, suggesting that these haplotypes had reached saturation of cis binding.
Interestingly, putative cis engagements of Ly49A with MHC class I correlate better with strength of licensing than does Ly49A tetramer binding, a measure of trans interactions (R2 = 0.83 and 0.31, respectively) (Fig. 3C and D). In particular, NK cell licensing and cis engagement of Ly49A have similar saturation thresholds, suggesting that cis interactions of Ly49A with MHC class I is a strong determinant of licensing, as recently proposed by Chalifour et al for Ly49A and H2Dd and H2Dk (26). Curiously, H2q, which only weakly licenses Ly49A+ NK cells, exhibits statistically significant levels of Ly49A tetramer binding and cis engagement of Ly49A as compared to the other weakly licensing MHC haplotypes, H2s and H2b. This finding suggests that a threshold of Ly49A binding beyond that of H2q must be achieved for strong licensing to occur, such as that observed with H2k.
NK cell licensing is insensitive to gene dosage changes in MHC class I expression
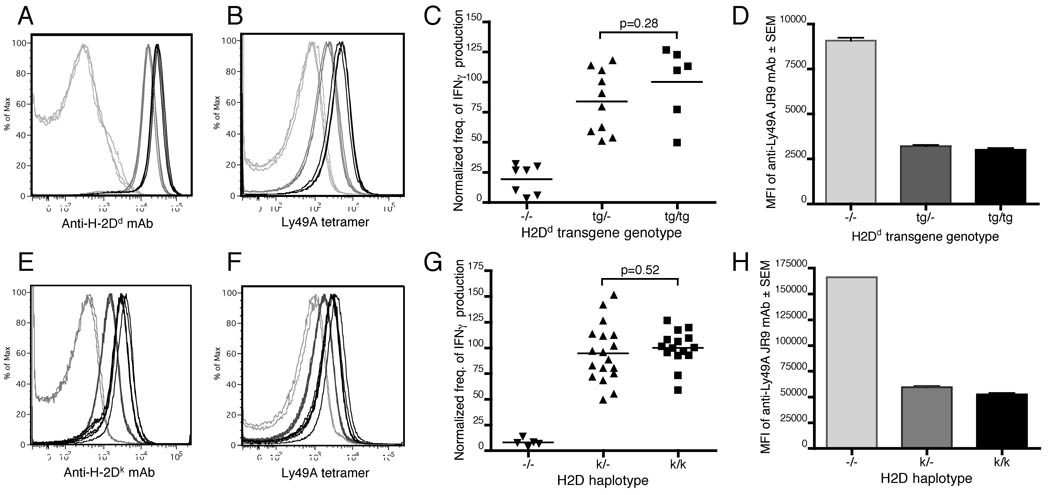
To further address the role of avidity of the Ly49A-MHC class I interaction in NK cell licensing, we used mice homozygous, hemizygous, or nullizygous for an H2Dd transgene (genomic Dd construct with Dd expression comparable to H2d mice) on an otherwise MHC class I-deficient background. Cells from mice homozygous for H2Dd (Dd tg/tg) express twice as much H2Dd as cells from hemizygous mice (Dd tg/−) (Fig. 4A). Accordingly, Dd tg/tg cells also bind more soluble Ly49A tetramer than Dd tg/− cells (Fig. 4B). However, the licensing of Ly49A+ NK cells from Dd tg/tg and Dd tg/− mice was not significantly different (Fig. 4C and supplemental Fig. 3A). These results indicate that saturation of NK cell licensing is maintained for high-affinity Ly49A-MHC class I interactions, even at lower avidities. Notably, Ly49A exhibited similar cis engagement in both Dd tg/tg and Dd tg/− mice (Fig. 4D), again demonstrating a strong correlation of cis engagement with strength of licensing.
Figure 4. Licensing of Ly49A+ NK cells is not affected by haploinsufficiency of the MHC class I ligand.
(A) H2Dd expression on splenocytes from mice homozygous (black), hemizygous (dark gray), or nullizygous (light gray) for a Dd transgene. Each histogram represents one mouse. Representative of four independent experiments. (B) Ly49A tetramer staining of splenocytes, histograms colored as in A, from a single experiment. (C) Frequency of IFNγ production by anti-NK1.1-stimulated (5 µg PK136) Ly49A+ NK cells from mice of the indicated Dd transgene genotype. Similar results were obtained when Ly49C, I, and G2+ cells were gated out of the analysis. Frequency was normalized to the average frequency of IFNγ production by Dd tg/tg Ly49A+ NK cells in each experiment. Each symbol represents one mouse, pooled from two independent experiments. (D) MFI ± SEM of anti-Ly49A staining (mAb JR9) on Ly49A+ NK cells from littermates of the indicated Dd transgene genotype. N=4 or 6 per group. Representative of three independent experiments. (E) H2Dk expression on splenocytes from mice homozygous (black), hemizygous (dark gray), or nullizygous (light gray) for the endogenous H2k class I genes. Each histogram represents one mouse. Representative of three independent experiments. (F) Ly49A tetramer staining of splenocytes, histograms colored as in E, from a single experiment. (G) Frequency of IFNγ production by anti-NK1.1-stimulated (5 µg PK136) Ly49A+ NK cells from mice of the indicated MHC class I genotype. Frequency was normalized to the average frequency of IFNγ production by H2k/k Ly49A+ NK cells in each experiment. Each symbol represents one mouse, pooled from three independent experiments. (H) MFI ± SEM of anti-Ly49A staining (mAb JR9) on Ly49A+ NK cells (NK1.1+ CD3− CD19−) from mice of the indicated MHC class I genotype. N=5 and 6 for the H2k/k and H2k/− groups, respectively. Results from a Kb−/− Db−/− mouse is shown for reference. Representative of three independent experiments.
Since cells of the H2d haplotype have a uniquely high affinity and/or avidity for Ly49A among the MHC haplotypes studied herein and we used a transgenic system for regulating H2Dd expression, we examined a different MHC class I ligand with a lower reactivity for Ly49A in MHC-congenic mice. We crossed B10.BR (H2k/k) mice with Kb−/− Db−/− (H2−/−) mice to create F1 hybrid mice hemizygous for H2Dk (H2k/−). As expected, H2k/k cells express twice the level of H2Dk as H2k/− cells and also bind more soluble Ly49A tetramer (Fig. 4E and F). However, there was no consistent statistically significant difference in licensing among Ly49A+ NK cells (Fig. 4G and supplemental Fig. 3B). Furthermore, cis engagement of Ly49A was similar in both H2k/k and H2k/− Ly49A+ NK cells (Fig. 4H), indicating that, as in the MHC-congenic system above, cis engagement of Ly49A has the same saturation threshold as NK cell licensing. Together, these results indicate that haploinsufficiency of self-MHC has little or no effect on NK cell licensing, further supporting a low saturation threshold for NK cell licensing.
Different thresholds of licensing and effector inhibition mediated by Ly49A
Ly49A has two functions on NK cells: (1) licensing, and (2) inhibition of effector function, i.e., the inhibition of target cell cytotoxicity in the presence of a target cell ligand for Ly49A. To determine if effector inhibition correlates with MHC haplotype in the same way as licensing, we assessed inhibition of Ly49A+ effector cells by Con A-activated blasts from MHC-congenic mice. We considered using naïve NK cells and/or CD107a-based degranulation assays, but we determined that these approaches are not robust enough against Con A blast target cells to provide meaningful results. For these reasons, we chose to assess effector inhibition using traditional 51Cr-release cytotoxicity assays. Specifically, Ly49A+ lymphokine-activated killer cells (LAK) cells from H2Dd-transgenic Kb−/− Db−/− mice (i.e., a licensing MHC class I context for Ly49A+ NK cells) were used in 51Cr-release assays with MHC-congenic Con A-activated splenocytes as target cells (Fig. 5A). To more easily visualize the data, we converted the percent specific lysis data to percent MHC-specific inhibition, normalizing to MHC class I-deficient cells (Fig. 5B). This inhibition is Ly49A dependent, since Ly49A− LAKs failed to produce a similar pattern of inhibition and addition of a blocking antibody against Ly49A restored Ly49A+ LAK killing of the MHC-congenic target cells (supplemental Fig. 4).
Figure 5. Ly49A-mediated inhibition of effector function is more sensitive than NK cell licensing.
(A) Aggregate results of three independent 51Cr-release assays of Ly49A+ LAK cells from H2Dd-transgenic Kb−/− Db−/− mice killing Con A-activated MHC-congenic splenocytes. Each condition was tested in triplicate in each experiment. (B) Specific inhibition ± SEM of killing of MHC-congenic Con A blasts by Ly49A+ LAK cells at an E:T of 8:1. Specific inhibition = 100 × (Specific lysis of Kb−/− Db−/− Con A blasts – Specific lysis of MHC-congenic Con A blasts) / Specific lysis of Kb−/− Db−/− Con A blasts. Aggregate results from three independent experiments in which each condition was assayed in triplicate. (C) Specific lysis of Con A blasts homozygous, hemizygous, or nullizygous for a Dd transgene by Ly49A+ LAK cells. Representative of two independent experiments. (D) Specific lysis of Con A blasts homozygous, hemizygous, or nullizygous for endogenous H2Kk and H2Dk genes by Ly49A+ LAK cells. Representative of two independent experiments. *: p<0.05; ***: p<0.001.
Interestingly, the relative levels of Ly49A-mediated effector inhibition by different MHC haplotypes produced the same hierarchy NK cell licensing (Fig. 5). However we also observed some differences from NK cell licensing. For example, the H2q haplotype robustly inhibited LAK killing yet had only a weak licensing effect (Fig. 1). Similarly, the H2s haplotype, which showed minimal licensing of Ly49A+ NK cells, still inhibited Ly49A+ LAK killing significantly more than the H2b MHC haplotype (Fig. 5B). These apparently different thresholds suggest that NK cell licensing and effector inhibition utilize separate molecular signaling pathways or, if mediated by the same signaling cascades, are controlled by differential threshold mechanisms. In addition, target cells hemizygous for a MHC class I ligand of Ly49A inhibited killing by Ly49A+ LAKs as well as their homozygous counterparts (Fig. 5C and D), indicating that effector inhibition, like licensing, is saturated by relatively low MHC class I avidity.
Discussion
This study represents the first test of the quantitative model of NK cell licensing in an array of MHC haplotype environments, other than H2b and H2d. Specifically, the experiments presented herein provide insight into the quantitative versus qualitative (“on-off”) nature of licensing of NK cells through a single Ly49 receptor. Moreover, the data provide evidence for Ly49A specificity for other MHC alleles.
Since NK cells may express more than one inhibitory receptor that may contribute to licensing, we need to consider the possibility that additional Ly49 receptors could influence the effects of the different MHC alleles on Ly49A+ NK cells. However, such effects are likely to be limited for several reasons. First, other Ly49 family members are generally expressed on individual NK cells in a stochastic manner such that only a fraction of Ly49A+ NK cells express another specific Ly49 receptor. Second, in C57BL/6 mice which have the same Ly49 gene complex as C57BL/10 mice (31), there are five potential inhibitory Ly49 receptors (Ly49A, C, F, G, I) expressed on adult NK cells. Other Ly49 genes either encode activation receptors (Ly49D, H), are primarily expressed on fetal NK cells (Ly49E) (32), are pseudogenes (Ly49J, K, L, N) (33), or expressed on non-NK cells (Ly49Q) (34). When we studied monopositive-Ly49A+ NK cells, by gating out all cells bearing other Ly49 receptors known to be expressed on adult NK cells (Ly49C, F, G, I) as well as NKG2A, we obtained data that are essentially indistinguishable from those without deliberate gating out of Ly49C/F/G/I/NKG2A+ cells. Third, MHC-specificity for Ly49A was also assessed by killing assays against Con A blasts from the same MHC-congenic mice. In these studies, we demonstrated that Ly49A has essentially the same hierarchy of MHC specificity as licensing of Ly49A+ NK cells. In the killing assays, Ly49A specificity was also verified by anti-Ly49A blocking antibody, which reversed the Ly49A-mediated, MHC-dependent inhibition. Finally, Ly49A tetramer binding to splenocytes from the same MHC-congenic mice also provided essentially the same hierarchy as the licensing effects. Therefore, the data indicate that a particular NK cell receptor exhibits different potencies on licensing, depending on the MHC class I context, and that NK cell licensing through a given receptor exhibits a low threshold for saturation.
Taken together, our findings are largely consistent with a quantitative (rheostat) model of NK cell licensing, with several new and important principles. First, NK cell licensing is saturated by a relatively low apparent “affinity” and “avidity” of Ly49A engagement, as determined by Ly49A tetramer binding. This is best exemplified by the H2d haplotype, whose uniquely high level of Ly49A engagement did not translate into stronger licensing compared to MHC haplotypes with more moderate binding to Ly49A. One possibility is that the H2d mice (B10.D2) have some unidentified genetic defect that dampens NK cell potency independent of NK cell licensing. However, H2Dd transgenic mice (Dd+/+) on a Kb−/− Db−/− C57BL/6 background bind Ly49A tetramers nearly as well as mice expressing the endogenous H2d/d locus and yet did not exhibit levels of licensing beyond that of B10.D2 mice. In addition, changes in MHC gene dosage, even of the moderate-binding MHC haplotype H2k, did not affect strength of licensing, indicating that the saturation threshold of Ly49A-mediated licensing encompasses low-avidity interactions.
Previous data regarding a saturation threshold for NK cell licensing were inconclusive when the effects of H2b and H2d alleles were studied. Joncker et al reported increased NK cell potency with up to three simultaneously expressed self-specific inhibitory receptors (16). However, Brodin et al observed saturation of NK cell licensing by NK cells from H2b mice that expressed three self-specific receptors: these NK cells were not more potent than NK cells expressing two of the three receptors (15). On the other hand, the inclusion of two additional receptors, i.e. the very small population of NK cells that expresses five inhibitory receptors, produced a boosted response to stimulation. In contrast, our data with additional MHC alleles demonstrate that licensing through Ly49A has an “affinity”-based saturation threshold when examined in different MHC contexts. Additional studies are needed to determine if this phenomenon is generalizable to other MHC class I-specific inhibitory receptors, which have different MHC specificities from Ly49A.
Interestingly, Ly49A surface accessibility by anti-Ly49A monoclonal antibodies, a surrogate measure of cis interactions of Ly49A and MHC class I (30), exhibited a much stronger correlation with NK cell licensing than did measures of trans interactions. Importantly, the saturation pattern of putative Ly49A cis engagements coincided with that of licensing of Ly49A+ NK cells. We cannot formally rule out that the observed pattern of anti-Ly49A antibody binding is due to actual differences in Ly49A surface expression. However, when interpreted as measures of cis engagements, as previously described, these findings are consistent with a recent study by Chalifour et al, which reported that cis interactions, but not trans interactions, of Ly49A and H2Dd or H2Dk are required for licensing (26). Indeed, our data provide additional evidence for an important role for cis interactions in licensing, and extend previous observations to additional MHC haplotypes.
The low threshold for saturation of cis interactions may be due to differences in relative surface expression levels of MHC class I and Ly49A. In studies of MHC class I (H2Dd) transfer from donor cells to Ly49A+ NK cells, H2Dd levels reached a maximum of 16 to 30% of endogenous levels (35, 36). These data suggest that endogenous MHC class I molecules outnumber Ly49A receptors by several-fold on the surface of NK cells. For cis engagement with Ly49A, high avidity may compensate for the moderate affinity of some MHC class I molecules for Ly49A such that even moderate Ly49A ligands induce full saturation of cis engagement. Furthermore, if cis binding determines licensing and cis binding is overwhelmed by the number of MHC class I molecules, then decreasing the number of MHC class I molecules while maintaining the same affinity should not significantly affect licensing. Indeed, these are the results observed in Ly49A+ NK cells from mice expressing one versus two copies of an H2Dd transgene or endogenous H2k class I locus. Future quantitative studies are needed to confirm this hypothesis.
In humans, genotyping analysis reveals an epidemiological association between disease outcomes and certain KIR and HLA allele pairs that can potentially be explained by licensing (37). For example, resolution of hepatitis C viral infection is associated with homozygosity of genotypes for both KIR2DL3 and its HLA ligand (38). However, KIR2DL3 homozygous individuals with HLA ligand heterozygosity did not show a protective phenotype, consistent with in vitro licensing assays of KIR3DL1+ NK cells from HLA ligand heterozygous individuals (10). If universal principles of licensing were solely responsible for these observations, we would expect poor licensing of Ly49A+ NK cells in mice hemizygous for its MHC ligand. However, Ly49A+ NK cells were equally licensed in hemizygous and homozygous mice. While it remains possible that other inhibitory receptor-ligand pairs may yield gene-dosage effects on licensing or that human NK cells behave differently, our data clearly indicate that for certain receptor-ligand pairs in mice, a single gene dose of MHC class I is sufficient for full licensing.
The specificity of Ly49A has been previously determined by several approaches (reviewed in (39)), including functional studies where transfection of a particular MHC class I allele into a target cell results in inhibition of killing by Ly49A+ IL-2-activated NK cells. Inhibition is reversed by monoclonal antibodies for either Ly49A or the MHC class I allele. Corroborating data have come from cell-cell adhesion assays where Ly49A+ transfectants bind to cells transfected with a specific MHC allele. Again, adhesion is blocked by antibodies against Ly49A or the MHC allele. Similarly, Ly49A or MHC class I tetramers bind cells expressing the cognate partner. Although all of these assays have their strengths and weaknesses, it is probably most important to note that Ly49A binding to MHC class I is through a site under the peptide binding domain, involving α1, α2, and α3 of the heavy chain as well as β2m (20). The β2m residues are species-specific such that human β2m cannot be bound by Ly49A (4, 20), which affects interpretation of prior data with tetramers of MHC class I refolded with human β2m. Moreover, binding studies using cells overexpressing either cognate partner by transfection may not fully recapitulate physiological binding of receptors and ligands expressed at native levels. With these caveats, it should be noted that we provide here information on new ligands for Ly49A based on more physiological assays of licensing and inhibition of cytotoxicity. While we have not yet identified the exact MHC class I allele(s) responsible for Ly49A interaction in the H2r, H2q, and H2s haplotypes, these considerations do reinforce the notion that Ly49A specificities may need to be considered in physiological contexts and that other assays of MHC specificities could be misleading.
Another interesting finding relates licensing to the function of inhibitory receptors like Ly49A in inhibition of effector functions, such as cytotoxicity (21, 40). Interestingly, Ly49A-dependent effector inhibition is even more sensitive to MHC class I engagement than is Ly49A-mediated NK cell licensing. This is best demonstrated by H2q, a MHC haplotype that can strongly inhibit cytotoxicity by Ly49A+ LAK cells yet produces only poorly licensed NK cells. In other words, the threshold of MHC class I engagement required for inhibition of effector function is lower than for NK cell licensing. Some caution should be exercised in comparing assays that use different output measures (i.e., cytokine production versus cytotoxicity). It is also possible that IL-2 activation affects the stimulation threshold of the NK cells, though similar effects likely occur during the pro-inflammatory phase of initial immune responses. Nonetheless, these findings reveal an interesting relationship between licensing and NK cell effector function.
The apparent differences in activation thresholds of NK cell licensing and effector function are reminiscent of T cell tolerance, in which the signal threshold for negative selection in the thymus is lower than for activation in the periphery (41–44). In this way, all T cells that have any chance of reacting to self-antigens in the periphery are deleted. A similar safeguard may govern the licensing of NK cells such that only NK cells capable of achieving a strong interaction with self-MHC become licensed. A more sensitive threshold of inhibition in the periphery ensures that licensed NK cells maintain tolerance to healthy self cells.
The difference in threshold of NK cell licensing and effector inhibition has important implications for our understanding of the signaling cascades that mediate these events. While Ly49A-mediated effector inhibition relies mainly upon signaling initiated by SHP1, the precise signaling pathway required for licensing remains unclear (45–47). However, the differences in threshold support the view that different signaling events or outcomes may underlie these two functions of Ly49A. While the ITIM is known to be required for both NK cell licensing and effector inhibition, it is increasingly evident that ITIMs can initiate a number of diverse signaling cascades (e.g., (48, 49)). Alternatively, similar molecular signaling mediators may be used but with a differentially regulated threshold mechanism. Both possibilities lead to further questions of how such a differential signaling or threshold mechanism might be regulated. In teleological terms, how does an NK cell know the difference between licensing and effector inhibition? These issues warrant further study.
Supplementary Material
Table.
Summary of phenotypes of MHC-congenic mice
| MHC haplotype |
% IFNγ+ ± SD of Ly49A+ NK cells1 |
% IFNγ+ ± SD of Ly49A- monopositive NK cells1 |
Ly49A tetramer MFI ± SEM |
A1 (anti-Ly49A) MFI ± SEM2 |
JR9 (anti-Ly49A) MFI ± SEM2 |
% Specific Inhibition of Ly49A+ LAKs ± SEM3 |
|---|---|---|---|---|---|---|
| H2d | 20.9 ± 6.0 | 25.9 ± 4.6 | 8232 ± 120 | 3601 ± 46 | 29978 ± 152 | 99.0 ± 1.0 |
| H2r | 29.2 ± 9.8 | 38.9 ± 16.3 | 3157 ± 22 | 4149 ± 71 | 22917 ± 1263 | 91.7 ± 6.2 |
| H2k | 24.3 ± 8.3 | 32.1 ± 13.3 | 2272 ± 193 | 4545 ± 296 | 23263 ± 916 | 89.5 ± 6.2 |
| H2q | 10.7 ± 5.3 | 14.8 ± 7.2 | 849 ± 5 | 10046 ± 593 | 41294 ± 534 | 85.3 ± 3.5 |
| H2s | 9.3 ± 5.4 | 13.9 ± 7.6 | 425 ± 8 | 16943 ± 610 | 63559 ± 1601 | 59.6 ± 8.8 |
| H2b | 8.2 ± 5.1 | 10.2 ± 5.5 | 332 ± 16 | 16788 ± 540 | 62624 ± 660 | 29.9 ± 0.9 |
| Kb−/− Db−/− | 2.4 ± 0.95 | 3.8 ± 2.0 | 363 ± 7 | 23194 ± 1348 | 83647 ± 5969 | n.a. |
Upon anti-NK1.1 (PK136) stimulation. See Fig. 1B and supplementary Fig. 1A for details.
Gated on Ly49A+ NK cells.
E:T ratio of 8:1. % specific inhibition = 100 × (specific lysis of Kb−/− Db−/− ConA blasts – specific lysis of MHC-congenic ConA blasts)/specific lysis of Kb−/− Db−/− ConA blasts.
n.a. Not applicable
Acknowledgements
We thank Danielle Atibalentja for helpful discussions of T cell tolerance, and Joseph Wahle and Megan Cooper for critical review of the manuscript. We also thank Nana Owusu-Boaitey for technical assistance.
Footnotes
Publisher's Disclaimer: "This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at a.www.jimmunol.org."
Work in the Yokoyama laboratory is supported by the Howard Hughes Medical Institute, Barnes Jewish Hospital Foundation, and grants from the National Institutes of Health. Microsatellite typing was supported by a Rheumatic Diseases Core Center grant from NIAMS.
References
- 1.Ljunggren HG, Karre K. In search of the 'missing self': MHC molecules and NK cell recognition. Immunol. Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. NK cell recognition. Annu. Rev. of Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 3.Hanke T, Takizawa H, McMahon CW, Busch DH, Pamer EG, Miller JD, Altman JD, Liu Y, Cado D, Lemonnier FA, Bjorkman PJ, Raulet DH. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 1999;11:67–77. doi: 10.1016/s1074-7613(00)80082-5. [DOI] [PubMed] [Google Scholar]
- 4.Michaelsson J, Achour A, Rolle A, Karre K. MHC class I recognition by NK receptors in the Ly49 family is strongly influenced by the beta(2)-microglobulin subunit. J. Immunol. 2001;166:7327–7334. doi: 10.4049/jimmunol.166.12.7327. [DOI] [PubMed] [Google Scholar]
- 5.Tormo J, Natarajan K, Margulies DH, Mariuzza RA. Crystal structure of a lectin-like natural killer cell receptor bound to its MHC class I ligand. Nature. 1999;402:623–631. doi: 10.1038/45170. [DOI] [PubMed] [Google Scholar]
- 6.Hoglund P, Ohlen C, Carbone E, Franksson L, Ljunggren HG, Latour A, Koller B, Kärre K. Recognition of beta 2-microglobulin- negative (beta 2m-) T-cell blasts by natural killer cells from normal but not from beta 2m- mice: nonresponsiveness controlled by beta 2m- bone marrow in chimeric mice. Proc. Natl. Acad. Sci. USA. 1991;88:10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama WM, Kim S. How do natural killer cells find self to achieve tolerance? Immunity. 2006;24:249–257. doi: 10.1016/j.immuni.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Kim S, Sunwoo JB, Yang L, Choi T, Song YJ, French AR, Vlahiotis A, Piccirillo JF, Cella M, Colonna M, Mohanakumar T, Hsu KC, Dupont B, Yokoyama WM. HLA alleles determine differences in human natural killer cell responsiveness and potency. Proc. Natl. Acad. Sci. USA. 2008;105:3053–3058. doi: 10.1073/pnas.0712229105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu J, Heller G, Chewning J, Kim S, Yokoyama WM, Hsu KC. Hierarchy of the human natural killer cell response is determined by class and quantity of inhibitory receptors for self-HLA-B and HLA-C ligands. J. Immunol. 2007;179:5977–5989. doi: 10.4049/jimmunol.179.9.5977. [DOI] [PubMed] [Google Scholar]
- 12.Carrington M, Wang S, Martin MP, Gao X, Schiffman M, Cheng J, Herrero R, Rodriguez AC, Kurman R, Mortel R, Schwartz P, Glass A, Hildesheim A. Hierarchy of resistance to cervical neoplasia mediated by combinations of killer immunoglobulin-like receptor and human leukocyte antigen loci. J Exp Med. 2005;201:1069–1075. doi: 10.1084/jem.20042158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulet S, Kleyman M, Kim JY, Kamya P, Sharafi S, Simic N, Bruneau J, Routy JP, Tsoukas CM, Bernard NF. A combined genotype of KIR3DL1 high expressing alleles and HLA-B*57 is associated with a reduced risk of HIV infection. AIDS (London, England) 2008;22:1487–1491. doi: 10.1097/QAD.0b013e3282ffde7e. [DOI] [PubMed] [Google Scholar]
- 14.Sentman CL, Barber MA, Barber A, Zhang T. NK cell receptors as tools in cancer immunotherapy. Adv. Cancer Res. 2006;95:249–292. doi: 10.1016/S0065-230X(06)95007-6. [DOI] [PubMed] [Google Scholar]
- 15.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 16.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J. Immunol. 2009;182:4572–4580. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yawata M, Yawata N, Draghi M, Partheniou F, Little AM, Parham P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK cell repertoires towards a balance of missing-self response. Blood. 2008;112:2369–2380. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto N, Tajima K, Mitsuki M, Yamamoto K. H-2 allele specificity of the NK cell C-type lectin-like MHC class I receptor Ly49A visualized by soluble Ly49A tetramer. Int. Immunol. 2001;13:615–623. doi: 10.1093/intimm/13.5.615. [DOI] [PubMed] [Google Scholar]
- 19.Belanger S, Tai LH, Anderson SK, Makrigiannis AP. Ly49 cluster sequence analysis in a mouse model of diabetes: an expanded repertoire of activating receptors in the NOD genome. Genes Immun. 2008;9:509–521. doi: 10.1038/gene.2008.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto N, Mitsuki M, Tajima K, Yokoyama WM, Yamamoto K. The functional binding site for the C-type lectin-like natural killer cell receptor Ly49A spans three domains of its major histocompatibility complex class I ligand. J Exp. Med. 2001;193:147–158. doi: 10.1084/jem.193.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karlhofer FM, Ribaudo RK, Yokoyama WM. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 1992;358:66–70. doi: 10.1038/358066a0. [DOI] [PubMed] [Google Scholar]
- 22.Mehta IK, Wang J, Roland J, Margulies DH, Yokoyama WM. Ly49A allelic variation and MHC class I specificity. Immunogenetics. 2001;53:572–583. doi: 10.1007/s002510100355. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Iizuka K, Kang HS, Dokun A, French AR, Greco S, Yokoyama WM. In vivo developmental stages in murine natural killer cell maturation. Nat. Immunol. 2002;3:523–528. doi: 10.1038/ni796. [DOI] [PubMed] [Google Scholar]
- 24.Deng L, Mariuzza RA. Structural basis for recognition of MHC and MHC-like ligands by natural killer cell receptors. Semin. Immunol. 2006;18:159–166. doi: 10.1016/j.smim.2006.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Natarajan K, Dimasi N, Wang J, Margulies DH, Mariuzza RA. MHC class I recognition by Ly49 natural killer cell receptors. Mol. Immunol. 2002;38:1023–1027. doi: 10.1016/s0161-5890(02)00031-7. [DOI] [PubMed] [Google Scholar]
- 26.Chalifour A, Scarpellino L, Back J, Brodin P, Devevre E, Gros F, Levy F, Leclercq G, Hoglund P, Beermann F, Held W. A role for cis interaction between the inhibitory Ly49A receptor and MHC class I for natural killer cell education. Immunity. 2009;30:337–347. doi: 10.1016/j.immuni.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Dong P, Hood L, McIndoe RA. Detection of a large RIII-derived chromosomal segment on chromosome 10 in the H-2 congenic strain B10.RIII(71NS)/Sn. Genomics. 1996;31:266–269. doi: 10.1006/geno.1996.0047. [DOI] [PubMed] [Google Scholar]
- 28.Nandakumar KS, Holmdahl R. A genetic contamination in MHC-congenic mouse strains reveals a locus on chromosome 10 that determines autoimmunity and arthritis susceptibility. Eur. J. Immunol. 2005;35:1275–1282. doi: 10.1002/eji.200425925. [DOI] [PubMed] [Google Scholar]
- 29.Andersson KE, Williams GS, Davis DM, Hoglund P. Quantifying the reduction in accessibility of the inhibitory NK cell receptor Ly49A caused by binding MHC class I proteins in cis. Eur. J. Immunol. 2007;37:516–527. doi: 10.1002/eji.200636693. [DOI] [PubMed] [Google Scholar]
- 30.Doucey MA, Scarpellino L, Zimmer J, Guillaume P, Luescher IF, Bron C, Held W. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat. Immunol. 2004;5:328–336. doi: 10.1038/ni1043. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama WM, Jacobs LB, Kanagawa O, Shevach EM, Cohen DI. A murine T lymphocyte antigen belongs to a supergene family of type II integral membrane proteins. J. Immunol. 1989;143:1379–1386. [PubMed] [Google Scholar]
- 32.Van Beneden K, Stevenaert F, De Creus A, Debacker V, De Boever J, Plum J, Leclercq G. Expression of Ly49E and CD94/NKG2 on fetal and adult NK cells. J. Immunol. 2001;166:4302–4311. doi: 10.4049/jimmunol.166.7.4302. [DOI] [PubMed] [Google Scholar]
- 33.McQueen KL, Lohwasser S, Takei F, Mager DL. Expression analysis of new Ly49 genes: most transcripts of Ly49j lack the transmembrane domain. Immunogenetics. 1999;49:685–691. doi: 10.1007/s002510050665. [DOI] [PubMed] [Google Scholar]
- 34.Toyama-Sorimachi N, Tsujimura Y, Maruya M, Onoda A, Kubota T, Koyasu S, Inaba K, Karasuyama H. Ly49Q, a member of the Ly49 family that is selectively expressed on myeloid lineage cells and involved in regulation of cytoskeletal architecture. Proc. Natl. Acad. Sci. USA. 2004;101:1016–1021. doi: 10.1073/pnas.0305400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sjostrom A, Eriksson M, Cerboni C, Johansson MH, Sentman CL, Karre K, Hoglund P. Acquisition of external major histocompatibility complex class I molecules by natural killer cells expressing inhibitory ly49 receptors. J. Exp. Med. 2001;194:1519–1530. doi: 10.1084/jem.194.10.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimmer J, Ioannidis V, Held W. H-2D ligand expression by Ly49A+ natural killer (NK) cells precludes ligand uptake from environmental cells: Implications for NK cell function. J. Exp. Med. 2001;194:1531–1539. doi: 10.1084/jem.194.10.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulkarni S, Martin MP, Carrington M. The Yin and Yang of HLA and KIR in human disease. Semin. Immunol. 2008;20:343–352. doi: 10.1016/j.smim.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O'Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 39.Yokoyama WM. Chapter 17. Natural killer cells. In: Paul WE, editor. Fundamental Immunology. 6th ed. New York: Lippincott-Raven; 2008. pp. 483–517. [Google Scholar]
- 40.Kim S, Yokoyama WM. NK cell granule exocytosis and cytokine production inhibited by Ly-49A engagement. Cell. Immunol. 1998;183:106–112. doi: 10.1006/cimm.1998.1252. [DOI] [PubMed] [Google Scholar]
- 41.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 42.Davey GM, Schober SL, Endrizzi BT, Dutcher AK, Jameson SC, Hogquist KA. Preselection thymocytes are more sensitive to T cell receptor stimulation than mature T cells. J. Exp. Med. 1998;188:1867–1874. doi: 10.1084/jem.188.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pircher H, Rohrer UH, Moskophidis D, Zinkernagel RM, Hengartner H. Lower receptor avidity required for thymic clonal deletion than for effector T-cell function. Nature. 1991;351:482–485. doi: 10.1038/351482a0. [DOI] [PubMed] [Google Scholar]
- 44.Lucas B, Stefanova I, Yasutomo K, Dautigny N, Germain RN. Divergent changes in the sensitivity of maturing T cells to structurally related ligands underlies formation of a useful T cell repertoire. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 45.Jonsson AH, Yokoyama WM. Natural killer cell tolerance: licensing and other mechanisms. Adv. Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 46.Long EO. Regulation of immune responses through inhibitory receptors. Annu. Rev. of Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura MC, Niemi EC, Fisher MJ, Shultz LD, Seaman WE, Ryan JC. Mouse Ly-49A interrupts early signaling events in natural killer cell cytotoxicity and functionally associates with the Shp-1 tyrosine phosphatase. J. Exp. Med. 1997;185:673–684. doi: 10.1084/jem.185.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peterson ME, Long EO. Inhibitory receptor signaling via tyrosine phosphorylation of the adapter CRK. Immunity. 2008;29:578–588. doi: 10.1016/j.immuni.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu MC, Su LL, Zou L, Liu Y, Wu N, Kong L, Zhuang ZH, Sun L, Liu HP, Hu JH, Li D, Strominger JL, Zang JW, Pei G, Ge BX. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat. Immunol. 2008;9:898–907. doi: 10.1038/ni.1635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.