Abstract
Background
In vivo, non-invasive optical coherence tomography (OCT) permits high-resolution imaging of tissue surfaces and subsurfaces, with the potential capability for detection and mapping of epithelial pathologies.
Purpose
To evaluate the clinical capability of non-invasive in vivo OCT for diagnosing oral dysplasia and malignancy.
Experimental Design
In 50 patients with oral lesions, conventional clinical examination was followed by OCT imaging, then standard biopsy and histopathology. Two blinded, pre-standardized investigators separately diagnosed each lesion based on (1) OCT and (2) histopathology.
Results
Intra- and inter-observer agreement between diagnoses based on histopathology and imaging data was excellent, with λ values between 0.844 and 0.896. Sensitivity and specificity were also very good.
Conclusions
These data demonstrate the excellent capability of in vivo OCT for detecting and diagnosing oral premalignancy and malignancy in human subjects.
Keywords: imaging, non-invasive diagnosis, oral dysplasia, squamous cell carcinoma
INTRODUCTION
Oral cancer will claim approximately 10,000 lives in the U.S. this year [1–3]. Despite significant advances in cancer treatment, early detection of oral cancer and its curable precursors remains the best way to ensure patient survival and quality of life. Accounting for 96% of all oral cancers, squamous cell carcinoma (SCC) is usually preceded by dysplasia presenting as white epithelial lesions on the oral mucosa (leukoplakia). Leukoplakias develop in 1–4% of the population [2]. Malignant transformation, which is quite unpredictable, occurs in 1–40% of leukoplakias over 5 years. Dysplastic lesions in the form of erythroplakias carry a risk for malignant conversion of 90% [2]. Tumor detection is complicated by a tendency towards field cancerization, leading to multicentric lesions [4]. Current techniques require surgical biopsy of lesions, which are often benign, yet they detect malignant change too late. Of all oral cancer cases documented by the National Cancer Institute Surveillance, Epidemiology and End Results Program, advanced lesions outnumbered localized lesions more than 2:1. Five-year survival rate is 75% for those with localized disease at diagnosis, but only 16% for those with cancer metastasis [2,3].
Optical Coherence Tomography (OCT)
OCT is a relatively new high-resolution optical technique that permits minimally invasive imaging of near-surface abnormalities in complex tissues. It has been compared to ultrasound scanning conceptually [5]. Both ultrasound and OCT provide real-time structural imaging, but unlike ultrasound, OCT is based on low-coherence interferometry, using broadband light to provide cross-sectional, high-resolution subsurface tissue images [6,7]. The engineering principles behind OCT have been described previously [8–11]. Cross-sectional images of tissues are constructed in real-time, at near histologic resolution (approximately 5–10 µm with our current technology). This permits in vivo non-invasive imaging of the macroscopic characteristics of epithelial and subepithelial structures, including: (1) depth and thickness, (2) histopathological appearance, and (3) peripheral margins. With a tissue penetration depth of 1–2 mm, the imaging range of our OCT technology is suitable for the oral mucosa [12–14]. The normal human oral mucosa is very thin, ranging from 0.2 to 1 mm. Previous studies using OCT have demonstrated the ability to evaluate macroscopic characteristics of epithelial, subepithelial, and basement membrane structures and show the potential for near histopathological-level resolution and close correlation with histologic appearance [5–34].
Goal of this study was to evaluate the clinical diagnostic capability of non-invasive in vivo OCT for oral dysplasia and malignancy.
MATERIALS AND METHODS
Patients referred to the UCI Department of Otolaryngology and UCLA Oral Medicine Clinic for evaluation of suspect white (Leukoplakia) or red and white patches (erythroplakia) intra-oral lesions were visually examined and photographed, then imaged along the long axis at the center of each lesion using either a fiberoptic high-resolution 3D OCT probe with a scan length of up to 10 mm or a commercially available (in-depth resolution 10–15 µm) 2D probe with a scan length of 2 mm Niris™ OCT imaging system by Imalux (Cleveland, OH). Contra lateral healthy tissues were scanned in a similar fashion. Acquisition required approximately 5–180 seconds per location for 3D scanning and 1.5 seconds for 2D scanning, totaling less than 15 minutes for each patient. Patients were given local anesthetic, an incisional or excisional biopsy (depending on the extent of the lesion) was performed and the specimens sent for routine histopathology. Patients retroactively identified by histopathology as NOT having healthy, dysplastic, or malignant lesions were excluded in these preliminary data, to achieve adequate numbers in each pathological group consistent with statistical usefulness. A total of 50 patients with healthy, dysplastic, or malignant lesions were included in this study (Table 1). This study was approved by UCI and UCLA Office of Research Protection Institutional Review Boards.
TABLE 1.
Pathologic Diagnosis for Patients Included in This Study (n = 50)
| Location | Healthy | Mild dysplasia |
Moderate dysplasia |
Severe dysplasia |
Carcinoma in situ |
Squamous cell carcinoma |
|---|---|---|---|---|---|---|
| Tongue | 7 | 3 | 4 | 3 | 3 | 6 |
| Buccal mucosa | 6 | 1 | 1 | 1 | 1 | 4 |
| Floor mouth | 4 | 1 | 2 | 2 | 0 | 1 |
| Total | 17 | 5 | 7 | 6 | 4 | 11 |
Pathologic diagnosis for patients included in this study (50 patients, 50 biopsies).
Histopathological Evaluation
From the 50 biopsies included in this study—1 for each lesion in each patient, the diagnosis of each tissue area that was scanned was provided by the histopathology service routinely used by our clinics. Histopathology was scored using a scale of 0 (normal)−6 (SCC) according to the criteria established by Macdonald [35]. In case of lesion heterogeneity, the highest (i.e., most severe) score was used.
OCT Imaging and Scoring
The same two blinded pre-trained scorers evaluated each OCT image diagnostically on a scale of 0 (normal)−6 (SCC). To facilitate OCT image scoring, this scale was designed to parallel the scale used for histopathological evaluation [35]. OCT diagnostic scores were based on changes in keratinization, epithelial thickening, epithelial proliferation and invasion, broadening of rete pegs, irregular epithelial stratification, and basal hyperplasia. Scorers were pre-trained using a standard set of 50 OCT and 50 matching histopathological images [12]. Total training time for each scorer was 2 hours. Each scorer evaluated all data in one session once all data accrual was complete. In case of lesion heterogeneity, scorers were instructed to use the highest (i.e., most severe) score. Scoring was repeated after 1 month to evaluate intra-observer agreement.
Statistical Analysis
Agreement between scorers and between modalities was assessed using Cohen’s λ statistics. Sensitivity and specificity of OCT diagnosis versus histopathology was also computed.
RESULTS
In the OCT images, epithelium, lamina propria, and basement membrane are clearly visible (Fig. 1).
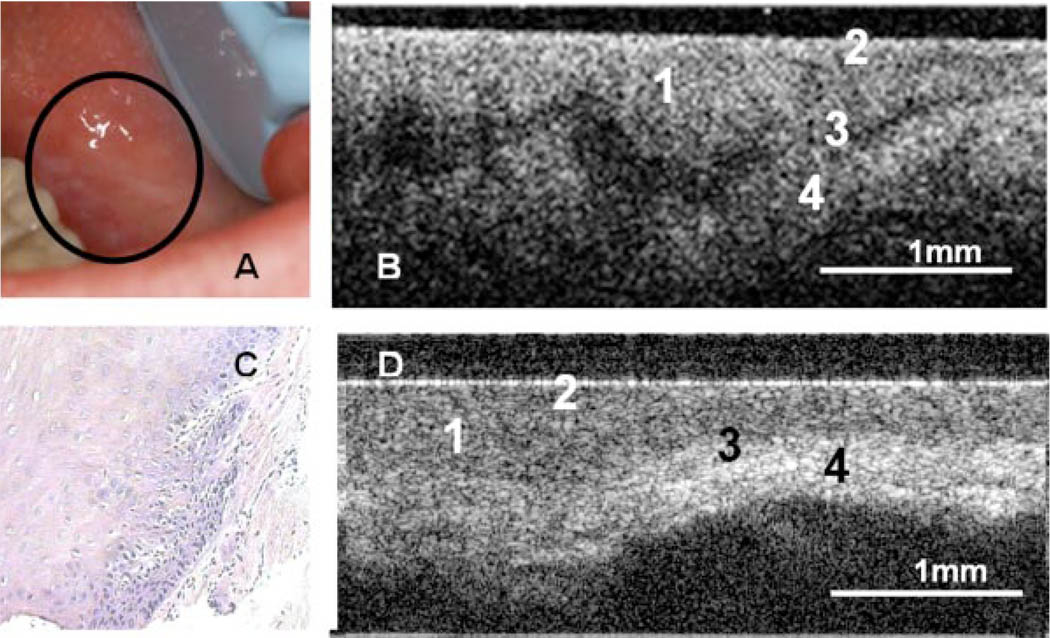
Fig. 1.
Photograph (A), in vivo OCT image (B), and H&E (10×) (C) of dysplastic buccal mucosa. D: In vivo OCT image of normal buccal mucosa. (1) Stratified squamous epithelium, (2) keratinized epithelial surface layer, (3) basement membrane, (4) submucosa.
The OCT image of a dysplastic lesion (Fig. 1B) parallels histolopathological status (Fig. 1C), showing epithelial thickening, loss of stratification in lower epithelial strata, epithelial downgrowth, and loss of epithelial stratification as compared to healthy oral mucosa (Fig. 1D).
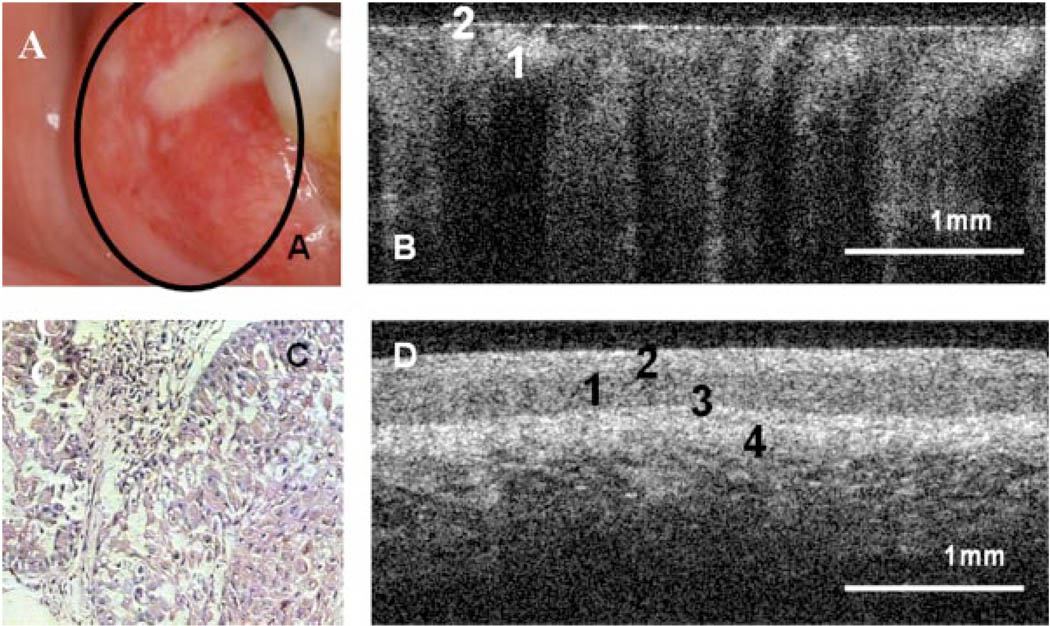
Figure 2A,C shows clinical appearance and histopathology, respectively, of an area of SCC on the buccal mucosa. In the OCT image (Fig. 2B), the epithelium is highly variable in thickness, with areas of erosion and extensive downgrowth and invasion into the subepithelial layers. The basement membrane is not visible as a coherent landmark.
Fig. 2.
Photograph (A), in vivo OCT image (B), and H&E (10×) (C) of alveolar ridge with squamous cell carcinoma. D: In vivo OCT image of normal alveolar mucosa. (1) Stratified squamous epithelium, (2) keratinized epithelial surface layer, (3) basement membrane, (4) submucosa.
Intra-observer agreement between OCT scores tabulated at the initial scoring event and scores allocated 1 month later was excellent, with a Cohen’s λ of 0.872 (SE = 0.053). Inter-observer agreement for OCT and for histopathological diagnosis and was also very good, with Cohen’s λ values of 0.870 (SE = 0.054) for OCT and 0.923 (SE = 0.042) for histopathology. Agreement between OCT and histopathology was excellent, averaging 0.858 (SE = 0.039) for both observers, and 0.896 (SE = 0.049) for both observers using the highest score as consensus. For detecting carcinoma in situ or SCC versus non-cancer, sensitivity was 0.931 and specificity was 0.931; for detecting SCC versus all other pathologies, sensitivity was 0.931 and specificity was 0.973.
DISCUSSION
Anticipated difficulties with movement artifacts were successfully avoided by seating patients in a reclining dental chair with headrest, neck, and arm support. OCT imaging was rapid, unproblematic and well received by all patients, with the imaging protocol adding only a few minutes to visit duration. Thus, the introduction of OCT imaging techniques to routine patient visits should be well received by patients and clinicians alike. OCT image scorers learned to read OCT images quickly and accurately, as evidenced by the excellent intra- and inter-observer agreement on OCT scores, as well as the close agreement between OCT and histopathology scores.
The close agreement between OCT-based diagnosis and histopathology for oral dysplasia and malignancy demonstrated in this human clinical study parallels data from previous animal imaging studies, where, typically λ values ranged from 0.85 to 0.95, sensitivity and specificity approximated 0.80–0.98 [12–14]. This compares well with existing forms of non-invasive diagnosis, primarily vital staining, and oral brush cytology. Although the sensitivity of staining agents such as Lugol iodine, Toluidine blue, and Tolonium chloride for detection of oral malignancy in the hands of experts generally approximates 90%, specificity of these agents is poor, diminishing rapidly when this modality is used by non-experts such as screeners in field units [36–41]. Extensive clinical experience is necessary to perform these examinations adequately. Using cytological examination of “brush biopsy” samples as a non-invasive method of oral diagnosis has been shown to provide moderate sensitivity levels of detection of oral epithelial dysplasia or SCC (70–90%), but poor specificity (3–44%). Thus this approach is of limited diagnostic value without augmentation by traditional biopsy [42–49].
The strong agreement between OCT-based diagnosis and histopathological diagnosis demonstrated in this study supports the concept that OCT will be a very useful tool for the early detection and diagnosis of oral lesions as well as regular monitoring of suspect lesions in the oral cavity and rapid, low-cost screening of high-risk populations. In its early stages, as OCT is introduced into a wider clinical setting and undergoes further testing and optimization, its initial primary use may be as an indicator of the need for biopsy. As the technology and techniques evolve, this modality may progressively reduce the need for biopsy, define surgical margins, and provide a direct evaluation of the effectiveness of cancer treatments.
ACKNOWLEDGMENTS
The authors thank Dr. William Armstrong, Dr. Brian Wong, and Dr. Terry Shibuya for their support in identifying subjects for this study. We also gratefully acknowledge the loan of a Niris™ OCT imaging system by Imalux (Cleveland, OH).
Contract grant sponsor: CA TRDRP; Contract grant number: 14IT-0097; Contract grant sponsor: DOE; Contract grant numbers: DE903-91ER, 61227; Contract grant sponsor: NIH; Contract grant numbers: EB-00293, CA91717; Contract grant sponsor: NSF; Contract grant number: BES-86924; Contract grant sponsor: NIH; Contract grant numbers: EB-00293, NCI-91717, RR-01192, EB0002SS, EB002494, AR47551; Contract grant sponsor: AFOSR; Contract grant numbers: F49620-00-1-0371, FA9550-04-1-0101.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Figures. Atlanta GA: American Cancer Society; 2008. p. 4. [Google Scholar]
- 2.Regezi J, Sciubba JWB, editors. Oral Pathology. Philadelphia: Saunders Co.; 1993. pp. 77–90. [Google Scholar]
- 3.California Department of Health Services. Cancer Surveillance Section Annual Report. 1999 March
- 4.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Tadrous PJ. Methods for imaging the structure and function of living tissues and cells: I. Optical coherence tomography. J Pathol. 2000;191(2):115–119. doi: 10.1002/(SICI)1096-9896(200006)191:2<115::AID-PATH589>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Izatt JA, Kulkarni MD, Kobayashi K, Sivak MV, Barton JK, Welch AJ. Optical coherence tomography for biodiagnostics. Opt Photon News. 1997;8:41–47. [Google Scholar]
- 7.Ding Z. High-resolution optical coherence tomography over a large depth range with an axicon lens. Opt Lett. 2002;27:4. doi: 10.1364/ol.27.000243. [DOI] [PubMed] [Google Scholar]
- 8.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson EA, Izatt JA, Hee MR, Huang D, Lin CP, Schuman JS, et al. In vivo retinal imaging by optical coherence tomography. Opt Lett. 1993;18(21):1864–1866. doi: 10.1364/ol.18.001864. [DOI] [PubMed] [Google Scholar]
- 10.Fujimoto JG, Hee MR, Izatt JA, Boppart SA, Swanson EA, Lin CP, et al. Biomedical imaging using optical coherent tomography. SPIE. 1999;3749:402. [Google Scholar]
- 11.Bouma B, Tearney GJ, Boppart SA, Hee MR, Brezinski ME, Fujimoto JG. High-resolution optical coherence tomographic imaging using a mode-locked Ti:Al/sub 2/O/sub 3/laser source. Opt Lett. 1995;20(13):1486–1488. doi: 10.1364/ol.20.001486. [DOI] [PubMed] [Google Scholar]
- 12.Wilder-Smith P, Jung WG, Brenner M, Osann K, Beydoun H, Messadi D, Chen Z. Optical coherence tomography for the diagnosis of oral malignancy. Lasers Surg Med. 2004;35:269–275. doi: 10.1002/lsm.20098. [DOI] [PubMed] [Google Scholar]
- 13.Matheny E, Mina-Araghy R, Hama N, El-Abbadi N, Jung WG, Chen Z, Wilder-Smith P, Brenner M. Optical coherence tomography of malignancy in the Hamster Cheek Pouch. J Biomed Opt. 2004;9(5):978–981. doi: 10.1117/1.1783897. [DOI] [PubMed] [Google Scholar]
- 14.Matheny ES, Hanna N, Mina-Araghi R, Jung WG, Chen Z, Wilder-Smith P, Brenner M. Optical coherence tomography of malignant Hamster Cheek Pouches. J Invest Med. 2003;51(1):S78. doi: 10.1117/1.1783897. [DOI] [PubMed] [Google Scholar]
- 15.Boppart SA, Hee MR, Fumimoto JG, Birngruber R, Toth CA, Swanson EA, et al. Dynamic evolution and in vivo tomographic imaging of laser-induced retinal lesions by using optical coherence tomography. Dynamic evolution and in vivo tomographic imaging of laser induced retinal lesions using optical coherence tomography. Baltimore, MD: IEEE/Proceeding CLEO; 1995. [Google Scholar]
- 16.Milner TE, Dave D, Zhongping C, Goodman DM, Nelson JS. Ch 3. In: Alfano RR, Fujimoto JG, editors. Optical Coherence Tomography as a Biomedical Monitor in Human Skin. Bellingham WA: SPIE Press Books; 1996. pp. 220–223. [Google Scholar]
- 17.Bamford KJ, James SW, Barr H, Tatam RP. Optical low coherence tomography of bronchial tissue. Advanced materials and optical systems for chemical and biological detection. SPIE. 1999;3858: Ch. 30:172–179. [Google Scholar]
- 18.Bohorfoush AG. New diagnostic methods for esophageal carcinoma. Recent Results Cancer Res. 2000;155:55–62. doi: 10.1007/978-3-642-59600-1_5. [DOI] [PubMed] [Google Scholar]
- 19.Brand S, Ponero JM, Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Optical coherence tomography in the gastrointestinal tract. Endoscopy. 2000;32(10):796–803. doi: 10.1055/s-2000-7714. [DOI] [PubMed] [Google Scholar]
- 20.Yazdanfar S, Kulkarni MD, Izatt JA. High resolution imaging of in vivo cardiac dynamics using color Doppler optical coherence tomography. Opt Expr. 1997;1(13):424–431. doi: 10.1364/oe.1.000424. [DOI] [PubMed] [Google Scholar]
- 21.Pitris C, Boppart SA, Brezinski ME, Bouma BE, Fujimoto JG. Cellular and neoplastic tissue imaging with optical coherence tomography. CLEO. 1998 May 3–8;:127–128. [Google Scholar]
- 22.Bouma BE, Poneros JM, Shishkov M, Schlendorf KH, Houser SL, Compton CC, et al. Diagnosis of specialized intestinal metaplasia of the esophagus with optical coherence tomography. SPIE. 2001;3916:307. [Google Scholar]
- 23.Poneros JM, Brand S, Bouma BE, Tearney GJ, Compton CC, Nishioka NS. Diagnosis of specialized intestinal metaplasia by optical coherence tomography. Gastroenterology. 2001;120(1):7–712. doi: 10.1053/gast.2001.20911. [DOI] [PubMed] [Google Scholar]
- 24.Poneros JM, Tearney GJ, Shiskov M, Kelsey PB, Lauwers GY, Nishioka NS, et al. Optical coherence tomography of the biliary tree during ERCP. Gastrointest Endosc. 2002;55(1):84–88. doi: 10.1067/mge.2002.120098. [DOI] [PubMed] [Google Scholar]
- 25.Feldchtein FI, Gelikonov GV, Gelikonov VM, Kuranov RV, Sergeev AM, Gladkova ND, Shakhov A, Shakhova N, Snopova L, Teventeva A, Zagainova E, Chumaka Y, Kuznetzova I. Endoscopic applications of optical coherence tomography. Opt Expr. 1998;3(6):257–270. doi: 10.1364/oe.3.000257. [DOI] [PubMed] [Google Scholar]
- 26.Colston BW, Everett MJ, DaSilva LB, Otis LL, Stroeve P, Nathel H. Imaging of hard and soft tissue in the oral cavity by optical coherence tomography. Appl Opt. 1998;37(16):3582–3585. doi: 10.1364/ao.37.003582. [DOI] [PubMed] [Google Scholar]
- 27.Otis LL, Everett MJ, Sathyam S, Colston BW. Optical coherence tomography: A new imaging technology for dentistry. J Am Dent Assoc. 2000;131:511–514. doi: 10.14219/jada.archive.2000.0210. [DOI] [PubMed] [Google Scholar]
- 28.Tearney GJ, Bouma BE, Boppart SA, Golubovic B, Swanson EA, Fujimoto JG. Rapid acquisition of in vivo biological images by use of optical coherence tomography. Opt Lett. 1996;21(17):1408–1410. doi: 10.1364/ol.21.001408. [DOI] [PubMed] [Google Scholar]
- 29.Drexler W, Morgner U, Kärtner F, Pitris C, Chen Y, Boppart SA, Li X, Ippen EP, Fujimoto JG. In vivo ultrahigh resolution optical coherence tomography. Opt Lett. 1999;24:1223–1224. doi: 10.1364/ol.24.001221. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto JG, Hee MR. Optical coherence tomography: Introduction and theory. In: Bouma BE, Tearney GJ, editors. Handbook of Optical Coherence Tomography. NY: Marcel Dekker, Inc; 2002. pp. 1–143. [Google Scholar]
- 31.Drexler W, Morgner U, Kartner FX, Pitris C, Boppart SA, Li XD, et al. In vivo ultrahigh-resolution optical coherence tomography. Opt Lett. 1999;24(17):1221–1223. doi: 10.1364/ol.24.001221. [DOI] [PubMed] [Google Scholar]
- 32.Podoleanu AG, Rogers JA, Cucu RC, Jackson DA, Wacogne B, Porte H, et al. Simultaneous low coherence interferometry imaging at two depths using an integrated optic modulator. Opt Commun. 2001;191(1–2):21–30. [Google Scholar]
- 33.Reiser BJ, Ignacio TS, Wang Y, Taban M, Graff JM, Sweet P, Chen Z, Chuck RS. In vitro measurement of rabbit corneal epithelial thickness using ultrahigh resolution optical coherence tomography. Vet Ophthalmol. 2005;8(2):85–88. doi: 10.1111/j.1463-5224.2005.00345.x. [DOI] [PubMed] [Google Scholar]
- 34.Feldchtein FI, Gelikonov GV, Gelikonov VM, Iksanov RR, Kuranov RV, Sergeev AM, Gladkova ND, Ourutina MN, Warren JA, Reitze DH. In vivo OCT imaging of hard and soft tissue of the oral cavity. Opt Expr. 1998;3(6):239–250. doi: 10.1364/oe.3.000239. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald DG. Comparison of epithelial dysplasia in hamster cheek pouch carcinogenesis and human oral mucosa. J Oral Pathol. 1981;10:186–191. doi: 10.1111/j.1600-0714.1981.tb01264.x. [DOI] [PubMed] [Google Scholar]
- 36.Epstein JB, Feldman R, Dolor RJ, Porter SR. The utility of tolonium chloride rinse in the diagnosis of recurrent or second primary cancers in patients with prior upper aerodigestive tract cancer. Head Neck. 2003;25(11):911–921. doi: 10.1002/hed.10309. [DOI] [PubMed] [Google Scholar]
- 37.Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral premalignant and malignant lesions. J Can Dent Assoc. 2002;68(10):617–621. [PubMed] [Google Scholar]
- 38.Silverman S, Migliorati C, Barbosa J. Toluidine blue staining in the detection of oral precancerous and malignant lesions. Oral Surg Oral Med Oral Pathol. 1984;57:379–382. doi: 10.1016/0030-4220(84)90154-3. [DOI] [PubMed] [Google Scholar]
- 39.Epstein J, Scully C, Spinelli U. Toluidine blue and Lugol’s iodine solution for the assessment of oral malignant disease and lesions at risk of malignancy. J Oral Pathol Med. 1992;21:160–163. doi: 10.1111/j.1600-0714.1992.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 40.Patton LL. The effectiveness of community-based visual screening and utility of adjunctive diagnostic aids in the early detection of oral cancer. Oral Oncol. 2003;39(7):708–723. doi: 10.1016/s1368-8375(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 41.Onofre MA, Sposto MR, Navarro CM. Reliability of toluidine blue application in the detection of oral epithelial dysplasia and in situ and invasive squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91(5):535–540. doi: 10.1067/moe.2001.112949. [DOI] [PubMed] [Google Scholar]
- 42.Poate TW, Buchanan JA, Hodgson TA, Speight PM, Barrett AW, Moles DR, Scully C, Porter SR. An audit of the efficacy of the oral brush biopsy technique in a specialist oral medicine unit. Oral Oncol. 2004;40(8):829–834. doi: 10.1016/j.oraloncology.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 43.Acha A, Ruesga MT, Rodriguez MJ, Martinez de Pancorbo MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Oral Med Oral Pathol Oral Cir Bucal. 2005;10(2):95–102. [PubMed] [Google Scholar]
- 44.Ogden GR, Cowpe JG, Green MW. Detection of field change in oral cancer using oral exfoliative cytologic study. Cancer. 1991;68:1611–1615. doi: 10.1002/1097-0142(19911001)68:7<1611::aid-cncr2820680724>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 45.Sciubba JJ. Improving detection of precancerosu and cancerous oral lesions: Computer-assisted analysis of the oral brush biopsy. J Am Dent Assoc. 1999;130:1445–1457. doi: 10.14219/jada.archive.1999.0055. [DOI] [PubMed] [Google Scholar]
- 46.Nichols ML, Quinn FB, Jr, Schnadig VJ, Zaharopoulos P, Hokanson JA, Des Jardins L, et al. Interobserver variability in the interpretation of brush cytologic studies from head and neck lesions. Arch Otolaryngol Head Neck Surg. 1991;117:1350–1355. doi: 10.1001/archotol.1991.01870240042006. [DOI] [PubMed] [Google Scholar]
- 47.Rick GM, Slater L. Oral brush biopsy: The problem of false positives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:252. doi: 10.1016/s1079-2104(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 48.Rosin MP, Epstein JB, Berean K, Durham S, Hay J, Cheng X, et al. The use of exfoliative cell samples to map clonal genetic alterations in the oral epithelium of high-risk patients. Cancer Res. 1997;57:5258–5260. [PubMed] [Google Scholar]
- 49.Huang MF, Chang YC, Liao PS, Huang TH, Tsay CH, Chou MY. Loss of heterozygosity of p53 gene of oral cancer detected by exfoliative cytology. Oral Oncol. 1999;35:296–301. doi: 10.1016/s1368-8375(98)00119-5. [DOI] [PubMed] [Google Scholar]