Abstract
ADAM8 is a member of the “a disintegrin and metalloproteinase” (ADAM) family of membrane-anchored metalloproteinases. ADAM8-deficient mice have no evident spontaneous developmental or pathological defects, and little is currently known about the role of ADAM8 in disease. Here, we investigated the contribution of ADAM8 to pathological neovascularization in mice using an oxygen-induced retinopathy (OIR) model and heterotopical injection of tumor cells. We found an increase in retinal re-vascularization but fewer neovascular tufts in the OIR model and increased growth of heterotopically injected tumor cells in Adam8−/− mice compared with wild-type controls. These results suggest that ADAM8 functions to limit both of these processes in wild-type mice. In cell-based assays, overexpression of ADAM8 increased the ectodomain shedding of several co-expressed membrane proteins with roles in angiogenesis (CD31, Tie-2, Flk-1, Flt-1, EphrinB2, EphB4, VE-cadherin, KL-1, E-selectin, and neuregulin-1β2). Thus, dysregulated expression of ADAM8 in endothelial cells in vivo could potentially increase the processing of these and other substrate proteins. Taken together, our findings suggest that inhibiting ADAM8 could be useful for promoting re-vascularization and thereby preventing formation of neovascular tufts in proliferative retinopathies. On the other hand, blocking ADAM8 could be detrimental in the context of rapidly growing tumors.
Keywords: ADAM8, Pathological neovascularization, Proliferative retinopathy, Oxygen-induced retinopathy, Protein ectodomain shedding
Introduction
The formation of new blood vessels is critical for normal development and tissue repair, as well as for pathological events such as retinal neovascularization, rheumatoid arthritis, and tumor growth [1–6]. During physiological angiogenesis, vascular endothelial growth factor (VEGF) expression in response to local hypoxia drives the development of new blood vessels [7–9]. In eye diseases, such as retinopathy of prematurity, proliferative diabetic retinopathy, and macular degeneration, hypoxia-driven neovascularization is generally considered a pathological response, leading to decreased vision and blindness [1–3]. In cancer, the growth of solid tumors also relies on pathological neovascularization.
This study is focused on the role of ADAM8, a member of the “a disintegrin and metalloprotease” (ADAM) protein family [10, 11], in pathological retinal neovascularization and in heterotopic tumor growth. ADAM8 is a membrane-anchored glycoprotein consisting of an N-terminal pro-domain, followed by a metalloproteinase-, disintegrin-, cysteine-rich-, EGF-like- and transmembrane domain and cytoplasmic tail [12, 13]. ADAM8 was initially identified in macrophages [12] and subsequently detected in neurons, osteoclasts, leukocytes, neutrophils, epithelial cells, and cancer cells [14–17]. ADAM8 may regulate cellular functions by processing membrane receptors that are crucial for cellular signaling pathways, and its catalytic activity has been demonstrated in biochemical and cell-based assays towards substrates that include HB-EGF, EGF, CD23, and L-selectin [18–24]. The dysregulation of ADAM8 could therefore influence pathological conditions such as rheumatoid arthritis, cancer, and asthma [25–27]. However, mice lacking ADAM8 are healthy with no evident major developmental or spontaneous pathological defects [17]. We have previously reported that ADAM9 and ADAM15 are overexpressed during pathological neovascularization and that both ADAMs are involved in new blood vessel formation in a mouse model of oxygen-induced retinopathy (OIR) and in the growth of tumors from heterotopically injected melanoma cells [28, 29]. To assess whether ADAM8 might also have a role in pathological neovascularization, we subjected Adam8−/− mice and wild-type controls to the OIR and heterotopic tumor model. Moreover, we investigated the catalytic activity of ADAM8 towards membrane-bound substrates with known roles in angiogenesis in order to gain insights into the potential substrate repertoire of ADAM8 during neovascularization.
Material and methods
Materials
All reagents and chemicals were from Sigma, unless otherwise indicated. The rabbit anti-mouse NG2 antibody was from Chemicon International (Temecula, CA), FITC-isolectin B4 from Vector Labs (Burlingame, CA), rat anti-CD31 from BD Biosciences/Pharmingen (San Diego, CA), anti-rabbit Texas Red and anti-rat Cy3 antibodies from Jackson Immunoresearch (West Grove, PA), rat anti-mouse F4/80 R-PE from Invitrogen (Carlsbad, CA), and the anti-mouse Alexa 488 antibody from Molecular Probes (Eugene, OR). Generation and use of the polyclonal anti-ADAM8 antibody was described previously [19, 21].
Oxygen-induced retinopathy
Adam8−/− mice and wild-type controls were subjected to the OIR model for retinopathy of prematurity, and the results were analyzed as described previously [28–30]. Statistical evaluation was performed using the unpaired Student’s t test (equal variation, two-sided). Since the animals were of mixed genetic background (129Ola/C57BL6), only wild-type (n=23) and Adam8−/− (n=21) littermates that were generated by matings of heterozygous Adam8+/− parents were analyzed.
Immunofluorescence and immunohistochemistry
Flat mounted retinas were incubated in modified LBB (0.5% Triton X-100) for 4 h, then washed 3× in PBS, incubated at 4°C overnight with either isolectin B4-FITC, lectin-TRITC, anti-F4/80-PE (macrophage marker), anti-NG2 (pericyte marker), or anti-CD31 (endothelial cell marker) antibodies, washed again with PBS, and incubated with anti-rabbit Texas Red or anti-rat Cy3 antibodies for 1 h, respectively. Subsequently, the retinas were washed with PBS, incubated with anti-ADAM8 monoclonal antibodies overnight, washed with PBS, and incubated for 1 h with anti-mouse Alexa488. After additional washes, retinas were mounted and photographed as described [29]. The number of tufts per retina was determined in a double blind analysis of photographs of whole mounts of retinas from wild-type and Adam8−/− mice subjected to the OIR model, counting only tufts that were clearly visible at 4× magnification to avoid scoring aggregates of macrophages. Immunohistochemistry of eye sections was performed as described [29].
Western blot
Cos7 cells were transfected with ADAM8 or the catalytically inactive E>Q mutant. Cell lysis, SDS-PAGE, and Western blot analysis using an anti-ADAM8 antibody were performed as described [19, 21].
Heterotopic injection of B16F0 mouse melanoma cells
1×106 B16F0 mouse melanoma cells were injected subcutaneously into age-matched Adam8−/− mice or wild-type controls. These animals were derived from matings of Adam8−/− mice or of wild-type controls that were offspring from the same pair of heterozygous Adam8+/− mice of mixed genetic background (129/Ola and C57BL6). Thus, the wild-type and Adam8−/− mice used for tumor injections shared the same grandparents and were therefore highly related. Tumor growth over time was monitored using a caliper (Scienceware, Pequannock, NJ, USA). Tumor volume was calculated as L (length)×W2 (width)×0.5. All animals used in a given experiment were sacrificed at the same time, between 2 to 3 weeks after injection of tumor cells, depending on the size of the tumors in the animals with the fastest tumor growth. The tumors were removed, weighed, and some processed for CD31 staining of frozen sections. Vascular density was evaluated by counting the number of vessels in 4× magnification fields throughout the entire tumor area, and presented as average number of vessels per field. The one-tailed unpaired Student’s t test was used for statistical evaluation (Prism 4.0a software).
Protein ectodomain shedding assays
The expression plasmids for alkaline phosphatase-tagged receptors with roles in angiogenesis (Flk-1, Flt-1, Tie-2, VE-cadherin, EphB4, EphrinB2, E-selectin, ICAM-1, VCAM-1, KL-1, TRANCE, NRG-1β1, and NRG-1β2), as well as for ADAM8 and the catalytically inactive E>Q mutant have been described previously [19, 29, 31–33]. An expression plasmid for alkaline phosphatase-tagged CD31 (PECAM) was generated by PCR (5′ primer: TACTCGAGCTAGTCCAGATTTCTATCCTGTCA; 3′ primer: ACAAGGGCCCCTAAGTTCCATCAAGGGAGCCT) and encodes amino acid residues 503 to 738 inserted in frame into the pAP-tag vector (Genehunter, Nashville, TN). All data are representative of at least three independent experiments. For shedding experiments, ADAM8 or ADAM8 E>Q were transfected together with AP-tagged candidate substrate proteins into Cos-7 cells. One day after transfection, the medium was replaced with fresh medium, conditioned for 4 h, and then removed. The level of released AP-tagged receptor proteins was determined by colorimetric analysis as described [29].
Results
Increased re-vascularization and decreased formation of neovascular tufts in Adam8−/− mice subjected to oxygen-induced retinopathy
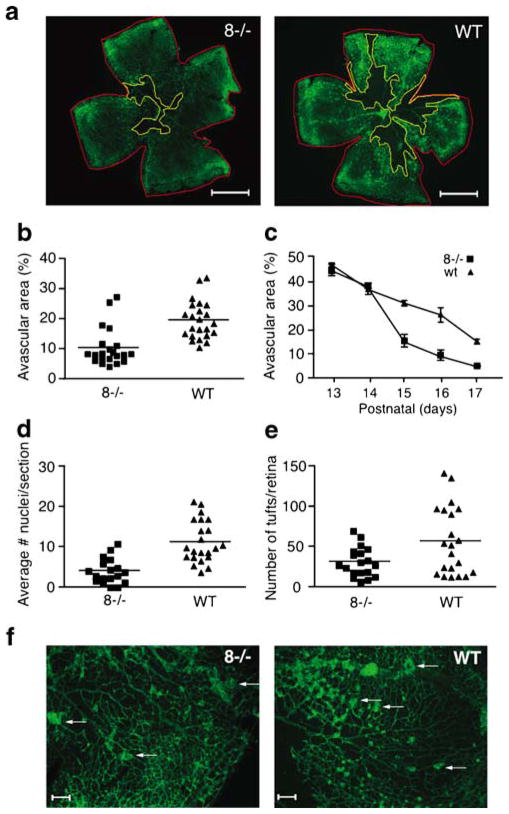
The first approach to evaluate pathological neovascularization in Adam8−/− mice was the OIR model for retinopathy of prematurity. Seven-day-old mice were placed together with their nursing mother in a chamber with 75% oxygen. The high concentration of oxygen during postnatal days P7–P12 induces regression of the retinal capillary bed, leading to a central avascular area. Returning the animals to room air (21% oxygen) results in a relative hypoxia, which triggers a neovascular response that includes re-growth of the capillary bed (days P13–P17) and the formation of neovascular tufts that are scored at P17. Retinal whole-mount preparations were stained with FITC-isolectin B4 to visualize endothelial cells in the capillary bed of the retina and to quantify the size of the central avascular area and detect the formation of neovascular tufts. A significantly smaller central avascular area was observed in Adam8−/− mice compared with wild-type controls at P17 (46.2% smaller, wild-type n=23, Adam8−/− n=21, p<0.0001, Fig. 1a, b). A time course of retinal re-vascularization after the OIR model (Fig. 1c) showed that Adam8−/− mice and wild-type controls had an avascular area of similar size at p12, whereas there was significantly more re-vascularization of the retina in Adam8−/− mice at P15, P16, and P17 compared with controls. Counting the number of endothelial cell nuclei that had traversed to the vitreal side of the internal limiting membrane revealed a 64.6% decrease in Adam8−/− mice compared with controls (Fig. 1d, wild-type n=22, Adam8−/− n=18, p=0.0001). Moreover, the number of neovascular tufts visible in a whole-mount analysis was also significantly reduced in Adam8−/− mice compared with controls (Fig. 1e, f). The enhanced re-vascularization in Adam8−/− mice could conceivably help prevent formation of neovascular tufts by increasing perfusion of the central avascular area after OIR.
Fig. 1.
Increased hypoxia-induced retinal neovascularization in Adam8−/− mice. Adam8−/− mice and wild-type controls were subjected to the oxygen-induced retinopathy (OIR) model (see Material and methods for details). a Analysis of whole-mount retinas prepared at P17 showed a smaller central avascular area in Adam8−/− mice compared with wild-type controls (representative examples are shown, scale bar=1 mm). b Quantification of the size of the central avascular area compared with the size of the retina corroborated that the central avascular area is smaller in Adam8−/− mice compared with controls (mean relative size of avascular area in Adam8−/− mice=10.5%±1.4% SEM, n=21; in wild-type controls=19.6%±1.3% SEM, n=23, Student’s t test, p<0.0001). c Adam8−/− and wild-type mice developed similar capillary regression at P12 of the OIR model, but starting at P15, re-vascularization of the retina was significantly increased in Adam8−/− mice compared with controls. d The average number of neovascular nuclei in tufts on the vitreous side of the internal limiting membrane (see Material and methods for details) was significantly reduced in the absence of ADAM8. Each point represents the average number of nuclei per retinal section of one animal. (Adam8−/− mice 4.0±1.1 SEM, n=18; wild-type mice 11.3±0.7 SEM, n=22, p<0.0001.) e The number of tufts that were clearly visible in retinal whole mounts at 4× magnification was decreased in Adam8−/− mice compared with wild-type controls (Adam8−/− mice 31.3±4.1 SEM, n=19; wild-type mice 56.8±9.1 SEM, n=21, p<0.0098). f Representative images of sections of a retina whole mount from an Adam8−/− mouse or wild-type control, stained with isolectin B4-FITC (arrows point to neovascular tufts, scale bar=100 μm)
ADAM8 expression in neovascular endothelial cells in the retina
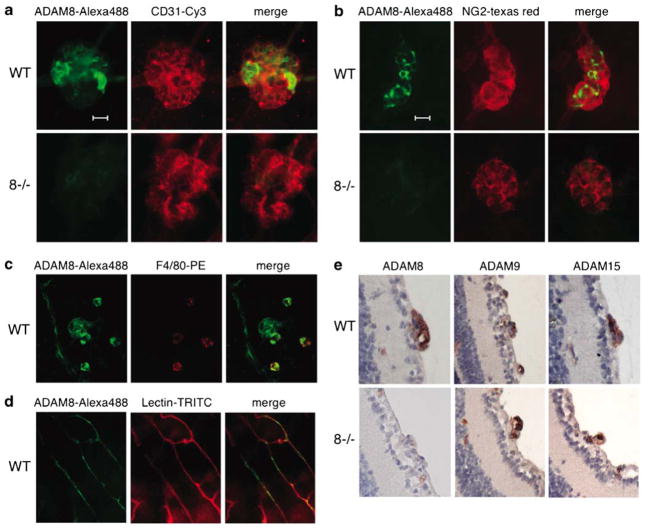
To determine whether ADAM8 is expressed in retinal neovascular tufts, whole-mount preparations of retinas of wild-type mice subjected to the OIR model were stained with antibodies against ADAM8 (Alexa 488 labeled secondary antibody, Fig. 2a, b), and with anti-CD31 (Cy3 labeled) to detect endothelial cells (Fig. 2a), or anti-NG2 to detect pericytes (Fig. 2b), or anti-F4/80 to detect macrophages (Fig. 2c). Immunofluorescence microscopy showed expression of ADAM8 in neovascular tufts; however, cells with the highest expression of ADAM8 did not stain strongly with anti-CD31, anti-NG2, or anti-F4/80 (Fig. 2a–c). In retinas from Adam8−/− mice subjected to the OIR model, only background ADAM8 staining was observed in neo-vascular tufts, even though these could be visualized with anti-CD31 or anti-NG2 (Fig. 2a, b, lower panels), corroborating that the ADAM8-staining in wild-type retinas was specific. In retinal capillaries of wild-type mice, predominantly intracellular expression of ADAM8 was seen in lectin-TRITC labeled endothelial cells (Fig. 2d). A histochemical analysis of the expression of ADAM8, 9, and 15 in retinal sections of mice subjected to the OIR model showed increased expression of all three ADAMs in neovascular tufts in wild-type mice, with sections of a tuft from an Adam8−/− mouse serving as control for the specificity of the anti-ADAM8 antibodies (Fig. 2e).
Fig. 2.
Expression of ADAM8 in retinas of mice subjected to oxygen-induced retinopathy. a–c Representative examples of a neovascular tuft from retinas of wild-type mice (top panels in a, b and all panels in c, or Adam8−/− mice (lower panels in a, b) stained with antibodies against ADAM8 (Alexa 488) in a, b, and c, or against the endothelial cell marker CD31 (Cy3 in a), the pericyte marker NG2 (Texas Red in b) or the macrophage marker F4/80 (PE in c). Merged images are shown in the right-hand panels in a–c, scale bar=20 μm. The expression of ADAM8 in neovascular tufts does not overlap with CD31, NG2, or F4/80, and no staining with the ADAM8 antibody was seen in neovascular tufts in Adam8−/− retinas. d Intracellular ADAM8 staining (Alexa 488) co-localizes with lectin-TRITC labeled capillaries in a wild-type retina. e Immunohistochemical analysis shows high expression of ADAMs 8, 9, and 15 in neovascular tufts in sections of retinas from wild-type mice subjected to the OIR model, but only ADAMs 9 and 15 are detected in neovascular tufts from retinas of Adam8−/− mice after OIR
Increased tumor growth of heterotopically injected B16F0 melanoma cells in Adam8−/− mice
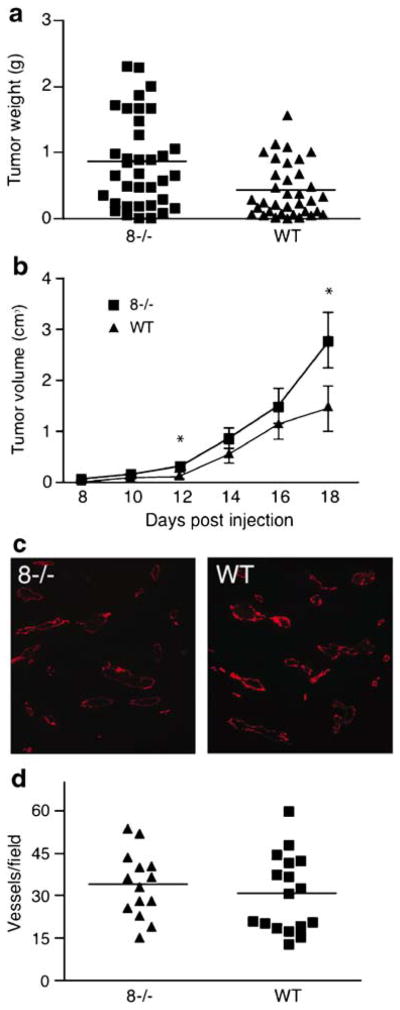
A commonly used approach to evaluate pathological neo-vascularization is to monitor the growth of heterotopically injected tumor cells in mice, which can also provide information on the contribution of host-derived factors and of other cell types besides endothelial cells to tumor growth. In our studies on how the lack of ADAM8 affects the growth of heterotopically injected tumor cells, the potential effects of the mouse genetic background was minimized by comparing tumor growth in litters of wild-type mice or Adam8−/− mice that had been bred from offspring of the same heterozygous grandparents (see Materials and methods). In five separate experiments, the average weight of tumors that developed after subcutaneous injection of 1×106 B16F0 cells in Adam8−/− mice was significantly increased compared with wild-type controls (Fig. 3a, increase in tumor weight in Adam8−/− versus wild-type controls=2-fold, wild-type n=35, Adam8−/− n=36, p<0.001). Caliper measurements showed an increase in tumor volume in Adam8−/− mice compared with controls as soon as the tumors could be detected around 12 days after injection (Fig. 3b). However, no difference in the distribution of CD31-stained blood vessels was observed in tumors of Adam8−/− mice compared with wild-type controls (Fig. 3c, representative section, Fig. 3d, quantification of vessels per field (Adam8−/− mice: 34.1±3.1 SEM, n=14; wild-type mice: 31.0±3.3 SEM, n=17; Student’s t test: p<0.25).
Fig. 3.
Increased growth of heterotopically injected B16F0 tumor cells in Adam8−/− mice. a Heterotopic injection of 106 B16F0 melanoma cells into the flanks of Adam8−/− mice yielded larger tumors compared with wild-type controls (data are from five separate experiments, mean tumor size in Adam8−/− mice 0.86 g±0.1 SEM, n=36; wild-type mice 0.43 g±0.06 SEM, n=35; Student’s t test p<0.001). b B16F0 heterotopic tumor growth measured with a caliper was accelerated in Adam8−/− mice compared with wild-type controls (at day 17 Adam8−/− mice volume 2.8 cm3±0.5 SEM, n=10; in wild-type mice 1.4 cm3± 0.4 SEM, n=10; Student’s t test p<0.044). c, d Staining of tumor sections with anti-CD31 did not reveal differences in the spacing or numbers of vessels per field between tumors from Adam8−/− mice and wild-type controls [representative example in c, quantitation of vessels per field by Prism4.0a software is shown in d (Adam8−/− mice vessels per field 34.1±3.1 SEM, n=14; in wild-type mice 31.0±3.3 SEM, n=17; Student’s t test p<0.25)]
ADAM8 can process receptors with roles in angiogenesis in cell-based assays
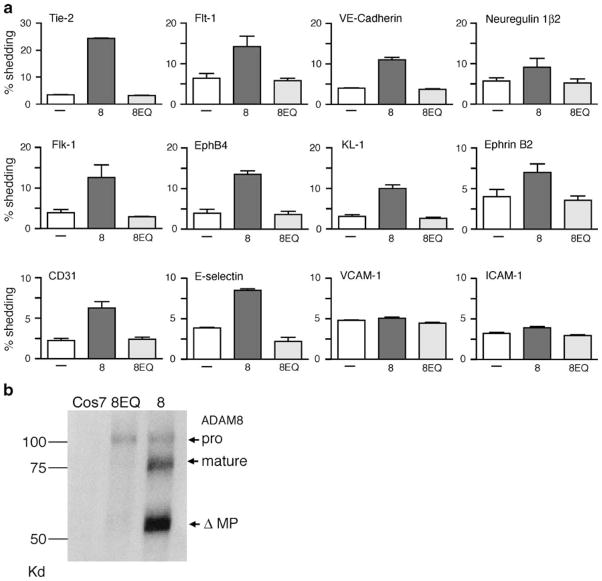
Since ADAM8 can function as a catalytically active membrane-anchored metalloproteinase [34, 35], we tested whether it can process membrane-bound proteins involved in angiogenesis. We found that overexpression of ADAM8 increased the shedding of alkaline phosphatase-tagged Tie-2, VE-cadherin, E-selectin, EphB4, EphrinB2, CD31, Flk-1, Flt-1, NRG-1β2, and KL-1 compared with cells overexpressing the inactive ADAM8 E>Q mutant with these substrates (Fig. 4a). Overexpression of ADAM8 did not increase the shedding of VCAM-1, ICAM-1 (Fig. 4a), NRG-1β1, or TRANCE (data not shown) when compared with ADAM8 E >Q under the conditions used here. A Western blot analysis confirmed the overexpression of ADAM8 and ADAM8E>Q in Cos-7 cells (Fig. 4b). These results demonstrate that ADAM8 is capable of processing several proteins with established functions in angiogenesis and neovascularization in “gain of function” overexpression experiments.
Fig. 4.
Ectodomain shedding of membrane receptors with roles in angiogenesis by ADAM8 in “gain of function” overexpression experiments. To identify potential substrates for ADAM8, several alkaline–phosphatase-tagged membrane proteins were overexpressed in Cos7 cells together with empty vector (−), wild-type mouse ADAM8 (8), or the catalytically inactive ADAM8E>Q mutant (8EQ; see Materials and methods for details). a Each graph shows the AP activity released into the supernatant as a percentage of the total AP activity in the cell lysate and supernatant and is representative of at least three separate experiments with two wells per experiment. Overexpressed ADAM8 can increase the ectodomain shedding of Tie-2, Flt-1, VE-cadherin, NRG-1β2, Flk-1, EphB4, KL-1, ephrinB2, CD31, and E-selectin, but does not affect the processing of VCAM-1 and ICAM-1, or NRG-1β1 and TRANCE (not shown). b Western blot analysis confirmed the overexpression of ADAM8 and ADAM8E>Q in Cos-7 cells
Discussion
In this study, we investigated the role of ADAM8 in pathological retinal neovascularization and the growth of heterotopically injected tumor cells using wild-type and Adam8−/− mice and evaluated the catalytic activity of ADAM8 in the ectodomain shedding of membrane receptors involved in angiogenesis. After exposure to the OIR model, we found that the hypoxia-induced neovascular response was altered in Adam8−/− mice compared with wild-type controls. The smaller central avascular area in Adam8−/− mice at P17 after the OIR model compared with controls was most likely caused by an enhanced re-vascularization of the central avascular area, since both wild-type and Adam8−/− mice showed a comparable avascular area after oxygen treatment on day P13. In a time course analysis, an increased vascular re-growth became apparent in Adam8−/− mice compared with wild-type controls starting at P15, suggesting that ADAM8 is a negative regulator of neovascularization. However, the significant decrease in neovascular tufts and in endothelial cells that had traversed the internal limiting membrane in Adam8−/− mice after OIR compared with controls appeared paradoxical, as one would expect an increase in both parameters in light of the increased re-vascularization of the central avascular area in Adam8−/− mice. It is tempting to speculate that the decrease in pathological neovascular tufts in Adam8−/− retinas resulted from an increased physiological neo- or re-vascularization of the central avascular area, which improved perfusion of the central retinal tissues and lead to a concomitant decrease in hypoxia and VEGF production in the OIR model.
A decrease in neovascular tufts has also been described in Adam9−/− or Adam15−/− mice [28, 29], yet the cause for this decrease is apparently very different than in Adam8−/− mice. In the Adam9−/− and Adam15−/− mice, the re-vascularization of the central avascular area following the OIR model is decreased, so formation of neovascular tufts is most likely prevented because there is an overall defect in the formation of new vessels that would be able to generate neovascular tufts. An increase in neovascularization in the absence of ADAM8 could also explain the increased growth of heterotopically injected tumor cells in Adam8−/− mice compared with controls. The comparable vessel density in tumors from Adam8−/− mice and controls suggests that the increased tumor growth in Adam8−/− mice most likely depended on vessels that grow faster, but nevertheless have a normal spacing. This notion is also supported by the observation that the re-vascularization of the central avascular area of the retina following the OIR model was more rapid in Adam8−/− mice compared with controls, even though there was no difference in the density in the newly developed retinal vessels (data not shown). Alternatively, ADAM8 might be involved in inhibiting tumor growth via a mechanism that is not related to its role in neovascularization, for example by processing or activating a tumor suppressive molecule in endothelial cells or other cell types, or through a tumor suppressive role in cell–cell interaction.
The high expression of ADAM8 in neovascular tufts of mice subjected to the OIR model further supports the notion that ADAM8 has a critical role in pathological retinal neovascularization. Interestingly, in cells expressing the highest levels of ADAM8 in neovascular tufts, there was no co-localization with the endothelial cell marker CD31 or the pericyte marker NG2 or the macrophage marker F4/80. However, since ADAM8 is expressed in normal retinal capillaries and can cleave CD31 when overexpressed in Cos-7 cells, it is possible that the high expression of ADAM8 in some cells in neovascular tufts leads to removal of the endothelial cell marker CD31 in those cells. In addition to CD31, overexpression of ADAM8 in cell-based assays also enhanced the shedding of Tie-2, VE-cadherin, E-selectin, EphB4, EphrinB2, Flk-1, Flt-1, NRG-1β2, and KL-1 compared with the inactive ADAM8 E>Q control. The consequences of enhanced shedding for these individual substrates remains to be determined, but to provide a hypothetical example, the processing of Flk-1 could reduce its concentration on the surface of endothelial cells, thereby diminishing Flk-1 signal transduction. In the Adam8−/− mice, decreased processing could preserve or even enhance the levels of Flk-1 and other tyrosine kinase receptors relative to wild-type mice, thereby perhaps increasing the likelihood of a signaling event and potentially also re-vascularization. However, it should be emphasized that overexpressed ADAM8 presumably has many more substrates on endothelial cells and other cell types, and so, the defects seen in Adam8−/− mice are most likely the consequence of the sum of effects caused by lack of processing of several different substrates. Additional studies will be necessary to assess how processing of the individual substrates of ADAM8 affects their function in angiogenesis and how this can be correlated to the changes in neovascularization observed in Adam8−/− mice. When we evaluated proliferation of purified endothelial cells and performed aortic ring sprouting assays, we found no significant difference between cells isolated from Adam8−/− mice compared with wild-type controls (not shown), presumably because the dysregulation of ADAM8 expression during neovascularization in vivo is not re-capitulated in these ex-vivo assays. Finally, it should be noted that other non-catalytic protein modules, such as the disintegrin-domain, cysteine-rich region, or cytoplasmic domain could also be important for the function of ADAM8 in neovascularization.
In summary, the analysis of the responses of Adam8−/− mice to the OIR model and the heterotopic tumor model suggest that ADAM8 has an important role in pathological neovascularization. Moreover, ADAM8 can process several membrane receptors with known functions in angiogenesis, suggesting that the processing of these molecules and potentially, other substrates could be involved in the regulation of neovascularization by ADAM8. The increased retinal re-vascularization following the OIR model in Adam8−/− mice suggests that ADAM8 normally functions to limit re-vascularization in this model. Moreover, the increased tumor growth suggests that ADAM8 in host cells restricts the growth of heterotopically injected tumor cells. It will now be interesting to test how the lack of ADAM8 affects other tumor models, such as those that are driven by transgenic expression of oncogenes. Collectively, these results suggest that ADAM8 should perhaps be considered as an anti-target [36] in the context of developing metalloproteinase inhibitors to block rapid tumor growth. On the other hand, the increased re-vascularization of retinas of Adam8−/− mice following the OIR model, which apparently protected from development of neovascular tufts, raises the possibility that ADAM8 could emerge as a useful target for treatment of ROP and potentially other diseases that would benefit from increased re-vascularization, such as myocardial infarction and diabetic vascular disease.
Acknowledgments
This work was supported by NIH/NEI grant EY015758 to CPB. We thank Dr. Andrew Docherty from UCB NewMedicines for providing Adam8−/− mice and Mrs. Elin Mogollon, Mr. Arash Shirazi, and Mr. Joshua Namm as well as the staff of the Center for Laboratory Animal Services of the Hospital for Special Surgery for excellent technical assistance.
Footnotes
Conflict of interest The authors reported no conflict of interest.
Contributor Information
Victor H. Guaiquil, Arthritis and Tissue Degeneration Program, Hospital for Special Surgery, Caspary Research Building, Room 426, 535 East 70th street, New York, NY 10021, USA
Steven Swendeman, Arthritis and Tissue Degeneration Program, Hospital for Special Surgery, Caspary Research Building, Room 426, 535 East 70th street, New York, NY 10021, USA.
Wenhui Zhou, Arthritis and Tissue Degeneration Program, Hospital for Special Surgery, Caspary Research Building, Room 426, 535 East 70th street, New York, NY 10021, USA. Gateways to the Laboratory Program, Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional, MD-PhD Program, New York, NY, USA.
Patricio Guaiquil, Arthritis and Tissue Degeneration Program, Hospital for Special Surgery, Caspary Research Building, Room 426, 535 East 70th street, New York, NY 10021, USA.
Gisela Weskamp, Arthritis and Tissue Degeneration Program, Hospital for Special Surgery, Caspary Research Building, Room 426, 535 East 70th street, New York, NY 10021, USA.
Jörg W. Bartsch, Pharmaceutical Science Research Division, King’s College London, London SE1 9NH, UK
Carl P. Blobel, Email: blobelc@hss.edu, Arthritis and Tissue Degeneration Program, Hospital for Special Surgery, Caspary Research Building, Room 426, 535 East 70th street, New York, NY 10021, USA. Departments of Medicine and of Physiology, Biophysics and Systems Biology, Weill Medical College of Cornell University, New York, NY 10021, USA
References
- 1.Friedlander M, Dorrell MI, Ritter MR, Marchetti V, Moreno SK, El-Kalay M, Bird AC, Banin E, Aguilar E. Progenitor cells and retinal angiogenesis. Angiogenesis. 2007;10:89–101. doi: 10.1007/s10456-007-9070-4. [DOI] [PubMed] [Google Scholar]
- 2.Bradley J, Ju M, Robinson GS. Combination therapy for the treatment of ocular neovascularization. Angiogenesis. 2007;10:141–148. doi: 10.1007/s10456-007-9069-x. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 4.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling—in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 5.Khong TL, Larsen H, Raatz Y, Paleolog E. Angiogenesis as a therapeutic target in arthritis: learning the lessons of the colorectal cancer experience. Angiogenesis. 2007;10:243–258. doi: 10.1007/s10456-007-9081-1. [DOI] [PubMed] [Google Scholar]
- 6.Sherris D. Ocular drug development—future directions. Angiogenesis. 2007;10:71–76. doi: 10.1007/s10456-007-9068-y. [DOI] [PubMed] [Google Scholar]
- 7.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 8.Holderfield MT, Hughes CC. Crosstalk between vascular endothelial growth factor, notch, and transforming growth factor-beta in vascular morphogenesis. Circ Res. 2008;102:637–652. doi: 10.1161/CIRCRESAHA.107.167171. [DOI] [PubMed] [Google Scholar]
- 9.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 10.Blobel CP. ADAMs: key players in EGFR-signaling, development and disease. Nat Rev Mol Cell Biol. 2005;6:32–43. doi: 10.1038/nrm1548. [DOI] [PubMed] [Google Scholar]
- 11.Murphy G. The ADAMs: signalling scissors in the tumour microenvironment. Nat Rev Cancer. 2008;12:929–941. doi: 10.1038/nrc2459. [DOI] [PubMed] [Google Scholar]
- 12.Yoshida S, Setoguchi M, Higuchi Y, Akizuki S, Yamamoto S. Molecular cloning of cDNA encoding MS2 antigen, a novel cell surface antigen strongly expressed in murine monocytic lineage. Int Immunol. 1990;2:586–591. doi: 10.1093/intimm/2.6.585. [DOI] [PubMed] [Google Scholar]
- 13.Yoshiyama K, Higuchi Y, Kataoka M, Matsuura K, Yamamoto S. CD156 (human ADAM8): expression, primary amino acid sequence, and gene location. Genomics. 1997;41:56–62. doi: 10.1006/geno.1997.4607. [DOI] [PubMed] [Google Scholar]
- 14.Choi SJ, Han JH, Roodman GD. ADAM8: a novel osteoclast stimulating factor. J Bone Miner Res. 2001;16:814–822. doi: 10.1359/jbmr.2001.16.5.814. [DOI] [PubMed] [Google Scholar]
- 15.Mandelin J, Li TF, Hukkanen MV, Liljestrom M, Chen ZK, Santavirta S, Kitti U, Konttinen YT. Increased expression of a novel osteoclast-stimulating factor, ADAM8, in interface tissue around loosened hip prostheses. J Rheumatol. 2003;30:2033–2038. [PubMed] [Google Scholar]
- 16.Hodgkinson CP, Ye S. Microarray analysis of peroxisome proliferator-activated receptor-gamma induced changes in gene expression in macrophages. Biochem Biophys Res Commun. 2003;308:505–510. doi: 10.1016/s0006-291x(03)01416-5. [DOI] [PubMed] [Google Scholar]
- 17.Kelly K, Hutchinson G, Klewe-Nebenius D, Smith A, Bartsch JW, Horiuchi K, Manova K, Docherty AJ, Blobel CP. Metalloprotese–disintegrin ADAM8: expression analysis and targeted deletion in mice. Dev Dyn. 2005;232:221–231. doi: 10.1002/dvdy.20221. [DOI] [PubMed] [Google Scholar]
- 18.Amour A, Knight C, English W, Webster A, Slocombe P, Knauper V, Docherty A, Becherer J, Blobel C, Murphy G. The enzymatic activity of ADAM8 and ADAM9 is not regulated by TIMPs. FEBS Lett. 2002;524:154–158. doi: 10.1016/s0014-5793(02)03047-8. [DOI] [PubMed] [Google Scholar]
- 19.Schlomann U, Wildeboer D, Webster A, Antropova O, Zeuschner D, Knight CG, Docherty AJ, Lambert M, Skelton L, Jockusch H, Bartsch JW. The metalloprotease disintegrin ADAM8. Processing by autocatalysis is required for proteolytic activity and cell adhesion. J Biol Chem. 2002;277:48210–48219. doi: 10.1074/jbc.M203355200. [DOI] [PubMed] [Google Scholar]
- 20.Fourie AM, Coles F, Moreno V, Karlsson L. Catalytic activity of ADAM8, ADAM15, and MDC-L (ADAM28) on synthetic peptide substrates and in ectodomain cleavage of CD23. J Biol Chem. 2003;278:30469–30477. doi: 10.1074/jbc.M213157200. [DOI] [PubMed] [Google Scholar]
- 21.Schlomann U, Rathke-Hartlieb S, Yamamoto S, Jockusch H, Bartsch JW. Tumor necrosis factor alpha induces a metalloprotease–disintegrin, ADAM8 (CD 156): implications for neuronglia interactions during neurodegeneration. J Neurosci. 2000;20:7964–7971. doi: 10.1523/JNEUROSCI.20-21-07964.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horiuchi K, Le Gall S, Schulte M, Yamaguchi T, Reiss K, Murphy G, Toyama Y, Hartmann D, Saftig P, Blobel C. Substrate selectivity of EGF-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol Biol Cell. 2007;18:176–188. doi: 10.1091/mbc.E06-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weskamp G, Ford J, Sturgill J, Martin S, Docherty A, Swendeman S, Broadway N, Hartmann D, Saftig P, Umland S, Sehara-Fujisawa A, Black R, Ludwig A, Becherer D, Conrad D, Blobel C. ADAM10 is a principal ‘sheddase’ of the low-affinity immunoglobulin E receptor CD23. Nat Immunol. 2006;7:1298–1393. doi: 10.1038/ni1399. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Gaviro M, Dominguez-Luis M, Canchado J, Calafat J, Janssen H, Lara-Pezzi E, Fourie A, Tugores A, Valenzuela-Fernandez A, Mollinedo F, Sanchez-Madrid F, Diaz-Gonzalez F. Expression and regulation of the metalloproteinase ADAM-8 during human neutrophil pathophysiological activation and its catalytic activity on L-selectin shedding. J Immunol. 2007;178:8053–8063. doi: 10.4049/jimmunol.178.12.8053. [DOI] [PubMed] [Google Scholar]
- 25.Ainola M, Li TF, Mandelin J, Hukkanen M, Choi SJ, Salo J, Konttinen YT. Involvement of a disintegrin and a metalloproteinase 8 (ADAM8) in osteoclastogenesis and pathological bone destruction. Ann Rheum Dis. 2009;68:427–434. doi: 10.1136/ard.2008.088260. [DOI] [PubMed] [Google Scholar]
- 26.Wildeboer D, Naus S, Amy Sang QX, Bartsch JW, Pagenstecher A. Metalloproteinase disintegrins ADAM8 and ADAM19 are highly regulated in human primary brain tumors and their expression levels and activities are associated with invasiveness. J Neuropathol Exp Neurol. 2006;65:516–527. doi: 10.1097/01.jnen.0000229240.51490.d3. [DOI] [PubMed] [Google Scholar]
- 27.King NE, Zimmermann N, Pope SM, Fulkerson PC, Nikolaidis NM, Mishra A, Witte DP, Rothenberg ME. Expression and regulation of a disintegrin and metalloproteinase (ADAM) 8 in experimental asthma. Am J Respir Cell Mol Biol. 2004;31:257–265. doi: 10.1165/rcmb.2004-0026OC. [DOI] [PubMed] [Google Scholar]
- 28.Horiuchi K, Weskamp G, Lum L, Hammes HP, Cai H, Brodie TA, Ludwig T, Chiusaroli R, Baron R, Preissner KT, Manova K, Blobel CP. Potential role for ADAM15 in pathological neovascularization in mice. Mol Cell Biol. 2003;23:5614–5624. doi: 10.1128/MCB.23.16.5614-5624.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guaiquil V, Swendeman S, Yoshida T, Chavala S, Campochiaro P, Blobel CP. ADAM9 is involved in pathological retinal neovascularization. Mol Cell Biol. 2009;29:2694–2703. doi: 10.1128/MCB.01460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammes HP, Brownlee M, Jonczyk A, Sutter A, Preissner KT. Subcutaneous injection of a cyclic peptide antagonist of vitronectin receptor-type integrins inhibits retinal neovascularization. Nat Med. 1996;2:529–533. doi: 10.1038/nm0596-529. [DOI] [PubMed] [Google Scholar]
- 31.Swendeman S, Mendelson K, Weskamp G, Horiuchi K, Deutsch U, Scherle P, Hooper A, Rafii S, Blobel CP. VEGF-A stimulates ADAM17-dependent shedding of VEGFR2 and crosstalk between VEGFR2 and ERK signaling. Circ Res. 2008;103:916–918. doi: 10.1161/CIRCRESAHA.108.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawaguchi N, Horiuchi K, Becherer JD, Toyama Y, Besmer P, Blobel CP. Different ADAMs have distinct influences on Kit ligand processing: phorbol-ester-stimulated ectodomain shedding of Kitl1 by ADAM17 is reduced by ADAM19. J Cell Sci. 2007;120:943–952. doi: 10.1242/jcs.03403. [DOI] [PubMed] [Google Scholar]
- 33.Zhou HM, Weskamp G, Chesneau V, Sahin U, Vortkamp A, Horiuchi K, Chiusaroli R, Hahn R, Wilkes D, Fisher P, Baron R, Manova K, Basson CT, Hempstead BL, Blobel CP. Essential role for ADAM19 in cardiovascular morphogenesis. Mol Cell Biol. 2004;24:96–104. doi: 10.1128/MCB.24.1.96-104.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naus S, Reipschlager S, Wildeboer D, Lichtenthaler SF, Mitterreiter S, Guan Z, Moss ML, Bartsch JW. Identification of candidate substrates for ectodomain shedding by the metalloprotease-disintegrin ADAM8. Biol Chem. 2006;387:337–346. doi: 10.1515/BC.2006.045. [DOI] [PubMed] [Google Scholar]
- 35.Naus S, Richter M, Wildeboer D, Moss M, Schachner M, Bartsch JW. Ectodomain shedding of the neural recognition molecule CHL1 by the metalloprotease-disintegrin ADAM8 promotes neurite outgrowth and suppresses neuronal cell death. J Biol Chem. 2004;279:16083–16090. doi: 10.1074/jbc.M400560200. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Otin C, Matrisian LM. Emerging roles of proteases in tumour suppression. Nat Rev Cancer. 2007;7:800–808. doi: 10.1038/nrc2228. [DOI] [PubMed] [Google Scholar]