Abstract
In this review, we highlight some of recent studies underscoring the importance of the tumor microenvironment, especially the role of bone marrow-derived myeloid cells, in restoring tumor growth after irradiation. Myeloid cells are hematopoietic cells that give rise to monocytes and macrophages in the peripheral blood and tissues. These cells have been shown to be proangiogenic in tumors promoting tumor growth. We also discuss our previously unpublished results on the effect of irradiation on the tumor vasculature including pericyte and basement membrane coverage to the endothelium of tumor blood vessels. We summarize the clinical significance of these studies including the use of MMP-9 inhibitors, administering white blood cell boosters, or planning safety margin of tumor volumes, in order to improve overall clinical benefits in cancer patients treated with radiotherapy.
Keywords: bone marrow-derived cells, myeloid cells, tumor vasculature, pericyte, basement membrane, radiation, solid tumors
Tumor Regrowth after Radiotherapy: Intrinsic Tumor Sensitivity versus Stromal Contribution
Radiotherapy is often given as the first line therapy to patients with many types of cancers, especially those with unresectable tumors. Compared to other treatment modalities, radiotherapy offers advantages including spatio-temporal flexibility in tumor-targeting, non-invasiveness and organ-preservation. However, despite recent advances in radiotherapy such as intensity-modulated (IMRT) and image-guided radiotherapy enabling reductions in dose to critical adjacent normal tissues,1 tumors relapse in many of the treated patients primarily due to an inability to control the primary tumors.2 Interestingly, many of these relapsed tumors appear within previous irradiated regions.3
Typically radiotherapy is delivered to the tumor as 2 Gy fractions given five times weekly to total doses of 50–70 Gy using IMRT,3,4 or 1–5 fractions delivering 15–67.5 Gy using image-guided robotic stereotactic radiosurgery.5 Experimental studies in vitro and with transplanted tumors show that such doses will kill the vast majority (>99%) of both the tumor and endothelial cells6–8 despite the fact that the presence of hypoxia in tumors affords some protection against radiation-induced cell killing.9 The fact that tumor growth following high doses of irradiation depends on a viable vasculature10 suggests that there is a mechanism by which the tumor vasculature can be restored despite the fact that most, if not all, the endothelial cells in the tumor have been sterilized. Recently, we demonstrated that bone marrow-derived myeloid cells may provide this mechanism: they enter irradiated tumors to restore the damaged tumor vasculature thereby allowing growth of the tumor following irradiation.11 Herein we discuss some recent studies supporting these observations.
There is no doubt that the intrinsic sensitivity of tumor cells is a major factor affecting the sensitivity of tumors to irradiation. For example, studies by Gerweck and colleagues demonstrated that expression of the DNA double strand break repair gene, DNA-PKcs in a tumor cell line made the tumor more resistant to irradiation than that without DNA-PKcs expression.12,13 Furthermore, genes that are not directly involved in DNA repair that are expressed by tumor cells can also play a role in affecting radiosensitivity of tumors. Overexpression of epidermal growth factor receptor (EGFR) in tumors, for example, is associated with radioresistance,14 and inhibition of EGFR by selective tyrosine kinase inhibitors including erlotinib15 and gefitinib,16 or by the monoclonal antibody cetuximab17 significantly sensitizes tumors in pre-clinical and clinical settings.
However, there is increasing evidence to suggest that stromal components in tumors can also contribute to the tumor response to irradiation. Recent studies have shown that tumors were much more sensitive to irradiation when grown in mice where endothelial cells are susceptible to undergo apoptosis following irradiation: such as mice deficient for p53,18 acid sphingomyelinase, or Bax.19 Furthermore, severe combined immunodeficient (SCID) mouse defective of DNA-dependent protein kinase has been an excellent model to demonstrate the impact of stromal cells on the tumor radiosensitivity. It is well documented that tumors grown in SCID mice, in which all the cells are highly radiosensitive, are much more sensitive to irradiation than those in other immunodeficient (e.g., nude) mice when the tumor response was assayed by growth delay.6,12,13,20 However, Budach and colleagues21 reported that the difference in the response between tumors transplanted in SCID and nude mice to irradiation disappears when the end point is measured as the radiation doses needed to achieve local control in 50% of the treated animals (TCD50). The former studies that examined the growth delay used radiation doses of up to 20 Gy6,13 while the latter study of TCD50 ranges from 35 to >100 Gy.21 These very high doses of irradiation used in the TCD50 studies (35–>100 Gy) would be expected to sterilize all of endothelial cells in the tumor and the immediately surrounding normal tissue, raising the question of how a functioning vasculature to develop to allow the surviving tumor cells to regrow the tumor. Are there circulating cells that would enter the irradiated tumors to help rebuilding the tumor vasculature? If there are, what are they?
Bone Marrow-Derived Hematopoietic Cells: More Than Just Blood
Tumor vasculature is formed by two mechanisms: angiogenesis and vasculogenesis. Angiogenesis is the process by which new blood vessels are formed by endothelial cell proliferation and sprouting from nearby existing blood vessels.22 Vasculogenesis involves colonization in the tissue or tumor of circulating cells primarily derived from the bone marrow to form new blood vessels in situ.22
Many studies have reported that the bone marrow-derived circulating cells that incorporate into the tumor vasculature are endothelial progenitor cells (EPCs).23–25 These cells express endothelial cell-specific markers such as CD31 (platelet-endothelial cell adhesion molecule-1; PECAM), VE-cadherin and/or VEGFR-2,23 and comprise 50–100% of all tumor vasculature in some studies.19,25 In these studies and others, EPCs were identified by transplanting into lethally irradiated non-transgenic mice whole bone marrow cells from transgenic mice that express genetic markers such as β-galactosidase (lacZ) or green fluorescent protein (GFP) either ubiquitously or under endothelial specific conditions. Although these transgenic mice have proven themselves to be useful in studying prenatal vasculogenesis,26,27 the extent to which the bone marrow-derived EPCs from these mice contributes to the tumor vasculature varies widely and is controversial.11,25,28–30 Nonetheless there is evidence that isolated human EPCs show endothelial cell characteristics such as the formation of endothelial tubes when cultured onto a matrigel basement membrane support or when admixed with matrigel and implanted into immunodeficient mice.31 In addition, it has been shown that genetic depletion of EPCs together with hematopoietic cells inhibits tumor growth in mice.25
Other populations of bone marrow-derived hematopoietic cells in the myeloid lineage, including Tie2-expressing mononuclear cells (TEMs), tumor associated macrophages (TAMs) and myeloid-derived immune suppressor cells (MDSCs), have been recognized for their roles in facilitating tumor growth.29,32,33 These bone marrow-derived myeloid cells express CD45 leukocyte antigen and myeloid-specific CD11b and/or Gr-1 surface markers, and eventually differentiate into monocytes, macrophages and granulocytes. Studies have shown that myeloid cells contribute to tumor angiogenesis when admixed with tumor cells,32 inhibit tumor growth when genetically depleted,29 and mediate refractoriness of the tumor to anti-VEGF therapies.34
In a recent study, we demonstrated that CD11b+ myeloid cells contribute to tumor growth in previously irradiated tissues by expressing MMP-9, an extracellular matrix-degrading enzyme.11 Implanting tumors into previously irradiated tissues is a widely used model system known as the ‘tumor bed effect’.35 Tumors grown in this way mimic human recurrent primary tumors after radiotherapy by showing increased hypoxic fractions and decreased radiocurability,36 as well as increased metastatic frequency.37 In this model we found that tumors could not grow in MMP-9 knockout (KO) mice while the growth was completely restored by transplanting wild-type bone marrow into the MMP-9 KO mice. Furthermore, by performing immunostaining for MMP-9 in tumors of these mice we found that the wild-type bone marrow cells gave rise to essentially all of the CD11b+ myeloid cells in the tumor.11 The importance of MMP-9 expressing myeloid cells has been also reported in studies where these cells were shown to initiate and promote tumor angiogenesis32,38 and to facilitate transition of multistep carcinogenesis39–41 in mouse models of cancers.
Despite the evidence for the involvement of bone marrow-derived myeloid cells in tumor angiogenesis and growth, the mechanisms by which these myeloid cells can mediate these effects are poorly understood. Studies have shown that these cells are recruited to sites of vasculogenesis by various cytokines and growth factors including VEGF,42,43 stromal derived factor-1 (SDF-1),43 placental growth factor44 and granulocyte/macrophage colony stimulating factor.45 The proangiogenic function of these cells is variously attributed to their expression of MMP-9,11,32,39,40 nitric oxide synthase,46 VEGF47 and transforming growth factor-β.48 We speculate that these features of myeloid cells provide powerful ways to remodel the tumor microenvironment that is necessary for growth and survival of tumors. However, further studies are needed to fully understand how myeloid cells contribute to tumor blood vessel growth.
Effect of Radiation on the Tumor Vasculature
In our study, we implanted tumors onto previously irradiated tissues to abrogate local angiogenesis thereby forcing the tumor to rely on vasculogenesis for its blood vessel formation. Tumors implanted into these irradiated tissues grow more slowly than corresponding tumors in non-irradiated tissues (the so-called “tumor bed effect”), likely because of a reduced ability of radiation-injured stroma to provide blood vessels to tumors.49 The degree to which the tumor bed effect develops is tumor type-dependent50 and the severity can be minimized by increasing the number of tumor cells, adding heavily irradiated tumor cells to the viable tumor cell inoculums, or by eliciting stronger neovascularization at the site of the implanted tumor cells.49 Importantly, the tumor bed effect is not observed when tumor fragments containing established tumor vasculature are implanted in previously irradiated tissues as opposed to single cell suspensions of the same tumor type.51
Milas and colleagues reported that the tumor bed effect appears following doses of 5–10 Gy and increases with radiation doses of up to 20–30 Gy at which it reaches a plateau. 50 Consistent with this, we also observed a small but significant tumor bed effect in animals given whole body radiation of 8–9 Gy followed by bone marrow transplantation (Fig. 1A). To determine that this was a manifestation of the tumor bed effect and not some effect of the bone marrow transplantation per se, we irradiated the tumor implantation site only at the same radiation dose (9 Gy) in one group of mice and in another group, we performed whole body radiation (9 Gy) followed by syngeneic bone marrow transplantation. We then implanted tumors 4 weeks later in both groups and observed an indistinguishable tumor growth in those mice (Fig. 1B). These results suggest that whole body irradiation in mice, often given at 8–12 Gy, could significantly compromise tumor growth and affect subsequent angiogenic and/or vasculogenic responses. Consistent with this, a number of studies noted similar findings of slowed tumor growth in the irradiated and bone marrow-reconstituted mice.19,52 However, the authors of these studies postulated that the effect was due to immune-deficiency associated with irradiation52 or reduction in the stem cell capacity that accompanies bone marrow transplantation.19 A recent study demonstrating that radiation-induced suppression of angiogenesis in the mouse cornea is independent of hematopoietic reconstitution,53 is in agreement with our results.
Figure 1.
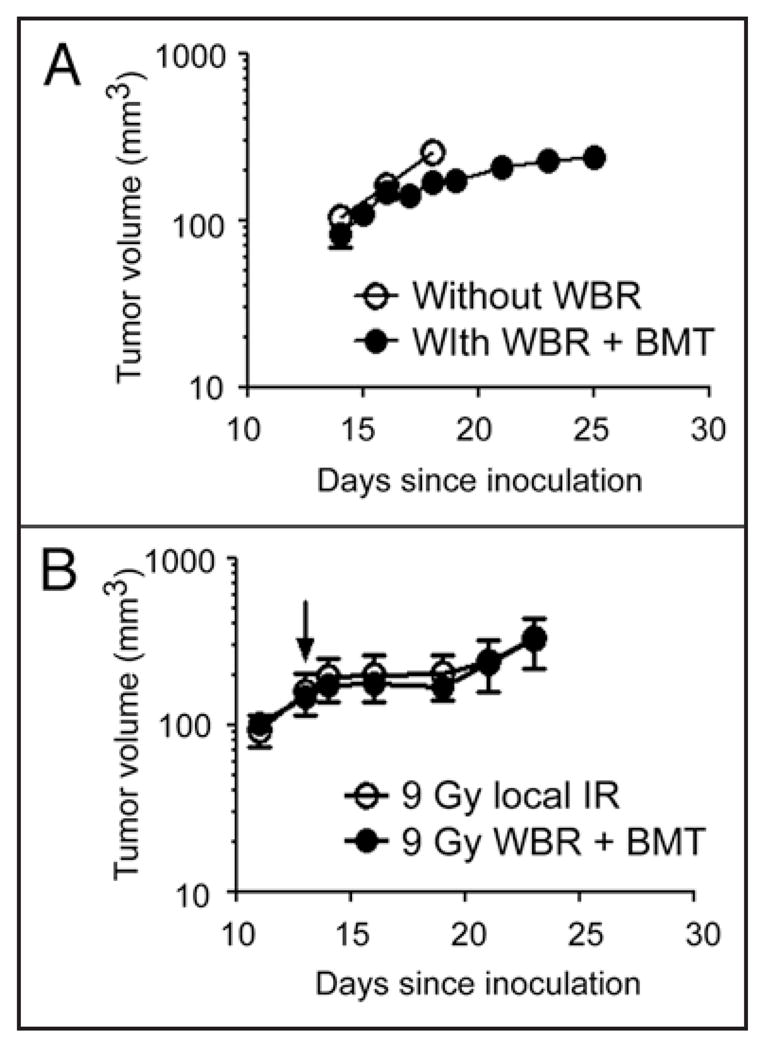
(A) Growth of MT1A2 tumor in FVB mice that did not receive whole body radiation (WBR) or bone marrow transplantation (BMT) (open circles) or in FVB mice that receive WBR at 9 Gy followed by BMT from Tie2lacZ transgenic mice (closed circles) 4 weeks prior. Tumors were implanted at the same time and monitored for their growth. (B) RIF tumor growth in female C3H mice that received 9 Gy local irradiation at the tumor implantation site (open circles) or in female C3H mice that received 9 Gy WBR followed by BMT from male C3H mice (closed circles) at the same time as the group in open circles. Tumors were implanted 4 weeks later, irradiated at approximately 200 mm3 with 20 Gy (arrow), and were monitored for their growth. Symbols in (A and B) are the mean ± s.e.m. for n = 5 per group.
What is the effect of irradiation on tumor vasculature? Despite many studies reporting that irradiation increases endothelial cell apoptosis and decreases microvessel density in tumors,53–56 the effect of irradiation on the tumor vasculature as a whole has not been studied as extensively. Zips and colleagues demonstrated that FaDu human tumors grown in the previously irradiated site responded better to an antiangiogenic therapy than those in non-irradiated controls.57 Although the authors of this study did not examine in great detail the histology of the tumor vasculature (such as pericyte coverage of the blood vessel), they noted a significant reduction in blood vessel area in the tumors grown in the previously irradiated bed compared to the control, which is consistent with our observations. A recent study by Kozin and colleagues reported that irradiated and subsequently regrowing lung tumor xenografts had less coverage with pericytes in blood vessels and these were more sensitive to VEGFR-2 blockade compared to unirradiated tumors.58 They also noted that VEGFR-2 blockade itself without irradiation did not affect the pericyte coverage of the tumor endothelium, indicating irradiation uniquely causes formation of tumor blood vessels without pericyte coverage in re-growing tumors (or tumors in previously irradiated tissues). Is there such an effect of irradiation on basement membrane? Mancuso and colleagues demonstrated that VEGFR-2 blockade causes tumor blood vessels to regress but leaves empty sleeves of basement membrane behind, on which tumor vessels grow back upon withdrawal of the treatment.59 There are only a limited number of studies investigating the effect of irradiation on basement membrane of the tumor vasculature. One such, a study by Kobayashi, reported a thickening of basement membrane by immunostaining for laminin in mouse mammary tumors on the 7th day of post-irradiation.60
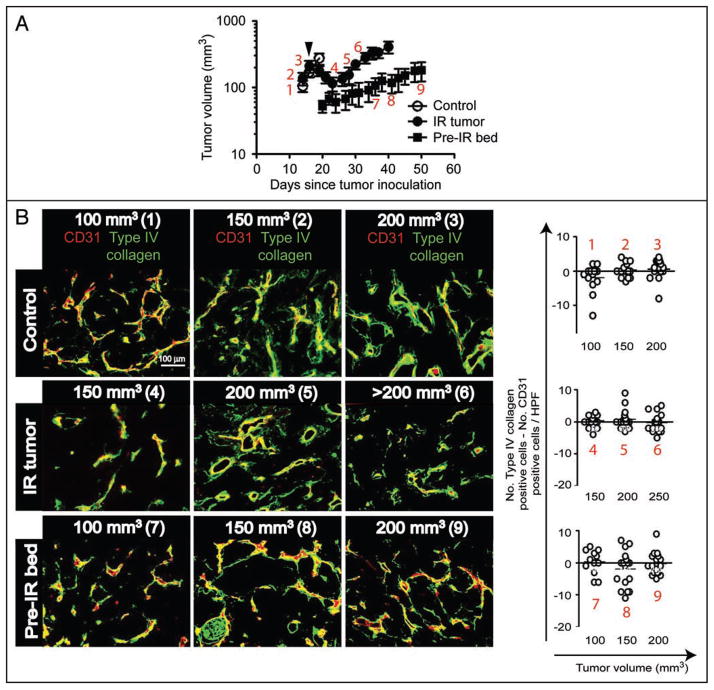
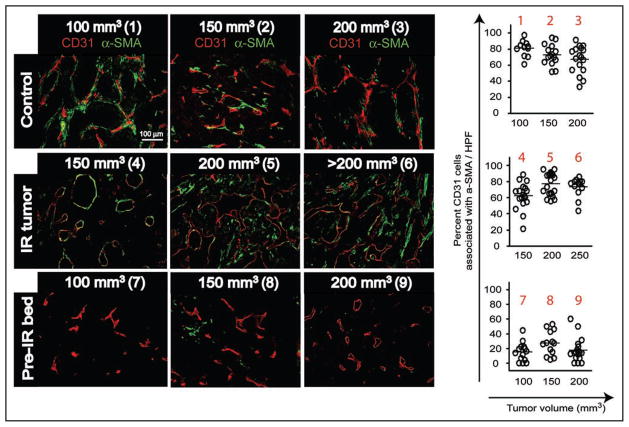
To further understand the effect of irradiation on the tumor vasculature, we examined pericytes and basement membrane of the endothelium in MT1A2 tumors that were either not irradiated, irradiated at 20 Gy single dose and allowed to re-grow, or grown in previously irradiated tissues at 20 Gy (Figs. 2 and 3). In these tumors we stained and measured the number of endothelial cells (CD31) that were associated with pericytes (α-smooth muscle actin; α-SMA) or with basement membrane (type IV collagen) at various tumor volumes. We found that there was no significant change in an association of basement membrane with endothelial cells in the tumor with or without irradiation (Fig. 2B). Furthermore, irradiation to neither the established tumors nor to the tumor bed caused a significant difference in the basement membrane coverage of the tumor endothelium (Fig. 2B). However, when we examined the pericytes coverage of the blood vessels, we observed extensive pericyte coverage of endothelial cells in the control tumors (no irradiation) and in irradiated and subsequently re-grown tumors (Fig. 3). In contrast, only sparsely distributed pericytes around endothelial cells were found in the tumors that were grown in the previously irradiated site regardless of the tumor volume (Fig. 3). These data indicate that radiation exerts a different mode of action from VEGFR2 blockade on basement membrane and pericytes of the tumor vasculature such that there may be a more substantial effect on pericytes than basement membrane within the endothelium. This also indicates that an immaturity of the tumor vessels (lack of pericyte coverage around the endothelium) and an imbalance between pericytes and endothelium signaling may cause the enhanced susceptibility of the tumor endothelium to VEGFR2 blockade. This also implies that pericytes may be an important target in combination with radiotherapy to achieve enhanced tumor response to irradiation.
Figure 2.
(A) Growth of MT1A2 tumors that were non-irradiated (control; open circles), irradiated and re-grown (IR tumor; closed circles), or grown in previously irradiated tissues (pre-IR bed; closed squares) in FVB mice. Arrowhead indicates local irradiation of 20 Gy to the ‘IR tumor group only. Numbers in red indicate points at which tumors were excised and examined histologically for basement membrane (B) and for pericytes (Fig. 3). Reproduced from11 with permission. (B) Left: Immunostaining of tumors in A for endothelial cells (CD31; red) or basement membrane (type IV collagen, green). Numbers in parentheses correspond to the numbers in red from (A). Right: The number of endothelial cells was subtracted from that of basement membrane to determine the presence of basement membrane empty sleeves (see the text). The difference in means between different volumes or treatment groups did not reach a statistical significance (p > 0.05).
Figure 3.
Immunostaining of tumors from Figure 2 for endothelial cells (CD31; red) or pericytes (α-smooth muscle actin; α-SMA; green). Numbers in parentheses are as in Figure 2. Percentage of endothelial cells associated with pericytes were calculated and shown on right. Note that pre-IR tumors showed the percent endothelial cells with pericyte coverage to be significantly lower than other groups (p < 0.001).
We hypothesize that the difference in the pericyte coverage of endothelial cells in irradiated tumors between our results and the study by Kozin and colleague58 may be associated with the type of tumors of different intrinsic sensitivity. We think that relatively radiation-resistant MT1A2 mouse mammary carcinoma in our study (approximately 5 days between tumor irradiation and re-growth) may not reflect much change in the tumor vasculature, especially with pericytes (Fig. 2A). On the other hand, human xenografts used in their study are more responsive to irradiation shown by the mean time between tumor irradiation and regrowth of approximately 20 days,58 which may have allowed sufficient time for the tumor vasculature to regress and remodel that is reflected by the reduced pericyte coverage around the perfused blood vessels.
Clinical Importance and Implications
Our recent study demonstrated that bone marrow-derived myeloid cells may modulate tumor response to irradiation by expressing the extracellular matrix-degrading enzyme MMP-9.11 Thus, MMP-9 could be a target for adjunct therapy to potentiate tumor response to irradiation. Indeed preclinical studies have reported that MMP inhibition significantly increases antitumor activity by irradiation.61,62 Our study also showed that despite the absence of MMP-9 from the bone marrow, tumors could still grow via low levels of MMP-9 provided by other stromal cells such as fibroblast- or smooth muscle-like cells.11 This indicates that even low levels of MMP-9 could catalyze restoration of tumor vasculature following irradiation. There are some limitations in inhibiting MMP in tumors as adjunct therapy to radiotherapy. First, currently available MMP inhibitors lack selectivity for targeting MMP-9 by inhibiting both MMP-9 and its closely related family member MMP-2 as shown by the study by Kaliski and colleagues.61 Second, the source of MMP-9 may be different depending on the type of tumors. For example, the MT1A2 tumors used in our study showed very little activity in MMP-9 with or without irradiation, and depended on supply of MMP-9 from stromal cells such as myeloid cells and fibroblast-like cells.11 In contrast, RIF tumors showed very strong activity of both MMP-2 and MMP-9 upon irradiation (irradiated tumors or the tumor bed; unpublished data, Ahn & Brown). Therefore it is important that the source and levels of MMP-9 activity in tumors should be monitored before an attempt of MMP-9 inhibition. Lastly, even if there was a MMP-9 specific inhibitor, one should carefully consider the extent to which MMP-9 can be inhibited. Due to physicochemical properties of the drug (diffusion properties) or tumor microenvironmental factors (leaky blood vessels) it may be difficult to achieve complete inhibition in MMP-9 within tumors. For some tumors, small levels of MMP-9 would be sufficient to allow tumors to growth, as in our study.11
Our recent study suggests that the proangiogenic property of myeloid cells is an attractive target for an enhancement of tumor response to irradiation. Use of granulocyte/macrophage-colony stimulating factor (GM-CSF) has been used to ameliorate mucositis in cancer patients treated with radiotherapy,63 and to treat chemotherapy-induced neutropenia in many patients treated with chemotherapy.64 Although it is critical for these patients to receive G-CSF/GM-CSF to avoid treatment delays, recent research indicates a potential danger involving the stimulation of tumor angiogenesis by myeloid cells. For example, Shojaei and colleagues demonstrated that myeloid cells contribute to tumor refractoriness to anti-angiogenic (anti-VEGF) therapy.34 Investigators have also reported that G-CSF positively regulate Bv8 (prokineticin 2), a proangiogenic factor, highly expressed in myeloid cells to influence tumor angiogenesis.65 Interestingly, a study of three randomized trials showed that chemotherapy-induced neutropenia was a good prognostic marker for increased survival in NSCLC patients, although they excluded patients treated with G-CSF in the analysis.66 It should be noted that this study utilized chemotherapy-induced neutropenia as a surrogate marker predicting adequate dosing of chemotherapeutic drug(s) rather than as a cause of the enhanced antitumor activity. However, a preclinical study has demonstrated that neutropenia caused by an antibody treatment to c-Kit (a hematopoietic stem cell marker) suppressed angiogenesis of various tumor xenograft models,67 underscoring the importance of myeloid cells for promoting tumor angiogenesis.
Despite the important role played by myeloid cells in tumor angiogenesis, the source of endothelial cells that restore tumor growth after irradiation is not likely to be the bone marrow. These cells may be non-bone marrow-derived progenitors68 or circulating endothelial cells69 as suggested by other investigators. Our previous study suggested that the bone marrow-derived myeloid cells could aid the (circulating) endothelial cells to rescue the tumor vasculature from radiation-induced collapse.11 If endothelial cells could access the irradiated tumors, it may be necessary to reconsider the small margins of the targeted tumor volume with IMRT on the basis that the narrow margin of the tumors may not necessarily be beneficial given the fact that surrounding endothelial cells may enter the tumor to reverse the treatment effect.
Overall, we believe that our study and others indicate that we need a better understanding of the functions and mechanisms by which myeloid cells (and other bone marrow-derived cells) interact with tumors. Such knowledge will enable us to develop novel therapeutic strategies that will improve cure rates in cancer patients.
Methods
Immunofluorescent staining was performed as previously.11 For basement membrane staining, a rabbit anti-mouse type IV collagen antibody (Biodesign, MA) was used.
Acknowledgments
This work was supported by National Institutes of Health grants CA118202 and CA128873 awarded to J.M.B. G-O.A. is a recipient of the Gary Slezak/American Brain Tumor Association Translational Research Grant.
References
- 1.Verellen D, De Ridder M, Linthout N, Tournel K, Soete G, Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–60. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 2.Chao KSC, Ozyigit G, Tran BN, Cengiz M, Dempsey JF, Low DA. Patterns of failure in patients receiving definitive and postoperative IMRT for head-and-neck cancer. Int J Rad Oncol Biol Phys. 2003;55:312–21. doi: 10.1016/s0360-3016(02)03940-8. [DOI] [PubMed] [Google Scholar]
- 3.Eisbruch A, Marsh LH, Dawson LA, Bradford CR, Teknos TN, Chepeha DB, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Rad Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Chao KSC, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Rad Oncol Biol Phys. 2004;59:43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 5.Brown WT, Wu X, Fayad F, Fowler JF, Amendola BE, García S, et al. CyberKnife radiosurgery for stage I lung cancer: results at 36 months. Clin Lung Cancer. 2007;8:488–92. doi: 10.3816/CLC.2007.n.033. [DOI] [PubMed] [Google Scholar]
- 6.Shinohara ET, Geng L, Tan J, Chen H, Shir Y, Edwards E, et al. DNA-dependent protein kinase is a molecular target for the development of noncytotoxic radiation-sensitizing drugs. Cancer Res. 2005;65:4987–92. doi: 10.1158/0008-5472.CAN-04-4250. [DOI] [PubMed] [Google Scholar]
- 7.Banáth JP, MacPhail SH, Olive PL. Radiation sensitivity, H2AX phosphorylation and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines. Cancer Res. 2004;64:7144–9. doi: 10.1158/0008-5472.CAN-04-1433. [DOI] [PubMed] [Google Scholar]
- 8.Zeng M, Narayanan L, Xu XS, Prolla TA, Liskay M, Glazer PM. Ionizing radiation-induced apoptosis via separate pms2- and p53-dependent pathways. Cancer Res. 2000;60:4889–93. [PubMed] [Google Scholar]
- 9.Brown JM, Wilson W. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–47. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 10.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–64. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 11.Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006;66:8352–85. doi: 10.1158/0008-5472.CAN-06-0533. [DOI] [PubMed] [Google Scholar]
- 13.Ogawa K, Boucher Y, Kashiwagi S, Fukumura D, Chen D, Gerweck LE. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. 2007;67:4016–21. doi: 10.1158/0008-5472.CAN-06-4498. [DOI] [PubMed] [Google Scholar]
- 14.Liang K, Ang KK, Milas L, Hunter N, Fan Z. The epidermal growth factor receptor mediates radioresistance. Int J Rad Oncol Biol Phys. 2003;57:246–54. doi: 10.1016/s0360-3016(03)00511-x. [DOI] [PubMed] [Google Scholar]
- 15.Chinnaiyan P, Huang S, Vallabhaneni G, Armstrong E, Varambally S, Tomlins SA, et al. Mechanisms of enhanced radiation response following epidermal growth factor receptor signaling inhibition by erlotinib (Tarceva) Cancer Res. 2005;65:3328–35. doi: 10.1158/0008-5472.CAN-04-3547. [DOI] [PubMed] [Google Scholar]
- 16.Gow C-H, Chien C-R, Chang Y-L, Chiu Y-H, Kuo S-H, Shih J-Y, et al. Radiotherapy in lung adenocarcinoma with brain metastases: effects of activating epidermal growth factor receptor mutations on clinical response. Clin Cancer Res. 2008;14:162–8. doi: 10.1158/1078-0432.CCR-07-1468. [DOI] [PubMed] [Google Scholar]
- 17.Baumann M, Krause M. Targeting the epidermal growth factor receptor in radiotherapy: radiobiological mechanisms, preclinical and clinical results. Radiother Oncol. 2004;72:257–66. doi: 10.1016/j.radonc.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Burdelya LG, Komarova EA, Hill JE, Browder T, Tararova ND, Mavrakis L, et al. Inhibition of p53 response in tumor stroma improves efficacy of anticancer treatment by increasing antiangiogenic effects of chemotherapy and radiotherapy in mice. Cancer Res. 2006;66:9356–61. doi: 10.1158/0008-5472.CAN-06-1223. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–9. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 20.Budach W, Hartford A, Gioioso D, Freeman J, Taghian A, Suit HD. Tumors arising in SCID mice share enhanced radiation sensitivity of SCID normal tissues. Cancer Res. 1992;52:6292–6. [PubMed] [Google Scholar]
- 21.Budach W, Taghian A, Freeman J, Gioioso D, Suit HD. Impact of stromal sensitivity on radiation response of tumors. J Natl Cancer Inst. 1993;85:988–93. doi: 10.1093/jnci/85.12.988. [DOI] [PubMed] [Google Scholar]
- 22.Rafii S, Lyden D, Benezra R, Hattori K, Heissig B. Vascular and haematopoietic stem cells: novel targets for anti-angiogenesis therapy? Nat Rev Cancer. 2002;2:826–35. doi: 10.1038/nrc925. [DOI] [PubMed] [Google Scholar]
- 23.Nolan DJ, Ciarrocchi A, Mellick AS, Jaggi JS, Bambino K, Gupta S, et al. Bone marrow-derived endothelial progenitor cells are a major determinant of nascent tumor neovascularization. Genes Dev. 2007;21:1546–58. doi: 10.1101/gad.436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells repsonsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 25.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 26.Schlaeger TM, Qin Y, Fujiwara Y, Magram J, Sato TN. Vascular endothelial cell lineage-specific promoter in transgenic mice. Development. 1995;121:1089–98. doi: 10.1242/dev.121.4.1089. [DOI] [PubMed] [Google Scholar]
- 27.Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, et al. Universal GFP reporter for the study of vascular development. Genesis. 2000;28:75–81. doi: 10.1002/1526-968x(200010)28:2<75::aid-gene50>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, et al. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci USA. 2008;105:6620–5. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–95. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 30.Gothert JR, Gustin SE, van Eekelen AM, Schmidt U, Hall MA, Jane SM, et al. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104:1769–77. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- 31.Bagley RG, Walter-Yohrling J, Cao X, Weber W, Simons B, Cook BP, et al. Endothelial precursor cells as a model of tumor endothelium: characterization and comparison with mature endothelial cells. Cancer Res. 2003;63:5866–73. [PubMed] [Google Scholar]
- 32.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-beaing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 33.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 34.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotech. 2007;25:911–20. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 35.Milas L, Hunter N, Peters LJ. The tumor bed effect: dependence of tumor take, growth rate and metastasis on the time interval between irradiation and tumor cell transplantation. Int J Rad Oncol Biol Phys. 1987;13:379–83. doi: 10.1016/0360-3016(87)90012-5. [DOI] [PubMed] [Google Scholar]
- 36.Milas L, Hunter N, Peters LJ. Tumor bed effect-induced reduction of tumor radiocurability through the increase in hypoxic cell fraction. Int J Rad Oncol Biol Phys. 1989;16:139–42. doi: 10.1016/0360-3016(89)90021-7. [DOI] [PubMed] [Google Scholar]
- 37.Rofstad EK, Mathiesen B, Henriksen K, Kindem K, Galappathi K. The tumor bed effect: increased dissemination from hypoxia-induced upregulation of metastasis-promiting gene products. Cancer Res. 2005;65:2387–96. doi: 10.1158/0008-5472.CAN-04-3039. [DOI] [PubMed] [Google Scholar]
- 38.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–20. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giraudo E, Inoue M, Hanahan D. An amino-bisphosphonate targets MMP-9-expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Invest. 2004;114:623–33. doi: 10.1172/JCI22087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contribute to skin carcinogenesis. Cell. 2000;103:481–90. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabbany SY, Heissig B, Hattori K, Rafii S. Molecular pathways regulating mobilization of marrow-derived stem cells for tissue revascularization. Trends Mol Med. 2003;9:109–17. doi: 10.1016/s1471-4914(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 43.Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S, et al. VEGF-induced adult neovascularization: recruitment, retention and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 44.Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1+ stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–9. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varney ML, Olsen KJ, Mosley RL, Singh RK. Paracrine regulation of vascular endothelial growth factor-α expression during macrophage-melanoma cell interaction: role of monocyte chemotactic protein-1 and macrophage colony-stimulating factor. J Interferon Cytokine Res. 2005;25:674–83. doi: 10.1089/jir.2005.25.674. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, et al. Regulation of HIF-1α stability through S-nitrosylation. Mol Cell. 2007;26:63–74. doi: 10.1016/j.molcel.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gianelli U, Vener C, Raviele PR, Savi F, Somalvico F, Calori R, et al. VEGF expression correlates with microvessel densitity in Philadelphia chromosome-negative chronic myeloproliferative disorders. Am J Clin Pathol. 2007;128:966–73. doi: 10.1309/FP0N3LC8MBJUFFA6. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, et al. Abrogation of TGFbeta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milas L. Tumor bed effect in murine tumors: relationship to tumor take and tumor macrophage content. Radiat Res. 1990;123:232–6. [PubMed] [Google Scholar]
- 50.Milas L, Ito H, Hunter N, Jones S, Peters LJ. Retardation of tumor growth in mice caused by radiation-induced injury of tumor bed stroma: dependency on tumor type. Cancer Res. 1986;46:723–7. [PubMed] [Google Scholar]
- 51.Baumann M, Wurschmidt F, Twardy A, Beck-Bornholdt H-P. Impact of tumor stroma on expression of the tumor bed effect in R1H rat rhabdomyosarcoma. Radiat Res. 1994;140:432–6. [PubMed] [Google Scholar]
- 52.Ershler WB, Moore AL, Shore H, Gamelli RL. Transfer of age-associated restrained tumor growth in mice by old-to-young bone marrow transplantation. Cancer Res. 1984;44:5677–80. [PubMed] [Google Scholar]
- 53.Udagawa T, Birsner AE, Wood M, D’Amato RJ. Chronic suppression of angiogenesis following radiation exposure is independent of hematopoietic reconsistution. Cancer Res. 2007;67:2040–5. doi: 10.1158/0008-5472.CAN-06-2877. [DOI] [PubMed] [Google Scholar]
- 54.Tsai JH, Makonnen S, Feldman M, Sehgal CM, Maity A, Lee WMF. Ionizing radiation inhibits tumor neovascularization by inducing ineffective angiogenesis. Cancer Biol Ther. 2005;4:1395–400. doi: 10.4161/cbt.4.12.2331. [DOI] [PubMed] [Google Scholar]
- 55.Abdollahi A, Lipson KE, Han X, Krempien R, Trinh T, Weber KJ, et al. SU5416 and SU6668 attenuates the angiogenic effects of radiation-induced tumor cell growth factor production and amplify the direct anti-endothelial action of radiation in vitro. Cancer Res. 2003;63:3755–63. [PubMed] [Google Scholar]
- 56.Itasaka S, Komaki R, Herbst RS, Shibuya K, Shintani T, Hunter NR, et al. Endostatin improves radioresponse and blocks tumor revascularization after radiation therapy for A431 xenografts in mice. Int J Rad Oncol Biol Phys. 2007;67:870–8. doi: 10.1016/j.ijrobp.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zips D, Eicheler W, Geyer P, Hessel F, Dorfler A, Thames HD, et al. Enhanced susceptibility of irradiated tumor vessels to vascular endothelial growth factor receptor tyrosine kinase inhibition. Cancer Res. 2005;65:5374–9. doi: 10.1158/0008-5472.CAN-04-3379. [DOI] [PubMed] [Google Scholar]
- 58.Kozin SV, Winkler F, Garkavtsev I, Hicklin DJ, Jain RK, Boucher Y. Human tumor xenografts recurring after radiotherapy are more sensitive to anti-vascular endothelial growth factor receptor-2 treatment than treatment-naive tumors. Cancer Res. 2007;67:5076–82. doi: 10.1158/0008-5472.CAN-06-3664. [DOI] [PubMed] [Google Scholar]
- 59.Mancuso MR, Davis R, Norberg SM, O’Brien S, Sennino B, Nakahara T, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116:2610–21. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kobayashi M. The irradiation effects on the cytoskeletons of C3H/He mouse mammary tumor cells and vascular basement membrane in relation to vascular invasion: a model of intraoperative radiotherapy. Tohoku J Exp Med. 1988;154:71–89. doi: 10.1620/tjem.154.71. [DOI] [PubMed] [Google Scholar]
- 61.Kaliski A, Maggiorella L, Cengel KA, Mathe D, Rouffiac V, Opolon P, et al. Angiogenesis and tumor growth inhibition by a matrix metalloproteinase inhibitor targeting radiation-induced invasion. Mol Cancer Ther. 2005;4:1717–28. doi: 10.1158/1535-7163.MCT-05-0179. [DOI] [PubMed] [Google Scholar]
- 62.McNally LR, Rosenthal EL, Zhang W, Buchsbaum DJ. Therapy of head and neck squamous cell carcinoma with replicative adenovirus expressing tissue inhibitor of metalloproteinase-2 and chemoradiation. Cancer Gene Ther. 2008;16:246–55. doi: 10.1038/cgt.2008.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patni N, Patni S, Bapna A. The optimal use of granulocyte macrophage colony stimulating factor in radiation induced mucositis in head and neck squamous cell carcinoma. J Cancer Res Ther. 2005;1:136–41. doi: 10.4103/0973-1482.19589. [DOI] [PubMed] [Google Scholar]
- 64.Kuzhan O, Ozet A, Ulutin C, Komurcu S, Arpaci F, Ozturk B, et al. Survival benefit with GM-CSF use after high-dose chemotherapy in high-risk breast cancer. Tumori. 2007;93:550–6. doi: 10.1177/030089160709300606. [DOI] [PubMed] [Google Scholar]
- 65.Shojaei F, Zhong C, Wu X, Yu L, Ferrara N. Role of myeloid cells in tumor angiogenesis and growth. Trends Cell Biol. 2008;18:372–8. doi: 10.1016/j.tcb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 66.Di Maio M, Gridelli C, Gallo C, Shepherd F, Piantedosi FV, Cigolari S, et al. Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncol. 2005;6:669–77. doi: 10.1016/S1470-2045(05)70255-2. [DOI] [PubMed] [Google Scholar]
- 67.Okamoto R, Ueno M, Yamada Y, Takahashi N, Sano H, Suda T, et al. Hematopoietic cells regulate the angiogenic switch during tumorigenesis. Blood. 2005;105:2757–63. doi: 10.1182/blood-2004-08-3317. [DOI] [PubMed] [Google Scholar]
- 68.Aicher A, Rentsch M, Sasaki K, Ellwart JW, Fandrich F, Siebert R, et al. Nonbone marrow-derived circulating progenitor cells contribute to postnatal neovascularization following tissue ischemia. Circ Res. 2007;100:581–9. doi: 10.1161/01.RES.0000259562.63718.35. [DOI] [PubMed] [Google Scholar]
- 69.Bertolini F. Chemotherapy and the tumor microenvironment: the contribution of circulating endothelial cells. Cancer Metastasis Rev. 2008;27:95–101. doi: 10.1007/s10555-007-9110-y. [DOI] [PubMed] [Google Scholar]