Abstract
One of the principle ways in which reef building corals are likely to cope with a warmer climate is by changing to more thermally tolerant endosymbiotic algae (zooxanthellae) genotypes. It is highly likely that hosting a more heat-tolerant algal genotype will be accompanied by tradeoffs in the physiology of the coral. To better understand one of these tradeoffs, growth was investigated in the Indo-Pacific reef-building coral Acropora millepora in both the laboratory and the field. In the Keppel Islands in the southern Great Barrier Reef this species naturally harbors nrDNA ITS1 thermally sensitive type C2 or thermally tolerant type D zooxanthellae of the genus Symbiodinium and can change dominant type following bleaching. We show that under controlled conditions, corals with type D symbionts grow 29% slower than those with type C2 symbionts. In the field, type D colonies grew 38% slower than C2 colonies. These results demonstrate the magnitude of trade-offs likely to be experienced by this species as they acclimatize to warmer conditions by changing to more thermally tolerant type D zooxanthellae. Irrespective of symbiont genotype, corals were affected to an even greater degree by the stress of a bleaching event which reduced growth by more than 50% for up to 18 months compared to pre-bleaching rates. The processes of symbiont change and acute thermal stress are likely to act in concert on coral growth as reefs acclimatize to more stressful warmer conditions, further compromising their regeneration capacity following climate change.
Introduction
Coral reefs are generally thought to be highly vulnerable to climate change as they live in a narrow range of thermal tolerance. Recent research however, indicates that scleractinian (reef-building) corals may have considerable scope for acclimatization to warmer conditions [1], [2], [3]. The key to acclimatization may be their capacity to ‘shuffle’ the levels of symbiotic zooxanthellae genotypes (taxonomic units below the clade level) that are now known to occur within the tissues of most corals [4], [5]. Symbiont shuffling occurs when the relative dominance of symbiont types changes. Under temperature stress, thermally sensitive symbiont types are displaced in favor of thermally tolerant types [6]. For instance, changing to thermally tolerant Symbiodinium type D in one study was found to increase thermal tolerance between 1.0–1.5°C in a common Indo-Pacific coral species, A. millepora [7]. Symbiont change on reefs must essentially involve a community shift in the symbionts of multiple coral species to realize an increase the thermal tolerance of the entire reef [3]. Field studies have yet to demonstrate how widespread the phenomenon of shuffling is, whether all corals have the ability to shuffle symbiont types, or what ecological benefits may result from ‘new’ host-symbiont combinations.
The symbiont type harbored by reef corals can influence the nutritional status and overall fitness of the holobiont. Corals rely heavily on their symbionts for their energy requirements through the translocation of photosynthetically fixed carbon (estimated to be as high as ∼95% of the total energy requirement) [8], [9]. In zooxanthellate corals, some of this energy is used to drive carbonate accretion [10]. This deposition of carbonate (calcification) is the process by which reef-builders form their hard skeletons. Processes that affect photosynthesis have the potential to have a simultaneous effect on host calcification [11]. The link between symbiont genotype, photosynthetic function and carbon fixation has already been established. For instance, Cantin et al. [12] found a positive correlation between the tissue incorporation of radio-labeled carbon (which represents photosynthetically-derived carbon-based energy) and the relative maximum rate of electron transport through photosystem II (rETRmax, a secondary measure of photosynthetic function) in A. millepora juveniles with C1 and D symbionts. Type D juveniles had lower rETRmax and fixed less 14C than those with type C1. Because photosynthesis is directly related to the amount of energy available to the host for calcification, this is likely to reflect in lower calcification and skeletal growth rate. This is supported by studies of growth in juvenile A. millepora in which increased skeletal growth has been demonstrated in type C1 compared to type D symbionts [13], [14]. Clearly, predominant symbiont genotype can influence host physiology. Symbiont community change by shuffling therefore has the potential to influence the growth dynamics of entire reef communities. While these studies show that symbiont type can affect the growth of juvenile corals, differences in the growth rates of adult corals with thermo-tolerant and –sensitive zooxanthellae types have so far not been studied.
The growth rate of reef-building corals has a substantial influence on the resilience and regeneration capacity of tropical reefs. The hard skeletons of scleractinian corals form the framework of reefs, providing food and habitat for other marine organisms [15]. Following disturbance, such as bleaching, the growth rate of the key structural corals, such as species belonging to the genera Acropora and Pocillopora, is the most important factor in reef recovery [16], [17]. Frequent disturbance without strong coral re-growth results in phase shifts to macro-algal and soft coral-dominated communities [18]. The growth rate of hard coral species is an important factor in preventing these phase shifts [19]. Factors that affect the process of calcium carbonate accretion in structural corals, such as Pocillopora and Acropora, have downstream influence on the habitat and food supply of other marine organisms [20]. The warmer and potentially more acidic marine conditions that are predicted to occur with climate change pose an as yet unquantifiable threat to the carbonate structure of coral reefs [21]. Retardation of coral re-growth by symbiont change could exacerbate these processes, accelerating the demise of coral reefs as we know them.
This study investigates one of the most important elements of reef resilience to climate change, namely skeletal growth. A. millepora was chosen for this study as this is an abundant and dominant reef-builder on the leeward shores of islands in the Keppel region of the Great Barrier Reef [6], [22]. Colonies on these reefs naturally host thermally-sensitive type C2 Symbiodinium, thermally-tolerant type D or a combination of both types. The results indicate that the growth rate of A. millepora is significantly affected in hosts containing the thermally tolerant symbiont type compared to those with the thermally sensitive type but that the stress of the bleaching confounds any costs or benefits of symbiont type.
Results
Laboratory study
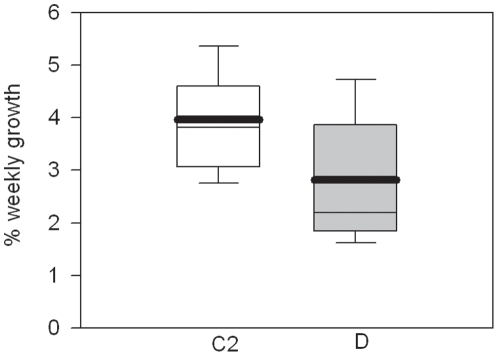
The skeletal growth rate of A. millepora explants in the laboratory varied significantly with predominant symbiont genotype. The buoyant weight gain of explants with type D symbionts for the four weeks of the study was 29% less than that gained by C2 explants (p<0.05, Figure 1, Table 1). There was no significant effect of temperature on explant growth and no significant interaction between symbiont type and temperature treatment.
Figure 1. Growth rates of Acropora millepora explants in the laboratory.
Acropora millepora explants with type D symbionts had significantly lower growth than explants with type C2 symbionts. Boxes represent the buoyant weight gained over a week expressed as a percentage of the initial buoyant weight of the explant. White boxes represent colonies with type C2 symbionts and grey boxes represent colonies with type D symbionts. Box boundaries represent the 75th and 25th percentiles. Thick lines within the boxplots represent the mean and thin lines represent the median. Whisker bars above and below the boxes represent the 95th and 5th percentiles.
Table 1. ANOVA of the growth rate of Acropora millepora with C2 and D symbionts.
| SS | df | Mean Square | F | Sig. | |
| Corrected Model | 47.69a | 3 | 15.90 | 8.3 | 0.000 |
| Intercept | 1449.72 | 1 | 1449.72 | 760.1 | 0.000 |
| Type | 41.17 | 1 | 41.17 | 21.6 | 0.000 |
| Temperature | 4.30 | 1 | 4.30 | 2.3 | 0.136 |
| Type * Temperature | 3.01 | 1 | 3.01 | 1.6 | 0.211 |
| Error | 236.51 | 124 | 1.91 | ||
| Total | 1819.63 | 128 | |||
| Corrected Total | 284.20 | 127 |
a. R2 = 0.168 (Adjusted R2 = 0.148).
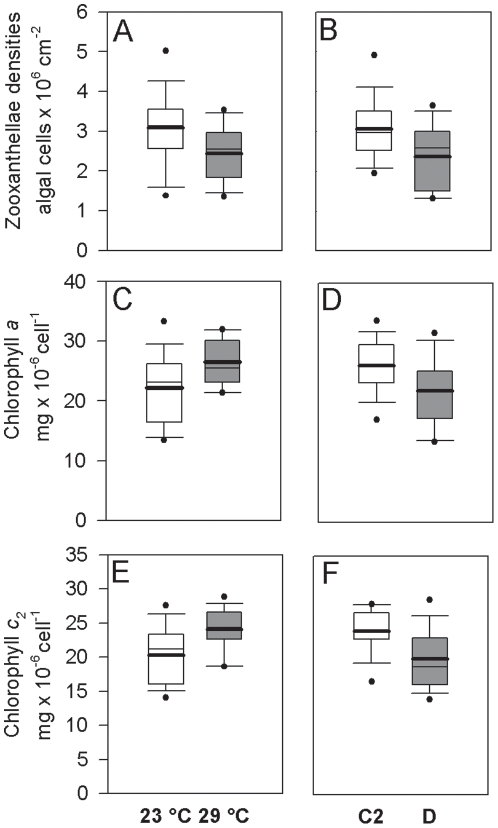
Chlorophyll and zooxanthellae density measurements varied significantly with the predominant symbiont genotype and with treatment temperature but there were no significant interactions (Figure 2 a–f, Table 2). Zooxanthellae densities for type D explants (averaged across temperature treatments) were 22% lower than densities for C2 explants (p<0.05). Zooxanthellae densities at 29°C were 21% lower than densities at 23°C (p<0.05). The algal cell chlorophyll a content of type D explants was 16% lower than for type C2 explants (p<0.05) while chlorophyll c2 for type D explants was 17% lower (p<0.05). At 29°C, the mean algal chlorophyll a was 20% higher than concentrations at 23°C (p<0.05) while chlorophyll c2 was 19% higher (p<0.05). At the end of the laboratory study, zooxanthellae densities and algal cell chlorophyll a and c2 compared well with values measured in nearby colonies sampled in the field at Miall Island (data not shown). Fv/Fm measurements of C2 and D colonies were stable between 0.6 and 0.8 throughout the experiment indicating that there was no measureable photo-damage and/or photo-inhibition [23], [24], [25], [26], [27], [28], [29].
Figure 2. Algal densities and chlorophyll pigments for Acropora millepora in the laboratory.
Boxplots showing the significant effects of (a, c, e) temperature on zooxanthellae densities and chlorophyll a and c2, and (b, d, f) symbiont genotype on zooxanthellae densities and chlorophyll a and c2, in Acropora millepora explants during a laboratory growth experiment. White boxes represent colonies with type C2 symbionts or those at 23°C and grey boxes represent colonies with type D symbionts or those at 29°C. Box boundaries represent the 75th and 25th percentiles. Thick lines within the boxplots represent the mean and thin lines represent the median. Whisker bars above and below the boxes represent the 95th and 5th percentiles. Missing and out of range values are not shown. Dots represent data that fall outside the confidence limits.
Table 2. ANOVA of symbiont densities and algal chlorophyll a and c2 for Acropora millepora in the laboratory.
| Dependent Variable | SS | df | Mean Square | F | Sig. | |
| Corrected Model | Zooxanthellae | 7.218a | 3 | 2.41 | 3.6 | 0.025 |
| Chlorophyll a | 348.15b | 3 | 116.05 | 5.2 | 0.005 | |
| Chlorophyll c2 | 262.35c | 3 | 87.45 | 8.0 | 0.001 | |
| Intercept | Zooxanthellae | 233.38 | 1 | 233.38 | 349.9 | 0.000 |
| Chlorophyll a | 18275.94 | 1 | 18275.94 | 822.0 | 0.000 | |
| Chlorophyll c2 | 15162.09 | 1 | 15162.09 | 1387.3 | 0.000 | |
| Type | Zooxanthellae | 3.77 | 1 | 3.77 | 5.7 | 0.025 |
| Chlorophyll a | 142.50 | 1 | 142.50 | 6.4 | 0.017 | |
| Chlorophyll c2 | 130.76 | 1 | 130.76 | 12.0 | 0.002 | |
| Temp | Zooxanthellae | 3.21 | 1 | 3.21 | 4.8 | 0.037 |
| Chlorophyll a | 174.25 | 1 | 174.25 | 7.8 | 0.009 | |
| Chlorophyll c2 | 125.34 | 1 | 125.34 | 11.5 | 0.002 | |
| Type * Temp | Zooxanthellae | 0.07 | 1 | 0.07 | 0.1 | 0.753 |
| Chlorophyll a | 51.99 | 1 | 51.99 | 2.3 | 0.137 | |
| Chlorophyll c2 | 15.07 | 1 | 15.07 | 1.4 | 0.250 | |
| Error | Zooxanthellae | 18.67 | 28 | 0.67 | ||
| Chlorophyll a | 622.57 | 28 | 22.23 | |||
| Chlorophyll c2 | 306.02 | 28 | 10.93 | |||
| Total | Zooxanthellae | 270.57 | 32 | |||
| Chlorophyll a | 19948.86 | 32 | ||||
| Chlorophyll c2 | 16330.80 | 32 | ||||
| Corrected Total | Zooxanthellae | 25.89 | 31 | |||
| Chlorophyll a | 970.72 | 31 | ||||
| Chlorophyll c2 | 568.38 | 31 |
a. R2 = 0.279 (Adjusted R2 = 0.202).
b. R2 = 0.359 (Adjusted R2 = 0.290).
c. R2 = 0.462 (Adjusted R2 = 0.404).
Field study
First experiment
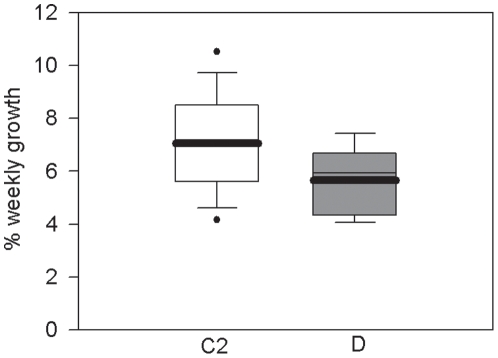
The first field growth experiment was conducted before a major bleaching event which affected the Keppel Islands in February 2006 [30]. At this time, the weekly growth rate of A. millepora colonies varied significantly with symbiont genotype (Figure 3, Table 3). The growth rate of D colonies was 38% lower than that of C2 colonies (p<0.05).
Figure 3. Growth of Acropora millepora with C2 and D symbionts in the field.
Boxplots showing the significantly higher weekly growth rate of Acropora millepora colonies with type C2 symbionts compared to colonies with type D symbionts before a bleaching event. Boxes represent the weekly buoyant weight gain as a percentage of the initial buoyant weight of the colony. White boxes represent colonies with type C2 symbionts and grey boxes represent colonies with type D symbionts. Box boundaries represent the 75th and 25th percentiles. Thick lines within the boxplots represent the mean and thin lines represent the median. Whisker bars above and below the boxes represent the 95th and 5th percentiles. Missing and out of range values are not shown. Dots represent data that fall outside the confidence limits.
Table 3. ANOVA of weekly growth of Acropora millepora colonies before a bleaching event.
| SS | df | Mean Square | F | Sig. | |
| Corrected Model | 0.71a | 1 | 0.71 | 4.9 | 0.032 |
| Intercept | 4.43 | 1 | 4.43 | 30.6 | 0.000 |
| Type | 0.71 | 1 | 0.71 | 4.9 | 0.032 |
| Error | 5.65 | 39 | 0.15 | ||
| Total | 23.94 | 41 | |||
| Corrected Total | 6.37 | 40 |
a. R2 = 0.112 (Adjusted R2 = 0.089).
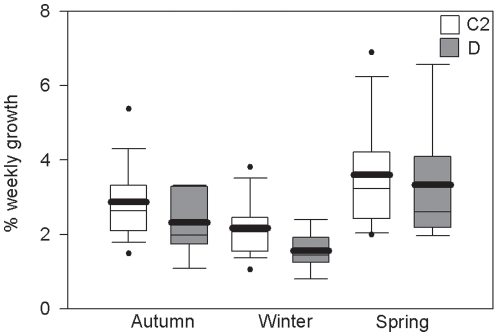
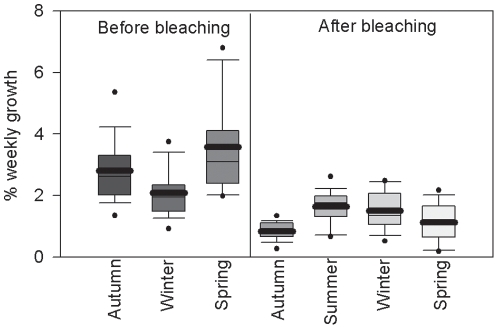
The growth rates of A. millepora colonies in the field also varied significantly with season (Figure 4, Table 4). Growth rates were higher in spring and autumn than in winter. Growth rates were 27% higher in spring than in autumn (p<0.05) and 71% higher in spring than in winter (p<0.0.5) and 34% higher in autumn than in winter (p<0.05). There was no interaction between symbiont type and season.
Figure 4. Seasonal growth of Acropora millepora colonies.
The growth rate of colonies of Acropora millepora varied significantly with season in the year before a bleaching event. Boxes represent the weekly buoyant weight gain expressed as a percentage of the initial buoyant weight of the colony. White boxes represent colonies with type C2 symbionts and grey boxes represent colonies with type D symbionts. Box boundaries represent the 75th and 25th percentiles. Thick lines within the boxplots represent the mean and thin lines represent the median. Whisker bars above and below the boxes represent the 95th and 5th percentiles. Missing and out of range values are not shown. Dots represent data that fall outside the confidence limits.
Table 4. ANOVA of seasonal growth of Acropora millepora before a bleaching event.
| SS | df | Mean Square | F | Sig. | |
| Season | 3.21 | 2 | 1.61 | 34.6 | 0.000 |
| Season * Type | 0.11 | 2 | 0.06 | 1.2 | 0.311 |
| Error(Season) | 3.43 | 74 | 0.05 |
Second experiment
The bleaching event in early 2006 severely affected A. millepora growth rates irrespective of symbiont genotype (which correlated with bleaching severity). The growth of A. millepora colonies followed a similar seasonal pattern to the first study before the bleaching however colonies gained only half of the buoyant weight (p<0.05, Figure 5, Table 5). The highest growth rate in 2006 was in spring, six months after the bleaching event, 76% lower than the spring of 2005 before the bleaching (p<0.05, Table 5). Growth rate in autumn 2006 (12 months after the bleaching) was 46% lower than in autumn 2005 before bleaching (p<0.05). The winter 2006 growth rate (nearly 18 months after the bleaching), was 47% lower than the growth rate in the winter 2005 before the bleaching (p<0.05). The highest growth rates of A. millepora colonies after the bleaching event were in spring and the lowest growth rates were in autumn and winter.
Figure 5. Growth of Acropora millepora colonies was severely affected by bleaching.
The weekly growth rate of Acropora millepora colonies was severely affected by a bleaching event in January/February 2006. Boxes represent the weekly buoyant weight gain expressed as a percentage of the initial buoyant weight of the colony. Box boundaries represent the 75th and 25th percentiles. Thick lines within the boxplots represent the mean and thin lines represent the median. Whisker bars above and below the boxes represent the 95th and 5th percentiles. Missing and out of range values are not shown. Dots represent data that fall outside the confidence limits.
Table 5. ANOVA of Acropora millepora growth during the combined field studies.
| SS | df | Mean Square | F | Sig. | ||
| Corrected Model | Hypothesis | 52.57a | 6 | 8.76 | 39.5 | 0.000 |
| Intercept | Error | 56.25 | 1 | 56.25 | 253.5 | 0.000 |
| Season | Hypothesis | 7.23 | 3 | 2.41 | 10.9 | 0.000 |
| Bleaching | Error | 41.96 | 1 | 41.96 | 189.1 | 0.000 |
| Season * Bleaching | Hypothesis | 5.84 | 2 | 2.92 | 13.2 | 0.000 |
| Error | Error | 47.48 | 214 | 0.22 | ||
| Total | Hypothesis | 168.16 | 221 | |||
| Corrected Total | Error | 100.05 | 220 |
a. R2 = 0.525 (Adjusted R2 = 0.512).
Because of the low number of type C2 colonies left by the end of the first field experiment and the shifting background population of symbiont types, it was not possible to conduct a robust statistical analysis of the effects of symbiont type on growth. However, the overall growth rate of all the colonies in the second experiment (after bleaching) was 47% lower than the lowest growth rate (that of type D colonies) in the first experiment.
Discussion
The growth rate of reef-building corals is likely to be significantly compromised by two separate and independent processes as they acclimatize to a warmer, more stressful, climate. Firstly, growth will be affected by symbiont population shuffling to more thermally tolerant types in response to thermal stress. The second process is the significant affect of bleaching stress itself on coral growth. Thermal stress involving bleaching is a key driver of symbiont shuffling. A community shift from thermally sensitive type C2 to thermally tolerant types D and C1 symbionts occurred in A. millepora colonies at Miall Island in the southern Great Barrier Reef following severe bleaching in 2006 [6]. Before the bleaching, colonies with predominantly type D symbionts had significantly lower growth rate than colonies with type C2 symbionts. Under normal (non-stressful) conditions, this shift is likely to have caused ∼38% lower growth in surviving colonies with predominantly type D symbionts. However, the effects of the bleaching event itself far out-weighed the effects of symbiont genotype, reducing growth in all colonies by 56% compared to the growth rate in the year before the bleaching. Even in corals with type D symbionts, that were apparently unaffected by heat stress at the peak of the bleaching event, growth was significantly reduced for at least 18 months after the bleaching event. It is unknown how long this stress event continued to impact coral growth as the experiment was terminated before there was any sign of recovery of growth rates to pre-bleaching levels.
This study provides conclusive evidence that symbiont genotype is a significant determinant of skeletal growth rate in A. millepora but suggests that although acclimation by symbiont shuffling may improve survival; it does not necessarily represent a long term benefit to host physiology, at least in terms of growth. Our finding that symbiont genotype influences adult coral growth supports previous studies that have demonstrated symbiont genotypic influences in juvenile corals in terms of their growth [14] and carbon acquisition [12]. While corals with thermally tolerant type D symbionts had a clear advantage over type C2 colonies in terms of survival [6], it is clear that at least in terms of skeletal growth, there are still significant heat stress effects on either the symbiont, the host or the holobiont. Our results suggest that acclimation by symbiont shuffling may not represent as beneficial an acclimatory response to warmer more stressful conditions as has previously been suggested. However, (and more importantly) if climate change causes reef-wide symbiont community change to more thermally tolerant types then there will be significant concurrent effects of symbiont change and thermal stress on reef growth and regeneration capacity.
Studies of scleractinian corals have previously found that bleaching can affect coral growth up to one year after bleaching. A correlation between growth variation and bleaching severity was found in A. millepora colonies in a study by Baird and Marshall [31]. While moderately bleached colonies grew ∼20%, severely bleached colonies either remained the same size or shrank over a six week period following a natural bleaching event [31]. Two earlier studies of Montastraea annularis confirm the effects of bleaching on skeletal extension rates for up to a year after the event. The growth rate of bleached M. annularis colonies was reduced by ∼80% [32] and the growth rates of both bleached and unbleached colonies was reduced by 66–98% [33]. While it is difficult to compare measurements of buoyant weight gain to those of skeletal extension and colony area used in these studies, this confirms that severe bleaching can have a debilitating effect on coral growth rate up to a year and possibly longer following recovery. Following the 2006 bleaching at Miall Island, there was also evidence of a shift back to thermally sensitive C2 symbionts in the colonies within 18 months of the bleaching [6]. In this case, the effects of bleaching are expected to persist even longer than symbiont community change, suggesting that the growth differences that are a result of symbiont identity are transitory and relatively minor in comparison to the effects of heat damage to cells and photosystems. However, if climate change causes repeated anomalously warm summers, type D symbionts could become more widespread and permanent on reefs, resulting in the additive effects of these two processes acting to depress coral growth.
Some of the growth differences in A. millepora explants in the laboratory study can be explained by the lower symbiont densities of type D explants. However, since a correlation between Symbiodinium genotype and zooxanthellae densities has not previously been demonstrated [34], a significant proportion of the growth anomaly (∼13% at 23°C) can be attributed solely to symbiont genotype. This was confirmed by re-analyzing the laboratory growth data after standardizing the percentage growth rate of explants to zooxanthellae densities. The model results were consistent with the unstandardized growth data, confirming the link between symbiont identity and growth rate.
The growth differences of adult A. millepora with symbionts of contrasting thermal tolerance in the field and laboratory were not as dramatic as those found for juveniles of this species. Little et al. [14] found a far greater (200–300%) growth difference between juvenile A. millepora with type D and those with C1 symbionts and Mieog et al. [13] found a 50% growth difference. Three factors may be responsible for this disparity. The first two factors may be the effect of isometric scaling with the size of the subject [35] and the age of the coral tissues [36]. As tissues age, cell senescence causes a reduction in the proportion of the coral's energy allocated to growth and an increase in the energy allocated to reproduction. Older, larger colonies will partition less of their resources into growth than smaller, younger colonies. Coral recruits invest all their energy in tissue and skeletal growth in the absence of reproductive effort. The third rationale may lie in the identity of the symbionts compared in the studies. Little et al. [14] and Mieog et al. [13] compared A. millepora juveniles with type D to those with C1, whereas in the present study, adult corals with type D symbionts were compared to those with type C2 symbionts. The growth differences between adult A. millepora and juveniles of this species may assume more parity in the context of these factors. A further factor may lie in the effects of environmental variables which can influence growth rates in the field. Little et al. [14] studied growth at Magnetic Island whereas the present study took place at Miall Island, 800 km south of Magnetic Island (along-shelf distance). Mieog et al. [13] found differences at Magnetic Island but not in the Keppels; clearly demonstrating that environmental factors are at play. Irrespective of these factors, the range of growth values found between and within studies [31] indicate that further studies are required before it will be possible to fully quantify the effects of symbiont genotypes on growth in the field as corals acclimatize to climate change.
In terms of symbiont effects on growth, one possible explanation for the lower growth of type D compared to type C2 A. millepora under normal conditions may lie in the photokinetics of the symbionts. Rapid light curves (RLC's) were used to show a strong positive correlation between rETRmax of photosystem II and the incorporation of radio-labelled carbon into host tissues in juvenile A. millepora with type D and C1 symbionts [12]. Corals with type C1 symbionts had 87% higher rETRmax which correlated with more than double 14C incorporation rates. While a direct link between reduced photosynthetic carbon fixation of thermally tolerant symbionts and lower holobiont growth rate has yet to be demonstrated, one of the key mechanisms of thermal tolerance involves the composition and fluidity of the thylakoid membranes that house the photosystems [37]. Because these mechanisms in plants [38], [39] and micro algae [40] are linked to reduced growth, it is likely that the lower electron transport rate of type D symbionts in the study by Cantin et al. [12] may provide an explanation for the 38% lower growth in the field (under non-stressful conditions) in the present study. Lower photosynthetic function may help type D symbionts to cope with excess electrons and reactive oxygen species during heat stress, thereby maintaining normal photosynthetic function. However, the growth differences found in the field and laboratory in this study and those of Little et al. [14] suggest that heat tolerance comes at a cost to growth rates even at non-stressful temperatures.
In the second field study, A. millepora colonies with type D Symbiodinium had reduced growth in spite of retaining their symbionts during the bleaching event. There are a number of possible explanations for this. The photosynthetically fixed carbon from intact type D symbionts may not be available to their hosts for skeletal growth. This is the concept of type D symbionts as ‘greedy’ partners under stressful conditions. It is possible that surviving stress tolerant symbiont genotypes retain a greater portion of their photosynthetically fixed carbon for cell metabolism and repair, thereby ensuring their own survival but effectively starving the host coral. This would not occur under non-stressful conditions (i.e. before the bleaching) during which photokinetics remain a more likely explanation for the observed growth differences. A second explanation may be that during the warmer conditions of the summer bleaching event, in spite of retaining their symbionts, A. millepora with type D symbionts used more energy for respiration, which is positively correlated with temperature [41]. The increased respiratory demand would have occurred in both bleached and unbleached corals, resulting in reductions in growth in both C2 and D corals, irrespective of symbiont losses. This does not explain why growth rates remained low throughout the following year as temperatures became less stressful. The third explanation may be that type D symbionts had increased rates of photo-inhibition during the bleaching event which reduced carbon fixation. At high temperatures (e.g. 32°C), type D Symbiodinium has been shown to undergo protective photoinhibition [1]. Diversion of photon energy via photoprotective processes is a mechanism to cope with heat stress as temperature can damage the algal cell's capacity to repair proteins [42]. Photoinhibition mimics reduced habitat irradiance, reducing photosynthesis [1]. Finally, the effects of heat stress on ‘host factors’ may play a part in reducing photosynthate translocation to the corals which retained their type D symbionts [43]. It is likely that a combination of these mechanisms may cause the loss of skeletal growth in type D corals. What is clear is that in spite of increasing the heat tolerance of A. millepora colonies, hosting type D Symbiodinium does not protect the coral from the more subtle effects of the bleaching on processes such as growth.
The relative differences in growth rate of A. millepora in the field and the laboratory (nearly double) are likely to be caused by the interactions of influences such as light, morphology and changes in heterotrophic feeding behaviour. Theoretically at least, increased heterotrophy in the field [44], where zooplankton and particulate matter are available, should reduce incorporation of the heavier carbon isotope 13C into the coral skeleton because zooplankton and particulate matter are lower in δ13C (ratio 13C:12C relative to Vienna Peedee Belemnite Limestone Standard) than seawater [45]. In the laboratory, corals were supplied with filtered seawater which has comparatively low δ13C due to the absence of zooplankton. A second explanation is that the field growth rates incorporate both winter and summer rates. When compared to the growth rate of in spring (when temperatures were most similar to those in the laboratory), laboratory growth rates (at 23°C) assume greater parity.
This study has provided some insights into the synergistic effects and magnitude of symbiont genotype and thermal stress on coral growth. These two influences are likely to have implications to the future resilience and regeneration capacity of reefs. However more work is required to determine how applicable these effects are to other coral/algal associations and localities. The results of the field studies suggest that symbiont genotype will affect the growth rate of some reef corals, and that this will be compounded by the long-term effects of severe heat stress on these corals if they survive. Predictions of annual bleaching events within the next 30–50 years could result in more frequent disturbances which have the potential to shift the community composition of some reefs from hard-coral to macro-algae and soft coral-dominated communities [18]. Some of the most structurally important scleractinian corals may be able to acclimatize to gradually warmer waters by hosting thermally tolerant symbionts [5] but, the pressures of annual heat stress, ocean acidification and permanent symbiont changes on growth may act synergistically in compromising the competitiveness of these species to recover and compete between events.
Materials and Methods
Ethics statement
This study followed the guidelines of the Central Queensland University (CQU) Code of Conduct for Researchers and was conducted in accordance with the Great Barrier Reef Marine Park Authority and CQU Memorandum of Understanding and the Great Barrier Reef Marine Park Regulation (1983). The study did not require clearance by the CQU Animal Ethics Committee.
Laboratory study
The growth rate of colonies with either C2 or D symbionts was measured in two studies. One study took place in the field on the reef slope at Miall Island in the Keppel region. To support the results of the field study, the second study took place under controlled laboratory conditions at two temperatures (23°C and 29°C). These temperatures represent the average stressful summer and non-stressful spring/autumn temperature ranges for corals at this site. The explants used in the laboratory experiment were sourced from the reef flat at Miall Island. The field experiment was repeated opportunistically following a natural bleaching event in February 2006 to further investigate the effect of bleaching on the growth differences between C2 and D corals.
Collection and maintenance of corals
In March 2005, 16 colonies of the Indo-Pacific stony coral A. millepora, Ehrenberg, 1834, with known Symbiodinium type C2 or type D were transplanted from the Keppel Islands region (a cool, clear southern inshore section of the Great Barrier Reef) to Magnetic Island (central Great Barrier Reef, ∼800 km north of Keppel). Corals were kept for a period of three months at Magnetic Island to allow recovery from transportation and acclimatization prior to the experiment. Temperatures ranged between 23°C and 27°C in the Keppels and between 24°C and 27°C at Magnetic Island during this time. Corals were kept on wire mesh racks at approximately the same depth that they were collected. In May 2005, the colonies were removed from the racks at Magnetic Island and transported to the Australian Institute of Marine Science (AIMS) where they were used for the growth experiment.
Experimental protocol
Six explants were cut from each of the 16 colonies (9 colonies with rDNA ITS1 type C2 and 7 colonies with ITS1 type D Symbiodinium) and distributed randomly and equally between three tanks (treatment replicates) within each of two temperature treatments (23°C and 29°C). Aerated seawater was supplied to the tanks at a flow rate of ∼1000 l h−1 and heated to the target temperatures (23°C and 29°C±1°C, mean ± S.D.). Coral explants were fixed to plastic stands with a cyanoacrylate-based adhesive (Loctite 454™ super glue gel) and then placed on elevated rotisseries. Each rotisserie was turned 180° twice daily to ensure even exposure to light and water flow. Corals were gradually acclimated for 10 days to light conditions in the tanks at the treatment temperatures. For the duration of the four week experiment, corals were supplied with 3.5 hours of shaded light (30–36 µmole photons m−2 s−1) followed by 5 hours of un-shaded light (87–107 µmole photons m−2 s−1), followed by another 3.5 hours of shaded light and 12 hours darkness each day to approximate their natural diurnal light cycle.
Light was provided by 10×400 W metal halide lamps (10,000°K colour temperature, BLV Germany) with a spectral quality suitable for coral photosynthesis. To monitor the health of explants with respect to the laboratory conditions, the dark-adapted maximum quantum yield of each explant was determined every second day by measuring Fv/Fm with a mini-PAM fluorometer (Heinz Walz, Germany) at the same time each morning after 8 hours of darkness. Measurements were made with a Diving-PAM fluorometer (Heinz Walz, Germany) sensor 5 cm underwater just above the coral explants with the tip of the fibre-optic probe touching the base of the explant surface on a vertical plane. Photosynthetically active radiation (PAR) measurements were made in the presence of a weak measuring light (F0) and then during a 1 s (8000 µmol photon m−2.s−1) saturating pulse of light (Fm). Damping and gain were set at 2 and the measuring light was set at 1 s µmol photons m−2.s−1.
Symbiodinium identification
The predominant Symbiodinium type in the colonies used in both field and laboratory experiments was verified just before the start of the experiments using Single Stranded Conformational Polymorphism (SSCP) analysis of the Inter-transcribed Spacer Region 1 (ITS1) of algal nuclear ribosomal DNA as described in Jones et al. [30]. Only colonies with intense SSCP bands representing type C2 and type D (EU189443, EU1894505) were chosen for the studies although the presence of other types below 5% abundance is not ruled out [46].
Buoyant weight determination
Coral explants were weighed to three decimal places at the end of each week for four weeks to determine equivalent skeletal buoyant weight using the methods described in Jokiel et al. [47].
Zooxanthellae densities and pigments
To determine the influence of zooxanthellae densities and algal pigment concentrations on coral growth, explants were snap-frozen in liquid nitrogen and stored at −20°C immediately following the experiment. Frozen branches were stripped of tissue using an air gun and the resultant slurry was macerated with a tissue homogenizer for 20 s. The homogenate volume was recorded and a 9 ml aliquot was drawn off and preserved with 1 ml of formalin (32% w.w−1). Zooxanthellae counts were made on eight independent drops (0.0001 mm3) from each sample using a New Improved Nuebauer haemocytomer under a compound light microscope. Zooxanthellae numbers were standardized to coral tissue surface area using the 3D digital image analysis method described in Jones et al. [48].
A separate 10 ml aliquot was drawn from the remaining tissue homogenate and the algal pellet was separated from the host tissue by centrifugation (3000 g for 5 min) at 4°C. Chlorophyll was extracted overnight from the algal pellet using 100% methanol at 4°C. The first 10 samples were extracted three times to determine the extraction efficiency. Absorbance at 668 nm and 635 nm was measured with a spectrophotometer (Hitachi U-3200). Total branch chlorophyll a was calculated from the equation of Jeffrey and Haxo [49] after adjustment for extraction efficiency and standardized to algal cells.
Field study
In March 2004, 43 pieces (15–20 cm) of A. millepora colonies from the Keppel region were cut from larger colonies from the reef flat and pruned to approximately similar sizes [36]. Colonies were genotyped in March 2004, at the start of the experiment using SSCP analysis of the algal nrDNA ITS1 region. Due to the low abundance of type D colonies at Miall Island when the study began, thirty six C2 colonies and only five D colonies were included in the first of the two field experiments. Initial buoyant weight measurements were made on the coral colonies in March 2004. Buoyant weight measurements (to the nearest gram) were performed by carefully transporting the colonies submerged in seawater to the weighing equipment a few 100 m from the study site. Colonies were carefully transported back to the study site after the buoyant weight measurements were completed and secured with plastic cable ties onto wire racks 75 cm above the sea bed at a depth of 3–4 m. Buoyant weight measurements were repeated seasonally every three months for a total of 9 months at the end of autumn (March to June 2005), winter (June to September 2005) and spring (September to December 2005).
The field growth experiment was repeated for another 12 months after a bleaching event in February 2006. Seven C2 and 15 D colonies were placed on the racks in May 2006 and allowed to recover from the bleaching until the experiment started in August 2006. The D colonies were sourced from the field while the C2 colonies were sourced from colonies in the first experiment that bleached but survived due to low abundance of C2 colonies in the field post-bleaching [6]. Symbiont genotypes were verified in May 2006 and then just before the start of the experiment in August 2006 using SSCP analysis. Only colonies with strong C2 or D SSCP bands were chosen for the experiment (verified by the intensity of the band) [46].
However, by the start of the study in August (3 months later), nearly all of the 22 colonies on the racks had undergone some change in symbiont proportions; gaining C2, D, or another thermally tolerant type, C1. The dynamic nature of the symbiont community after bleaching made a comparison of the growth rate of colonies as a function of symbiont genotypes difficult. Nevertheless, results from the second field experiment are included because they provide an insight into the overall growth performance of A. millepora pre-and post bleaching.
Colonies were weighed every three months at the end of spring (August to November 2006), summer (November to January 2007), autumn (January to May 2007) and winter (May to August 2007). Colonies used in the growth studies were not sampled for symbionts density or chlorophyll content to avoid compromising skeletal weight changes. Nearby C2 colonies showed ∼80% decline in symbiont densities following the bleaching. Nearby colonies also had lower algal chlorophyll a and c2 content irrespective of symbiont genotype (data not shown).
Statistical analysis
Laboratory study
To examine overall growth, the weekly buoyant weight measurements were expressed as a percentage of the initial buoyant weight of the explant and averaged over the four weeks of the study. Data for the percent average weekly buoyant weight gain of the explants were analyzed with a nested ANOVA using symbiont Type (fixed, two levels), Temperature (fixed, two levels), Tank (random, three levels, nested within temperature) as factors in the model. There were no significant differences between growth in the treatment tanks and data were averaged across the three tanks and the model re-run with an orthogonal ANOVA model using the fixed factors symbiont Type and Temperature. Unstandardized predicted values and standardized residuals were used to check the assumption of normality. Levene's test was used to verify homogeneity of variances.
To examine their influence on explant growth, data for zooxanthellae densities and chlorophyll a and c2 concentrations in the laboratory experiment were analyzed with separate multivariate ANOVA's using symbiont Type (two levels) and Temperature (two levels) as fixed factors in the models and Tank (three levels) as a random factor nested within temperature. Unstandardized predicted values and standardized residuals were used to check the assumptions of normality. Levene's test was used to verify homogeneity of variances. Zooxanthellae densities and chlorophyll a and c2 values were aggregated across all three treatment tanks. Zooxanthellae densities and algal cell chlorophyll a and c2 concentrations were examined with an orthogonal multivariate ANOVA using Temperature (two levels) and Type (two levels) as fixed factors in the analysis.
Field study
To examine growth variation of A. millepora colonies with respect to symbiont type in the first field study, before the bleaching, a one-factor ANOVA was performed on the weekly growth rates using symbiont Type as the fixed, predictor variable (two levels). Growth for each colony was expressed as the weekly buoyant weight gain as a percentage of the initial buoyant weight of the colony at the start of the study. The assumption of normality was verified using plots of the unstandardized predicted values by the standardized residuals and Levene's test was used to verify the homogeneity of variances. Data were natural-log transformed to improve the normality of the distribution.
To examine the seasonal variations in growth of A. millepora colonies with different symbionts types in the first field experiment, data for the weekly buoyant weight gain during each three-month season were analyzed with a repeated-measures ANOVA using symbiont Type as the fixed variable (two levels) and Season (three levels) as the repeated measure in the model. The growth rate was expressed as the weekly buoyant weight gain of each colony in the study over the three month season as a percentage of the initial buoyant weight of the colony. The model residuals were examined to verify the validity of the assumption of normality and Levene's test was used to verify the homogeneity of variances. Data were natural-log transformed to improve the normality of the distribution.
To examine the variation in seasonal growth of A. millepora colonies as a result of the bleaching event in early 2006, the weekly growth rates for each season in the two studies were analyzed with ANOVA using the fixed factor Bleaching (before or after bleaching), and the random factor Season (three levels) as predictor variables. The growth rate was expressed as the weekly buoyant weight gain as a percentage of the initial buoyant weight of the colony. The assumption of normality was verified using plots of the unstandardized predicted values by the standardized residuals. Levene's test was used to verify the homogeneity of variances. Data were natural-log transformed to improve the normality of the distribution. Simple pair-wise comparisons were performed to further investigate significant differences in growth using Sidak's adjustment for multiple comparisons [50]. All statistical tests were completed using SPSS Version 15.0.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was made possible by funding from the Australian Institute of Marine Science and Central Queensland University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rowan R. Thermal adaptation in reef coral symbionts. Nature. 2004;430:742. doi: 10.1038/430742a. [DOI] [PubMed] [Google Scholar]
- 2.van Oppen MJH, Little A, Willis BL. Algal shuffling helps corals cope with environmental change. Australasian Science. 2004;25:41. [Google Scholar]
- 3.Baker AC, Starger CJ, McClanahan TR, Glynn PW. Coral reefs: Corals' adaptive response to climate change. Nature. 2004;430:741. doi: 10.1038/430741a. [DOI] [PubMed] [Google Scholar]
- 4.Baker AC, Romanski AM. Multiple symbiotic partnerships are common in scleractinian corals, but not in octocorals: Comment on Goulet (2006). Marine Ecology Progress Series. 2007;335:237–242. [Google Scholar]
- 5.Mieog JC, Van Oppen MJH, Cantin N, Stam DWT, Olsen JL. Real-time PCR reveals a high incidence of Symbiodinium clade D at low levels in four scleractinian corals across the Great Barrier Reef: implications for symbiont shuffling. Coral Reefs. 2007;26:449–457. [Google Scholar]
- 6.Jones AM, Berkelmans R, Mieog JC, Van Oppen MJH, Sinclair W. A community change in the symbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proceedings of the Royal Society of London Series B, Biological Sciences (1934–1990) 2008;275:1359–1365. doi: 10.1098/rspb.2008.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berkelmans R, van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proceedings of the Royal Society of London Series B, Biological Sciences (1934–1990) 2006 doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muscatine L. The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky, editor. Ecosystems of the world 25: Coral reefs. New York: Elsiever; 1990. pp. 75–87. [Google Scholar]
- 9.Davies PS. The role of zooxanthellae in the nutritional energy requirements of Pocillopora eydouxi. Coral Reefs. 1984;2:181–186. [Google Scholar]
- 10.Vago E, Gill E, Collingwood JC. Laser measurements of coral growth. Nature. 1997;386:30–31. [Google Scholar]
- 11.Gladfelter EH. Skeletal development in Acropora cervicornis III. A comparison of monthly rates of linear extension and calcium carbonate accretion measured over a year. Coral Reefs. 1984;3:51–57. [Google Scholar]
- 12.Cantin N, van Oppen M, Willis B, Mieog J, Negri A. Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs. 2009;28:405–414. [Google Scholar]
- 13.Mieog JC, Olsen JL, Berkelmans R, Bleuler-Martinez SA, Willis BL, et al. The roles and interactions of symbiont, host and environment in defining coral fitness. PLoS ONE. 2009;4:e6364. doi: 10.1371/journal.pone.0006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little AF, van Oppen MJH, Willis BL. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;304:1492–1494. doi: 10.1126/science.1095733. [DOI] [PubMed] [Google Scholar]
- 15.Henry L-A, Hart M. Regeneration from injury and resource allocation in sponges and corals - a review. International Review of Hydrobiology. 2005;90:125–158. [Google Scholar]
- 16.Smith LD, Gilmour JP, Heyward AJ. Resilience of coral communities on an isolated system of reefs following catastrophic mass-bleaching. Coral Reefs. 2008;27:197–205. [Google Scholar]
- 17.Wakeford M, Done TJ, Johnson CR. Decadal trends in a coral community and evidence of changed disturbance regime. Coral Reefs. 2008;27:1–13. [Google Scholar]
- 18.Done T. Phase shifts in coral reef communities and their ecological significance. Hydrobiologia. 1992;247:121–132. [Google Scholar]
- 19.Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, et al. Doom and boom on a resilient reef: Climate change, algal overgrowth and coral recovery. PLoS ONE. 2009;4:e5239. doi: 10.1371/journal.pone.0005239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pratchett MS, Wilson SK, Beruman ML, McCormick MI. “Sublethal effects of coral bleaching on an obligate coral feeding butterflyfish”. Coral Reefs. 2004;23:352–356. [Google Scholar]
- 21.Hoegh-Guldberg O, Mumby PJ, Hooten AJ, Steneck RS, Greenfield P, et al. Coral reefs under rapid climate change and ocean acidification. Science. 2007;318:1737–1742. doi: 10.1126/science.1152509. [DOI] [PubMed] [Google Scholar]
- 22.van Woesik R. An assessment of the coral reef communities of the Keppel Isles, report to the Great Barrier Reef Marine Park Authority. Townsville 1991 [Google Scholar]
- 23.Hoegh-Guldberg O, Jones RJ. Photoinhibition and photoprotection in symbiotic dinoflagellates from reef-building corals. Marine Ecology Progress Series. 1999;183:73–86. [Google Scholar]
- 24.Jones RJ, Kildea T, Hoegh-Guldberg O. PAM chlorophyll fluorometry: a new in situ technique for stress assessment in scleractinian corals, used to examine the effects of cyanide from cyanide fishing. Marine Pollution Bulletin. 1999;38:864–874. [Google Scholar]
- 25.Jones RJ, Ward S, Amri AY, Hoegh-Guldberg O. Changes in quantum efficiency of Photosystem II of symbiotic dinoflagellates of corals after heat stress, and of bleached corals sampled after the 1998 Great Barrier Reef mass bleaching event. Marine and Freshwater Research. 2000;51:63–71. [Google Scholar]
- 26.Ralph PJ, Gademann R, Larkum AWD. Zooxanthellae expelled from bleached corals at 33°C are photosynthetically competent. Marine Ecology Progress Series. 2001;220:163–168. [Google Scholar]
- 27.Ralph PJ, Gademann R, Larkum AWD, Schreiber U. In situ underwater measurements of photosynthetic activity of coral zooxanthellae and other reef-dwelling dinoflagellate endosymbionts. Marine Ecology Progress Series. 1999;180:139–147. [Google Scholar]
- 28.Warner ME, Fitt WK, Schmidt GW. The effects of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: A novel approach. Plant Cell and Environment. 1996;19:291–299. [Google Scholar]
- 29.Krause GH, Weis E. Chlorophyll fluorescence and photosynthesis: the basics. Annual Review of Plant Physiology and Plant Molecular Biology. 1991;42:313–349. [Google Scholar]
- 30.Jones A, Berkelmans R, van Oppen MJH, Mieog JC, Sinclair B. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. In: Mieog JC, editor. Flexibility of the coral-algal symbiosis in the face of climate change: investigating the adaptive bleaching hypothesis. Enschede, The Netherlands: Ipskamp Drukkers; 2009. 200 [Google Scholar]
- 31.Baird AH, Marshall PA. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Marine Ecology Progress Series. 2002;237:133–141. [Google Scholar]
- 32.Mendes JM, Woodley JD. Effect of the 1995-1996 bleaching event on polyp tissue depth, growth, reproduction and skeletal band formation in Montastraea annularis. Marine Ecology Progress Series. 2002;235:93–102. [Google Scholar]
- 33.Leder J, Szmant A, Swart P. The effect of prolonged “bleaching” on skeletal banding and stable isotopic composition in Montastrea annularis. Preliminary observations. Coral Reefs. 1991;10:19–27. [Google Scholar]
- 34.Chen CA, Wang J-T, Fang L-S, Ya-Wen Y. Fluctuating algal symbiont communities in Acropora palifera (Scleractinia: Acroporidae) from Taiwan. Marine Ecology Progress Series. 2005;295:113–121. [Google Scholar]
- 35.Leuzinger S, Anthony K, Willis BL. Reproductive energy investment in corals: scaling with module size. Oecologia. 2003;136:524–531. doi: 10.1007/s00442-003-1305-5. [DOI] [PubMed] [Google Scholar]
- 36.Elahi R, Edmunds PJ. Tissue age affects calcification in the scleractinian coral Madracis mirabilis. Biological Bulletin. 2007;212:20–28. doi: 10.2307/25066577. [DOI] [PubMed] [Google Scholar]
- 37.Tchernov D, Gorbunov MY, de Vargas C, Narayan Yadav S, Milligan AJ, et al. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proceedings of the National Academy of Sciences. 2004:13531–13535. doi: 10.1073/pnas.0402907101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hugly S, Kunst L, Browse J, Somerville C. Enhanced thermal tolerance of photosynthesis and altered chloroplast ultrastructure in a mutant of Arabidopsis deficient in lipid desaturation. Plant Physiology. 1989;90:1134–1142. doi: 10.1104/pp.90.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vigh L, Joo F, Droppa M, Horvath LI, Horvath G. Modulation of chloroplast membrane lipids by homogenous catalytic hydrogenation. European Journal of Biochemistry. 1985;147:477–481. doi: 10.1111/j.0014-2956.1985.00477.x. [DOI] [PubMed] [Google Scholar]
- 40.Sato N, Sonoike K, Kawaguchi A, Tsuzuki M. Contribution of lowered unsaturation levels of chloroplast lipids to high temperature tolerance of photosynthesis in Chlamydomonas reinhardtii. Journal of Photochemistry and Photobiology B: Biology. 1996;36:333–337. [Google Scholar]
- 41.Fitt WK, McFarland FK, Warner ME, Chilcoat GC. Seasonal patterns of tissue biomass and densities of symbiotic dinoflagellates in reef corals and relation to coral bleaching. Limnology and Oceanography. 2000;45:677–685. [Google Scholar]
- 42.Takahashi S, Nakamura T, Sakamizu M, van Woesik R, Yamasaki H. Repair machinery of symbiotic photosynthesis as the primary target of heat stress for reef-building corals. Plant and Cell Physiology. 2004;45:251–255. doi: 10.1093/pcp/pch028. [DOI] [PubMed] [Google Scholar]
- 43.Stat M, Morris E, Gates RD. Functional diversity in coral-dinoflagellate symbiosis. Proceedings of the National Academy of Sciences. 2008;105:9256–9261. doi: 10.1073/pnas.0801328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anthony K, Fabricius K. Shifting roles of heterotrophy and autotrophy in coral energetics under varying turbidity. Journal of Experimental Marine Biology and Ecology. 2000;252:221–253. doi: 10.1016/s0022-0981(00)00237-9. [DOI] [PubMed] [Google Scholar]
- 45.Grottoli AG, Wellington GM. Effect of light and zooplankton on skeletal δ 13C values in the eastern Pacific corals Pavona clavus and Pavona gigantea. Coral Reefs. 1999;18:29–41. [Google Scholar]
- 46.Fabricius KE, Mieog JC, Colin PL, Idip D, van Oppen MJ. Identity and diversity of coral endosymbionts (zooxanthellae) from three Palauan reefs with contrasting bleaching, temperature and shading histories. Molecular Ecology. 2004;13:2445–2458. doi: 10.1111/j.1365-294X.2004.02230.x. [DOI] [PubMed] [Google Scholar]
- 47.Jokiel PL, Margos JE, Franzisket L. Coral growth: Buoyant weight technique. In: Stoddart DR, Johannes RE, editors. Coral Reefs: Research methods. 5 ed. Paris: UNESCO; 1978. pp. 529–542. [Google Scholar]
- 48.Jones A, Cantin N, Berkelmans R, Sinclair B, Negri A. A 3D modeling method to calculate the surface areas of coral branches. Coral Reefs. 2008;27:521–526. [Google Scholar]
- 49.Jeffrey SW, Haxo FT. Photosynthetic pigments of symbiotic dinoflagellates (zooxanthellae) from corals and clams. Biological Bulletin. 1968;135:149–165. [Google Scholar]
- 50.Weinberg SL, Abramowitz SK. New York: Cambridge University Press; 2002. Data analysis for the Behavioral Sciences Using SPSS. [Google Scholar]