Abstract
Background
Recently, germline variants of the melanocortin-1 receptor (MC1R) have been shown to be associated with an increased risk for BRAF mutant but not BRAF wild-type cutaneous melanoma. Similar to melanoma, BRAF mutations are also commonly found in papillary thyroid carcinomas. Furthermore, patients with melanoma have an increased risk for thyroid carcinoma and vice versa.
Methods
To determine whether MC1R variation also represents a risk factor for BRAF mutant thyroid carcinomas, we sequenced BRAF and MC1R in two separate case-control cohorts.
Results
We demonstrate that MC1R is expressed in normal and neoplastic thyroid epithelial cells, albeit at lower levels than in melanocytes. In the first cohort of 66 follicular (FTC) and 62 papillary thyroid carcinomas (PTC), and 128 matched controls from the San Francisco Bay Area we found no association between the number of MC1R variant alleles and thyroid cancer. Patients with BRAF mutated tumors had a higher frequency of MC1R variant alleles than their matched controls (p=0.039). However contrary to the findings in melanoma, the odds ratio for having a BRAF mutant cancer decreased from 3.9 for carriers of one MC1R allele to 1.5 for carriers of two or more alleles. As the frequency of MC1R alleles varies highly among different ethnic populations, we analyzed a second, ethnically more homogeneous cohort from Spain and Portugal, and found no association with PTC nor with BRAF mutated PTC.
Conclusion
Our data indicates that the strong association between BRAF mutations and MC1R variants previously found in melanoma does not extend to thyroid cancer.
Keywords: MC1R, BRAF, thyroid carcinoma, melanoma, susceptibility gene
Introduction
BRAF is a serine/tyrosine kinase in the MAP kinase signaling pathway that is frequently mutated in different types of cancer (1). In cutaneous melanoma its mutation frequency varies dramatically depending on anatomic site and sun exposure (2). We and collaborators recently showed that germline variants of the melanocortin-1 receptor (MC1R), a G-protein coupled receptor that responds to alpha-melanocyte stimulating hormone (α-MSH), confer a highly increased risk for the development of BRAF mutated melanomas but not BRAF wild-type melanomas in intermittently sun-exposed skin (3;4). MC1R is highly polymorphic throughout all ethnicities, with about 50% Caucasians carrying at least one variant allele, and a spectrum of less frequent and different variations in Asians and Africans (5–10). Variants have reduced signaling ability, contributing to distinct phenotypic traits such as fair skin, freckling, and red hair. MC1R variations have been shown to be melanoma risk factors (10), even beyond their effect on pigmentation (11;12).
The mechanism of the gene-gene interaction between germline MC1R variants and somatic BRAF mutations in melanoma rendering MC1R variants a critical predisposing factor for BRAF-driven melanomas is currently unclear. Several lines of evidence suggested that the gene-gene interaction between MC1R and BRAF in melanoma may extend to other cancers. Animal models show that reduced MC1R signaling results in an increased cancer incidence. For example, in mice, activating mutations of the agouti signaling protein gene (ASIP), an inhibitor of MC1R, result in yellow-orange coat color and a significantly increased incidence of mammary-gland tumors and liver carcinoma (13–16). In the human, BRAF mutations are also commonly found in papillary thyroid carcinomas (17), which occur more frequently in Caucasians. Most interestingly, patients with melanoma have a higher risk of thyroid cancer, and vice versa (18;19), indicating a possible common susceptibility mechanism. MC1R is expressed in the thyroid gland, and other tissues in levels comparable to the skin (20). We hypothesized that impaired MC1R signaling may have more far reaching cancer promoting consequences than its impact on skin pigmentation.
Material and Methods
Real-Time Quantitative PCR
To test whether MC1R is expressed in thyroid epithelial cells, we performed real-time quantitative PCR (qPCR) on mRNA from 11 normal thyroid tissue samples, 20 papillary thyroid carcinoma samples, both extracted from frozen thyroid cancer surgery specimen, and from two foreskin melanocyte cell cultures. Total RNA was prepared by TRIZOL extraction (Invitrogen, Carlsbad, CA). A total of 123 ng of total RNA were reverse-transcribed using the RT script cDNA synthesis kit (USB, Cleveland, OH). Real-time quantitative qPCR was used to measure mRNA expression levels normalized to GUS mRNA expression. The PCR primers and probes for the genes were purchased from Applied Biosystems (Assay-on-Demand kit; Foster City, CA). All quantitative qPCR reactions were performed in triplicate.
Patients and Control Cohorts
A first cohort of 128 thyroid carcinomas (66 follicular thyroid carcinomas (FTC), and 62 papillary thyroid carcinomas (PTC), median age 49, range 15 to 96) from the archives of the Department of Pathology at UCSF, and 128 gender and ethnicity matched controls from the DNA bank at UCSF (103 female, 25 male; 6 African American, 22 Asian, 87 Caucasian, 13 Latino) was assembled. The study was approved by the institutional review board of the University of California, San Francisco.
The second cohort of 74 PTC and 74 age- and gender-matched controls (57 female, 17 male, median age PTC 41 years, controls 44 years) was from Portugal and Spain and previously described (21). The study was approved by the institutional review board of the University of Porto, Portugal.
Sequence analysis
For cases, DNA was extracted from micro-dissected paraffin embedded tissue sections, and BRAF exon 15 and the entire MC1R gene (5 amplicons) were amplified from tumor DNA and neighboring normal tissue DNA, respectively, as published previously (3). Micro-dissection of the first cohort was performed in San Francisco, for the second cohort in Porto. For the controls, MC1R was amplified form peripheral blood lymphocyte DNA. PCR products were purified using ExoSAP-IT (USB Corporation, Cleveland, OH) and sequenced directly using the ABI BigDye v3.1 dye terminator sequencing chemistry and an ABI PRISM 3730xl capillary DNA analyzer (Applied Biosystems, Foster City, CA).
Statistical analysis
Standard univariate statistical tests were performed using STATA 9.2 (StataCorp, College Station, TX). All p-values were two-sided, and p<0.05 was considered statistically significant. As the functional impairment of individual MC1R variants is not entirely clear, we classified the MC1R genotype for statistical analysis as “0” for consensus sequence, “1” for one for any one variant allele, and “2” for two or more variants. In a second, alternative analysis we classified MC1R variants according to their associated phenotype as red-hair (R151C, R160W, and D294H), non-red-hair (all other variants), and consensus sequence.
Results
Expression of MC1R in normal thyroid and thyroid cancer
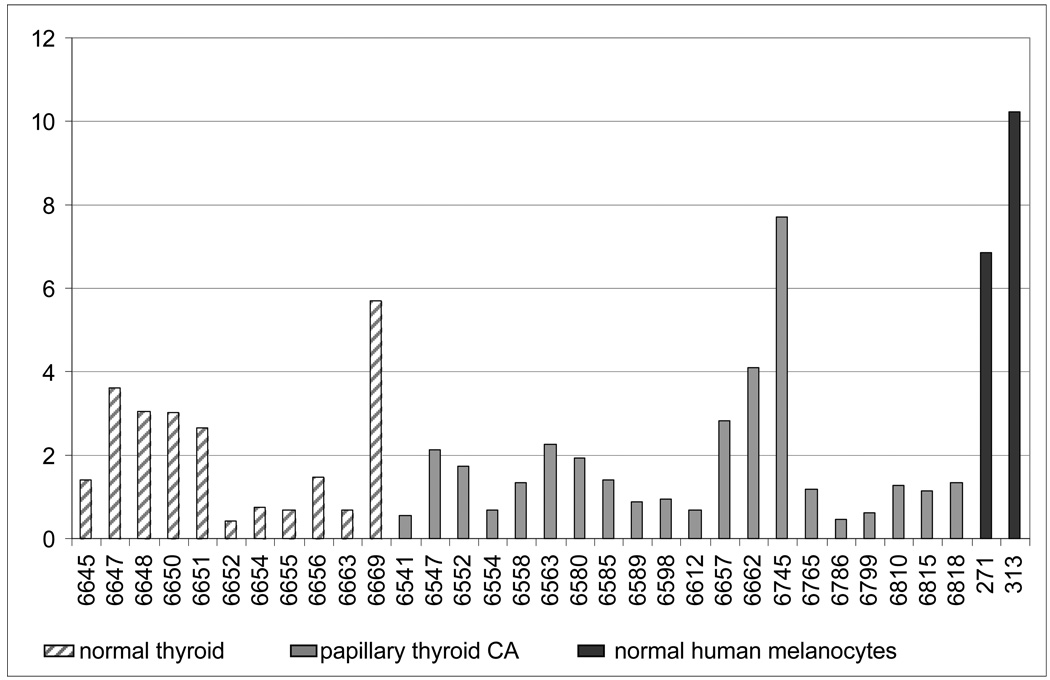
Overall, in benign and malignant thyroid tissue the expression levels of MC1R were lower than in cultures of normal human melanocytes, with mean MC1R expression levels of 2.1 in normal thyroid tissue, 1.8 in PTC, and 8.5 in foreskin melanocyte cultures (Figure 1). In spite of the fact that different sample preparation techniques might have added certain variability to our results (frozen tissue vs. cultured cells), we conclude that MC1R is expressed in benign and malignant thyroid tissue, however at lower levels than in melanocyte cultures. Hence we decided to proceed and investigate the possibility of MC1R variation as a risk factor for BRAF mutant thyroid cancers.
Figure 1.
qPCR analysis of MC1R mRNA expression relative to GUS mRNA in 11 normal thyroid tissue samples, 20 papillary thyroid carcinoma, and normal human melanocytes.
Sequence Analysis
First study cohort from the San Francisco Bay Area
As expected, the frequency of BRAF mutations was significantly higher in papillary than follicular thyroid carcinomas (69.4% vs. 3.0%, respectively). All mutations in exon 15 were V600E mutations. There was no association between MC1R genotype and thyroid cancer as a whole or within the PTC or FTC subgroups. Similarly, no association was found when the analysis was restricted to Caucasians or Asians (Table 1). Other ethnic groups were too small to be analyzed.
Table 1.
Analysis of the San Francisco Bay Area cohort independently of ethnicity and BRAF mutation status.
| Tumors | Papillary thyroid carcinoma | Follicular thyroid carcinoma | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) |
Cases | Controls | OR (95% CI) |
|
|
MC1R consensus |
12 | 20 | reference | 13 | 10 | reference |
| 1 MC1R variant |
26 | 16 | 2.708 (1.049 to 6.994) |
29 | 26 | 0.858 (0.322 to 2.286) |
| ≥ 2 MC1R variants |
24 | 26 | 1.539 (0.622 to 3.805) |
24 | 30 | 0.615 (0.230 to 1.646) |
| total | 62 | 62 | p = 0.108* | 66 | 66 | p = 0.543* |
Given are odds ratios for papillary (PTC) and follicular (FTC) thyroid carcinoma depending on the number of MC1R variants compared to the matched control cohort.
Pearson chi-square test.
By contrast, we found a higher frequency of MC1R alleles in patients with BRAF mutated tumors compared to their matched controls (Pearson chi-square test, p=0.039). The corresponding odds ratios were 3.9 (95% confidence interval 1.3 to 12.3) for carriers of one MC1R variant allele compared to MC1R consensus sequence carriers, but only 1.5 (95% confidence interval 0.5 to 4.4) for two or more variants (Table 2) When this analysis was restricted to Caucasians a similar trend was seen (p=0.062). By contrast, when MC1R alleles were stratified by red-hair or non-red-hair alleles no significant association was found.
Table 2.
Analysis of the San Francisco Bay Area cohort independently of ethnicity and tumor type (PTC and FTC included).
| Tumors | BRAF mutant | BRAF wild-type | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) |
Cases | Controls | OR (95% CI) |
|
|
MC1R consensus |
8 | 15 | reference | 17 | 15 | reference |
| 1 MC1R variant |
21 | 10 | 3.938 (1.2572 to 12.3322) |
34 | 32 | 0.938 (0.4024 to 2.1839) |
| ≥ 2 MC1R variants |
16 | 20 | 1.5 (0.5089 to 4.4213) |
32 | 36 | 0.784 (0.3379 to 1.8202) |
| total | 45 | 45 | p = 0.039* | 83 | 83 | p = 0.810* |
Given are odds ratios for BRAF mutant and BRAF wild-type thyroid carcinoma depending on the number of MC1R variants compared to the matched control cohort.
Pearson chi-square test.
Second study cohort from Portugal and Spain
In the second, ethnically more homogeneous cohort 32 of 74 (43.2%) PTCs harbored a V600E BRAF mutation. The frequency of MC1R variants was almost identical between cases and controls (Table 3). Similarly, no significant differences were found when the MC1R genotype was stratified as red or non-red hair variants.
Table 3.
Analysis of the Portuguese and Spanish cohort consisting of PTC cases only.
| Tumors | BRAF mutant or wild-type | BRAF mutant | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI) |
Cases | Controls | OR (95% CI) | |
|
MC1R consensus |
41 | 40 | reference | 21 | 22 | reference |
| 1 MC1R variant |
26 | 26 | 0.976 (0.486 to 1.958) |
8 | 8 | 1.048 (0.3324 to 3.302) |
| ≥ 2 MC1R variants |
7 | 8 | 0.854 (0.283 to 2.575) |
3 | 2 | 1.571 (0.2382 to 10.3653) |
| total | 74 | 74 | p = 0.961* | 32 | 32 | p = 0.894* |
Given are odds ratios for any BRAF mutation status and for BRAF mutant cases only, depending on the number of MC1R variants compared to the matched control cohort.
Pearson chi-square test.
Discussion
Our analysis of the effect of germline variations of MC1R on somatic mutations of BRAF in thyroid tissue indicate that the strong association previously found in melanoma does not exist in thyroid cancer. Whereas patients from the San Francisco Bay Area with BRAF-mutant carcinomas tended to have a higher frequency of MC1R alleles than the corresponding controls, this association did not replicate in the ethnically more homogenous cohort of PTCs from Portugal and Spain. Further, the pattern of association found in the first cohort differs significantly from our prior findings in melanoma, where the odds ratios increased with the number of alleles as well as from weak (non-red-hair) to strong (red-hair) alleles. It is therefore likely that the well documented strong variation of MC1R alleles among ethnic populations has resulted in a spurious association between MC1R and BRAF in the first cohort. The sample sizes of the two cohorts analyzed in the present study were larger than the two cohorts that were used to show a statistically significant correlation between BRAF mutation and MC1R variants in melanoma (3). Based on the hypothesis of an equally strong association in thyroid carcinoma, the present study should have been able to show a correlation, or at least a clear trend.
In summary, we conclude that although MC1R is expressed in the thyroid it is unlikely to affect the mutation frequency of BRAF present in thyroid carcinoma.
Table 4.
Frequency of MC1R variant alleles in the two cohorts from the San Francisco Bay Area and Portugal and Spain.
| n (individuals) | San Francisco Cases | San Francisco Controls | Portugal & Spain | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| African American |
Asian | Caucasian | Latino | All ethnicities |
African American |
Asian | Caucasian | Latino | All ethnicities |
Cases | Controls | |
| 6 | 22 | 87 | 13 | 128 | 6 | 22 | 87 | 13 | 128 | 74 | 74 | |
| Variants | ||||||||||||
| V60L | - | - | 22 | 3 | 25 | - | - | 26 | 3 | 29 | 15 | 22 |
| R67Q | - | - | - | - | - | - | 1 | - | - | 1 | - | - |
| D84E | - | - | 3 | - | 3 | - | - | 1 | - | 1 | - | 1 |
| V92M | - | 16 | 19 | 1 | 36 | - | 9 | 15 | 5 | 29 | 13 | 5 |
| I120T | - | 4 | - | - | 4 | - | 3 | - | - | 3 | 1 | - |
| V122M | - | - | 1 | - | 1 | - | - | - | - | - | - | 1 |
| S131N | - | - | - | - | - | - | - | 1 | - | 1 | - | - |
| R142H | - | - | - | - | - | - | - | 2 | - | 2 | 1 | 1 |
| R151C | - | - | 10 | - | 10 | - | - | 16 | 1 | 17 | 4 | 3 |
| I155T | - | - | - | - | - | - | - | 1 | - | 1 | - | - |
| V156L | - | - | - | - | - | 1 | - | - | - | 1 | - | - |
| R160W | - | - | 13 | - | 13 | - | - | 14 | - | 14 | 1 | - |
| R163Q | 1 | 22 | 14 | 9 | 46 | - | 32 | 9 | 9 | 50 | 3 | 6 |
| A166G | - | - | - | - | - | - | 1 | - | - | 1 | - | - |
| S172I | - | - | - | - | - | - | - | 1 | - | 1 | - | - |
| Y183D | - | - | - | - | - | - | - | 1 | - | 1 | - | - |
| A198P | - | - | - | - | - | - | - | - | 1 | 1 | - | - |
| R213W | - | - | - | - | - | - | - | - | - | - | 1 | - |
| G248D | - | - | - | - | - | - | - | - | - | - | - | 2 |
| P256S | - | - | - | 1 | 1 | - | - | - | - | - | - | - |
| D294H | 1 | - | 3 | 1 | 5 | - | - | 2 | - | 2 | 3 | 1 |
| T308M | - | 1 | - | - | 1 | - | - | - | - | - | - | - |
| C315S | - | - | 1 | - | 1 | - | - | - | - | - | - | - |
| insA726 | - | - | - | - | - | - | - | 1 | - | 1 | - | - |
| all variants | 2 | 43 | 86 | 15 | 146 | 1 | 46 | 90 | 19 | 156 | 42 | 42 |
Highlighted in grey are the “red-hair variants”.
Acknowledgements
Grant support: UCSF Stewart Trust Cancer Research Program Award to BCB, Deutsche Forschungsgemeinschaft stipend (BA 2852/1-1) to JB, Portuguese Science and Technology Foundation (FCT) PhD grant (SFRH/BD/13055/2003) to VT, and FCT project fund (POCI/SAU-OBS/56175/2004) to PS.
References
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J.Natl.Cancer Inst. 2003;95:1878–1890. doi: 10.1093/jnci/djg123. [DOI] [PubMed] [Google Scholar]
- 3.Landi MT, Bauer J, Pfeiffer RM, et al. MC1R Germline Variants Confer Risk for BRAF-Mutant Melanoma. Science. 2006;313:521–522. doi: 10.1126/science.1127515. [DOI] [PubMed] [Google Scholar]
- 4.Fargnoli MC, Pike K, Pfeiffer RM, et al. MC1R Variants Increase Risk of Melanomas Harboring BRAF Mutations. J.Invest Dermatol. 2008 doi: 10.1038/jid.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altshuler D, Brooks LD, Chakravarti A, Collins FS, Daly MJ, Donnelly P. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerstenblith MR, Goldstein AM, Fargnoli MC, Peris K, Landi MT. Comprehensive evaluation of allele frequency differences of MC1R variants across populations. Hum.Mutat. 2007;28:495–505. doi: 10.1002/humu.20476. [DOI] [PubMed] [Google Scholar]
- 7.John PR, Makova K, Li WH, Jenkins T, Ramsay M. DNA polymorphism and selection at the melanocortin-1 receptor gene in normally pigmented southern African individuals. Ann.N Y.Acad.Sci. 2003;994:299–306. doi: 10.1111/j.1749-6632.2003.tb03193.x. [DOI] [PubMed] [Google Scholar]
- 8.Motokawa T, Kato T, Hongo M, et al. Characteristic MC1R polymorphism in the Japanese population. J Dermatol Sci. 2006;41:143–145. doi: 10.1016/j.jdermsci.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Peng S, Lu XM, Luo HR, Xiang-Yu JG, Zhang YP. Melanocortin-1 receptor gene variants in four Chinese ethnic populations. Cell Res. 2001;11:81–84. doi: 10.1038/sj.cr.7290070. [DOI] [PubMed] [Google Scholar]
- 10.Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat.Genet. 1995;11:328–330. doi: 10.1038/ng1195-328. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy C, ter Huurne J, Berkhout M, et al. Melanocortin 1 receptor (MC1R) gene variants are associated with an increased risk for cutaneous melanoma which is largely independent of skin type and hair color. J Invest Dermatol. 2001;117:294–300. doi: 10.1046/j.0022-202x.2001.01421.x. [DOI] [PubMed] [Google Scholar]
- 12.Brudnik U, Branicki W, Wojas-Pelc A, Kanas P. The contribution of melanocortin 1 receptor gene polymorphisms and the agouti signalling protein gene 8818A>G polymorphism to cutaneous melanoma and basal cell carcinoma in a Polish population. Exp.Dermatol. 2008 doi: 10.1111/j.1600-0625.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 13.Heston WE, Vlahaki G. Influence of the Ay gene on mammary-gland tumors, hepatomas, and normal growth in mice. J Natl.Cancer Inst. 1961;26:969–983. [PubMed] [Google Scholar]
- 14.Kuklin AI, Mynatt RL, Klebig ML, et al. Liver-specific expression of the agouti gene in transgenic mice promotes liver carcinogenesis in the absence of obesity and diabetes. Mol.Cancer. 2004;3:17. doi: 10.1186/1476-4598-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolff GL, Roberts DW, Galbraith DB. Prenatal determination of obesity, tumor susceptibility, and coat color pattern in viable yellow (Avy/a) mice. The yellow mouse syndrome. J Hered. 1986;77:151–158. doi: 10.1093/oxfordjournals.jhered.a110206. [DOI] [PubMed] [Google Scholar]
- 16.Klebig ML, Wilkinson JE, Geisler JG, Woychik RP. Ectopic expression of the agouti gene in transgenic mice causes obesity, features of type II diabetes, and yellow fur. Proc.Natl.Acad.Sci.U.S.A. 1995;92:4728–4732. doi: 10.1073/pnas.92.11.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr.Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 18.Canchola AJ, Horn-Ross PL, Purdie DM. Risk of second primary malignancies in women with papillary thyroid cancer. Am.J Epidemiol. 2006;163:521–527. doi: 10.1093/aje/kwj072. [DOI] [PubMed] [Google Scholar]
- 19.Goggins W, Daniels GH, Tsao H. Elevation of thyroid cancer risk among cutaneous melanoma survivors. Int.J Cancer. 2006;118:185–188. doi: 10.1002/ijc.21300. [DOI] [PubMed] [Google Scholar]
- 20.Su AI, Wiltshire T, Batalov S, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc.Natl.Acad.Sci.U.S.A. 2004;101:6062–6067. doi: 10.1073/pnas.0400782101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trovisco V, Soares P, Preto A, et al. Type and prevalence of BRAF mutations are closely associated with papillary thyroid carcinoma histotype and patients' age but not with tumour aggressiveness. Virchows Arch. 2005;446:589–595. doi: 10.1007/s00428-005-1236-0. [DOI] [PubMed] [Google Scholar]